Pretreatment of Sweet Sorghum Bagasse for Ethanol Production Using Na2CO3 Obtained by NaOH Absorption of CO2 Generated in Sweet Sorghum Juice Ethanol Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
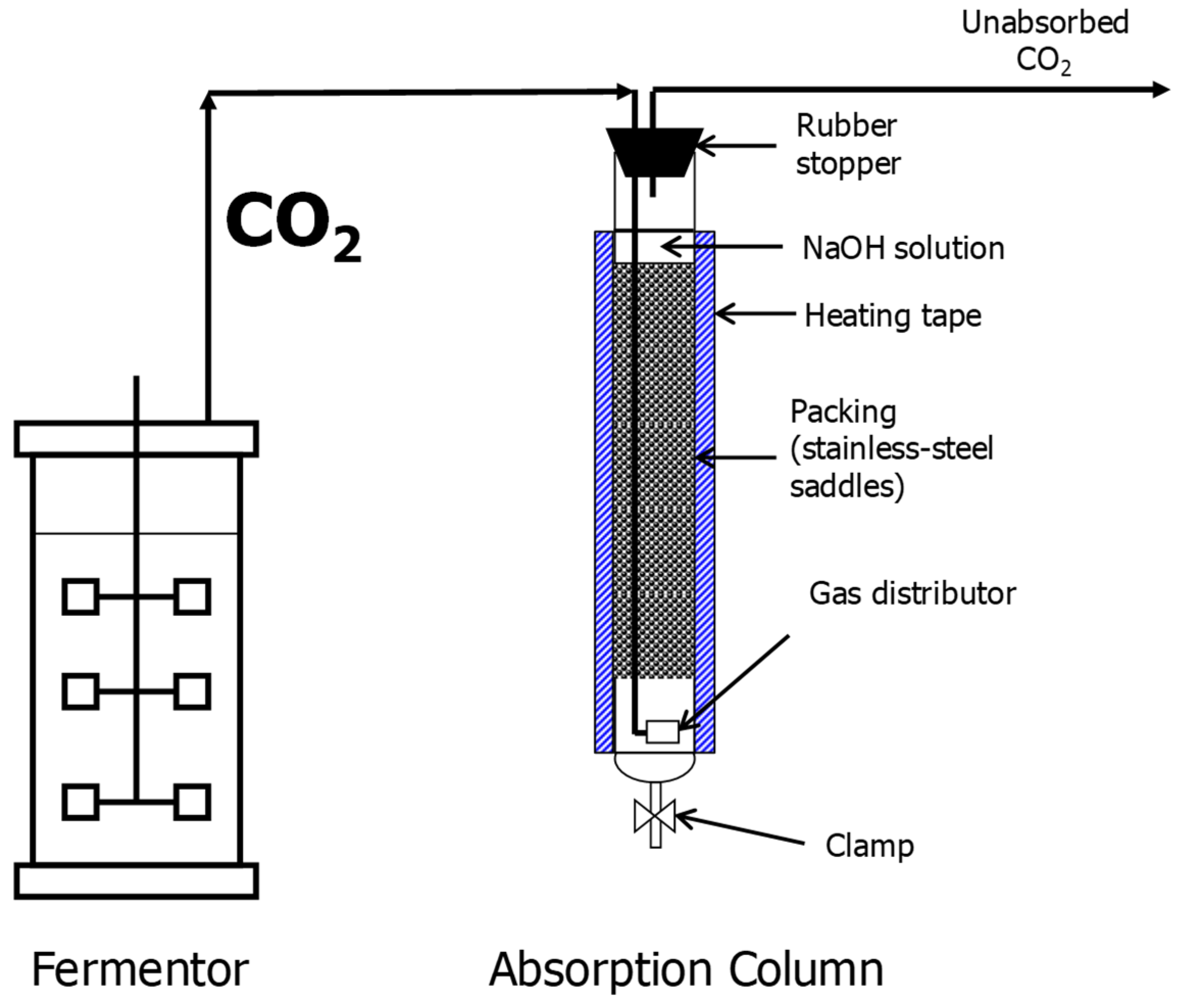
2.2.1. SSJ Fermentation and CO2 Capture
2.2.2. Pretreatment of SSB
2.2.3. Fermentation of Pretreated SSB in SSJ
2.2.4. Fermentation of Untreated SSB in SSJ
2.2.5. Analytical Methods
3. Results
3.1. SSJ Fermentation and CO2 Capture
3.2. Pretreatment of SSB
3.3. Fermentation of Pretreated SSB in SSJ
4. Discussion
5. Conclusions
Declaring
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Drapcho, C.M.; Nghiem, N.P.; Walker, T.H. Biofuels Engineering Process Technology, 1st ed.; McGraw-Hill: New York, NY, USA, 2008; pp. 133–143. [Google Scholar]
- Kazi, F.K.; Fortman, J.; Anex, R. Techno-Economic Analysis of Biochemical Scenarios for Production of Cellulosic Ethanol; Technical Report NREL/TP-6A2-46588; National Renewable Energy Laboratory: Lakewood, CO, USA, 2010.
- Macrelli, S.; Mogensen, A.; Zacchi, G. Techno-economic evaluation of 2nd generation bioethanol production from sugar cane bagasse and leaves integrated with the sugar-based ethanol process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Frankó, B.; Galbe, M.; Wallberg, O. Bioethanol production from forestry residues: A comparative techno-economic analysis. Appl. Energy 2016, 184, 727–736. [Google Scholar] [CrossRef]
- Jin, Y.; Jameel, H.; Chang, H.-M. Green liquor pretreatment of mixed hardwood for ethanol production in a repurposed kraft pulp mill. J. Wood Chem. Technol. 2013, 30, 86–104. [Google Scholar] [CrossRef]
- Wu, S.; Chang, H.-M.; Jameel, H. Novel green liquor pretreatment of loblolly pine chips to facilitate enzymatic hydrolysis into fermentable sugars for ethanol production. J. Wood Chem. Technol. 2013, 30, 205–218. [Google Scholar] [CrossRef]
- Zhou, Z.; Xue, W.; Lei, F. Kraft GL-ethanol pretreatment on sugarcane bagasse for effective enzymatic hydrolysis. Ind. Crops Prod. 2016, 90, 100–109. [Google Scholar] [CrossRef]
- Gu, F.; Yang, L.; Jin, Y. Green liquor pretreatment for improving enzymatic hydrolysis of corn stover. Bioresource Technol. 2012, 124, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.T.; Nghiem, N.P.; Kim, T.H. Near theoretical saccharification of sweet sorghum bagasse using simulated green liquor pretreatment and enzymatic hydrolysis. Energy 2018, 157, 894–903. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Senske, G.E. Capture of carbon dioxide from ethanol fermentation by liquid absorption for use in biological production of succinic acid. Appl. Biochem. Biotechnol. 2015, 175, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.L.; Johnson, N.; McCoy, S.T. Near-term deployment of carbon capture and sequestration from biorefineries in the United States. Proc. Natl. Acad. Sci. USA 2018, 115, 4875–4880. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Montanti, J.; Johnston, D.B. Sorghum as a renewable feedstock for production of fuels and industrial chemicals. AIMS Bioeng. 2016, 3, 75–91. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Lakewood, CO, USA, 2012.
- Sutton, F.; Sutton, W.L.; Johnson, A.E. Systematic Handbook of Volumetric Analysis, 10th ed.; P. Blakiston’s Son and Co.: Philadelphia, PA, USA, 1911; p. 61. [Google Scholar]
- Cao, W.; Sun, C.; Liu, R. Comparison of the effects of five pretreatment methods on enhancing the enzymatic digestibility and ethanol production from sweet sorghum bagasse. Bioresource Technol. 2012, 111, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Ellis, C.W.; Montanti, J. The effects of ethanol on hydrolysis of cellulose and pretreated barley straw by some commercial cellulolytic enzyme products. AIMS Bioeng. 2016, 3, 441–453. [Google Scholar] [CrossRef]

| Results of Ethanol Fermentation | |||
| Final Ethanol Concentration (g/L) | Ethanol Yield (% theoretical) | Total Ethanol Production (g) | Total CO2 Production (g) |
| 64.7 ± 1.4 | 85.2 | 334.2 | 319.5 (7.3 mol) |
| Characteristics of Na2CO3 Solution | |||
| pH | Na2CO3 concentration (M) | Absorption efficiency (%) | CO2 removal efficiency (%) |
| 11.2 | 2.30 ± 0.01 | 92.0 | 63.0 |
| Glucan (%) | Xylan (%) | Arabinan (%) | AI lignin (%) | AS lignin (%) | Total Lignin (%) | |
|---|---|---|---|---|---|---|
| Raw SSB | 38.3 ± 3.9 | 20.6 ± 1.9 | 2.3 ± 0.3 | 17.7 ± 1.7 | 1.2 ± 0.2 | 18.9 ± 1.8 |
| Pretreated SSB in small reactor | 47.0 ± 0.9 | 23.1 ± 0.4 | 2.1 ± 0.1 | 13.5 ± 0.3 | 1.0 ± 0.0 | 14.4 ± 0.3 |
| Pretreated SSB in large reactor | 44.6 ± 1.5 | 24.4 ± 1.0 | 2.8 ± 0.1 | 14.7 ± 0.8 | 1.5 ± 0.0 | 16.2 ± 0.8 |
| Loss during pretreatment in small reactor (%) | 0 | 8.1 | 24.7 | 37.8 | 35.0 | 37.7 |
| Ethanol (g/L) | Xylose (g/L) | Ethanol Produced from SSB (g/g Pretreated SSB) | Xylose Yield from Xylan in SSB (% Theoretical) | Ethanol Yield from Glucan in SSB (% Theoretical) | |
|---|---|---|---|---|---|
| SSJ only (control) | 65.4 ± 2.1 | ||||
| SSJ + Pretreated SSB | 75.3 ± 1.3 | 7.5 ± 0.7 | 0.15 | 56.9 | 81.7 |
| Ethanol (g/L) | Xylose (g/L) | Ethanol Produced from SSB (g/g Pretreated SSB) | Xylose Yield from Xylan in SSB (% Theoretical) | Ethanol Yield from Glucan in SSB (% Theoretical) | |
|---|---|---|---|---|---|
| SSJ only (control) | 68.2 ± 0.1 | ||||
| SSJ + Pretreated SSB | 69.5 ± 0.6 | 1.3 ± 0.1 | 0 | 11.0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nghiem, N.P.; Toht, M.J. Pretreatment of Sweet Sorghum Bagasse for Ethanol Production Using Na2CO3 Obtained by NaOH Absorption of CO2 Generated in Sweet Sorghum Juice Ethanol Fermentation. Fermentation 2019, 5, 91. https://doi.org/10.3390/fermentation5040091
Nghiem NP, Toht MJ. Pretreatment of Sweet Sorghum Bagasse for Ethanol Production Using Na2CO3 Obtained by NaOH Absorption of CO2 Generated in Sweet Sorghum Juice Ethanol Fermentation. Fermentation. 2019; 5(4):91. https://doi.org/10.3390/fermentation5040091
Chicago/Turabian StyleNghiem, Nhuan P., and Matthew J. Toht. 2019. "Pretreatment of Sweet Sorghum Bagasse for Ethanol Production Using Na2CO3 Obtained by NaOH Absorption of CO2 Generated in Sweet Sorghum Juice Ethanol Fermentation" Fermentation 5, no. 4: 91. https://doi.org/10.3390/fermentation5040091
APA StyleNghiem, N. P., & Toht, M. J. (2019). Pretreatment of Sweet Sorghum Bagasse for Ethanol Production Using Na2CO3 Obtained by NaOH Absorption of CO2 Generated in Sweet Sorghum Juice Ethanol Fermentation. Fermentation, 5(4), 91. https://doi.org/10.3390/fermentation5040091





