1. Introduction
The global demand of energy is expected to rise sharply in the next decades as a consequence of the increase in worldwide population. The energy demand is projected to increase by 50% or more by 2030 [
1]. The depletion of conventional fossil fuel reserves together with the growing concerns for environmental protection have aroused considerable interest in promoting alternative and renewable sources of energy. The shift towards more sustainable energy sources is thus unavoidable, and in this context, biofuels are seen as promising candidates to replace fossil fuels in the short term [
2]. Biofuels containing energy from geologically recent carbon fixation offer environmental benefits as their employment reduces the atmospheric dioxide carbon concentration, the emission of hydrocarbons and particulate matter, and the discharge of sulfur compounds [
3].
Bioethanol is especially attractive as an alternative to fossil fuels, reason why its global production has significantly increased in the last years. Bioethanol is an environment-friendly oxygenated fuel containing over 34% of oxygen, with enhanced combustion efficiency (15% higher) in comparison to gasoline. The renewable sources for bioethanol generation largely include sugars, starch, lignocellulosic biomass, and algae [
4,
5]. While third-generation bioethanol obtained from algal biomass is still under development and has been demonstrated at laboratory scale, sugars, starch, and lignocellulosic biomass have already shown their potential as commercial feedstock for bioethanol. Nevertheless, their treatment requires different approaches, since raw materials such as cane need a single extraction process to obtain fermentable sugars, while starchy crops such as wheat and corn require a previous hydrolysis stage to transform starch into sugar [
6,
7,
8].
One of the agricultural crops with high carbohydrate content is
Ceratonia siliqua, also known as carob [
9,
10]. The carob tree is native of the Mediterranean region, and its cultivation has been promoted for the revitalization of coastal agriculture in dryland areas [
11]. The average worldwide production of carob pods for the period 2010–2013 was 165,990 tons, and the main producers were Spain (26.31%), Italy (16.62%), Portugal (13.77%), Morocco (12.27%), Greece (12.05%), and Turkey (8.53%) [
10].
Carob is a drought-resistance evergreen tree requiring low maintenance and producing several products like seeds and pods. The carob pod can be employed for animal feeding or to produce carob powder that in turn can be exploited for human consumption. However, the high content of tannin in carob pods greatly limits this application. Carob pods can be also used for bioethanol production through fermentation with
Saccharomyces cerevisiae, as has been shown in previous works. Sugars needs to be extracted from carob pods, and this can be achieved with water extraction before the subsequent fermentation process [
12].
On the other hand, the energy requirements in the process of bioethanol production offer a future challenge. Bioalcohol needs to be separated from the aqueous medium in which fermentation is performed [
13,
14]. To achieve the desired purity of bioethanol, distillation is usually employed, but this stage is highly energy-consuming. Liquid–liquid extraction of bioethanol has been proposed as an alternative method, but the solvent used has to display at the same time water immiscibility and high polarity for the extraction of alcohol molecules. Both features are rarely found in conventional organics. Unique compounds such as ionic liquids (ILs), however, have shown a promising capacity to separate polar substances such as ethanol from aqueous media [
15].
Room-temperature ionic liquids (RTILs) are organic salts usually formed by an organic cation and an inorganic anion that remain in the liquid state at room temperature. RTILs offer advantageous properties versus organic solvents, such as negligible vapor pressure, physical-chemical tunability, and non-flammability [
16,
17]. Because of these features, they are regarded as a real option to substitute organic solvents in liquid–liquid extraction processes both in bulk form and in membrane-based technologies [
18,
19].
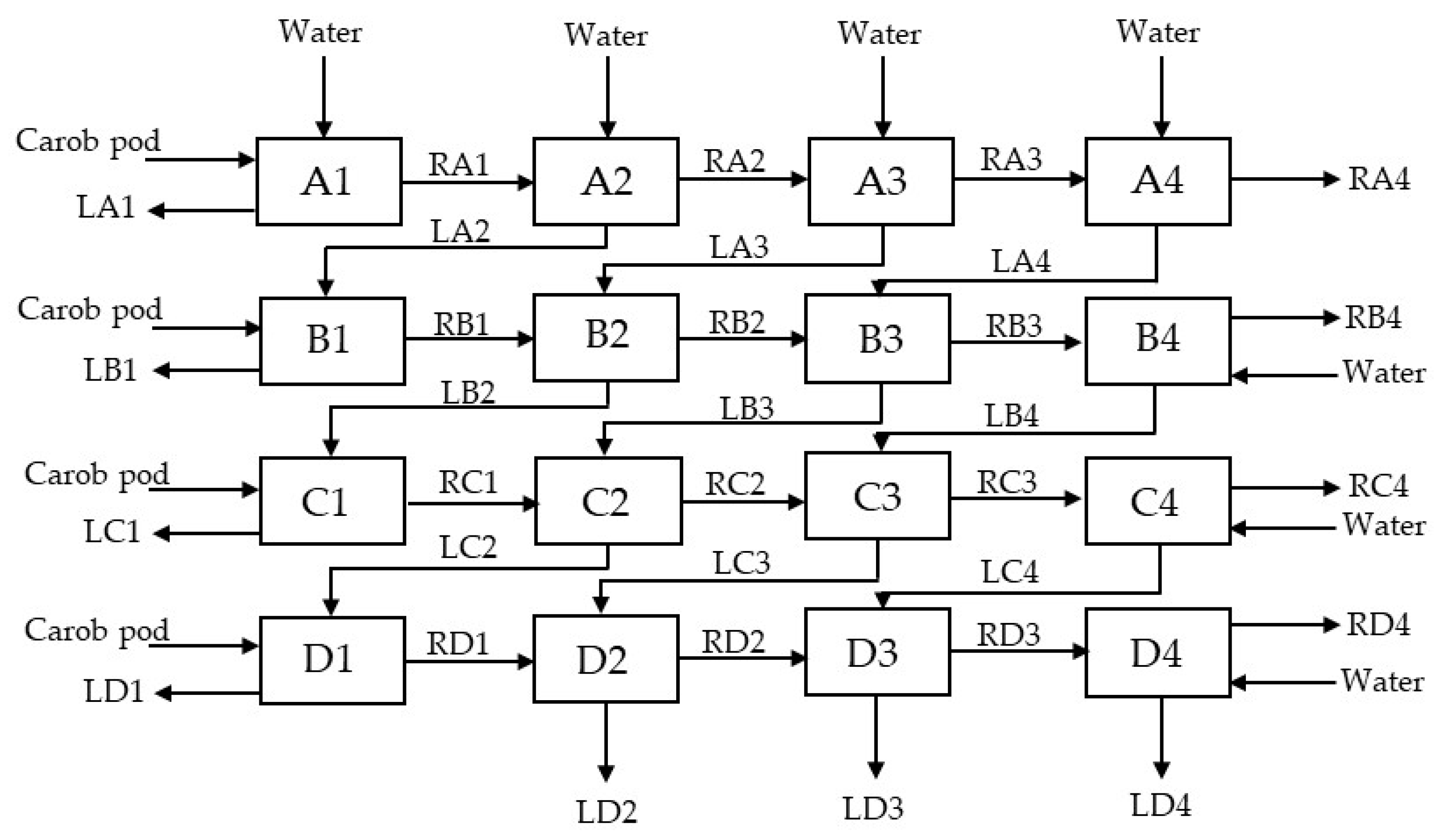
This work discusses the potential development of an integrated process for bioethanol production using ionic liquids and C. siliqua as bioethanol feedstock. Firstly, the sugar extraction process from carob pods is analyzed. Sugar extraction was performed at different liquid/solid ratios (L/S) for 30 min at 25 °C. A four-stage counter-current system was designed to improve the sugar extraction efficiency from carob pods. After the fermentation of carob pod extracts with S. cerevisiae, bioethanol recovery was approached through simulation tests. Finally, several ionic liquids are proposed and analyzed as potential solvents to recover bioethanol for the design of an integrated extraction–fermentation–separation process.
2. Materials and Methods
2.1. Characterization of the Carob Pod
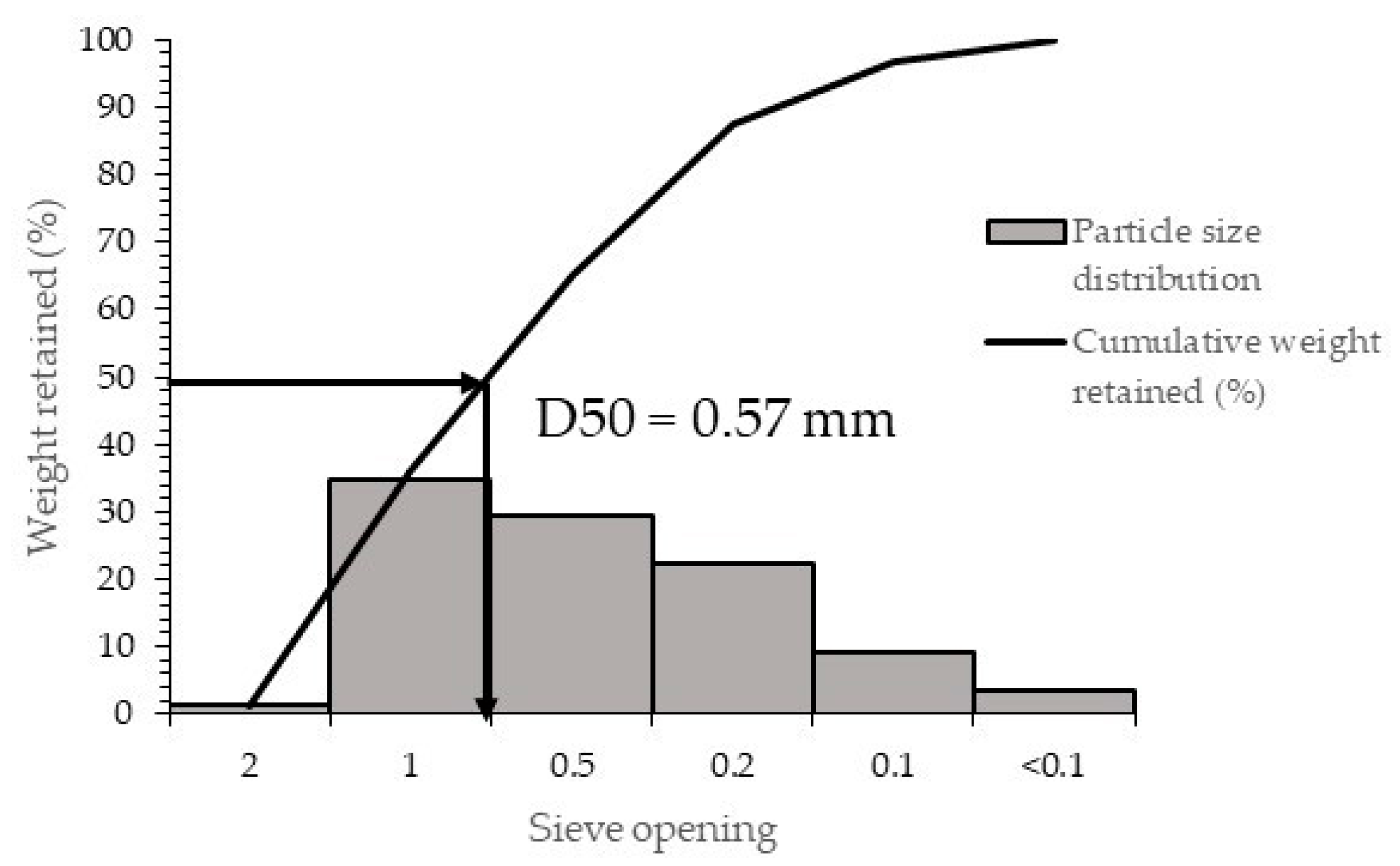
Carob pods purchased from a local supplier were physically and chemically characterized. The physical characterization consisted in the determination of their particle size distribution by sieve analysis. For that, 100 g of carob pod was sieved using for 10 min a set of sieves with 2.0, 1.0, 0.5, 0.2, and 0.1 mm mesh opening and a vibrating sieve machine. Sugar, ash, protein, and fibre analyses were carried out according to the methodology proposed by Mahtout et al. [
20].
2.2. Sugar Extraction
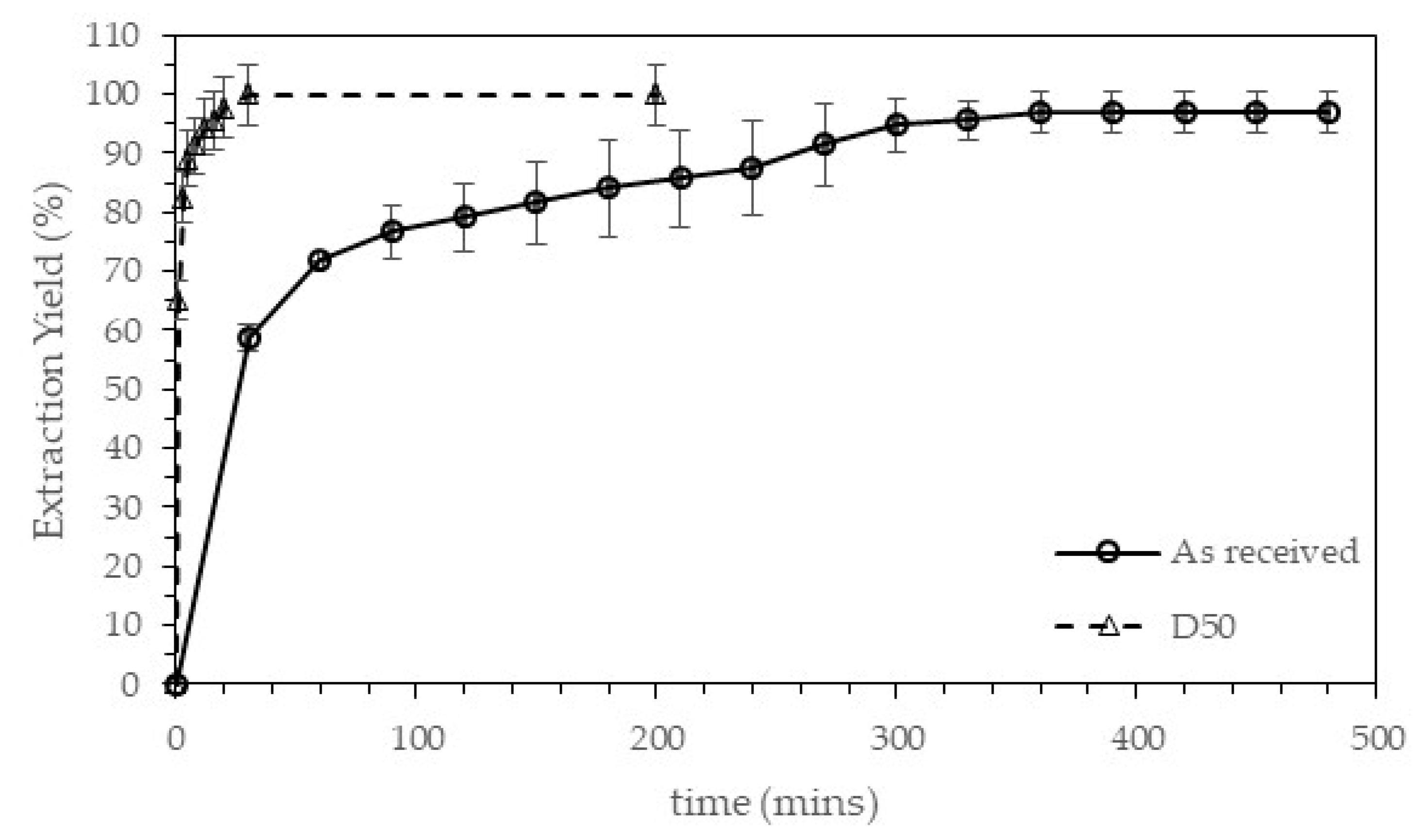
The sugar extraction was carried out using 250 g of carob pod and 750 mL of water at a fix stirring speed of 1000 r.p.m in a stirred tank reactor at room temperature. The as-received carob pod and the average size fraction of the sieved carob pod (D50) were tested until the extraction yield was constant, with the aim to study the influence of the particle size over the extraction time and to ascertain a suitable particle size for the as-received material. Samples were withdrawn at periodic time intervals to analyze the total sugars content.
2.3. Fermentation Test
The anaerobic fermentation was carried out in a 3 L fermenter with several sampling devices and temperature and stirring control. The aqueous extract from the sugar extraction tests with a concentration of 200 g/L was used as fermentation medium supplemented with ammonium phosphate (3.2 g/L), potassium sulphate (1 g/L), and magnesium sulphate (1.8 g/L). The pH was adjusted to 3.5–4, using diluted sulphuric acid. The resulting solution was sterilized by heating up to its boiling point and then cooled at 35 °C. This solution was fed into the fermenter at a fixed temperature of 35 °C and at a fix stirring speed of 125 r.p.m. Free cells of S. cerevisiae at concentration sof 10, 15, and 25 g/L were used for ethanol production. The evolution of the fermentation process was determined by density measurement of the hydro-alcoholic solutions obtained and by gas chromatography using an HP-INNOWAX column (30 m × 0.53 mm × 0.25 μm, Agilent). A medium consisting of 200 g/L of pure saccharose prepared in the same conditions as described above and 15 g/L of S. cerevisiae cells was used as a control test.
2.4. Ethanol Recovery Simulation
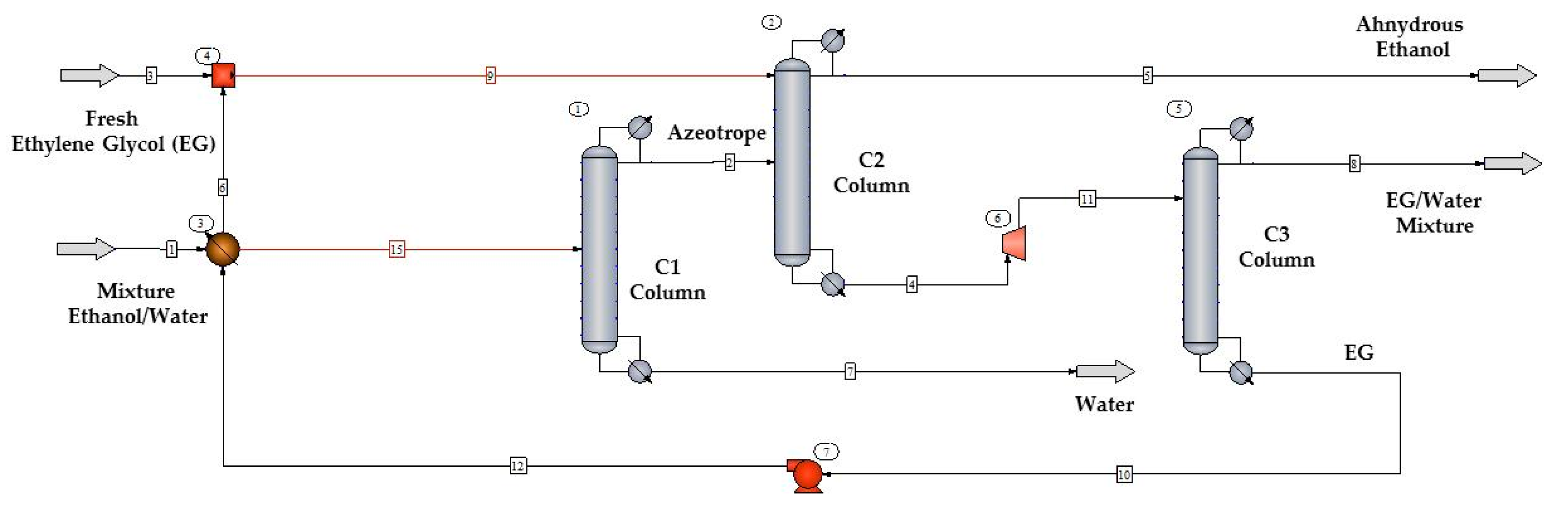
Ethanol recovery from the fermentation broth through extractive distillation with ethylene glycol (EG) was simulated using CHEMCAD software and the NRTL thermodynamic model, according to the flowsheet shown in
Figure 1. The ethanol/water mixture with 10% mol of ethanol was distilled in the column C1 to produce an ethanol/water mixture with azeotropic composition.
Ethanol was dehydrated in column C2 with the aid of EG and recovered as a head product. The bottom product of column C2 was expanded from 100 to 26.34 kPa and fed into column C3 for EG recovery as a bottom product. The decision variables used in the simulation after sensitivity analysis of the simulation flowsheet in
Figure 1 are shown in
Table 2.
2.5. Evaluation of Ionic Liquids as Potential Solvents for the Development of In Situ Alcoholic Fermentation Processes
In order to address the use of ILs as in situ extraction agents to recover ethanol from fermentation broths, it is required to study their water solubility, microorganism biocompatibility, and ethanol extraction power. The ionic liquids used in this study were: 1-octyl-3-methylimidazolium{Bis(trifluoromethyl)sulfonyl}imide[OMIM+][NTf2−], Methyltrioctylammonium{Bis(trifluoromethyl)sulfonyl}imide[MTOA+][NTf2−], 1-butyl-3-methylimidazolium{Bis(trifluoromethyl)sulfonyl}imide[BMIM+][NTf2−], 1-butyl-3-methylimidazolium hexafluorophosphate[BMIM+][PF6−], 1-octyl-3-methylimidazolium tetrafluoroborate[OMIM+][BF4−], tetradecyl(trihexyl)phosphonium dicyanamide [Hex3TDP+][dca−], tetradecyl(trihexyl)phosphonium bromide [Hex3TDP+][Br−], tetradecyl(trihexyl)phosphonium chloride [Hex3TDP+][Cl−], ethylpyridinium {bis(trifluoromethyl)sulfonyl}imide [EPy+][NTf2−]. These liquids were used as received without further purification.
2.5.1. Water Solubility Tests
Water solubility tests were carried out using the cloud-point method. For that, 2 µL of IL was added to 1 mL of water until complete dissolution after vigorous stirring. The additions stopped when saturation was not reached after adding more than 100 µL of IL.
2.5.2. Ionic Liquids Biocompatibility with S. cerevisiae
Ionic liquids biocompatibility was measured through growth inhibition in liquid medium and the agar diffusion test. For the growth inhibition tests, a liquid culture medium composed of D-glucose (20 g/L), peptone (20 g/L), yeast extract (10 g/L), and 3% (v/v) of ionic liquid was used. The ionic liquid content of the liquid medium was selected on the basis of previous research works, which report that a significant toxicity is observed for concentrations of ILs ranged between 2% and 5% (v/v) [
21,
22]. The liquid medium with 1 g/L of
S. cerevisiae was incubated at 30 °C with continuous shaking. Samples were withdrawn at regular time intervals to measure the OD
660 using a 1650 PC Shimadzu UV–Vis spectrophotometer and calculate the specific growth rate by selecting two time points in the exponential growth phase.
For the agar diffusion tests, a solid medium with the same composition mentioned above was placed in a Petri dish. Wells of 6 mm diameter were impregnated with 50 µL of each IL in sterile conditions and placed over dish. The radius of the inhibition zone around the wells was recorded.
2.5.3. Ethanol Extraction Tests
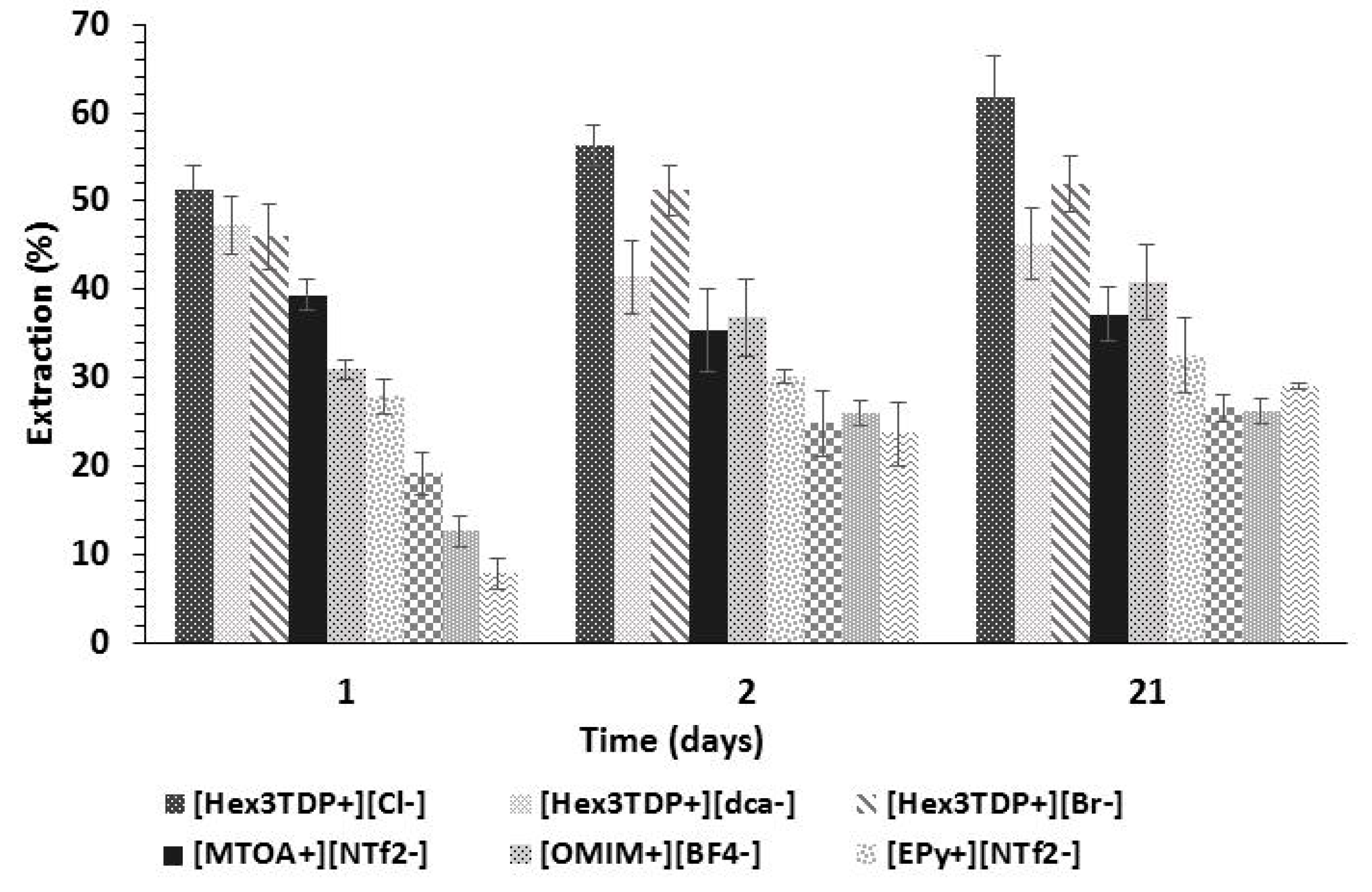
For the extraction tests, a 10% v/v ethanol aqueous solution was put in contact with the same volume of water-insoluble IL at 30 °C. The mixture was shaken for 2 min to facilitate ethanol transfer into the IL phase and left at a constant temperature to complete the phase separation. Samples from the aqueous phase were taken at 24, 48 h, and 21 days and analyzed through gas chromatography as described in
Section 2.3. The ethanol extraction percentage was calculated according to Equation (1).
where C
IL and C
W are the ethanol equilibrium concentrations in the ionic liquid and the aqueous phase, respectively. Measurements were carried out in triplicate, and the ethanol content in the aqueous phase was determined by gas chromatography following the same procedure described above. The ethanol concentration in the ionic liquid was determined by taking the difference between the initial and the final ethanol concentrations in the aqueous phase.
4. Conclusions
The present study revealed the feasibility of sugar extraction from carob pod using a four-stage counter-current system and an L/S ratio of 3.75, with sugar losses below 2%. The alcoholic fermentation of the aqueous extracts with a sugar concentration of 168 g/L using S. cerevisiae showed that a maximum ethanol concentration of 95 g/L was achieved after 20 h, independently of the initial concentration of the microorganism.
According to the biocompatibility and ethanol extraction tests, the ILs [Hex3TDP+][Cl−] and [MTOA+][NTf2−] are potential candidates for the development of in situ extraction–fermentation processes.
The extractive ethanol recovery simulation using ethylene glycol showed that 62% of the energy consumed in the process can be attributed to the production of the azeotropic ethanol–water mixture. Current research works have used ILs as entrainers to replace ethylene glycol and other solvents commonly employed in ethanol extractive distillation to produce anhydrous ethanol [
28,
29]. However, there are not many data and experimental studies about ethanol–ionic liquids binary mixtures. These data are of crucial importance to develop single distillation processes for the production of anhydrous ethanol.

 ) and radius of inhibition in solid media (
) and radius of inhibition in solid media ( ).
).
 ) and radius of inhibition in solid media (
) and radius of inhibition in solid media ( ).
).