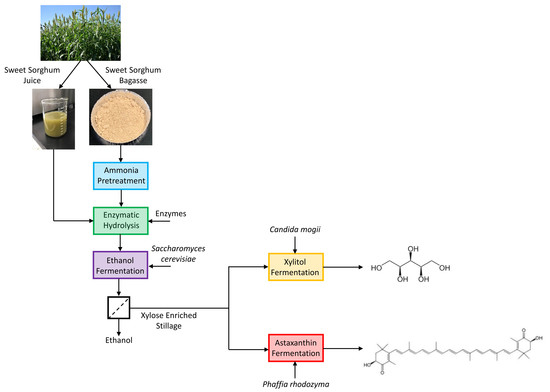
Xylose-Enriched Ethanol Fermentation Stillage from Sweet Sorghum for Xylitol and Astaxanthin Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Yeast Strains
2.2. Feedstock
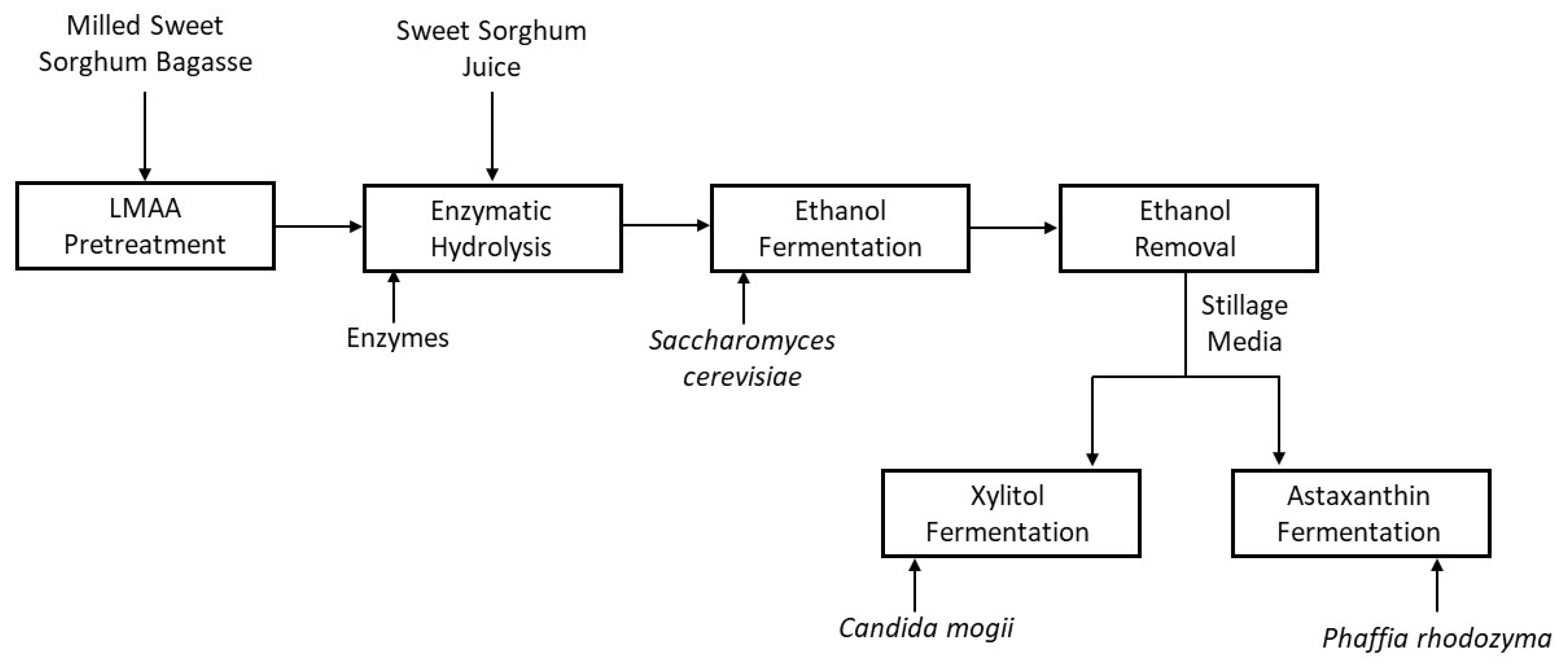
2.3. Pretreatment
2.4. Enzymatic Hydrolysis and Ethanol Fermentation
2.5. Ethanol Removal and Stillage Detoxification
2.6. Xylitol Fermentation
2.6.1. Xylitol Production in Synthetic Media
2.6.2. Xylitol Production in Stillage Media without Detoxification
2.6.3. Xylitol Production in Stillage Media with Detoxification
2.7. Astaxanthin Fermentation
2.7.1. Astaxanthin Production in Stillage Media with and without Detoxification
2.7.2. Astaxanthin Production in Stillage Media with Increased Nitrogen and Nutrient Supplementation
2.8. Analytical Methods
3. Results and Discussion
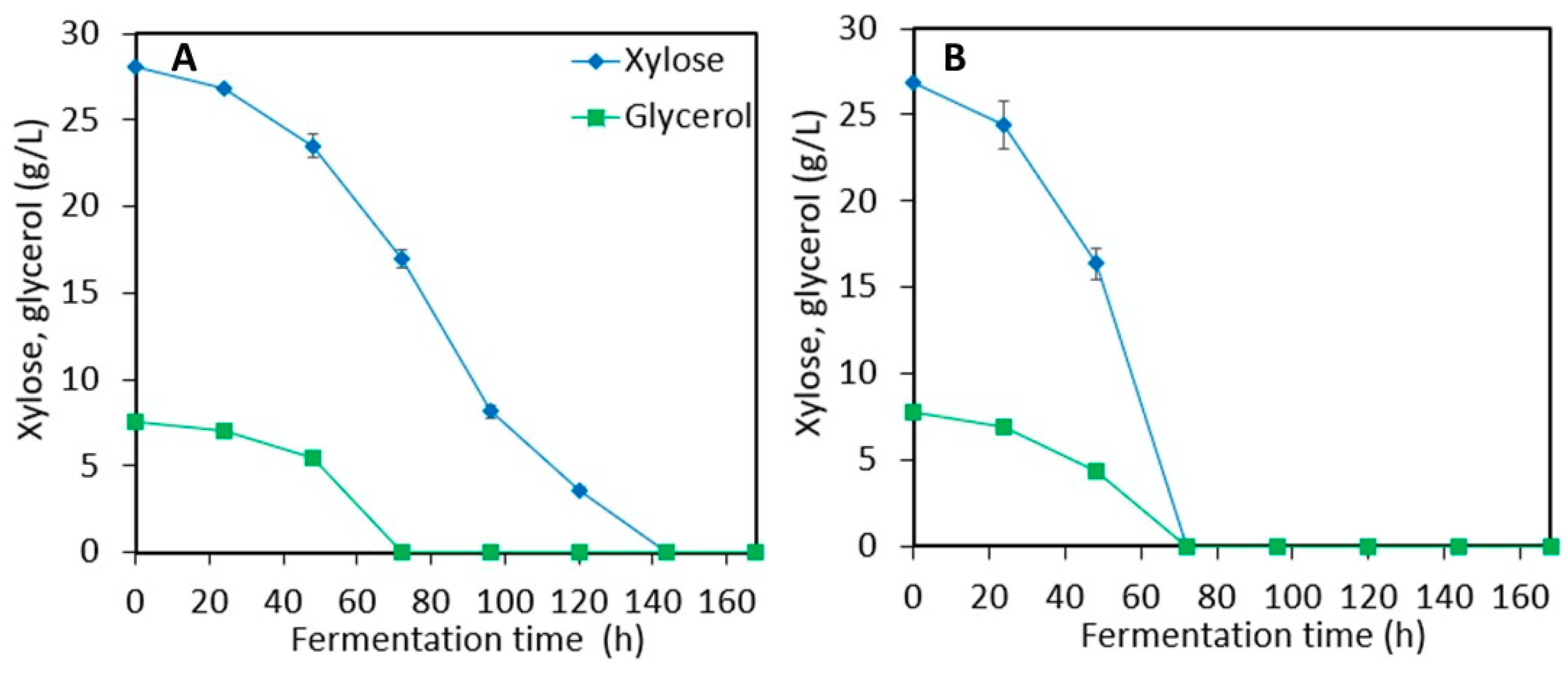
3.1. Ethanol Production from Sweet Sorghum Juice (SSJ) and LMAA-treated Sweet Sorghum Bagasse (SSB)
3.2. Xylitol Production in Post-Ethanol Media
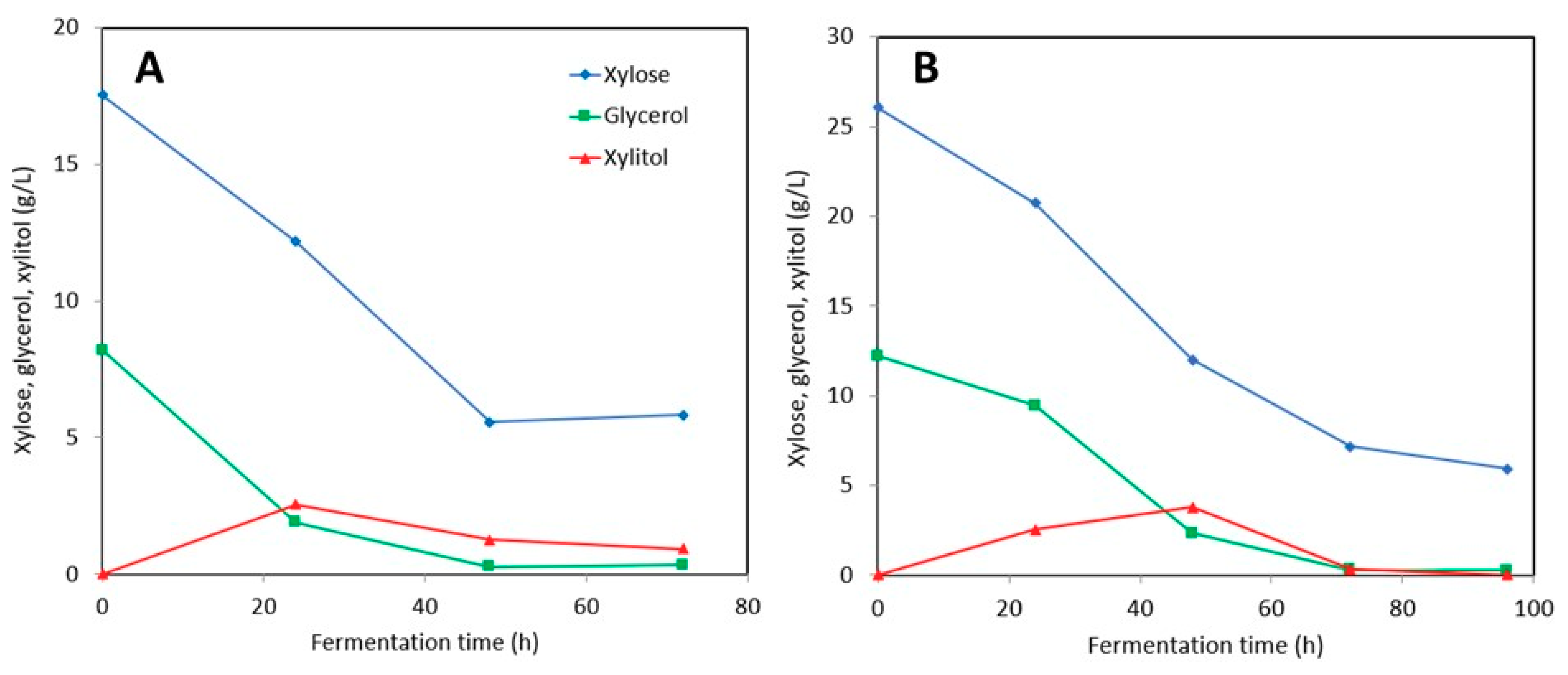
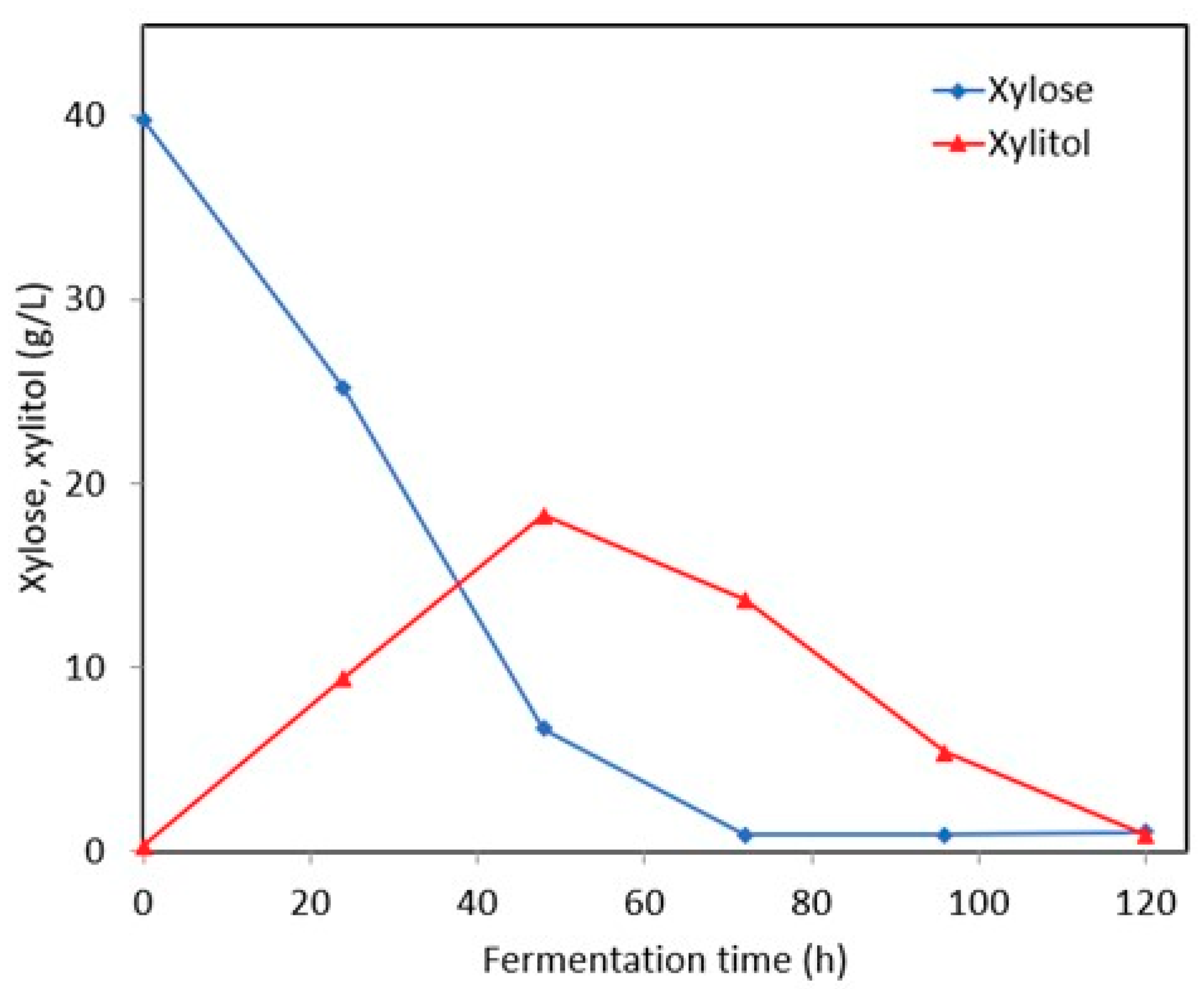
3.2.1. Synthetic Media
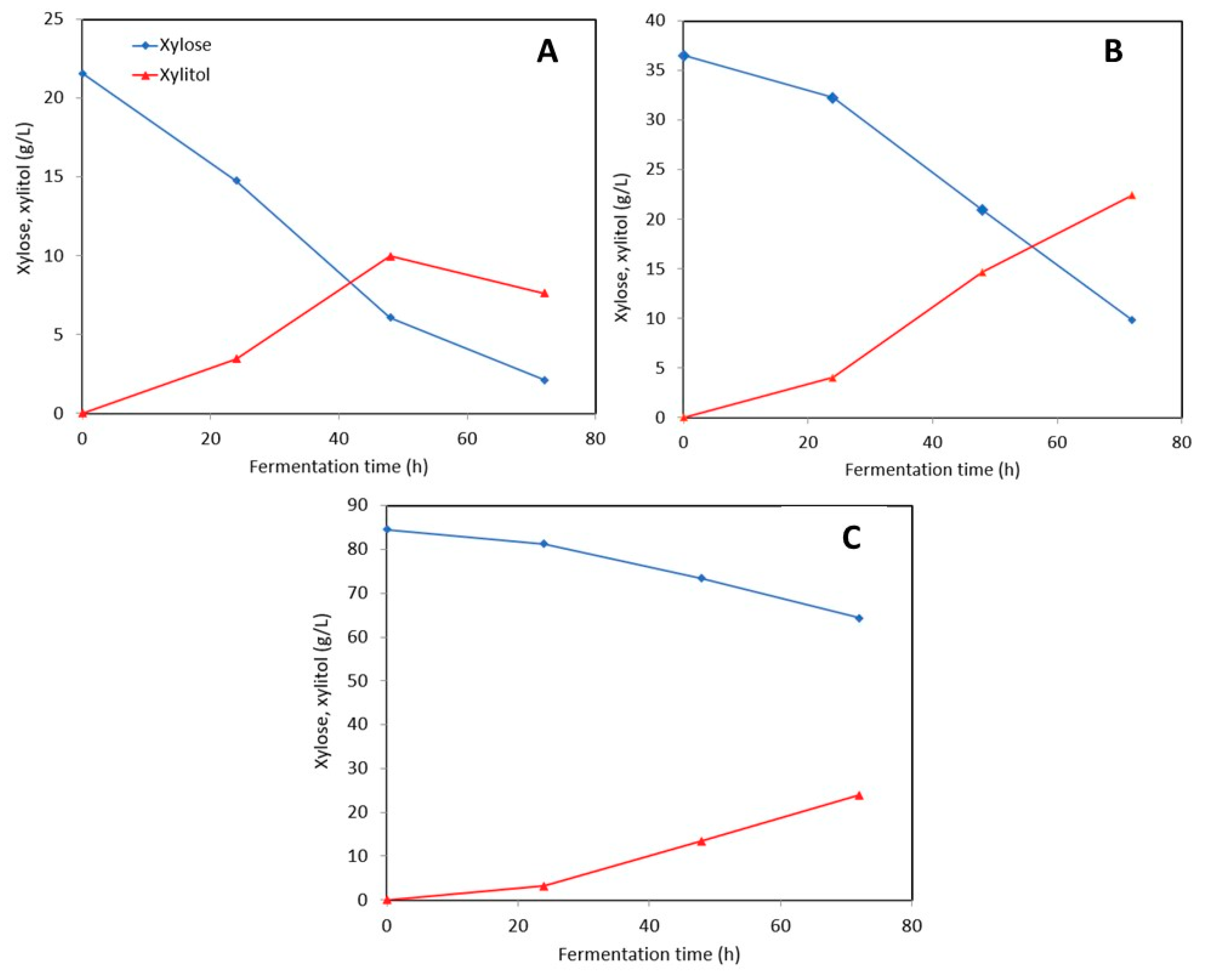
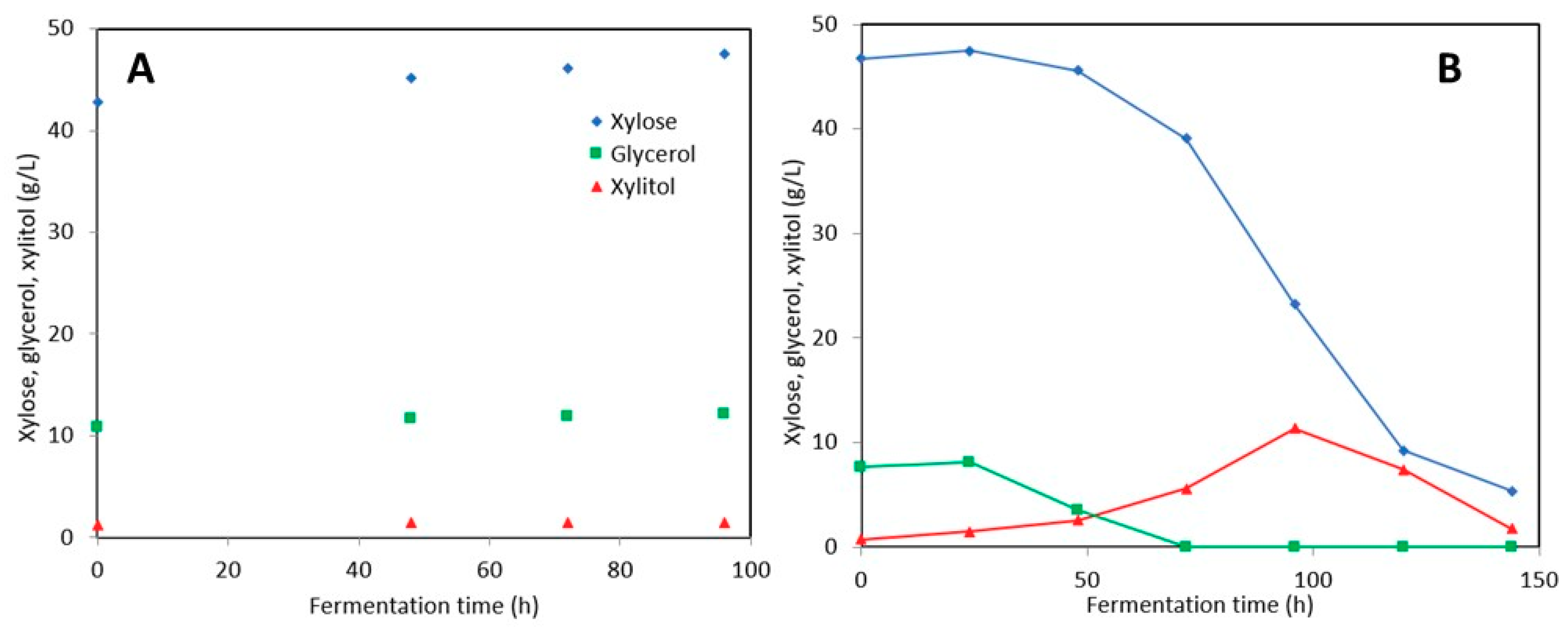
3.2.2. Stillage Media with Detoxification
3.2.3. Stillage Media without Detoxification
3.3. Astaxanthin Production in Post-Ethanol Media
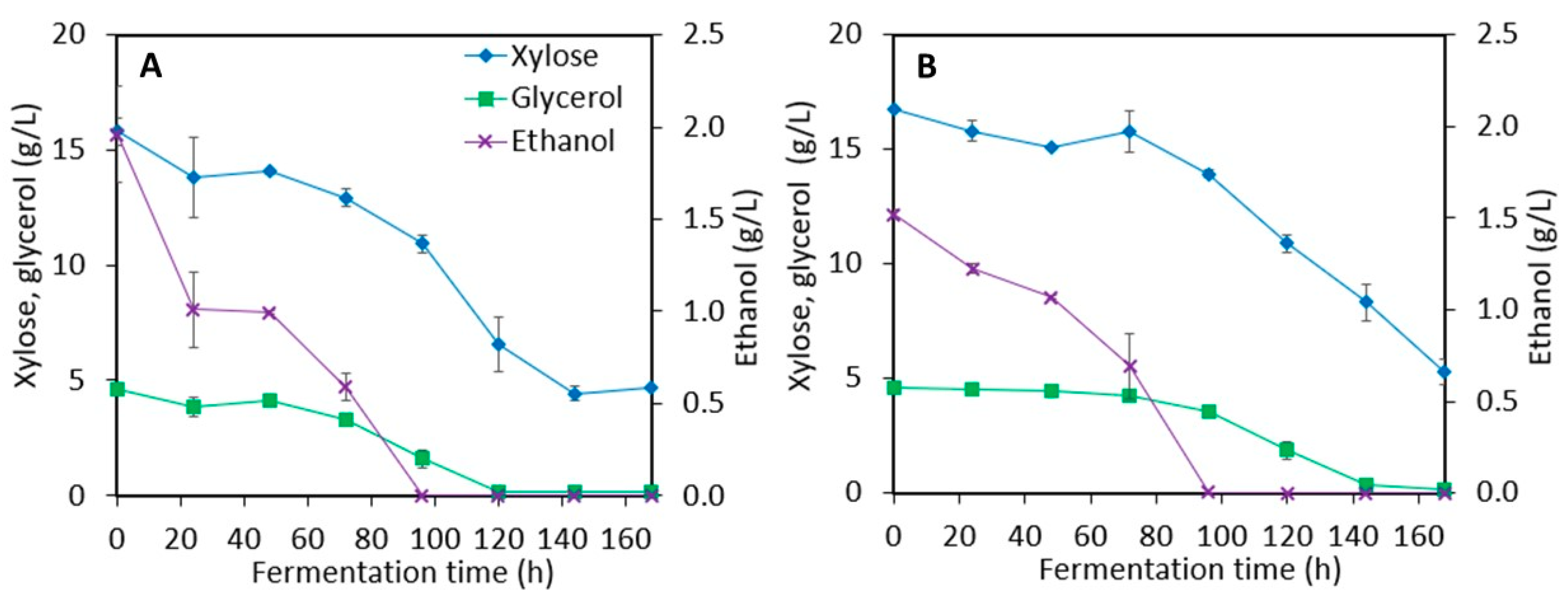
3.3.1. Stillage Media with and without Detoxification
3.3.2. Stillage Media with Increased Nitrogen and Nutrient Supplementation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Al-Alawi, B.M.; Bradley, T.H. Review of hybrid, plug-in hybrid, and electric vehicle market modeling studies. Renew. Sustain. Energy Rev. 2013, 21, 190–203. [Google Scholar] [CrossRef]
- Fulton, L.M.; Lynd, L.R.; Körner, A.; Greene, N.; Tonachel, L.R. The need for biofuels as part of a low carbon energy future. Biofuels Bioprod. Biorefin. 2015, 9, 476–483. [Google Scholar] [CrossRef]
- Rausch, K.D.; Hummel, D.; Johnson, L.A.; May, J.B. Wet Milling: The Basis for Corn Biorefineries. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 501–535. [Google Scholar] [CrossRef]
- Hess, J.R.; Kenney, K.L.; Wright, C.T.; Perlack, R.; Turhollow, A. Corn stover availability for biomass conversion: Situation analysis. Cellulose 2009, 16, 599–619. [Google Scholar] [CrossRef]
- Lamers, P.; Roni, M.S.; Tumuluru, J.S.; Jacobson, J.J.; Cafferty, K.G.; Hansen, J.K.; Kenney, K.; Teymouri, F.; Bals, B. Techno-economic analysis of decentralized biomass processing depots. Bioresour. Technol. 2015, 194, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M. Process design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Laboratory: Lakewood, CO, USA, 2011. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; da Costa Sousa, L.; Schwartz, C.; He, Y.; Sarks, C.; Gunawan, C.; Balan, V.; Dale, B.E. Toward lower cost cellulosic biofuel production using ammonia based pretreatment technologies. Green Chem. 2016, 18, 957–966. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Hodge, D.B. Fractionation and improved enzymatic deconstruction of hardwoods with alkaline delignification. Bioenergy Res. 2015, 8, 1224–1234. [Google Scholar] [CrossRef]
- Moysés, D.; Reis, V.; Almeida, J.; Moraes, L.; Torres, F. Xylose fermentation by Saccharomyces cerevisiae: Challenges and prospects. Int. J. Mol. Sci. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.D.; Rosa, C.A.; Hector, R.E.; Dien, B.S.; Mertens, J.A.; Ayub, M.A.Z. Influence of genetic background of engineered xylose-fermenting industrial Saccharomyces cerevisiae strains for ethanol production from lignocellulosic hydrolysates. J. Ind. Microbiol. Biotechnol. 2017, 44, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.W.; Bals, B.D.; Chundawat, S.P.; Jin, M.; Gunawan, C.; Balan, V.; Jones, A.D.; Dale, B.E. An integrated paradigm for cellulosic biorefineries: Utilization of lignocellulosic biomass as self-sufficient feedstocks for fuel, food precursors and saccharolytic enzyme production. Energy Environ. Sci. 2012, 5, 7100–7110. [Google Scholar] [CrossRef]
- Baptista, S.L.; Cunha, J.T.; Romaní, A.; Domingues, L. Xylitol production from lignocellulosic whole slurry corn cob by engineered industrial Saccharomyces cerevisiae PE-2. Bioresour. Technol. 2018, 267, 481–491. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, T.L.; da Silva, I.J., Jr.; de Macedo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process. Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Laboratory: Lakewood, CO, USA, 2004. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Parajo, J.C.; Santos, V.; Vazquez, M. Production of carotenoids by Phaffia rhodozyma growing on media made from hemicellulosic hydrolysates of Eucalyptus globulus wood. Biotechnol. Bioeng. 1998, 59, 501–506. [Google Scholar] [CrossRef]
- Vazquez, M.; Santos, V.; Parajo, J.C. Fed-batch cultures of Phaffia rhodozyma in xylose-containing media made from wood hydrolysates. Food Biotechnol. 1998, 12, 43–55. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Montanti, J.; Johnston, D.B. Sorghum as a renewable feedstock for production of fuels and industrial chemicals. Aims Bioeng. 2016, 3, 75–91. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Johnston, D.B.; Nghiem, N.P. Utilization of Sweet Sorghum Juice for the Production of Astaxanthin as a Biorefinery Co-Product by Phaffia rhodozyma. ACS Sustain. Chem. Eng. 2018, 6, 3124–3134. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Johnston, D.B.; Nghiem, N.P. Phaffia rhodozyma cultivation on structural and non-structural sugars from sweet sorghum for astaxanthin generation. Process. Biochem. 2019, 83, 9–17. [Google Scholar] [CrossRef]
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Pretreatment of corn stover using low-moisture anhydrous ammonia (LMAA) process. Bioresour. Technol. 2011, 102, 10028–10034. [Google Scholar] [CrossRef] [PubMed]
- Montanti, J.; Nghiem, N.P.; Johnston, D.B. Production of astaxanthin from cellulosic biomass sugars by mutants of the yeast Phaffia rhodozyma. Appl. Biochem. Biotechnol. 2011, 164, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Montanti, J.; Johnston, D. Production of Astaxanthin from Corn Fiber as a Value-Added Co-product of Fuel Ethanol Fermentation. Appl. Biochem. Biotechnol. 2009, 154, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Lakewood, CO, USA, 2011. [Google Scholar]
- Koo, B.; Park, J.; Gonzalez, R.; Jameel, H.; Park, S. Two-stage autohydrolysis and mechanical treatment to maximize sugar recovery from sweet sorghum bagasse. Bioresour. Technol. 2019, 276, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, C.A.; Maeda, R.N.; Santa Anna, L.M.M.; Pereira, N., Jr. Sweet sorghum as a whole-crop feedstock for ethanol production. Biomass Bioenergy 2016, 94, 46–56. [Google Scholar] [CrossRef]
- Tochampa, W.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Srinophakun, P.; Bakker, H.H.; Chisti, Y. A model of xylitol production by the yeast Candida mogii. Bioprocess. Biosyst. Eng. 2005, 28, 175–183. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Xylitol production by Candida species grown on a grass hydrolysate. World J. Microbiol. Biotechnol. 2009, 25, 913–916. [Google Scholar] [CrossRef]
- De Mancilha, I.M.; Karim, M.N. Evaluation of ion exchange resins for removal of inhibitory compounds from corn stover hydrolyzate for xylitol fermentation. Biotechnol. Prog. 2003, 19, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Camargo, D.; Sene, L.; Variz, D.I.; de Almeida Felipe, M.D.G. Xylitol bioproduction in hemicellulosic hydrolysate obtained from sorghum forage biomass. Appl. Biochem. Biotechnol. 2015, 175, 3628–3642. [Google Scholar] [CrossRef] [PubMed]
- Sene, L.; Arruda, P.V.; Oliveira, S.M.M.; Felipe, M.G.A. Evaluation of sorghum straw hemicellulosic hydrolysate for biotechnological production of xylitol by Candida guilliermondii. Braz. J. Microbiol. 2011, 42, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-C.; Kwon, Y.-J. Optimization of the pretreatment of rice straw hemicellulosic hydrolyzates for microbial production of xylitol. Biotechnol. Bioprocess. Eng. 2007, 12, 404. [Google Scholar] [CrossRef]
- Buhner, J.; Agblevor, F. Effect of detoxification of dilute-acid corn fiber hydrolysate on xylitol production. Appl. Biochem. Biotechnol. 2004, 119, 13–30. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.; Lopes, S.; Parajó, J.; Pereira, H.; Gırio, F. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process. Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Vázquez, M.; Santos, V.; Parajó, J.C. Effect of the carbon source on the carotenoid profiles of Phaffia rhodozyma strains. J. Ind. Microbiol. Biotechnol. 1997, 19, 263–268. [Google Scholar] [CrossRef]
- Clark, T.A.; Mackie, K.L. Fermentation inhibitors in wood hydrolysates derived from the softwood Pinus radiata. J. Chem. Technol. Biotechnol. 1984, 34, 101–110. [Google Scholar] [CrossRef]
- Yoshioka, K.; Daidai, M.; Matsumoto, Y.; Mizuno, R.; Katsura, Y.; Hakogi, T.; Yanase, H.; Watanabe, T. Self-sufficient bioethanol production system using a lignin-derived adsorbent of fermentation inhibitors. ACS Sustain. Chem. Eng. 2018, 6, 3070–3078. [Google Scholar] [CrossRef]
- Flores-Cotera, L.; Martin, R.; Sanchez, S. Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma: Influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium. Appl. Microbiol. Biotechnol. 2001, 55, 341–347. [Google Scholar] [CrossRef]
- Ramírez, J.; Gutierrez, H.; Gschaedler, A. Optimization of astaxanthin production by Phaffia rhodozyma through factorial design and response surface methodology. J. Biotechnol. 2001, 88, 259–268. [Google Scholar] [CrossRef]
- Pan, X.; Wang, B.; Gerken, H.G.; Lu, Y.; Ling, X. Proteomic analysis of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous in response to low carbon levels. Bioprocess. Biosyst. Eng. 2017, 40, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Cabrera, C.; Flores-Bustamante, Z.R.; Marsch, R.; Montes, M.D.C.; Sánchez, S.; Cancino-Díaz, J.C.; Flores-Cotera, L.B. ATP-citrate lyase activity and carotenoid production in batch cultures of Phaffia rhodozyma under nitrogen-limited and nonlimited conditions. Appl. Microbiol. Biotechnol. 2010, 85, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.-L.; An, G.-H.; Johnson, E.A. Ethanol increases carotenoid production in Phaffia rhodozyma. J. Ind. Microbiol. Biotechnol. 1997, 19, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Nunez, M.L.; Valdivia, R. Increased astaxanthin production by a Phaffia rhodozyma mutant grown on date juice from Yucca fillifera. J. Ind. Microbiol. Biotechnol. 2000, 24, 187–190. [Google Scholar] [CrossRef]
- An, G.H.; Jang, B.G.; Cho, M.H. Cultivation of the carotenoid-hyperproducing mutant 2A2N of the red yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma) with molasses. J. Biosci. Bioeng. 2001, 92, 121–125. [Google Scholar] [CrossRef]
- Schewe, H.; Kreutzer, A.; Schmidt, I.; Schubert, C.; Schrader, J. High concentrations of biotechnologically produced astaxanthin by lowering pH in a Phaffia rhodozyma bioprocess. Biotechnol. Bioprocess. Eng. 2017, 22, 319–326. [Google Scholar] [CrossRef]
- Ducrey Sanpietro, L.M.; Kula, M.R. Studies of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Effect of inhibitors and low temperature. Yeast 1998, 14, 1007–1016. [Google Scholar] [CrossRef]
- Xue, S.; Jones, A.D.; Sousa, L.; Piotrowski, J.; Jin, M.; Sarks, C.; Dale, B.E.; Balan, V. Water-soluble phenolic compounds from extractive ammonia pretreatment exerted binary inhibitory effects on yeast fermentation using synthetic hydrolysate. PLoS ONE 2018, 13, e0194012. [Google Scholar] [CrossRef]


| Glucan | Xylan | Arabinan | AI Lignin | AS Lignin | Total Lignin | Ash |
|---|---|---|---|---|---|---|
| 38.3 ± 2.3 | 22.4 ± 1.5 | 3.0 ± 0.3 | 13.4 ± 3.9 | 3.1 ± 1.4 | 16.6 ± 4.7 | 0.2 ± 0.0 |
| SSJ Only | SSJ + SSB | SSJ + SSB (Ethanol Removed) | |
|---|---|---|---|
| Xylose (g/L) | 0 | 18.1 ± 0.1 | 23.4 ± 1.4 |
| Glycerol (g/L) | 6.4 ± 0.1 | 7.1 ± 0.2 | 9.5 ± 1.1 |
| Ethanol (g/L) | 60.9 ± 0.4 | 69.6 ± 0.1 | 2.3 ± 0.1 |
| X (g/L) | P (mg/L) | YP/S (mg/g) | Yx/S (g/g) | YP/X (mg/g) | QP (mg/L/h) | |
|---|---|---|---|---|---|---|
| Non-Detoxified | 17.5 ± 0.9 | 17.5 ± 1.8 | 0.47 ± 0.05 | 0.47± 0.02 | 1.0 ± 0.1 | 0.10 ± 0.01 |
| Detoxified | 18.2 ± 1.3 | 71.7 ± 3.3 | 1.9 ± 0.1 | 0.5 ± 0.03 | 3.9 ± 0.3 | 0.43 ± 0.02 |
| X (g/L) | P (mg/L) | YP/S (mg/g) | Yx/S (g/g) | YP/X (mg/g) | QP (mg/L/h) | ||
|---|---|---|---|---|---|---|---|
| 36% Concentrated | 6.0 g/L YE | 8.5 ± 0.3 | 17.3 ± 1.0 | 0.87 ± 0.05 | 0.43 ± 0.01 | 2.0 ± 0.1 | 0.10 ± 0.01 |
| 1.0 g/L AS | 6.0 ± 0.2 | 14.2 ± 1.0 | 0.72 ± 0.05 | 0.31 ± 0.01 | 2.4 ± 0.1 | 0.08 ± 0.01 | |
| 60% Concentrated | 6.0 g/L YE | 8.6 ± 0.2 | 18.1 ± 1.8 | 0.98 ± 0.1 | 0.46 ± 0.01 | 2.1 ± 0.3 | 0.11 ± 0.01 |
| 1.0 g/L AS | 7.9 ± 0.5 | 15.3 ± 1.6 | 0.87 ± 0.1 | 0.45 ± 0.03 | 1.9 ± 0.2 | 0.09 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoklosa, R.J.; Nghiem, N.P.; Latona, R.J. Xylose-Enriched Ethanol Fermentation Stillage from Sweet Sorghum for Xylitol and Astaxanthin Production. Fermentation 2019, 5, 84. https://doi.org/10.3390/fermentation5040084
Stoklosa RJ, Nghiem NP, Latona RJ. Xylose-Enriched Ethanol Fermentation Stillage from Sweet Sorghum for Xylitol and Astaxanthin Production. Fermentation. 2019; 5(4):84. https://doi.org/10.3390/fermentation5040084
Chicago/Turabian StyleStoklosa, Ryan J., Nhuan P. Nghiem, and Renee J. Latona. 2019. "Xylose-Enriched Ethanol Fermentation Stillage from Sweet Sorghum for Xylitol and Astaxanthin Production" Fermentation 5, no. 4: 84. https://doi.org/10.3390/fermentation5040084
APA StyleStoklosa, R. J., Nghiem, N. P., & Latona, R. J. (2019). Xylose-Enriched Ethanol Fermentation Stillage from Sweet Sorghum for Xylitol and Astaxanthin Production. Fermentation, 5(4), 84. https://doi.org/10.3390/fermentation5040084