High Gravity Fermentation of Sugarcane Bagasse Hydrolysate by Saccharomyces pastorianus to Produce Economically Distillable Ethanol Concentrations: Necessity of Medium Components Examined
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Medium Components
2.3. E. coli Extract (ECE)
2.4. Sugarcane Bagasse and the Pre-Treatment Process
2.5. Enzyme Hydrolysis Conditions
2.6. S. Pastorianus Fermentations
2.7. Analytical Methods
2.8. Statistical Analyses
3. Results
3.1. Medium Component Effects on Ethanol Productivity
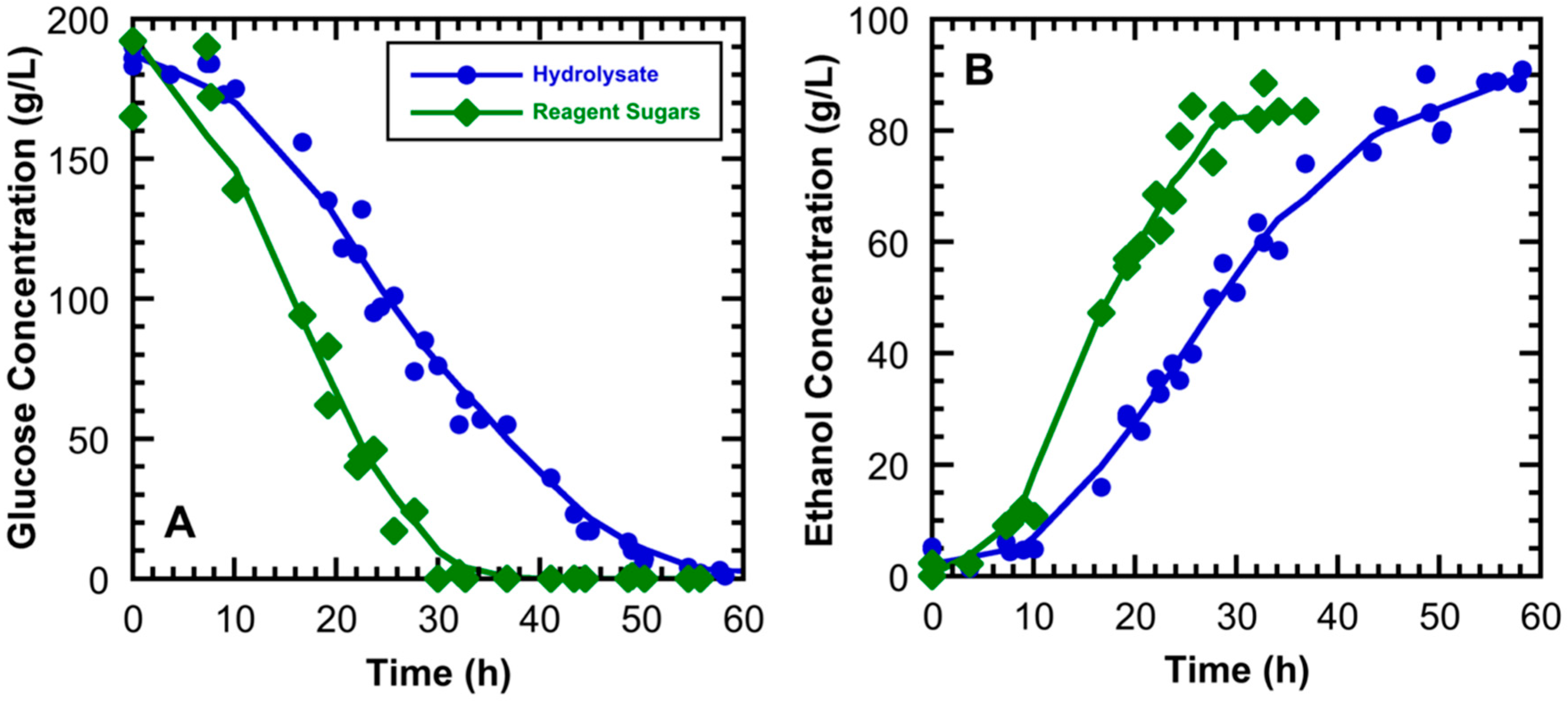
3.2. High Gravity Fermentations Using Sugarcane Bagasse Hydrolysate
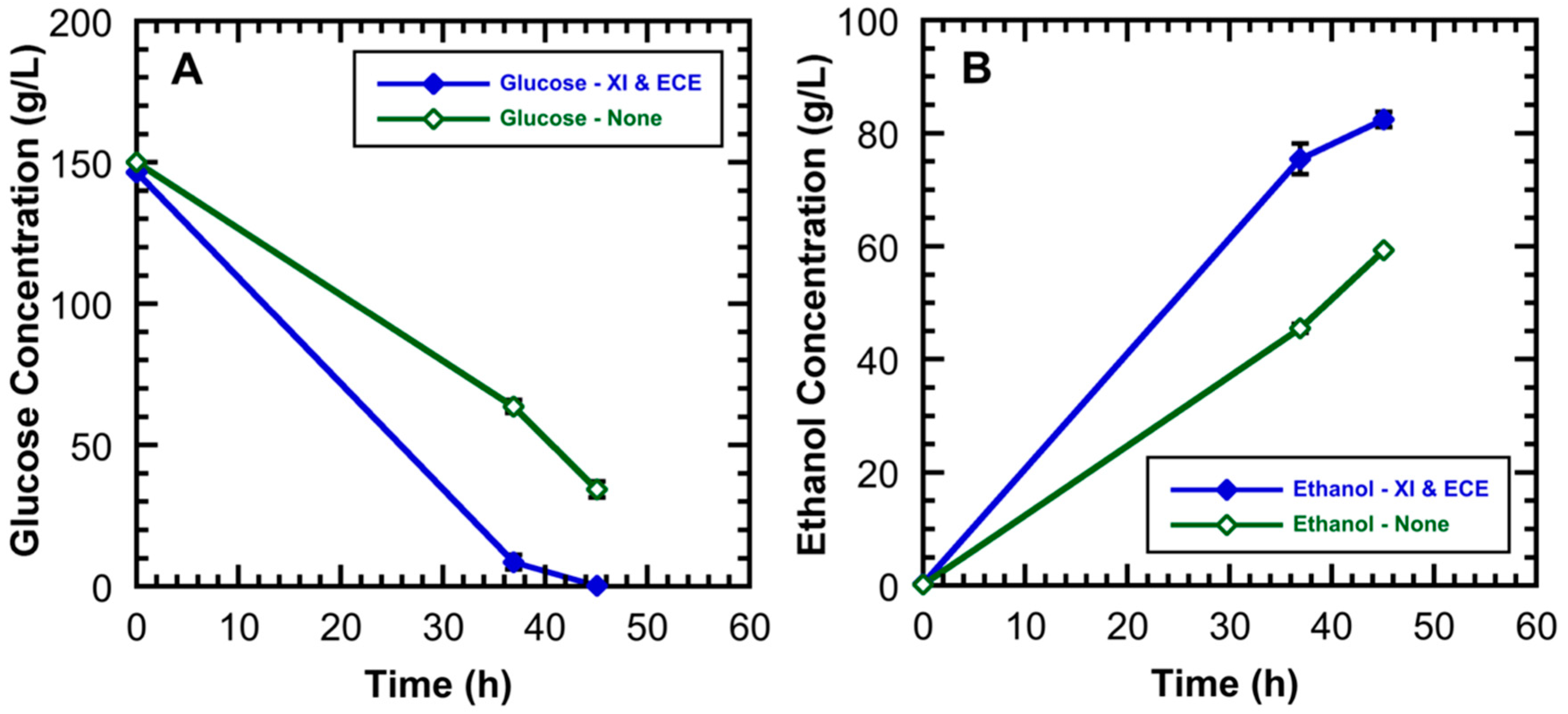
3.3. Effect of ECE and Xylose Isomerase on the Hydrolysate Fermentation Kinetics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.L. Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: A systematic review. J. Ind. Microbiol. Biotechnol. 2011, 38, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; McBride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef]
- Margeot, A.; Hahn-Hagerdal, B.; Edlund, M.; Slade, R.; Monot, F. New improvements for lignocellulosic ethanol. Curr. Opin. Biotechnol. 2009, 20, 372–380. [Google Scholar] [CrossRef]
- Prior, B.A.; Kilian, S.G.; Preez, J.C.d. Fermentation of D-xylose by the yeasts Candida shehatae and Pichia stipitis. Process Biochem. 1989, 24, 21–32. [Google Scholar]
- Chen, R.; Dou, J. Biofuels and bio-based chemicals from lignocellulose: Metabolic engineering strategies in strain development. Biotechnol. Lett. 2016, 38, 213–221. [Google Scholar] [CrossRef]
- Gowtham, Y.K.; Miller, K.P.; Hodge, D.B.; Henson, J.M.; Harcum, S.W. Novel two-stage fermentation process for bioethanol production using Saccharomyces pastorianus. Biotechnol. Prog. 2014, 30, 300–310. [Google Scholar] [CrossRef]
- Okuno, M.; Kajitani, R.; Ryusui, R.; Morimoto, H.; Kodama, Y.; Itoh, T. Next-generation sequencing analysis of lager brewing yeast strains reveals the evolutionary history of interspecies hybridization. DNA Res. 2016, 23, 67–80. [Google Scholar] [CrossRef]
- Quain, D.E. Yeast Genetics in Brewing: New Insights and Opportunies. In Brewing: New Technologies; Bamforth, C.W., Ed.; Woodhead Publishing Limited: Cambridge, England, 2006; pp. 149–166. [Google Scholar]
- Dunn, B.; Sherlock, G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008, 18, 1610–1623. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Duong, C.T.; Nevoigt, E. Genetic improvement of brewer’s yeast: Current state, perspectives and limits. Appl. Microbiol. Biotechnol. 2010, 86, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, P.; de Sousa, H.R.; Spencer-Martins, I. FSY1, a novel gene encoding a specific fructose/H(+) symporter in the type strain of Saccharomyces carlsbergensis. J. Bacteriol. 2000, 182, 5628–5630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, K.P.; Gowtham, Y.K.; Henson, J.M.; Harcum, S.W. Xylose isomerase improves growth and ethanol production rates from biomass sugars for both Saccharomyces pastorianus and Saccharomyces cerevisiae. Biotechnol. Prog. 2012, 28, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ladisch, M.R.; Svarczkopf, J.A. Ethanol production and the cost of fermentable sugars from biomass. Bioresource Technol. 1991, 36, 83–95. [Google Scholar] [CrossRef]
- Joelsson, E.; Erdei, B.; Galbe, M.; Wallberg, O. Techno-economic evaluation of integrated first- and second-generation ethanol production from grain and straw. Biotechnol. Biofuels 2016, 9, 1. [Google Scholar] [CrossRef]
- Ingledew, W.M. The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries; Nottingham University Press: Nottingham, UK, 2009. [Google Scholar]
- Son, C.K.; Pham, T.H.; Bui, K.L.T.; Nguyen, T.T.; Pham, K.D.; Nguyen, H.D.T.; Luong, H.N.; Tu, V.P.; Nguyen, T.H.; Ho, P.H.; et al. Simultaneous liquefaction, saccharification and fermentation at very high gravity of rice at pilot scale for potable ethanol production and distillers dried grains composition. Food Bioprod. Process. 2016, 98, 79–85. [Google Scholar] [CrossRef]
- De Bari, I.; Cuna, D.; Di Matteo, V.; Liuzzi, F. Bioethanol production from steam-pretreated corn stover through an isomerase mediated process. New Biotechol. 2014, 31, 185–195. [Google Scholar] [CrossRef]
- Yuan, D.W.; Rao, K.; Relue, P.; Varanasi, S. Fermentation of biomass sugars to ethanol using native industrial yeast strains. Bioresource Technol. 2011, 102, 3246–3253. [Google Scholar] [CrossRef]
- Wei, S.; Bai, P.; Liu, Y.; Yang, M.; Ma, J.; Hou, J.; Liu, W.; Bao, X.; Shen, Y. A Thi2p Regulatory Network Controls the Post-glucose Effect of Xylose Utilization in Saccharomyces cerevisiae. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Niu, Y.; Wu, L.; Shen, Y.; Zhao, J.; Zhang, J.; Yi, Y.; Li, H.; Bao, X. Coexpression of beta-xylosidase and xylose isomerase in Saccharomyces cerevisiae improves the efficiency of saccharification and fermentation from xylo-oligosaccharides. Cellulose 2019, 26, 7923–7937. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Xiong, L.; Tang, Y.-J.; Mehmood, M.A.; Zhao, Z.K.; Bai, F.-W.; Zhao, X.-Q. Enhanced acetic acid stress tolerance and ethanol production in Saccharomyces cerevisiae by modulating expression of the de novo purine biosynthesis genes. Biotechnol. Biofuels 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.K.; Ho, N.W.Y.; Khan, A.; Sedlak, M. A genetic overhaul of Saccharomyces cerevisiae 424A(LNH-ST) to improve xylose fermentation. J. Ind. Microbiol. Biotechnol. 2011, 38, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Kricka, W.; James, T.C.; Fitzpatrick, J.; Bond, U. Engineering Saccharomyces pastorianus for the co-utilisation of xylose and cellulose from biomass. Microb. Cell Fact. 2015, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Um, Y.; Woo, H.M.; Kim, K.H.; Lee, S.-M. Ethanol production from lignocellulosic hydrolysates using engineered Saccharomyces cerevisiae harboring xylose isomerase-based pathway. Bioresource Technol. 2016, 209, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Moyses, D.N.; Branco Reis, V.C.; Moreira de Almeida, J.R.; Pepe de Moraes, L.M.; Goncalves Torres, F.A. Xylose fermentation by Saccharomyces cerevisiae: Challenges and prospects. Int. J. Mol. Sci. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, F.; Zhang, S.; Gong, Q.; Chen, G. Ethanol production by simultaneous saccharification and cofermentation of pretreated corn stalk. J. Basic Microbiol. 2019, 59, 744–753. [Google Scholar] [CrossRef]
- Li, Y.-C.; Gou, Z.-X.; Zhang, Y.; Xia, Z.-Y.; Tang, Y.-Q.; Kida, K. Inhibitor tolerance of a recombinant flocculating industrial Saccharomyces cerevisiae strain during glucose and xylose co-fermentation. Braz. J. Microbiol. 2017, 48, 791–800. [Google Scholar] [CrossRef]
- Ha, S.J.; Galazka, J.M.; Kim, S.R.; Choi, J.H.; Yang, X.M.; Seo, J.H.; Glass, N.L.; Cate, J.H.D.; Jin, Y.S. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc. Natl. Acad. Sci. USA 2011, 108, 504–509. [Google Scholar] [CrossRef]
- Liu, T.; Huang, S.; Geng, A. Recombinant Diploid Saccharomyces cerevisiae Strain Development for Rapid Glucose and Xylose Co-Fermentation. Fermentation-Basel 2018, 4, 59. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Yang, S.; Jiang, Y. Unraveling the genetic basis of fast L-arabinose consumption on top of recombinant xylose-fermenting Saccharomyces cerevisiae. Biotechnol. Bioeng. 2018. [Google Scholar] [CrossRef]
- Jayakody, L.N.; Turner, T.L.; Yun, E.J.; Kong, I.I.; Liu, J.-J.; Jin, Y.-S. Expression of Gre2p improves tolerance of engineered xylose-fermenting Saccharomyces cerevisiae to glycolaldehyde under xylose metabolism. Appl. Microbiol. Biotechnol. 2018, 102, 8121–8133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Zhu, B.; Zhang, G.; Wei, N. Co-fermentation of cellobiose and xylose by mixed culture of recombinant Saccharomyces cerevisiae and kinetic modeling. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Mokomele, T.; Sousa, L.D.C.; Balan, V.; van Rensburg, E.; Dale, B.E.; Gorgens, J.F. Ethanol production potential from AFEX (TM) and steam-exploded sugarcane residues for sugarcane biorefineries. Biotechnol. Biofuels 2018, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Jung, J.H.; Altpeter, F.; Kannan, B.; Kim, H.E.; Kim, K.H.; Alper, H.S.; Um, Y.; Lee, S.-M. Largely enhanced bioethanol production through the combined use of lignin-modified sugarcane and xylose fermenting yeast strain. Bioresource Technol. 2018, 256, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Korz, D.J.; Rinas, U.; Hellmuth, K.; Sanders, E.A.; Deckwer, W.D. Simple fed-batch technique for high cell-density cultivation of Escherichia coli. J. Biotechnol. 1995, 39, 59–65. [Google Scholar] [CrossRef]
- Sharma, S.S.; Blattner, F.R.; Harcum, S.W. Recombinant protein production in an Escherichia coli reduced genome strain. Metab. Eng. 2007, 9, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.S.; Chen, L.F.; Flickinger, M.C.; Chiang, L.C.; Tsao, G.T. Production of ethanol from D-xylose by using D-xylose isomerase and yeasts. Appl. Environ. Microbiol. 1981, 41, 430–436. [Google Scholar] [CrossRef]
- Xu, B.; Jahic, M.; Blomsten, G.; Enfors, S.O. Glucose overflow metabolism and mixed-acid fermentation in aerobic large-scale fed-batch processes with Escherichia coli. Appl. Microbiol. Biotechnol. 1999, 51, 564–571. [Google Scholar] [CrossRef]
- Akesson, M.; Hagander, P.; Axelsson, J.P. A probing feeding strategy for Escherichia coli cultures. Biotechnol. Tech. 1999, 13, 523–528. [Google Scholar] [CrossRef]
- Taymaz-Nikerel, H.; Borujeni, A.E.; Verheijen, P.J.T.; Heijnen, J.J.; van Gulik, W.M. Genome-Derived Minimal Metabolic Models for Escherichia coli MG1655 With Estimated In Vivo Respiratory ATP Stoichiometry. Biotechnol. Bioeng. 2010, 107, 369–381. [Google Scholar] [CrossRef]
- Delgado, F.F.; Cermak, N.; Hecht, V.C.; Son, S.; Li, Y.; Knudsen, S.M.; Olcum, S.; Higgins, J.M.; Chen, J.; Grover, W.H.; et al. Intracellular Water Exchange for Measuring the Dry Mass, Water Mass and Changes in Chemical Composition of Living Cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Neidhardt, F.C.; Umbarger, H.E. Chemical Composition of Escherichia coli. In Escherichia coli and Salmonella, 2nd ed.; Neidhardt, F.C., Curtiss, R., Lin, E.C.C., Low, K.B., Magasanik, B., Reanikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996; Volume 1, pp. 13–16. [Google Scholar]
- Bremer, H.; Dennis, P.P. Modulation of Chemical Composition and Other Parameters of the Cell by Growth Rate. In Escherichia coli and Salmonella, 2nd ed.; Neidhardt, F.C., Curtiss, R., Lin, E.C.C., Low, K.B., Magasanik, B., Reanikoff, W.S., Riley, M., Schaechter, M., Umbarger, H.E., Eds.; ASM Press: Washington, DC, USA, 1996; Volume 2, pp. 1553–1569. [Google Scholar]
- Gonzalez, J.E.; Long, C.P.; Antoniewicz, M.R. Comprehensive analysis of glucose and xylose metabolism in Escherichia coli under aerobic and anaerobic conditions by C-13 metabolic flux analysis. Metab. Eng. 2017, 39, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Wei, Y.; Tietje, A. Biochemical conversion of sugarcane bagasse into bioproducts. Biomass Bioenergy 2016, 93, 227–242. [Google Scholar] [CrossRef]
- Jain, A.; Walker, T.H. Pretreatment Composition for Biomass Conversion Process. U.S. Patent 20150087030A1, 26 March 2015. [Google Scholar]
- Miller, K.P. Biofuel Ethanol Production by Saccharomyces bayanus, the Champagne Yeast. Master’s Thesis, Clemson University, Clemson, SC, USA, 2010. [Google Scholar]
- Ingledew, W.M. Alcohol production by Saccharomyces cerevisiae: A yeast primer. In The Alcohol Textbook, 3rd ed.; Nottingham University Press: Nottingham, UK, 1999; pp. 49–86. [Google Scholar]
- Smith, J.; van Rensburg, E.; Görgens, J.F. Simultaneously improving xylose fermentation and tolerance to lignocellulosic inhibitors through evolutionary engineering of recombinant Saccharomyces cerevisiae harbouring xylose isomerase. BMC Biotechnol. 2014, 14, 1–17. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sahara, T.; Ohgiya, S.; Kamagata, Y.; Fujimori, K.E. Systematic optimization of gene expression of pentose phosphate pathway enhances ethanol production from a glucose/xylose mixed medium in a recombinant Saccharomyces cerevisiae. Amb Express 2018, 8, 139. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A review of recent advances in high gravity ethanol fermentation. Renew. Energy 2019, 133, 1366–1379. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Zhang, J.; Bao, J. High ethanol fermentation performance of the dry dilute acid pretreated corn stover by an evolutionarily adapted Saccharomyces cerevisiae strain. Bioresource Technol. 2015, 189, 399–404. [Google Scholar] [CrossRef]
- Zhao, X.; Moates, G.K.; Elliston, A.; Wilson, D.R.; Coleman, M.J.; Waldron, K.W. Simultaneous saccharification and fermentation of steam exploded duckweed: Improvement of the ethanol yield by increasing yeast titre. Bioresource Technol. 2015, 194, 263–269. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Den Abt, T.; Bonini, B.M.; Lidén, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef]
- Casey, G.P.; Magnus, C.A.; Ingledew, W.M. High-Gravity Brewing Effects Of Nutrition on Yeast Composition, Fermentative Ability, and Alcohol Production. Appl. Environ. Microbiol. 1984, 48, 639–646. [Google Scholar] [CrossRef]
- Rao, K.; Chelikani, S.; Relue, P.; Varanasi, S. A novel technique that enables efficient conduct of simultaneous isomerization and fermentation (SIF) of xylose. Appl. Biochem. Biotechnol. 2008, 146, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Q.; Tan, X.; Qi, W.; Yu, Q.; Zhou, G.; Zhuang, X.; Yuan, Z. High conversion of sugarcane bagasse into monosaccharides based on sodium hydroxide pretreatment at low water consumption and wastewater generation. Bioresource Technol. 2016, 218, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
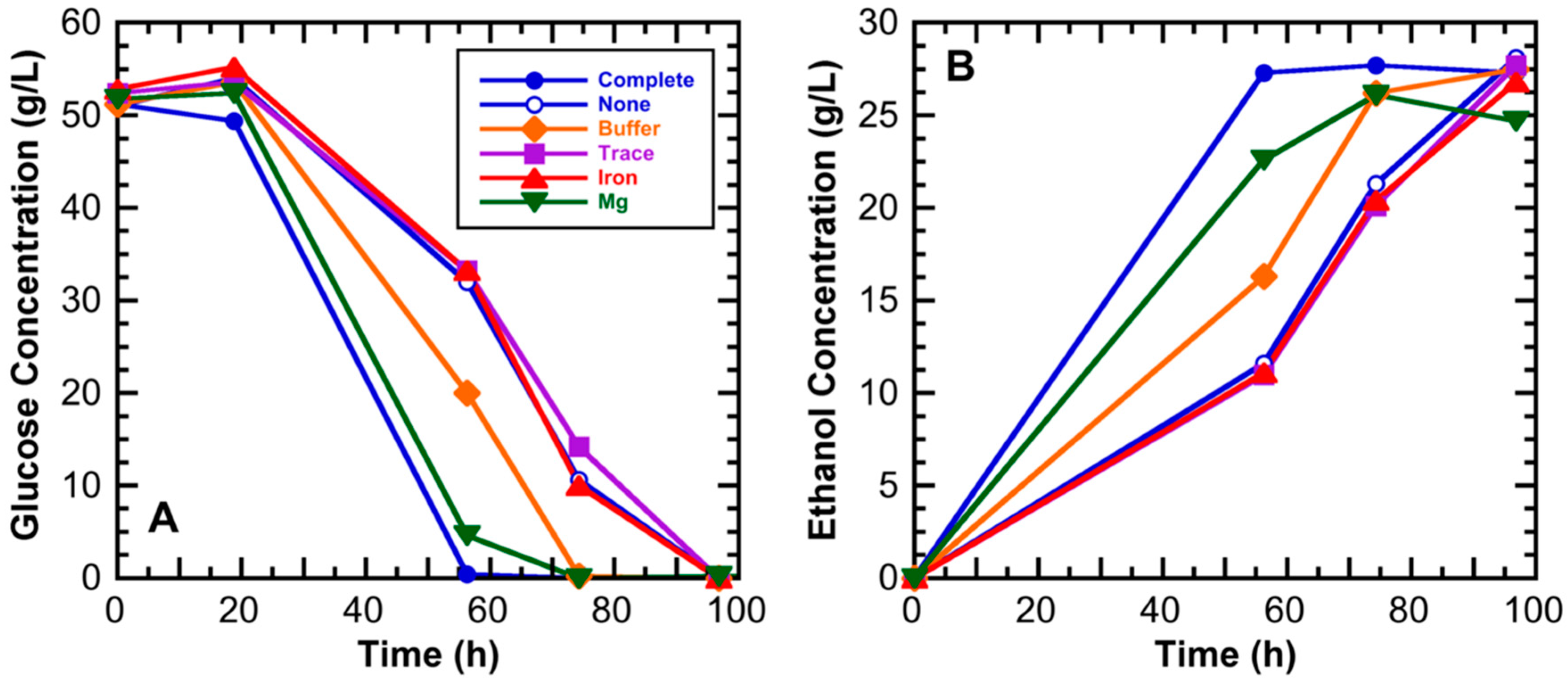
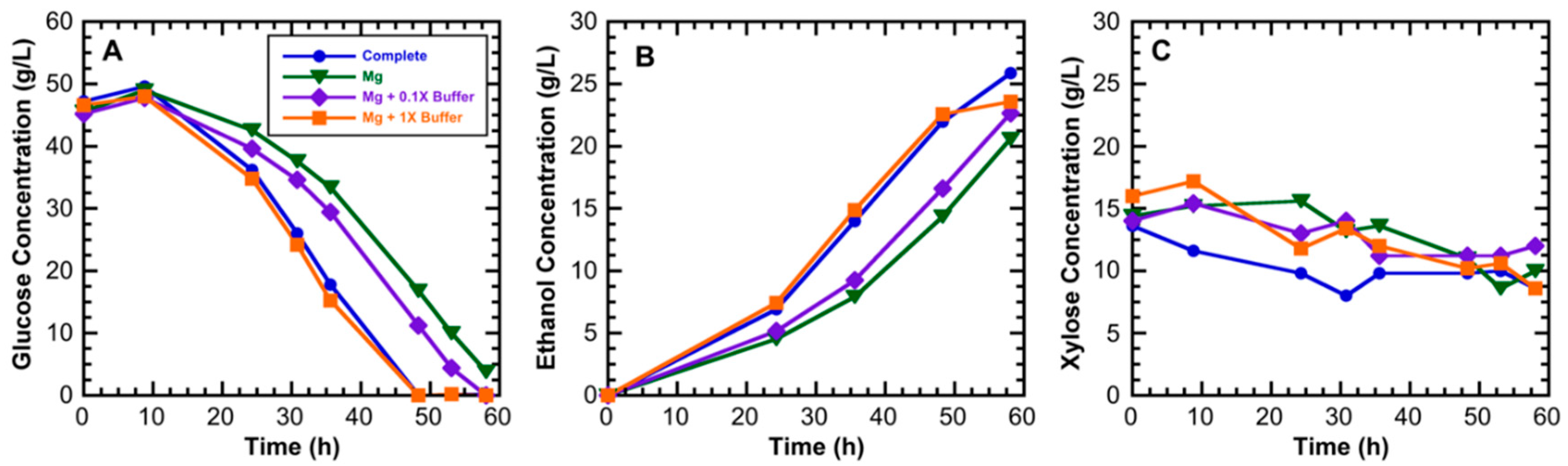
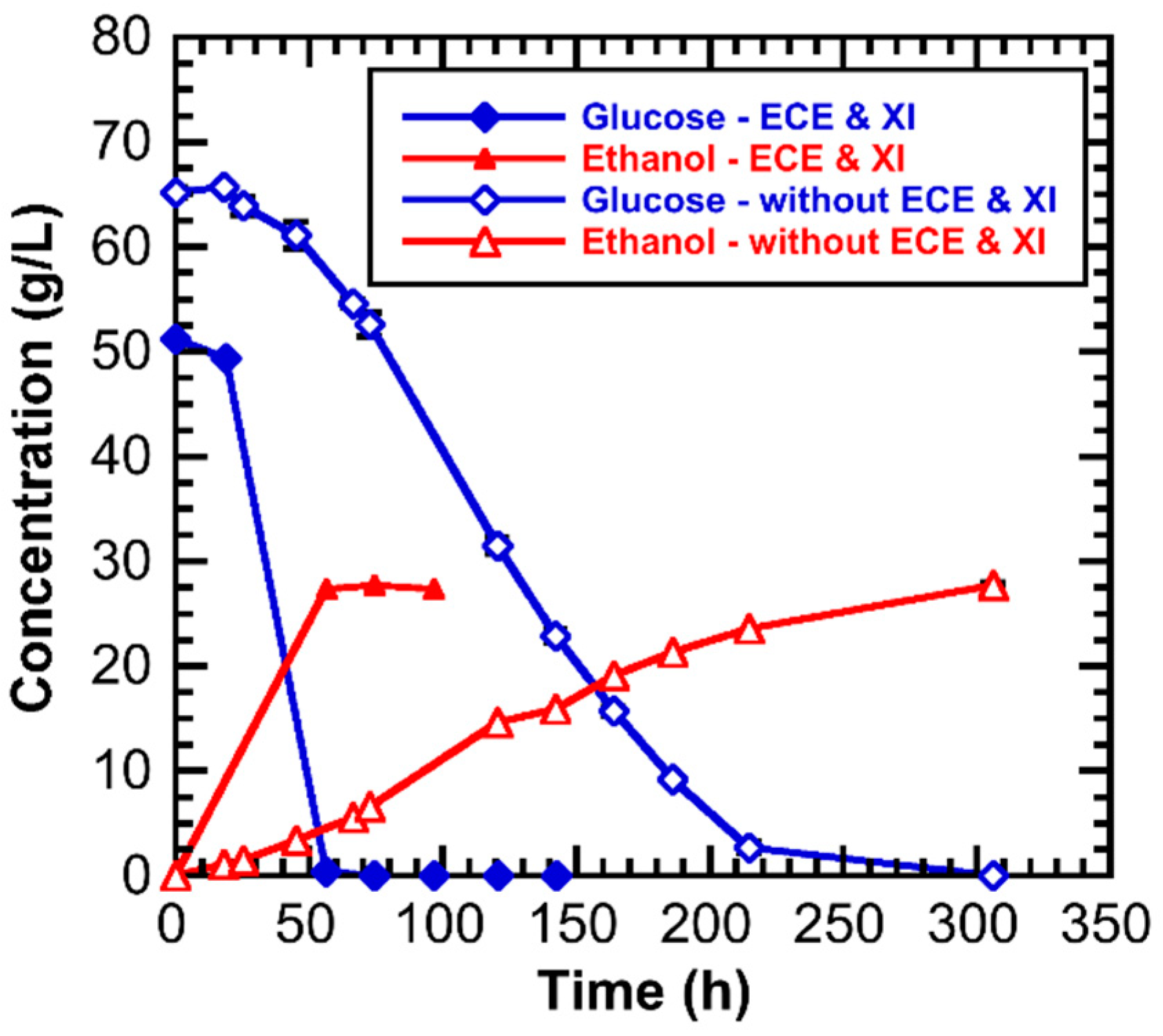
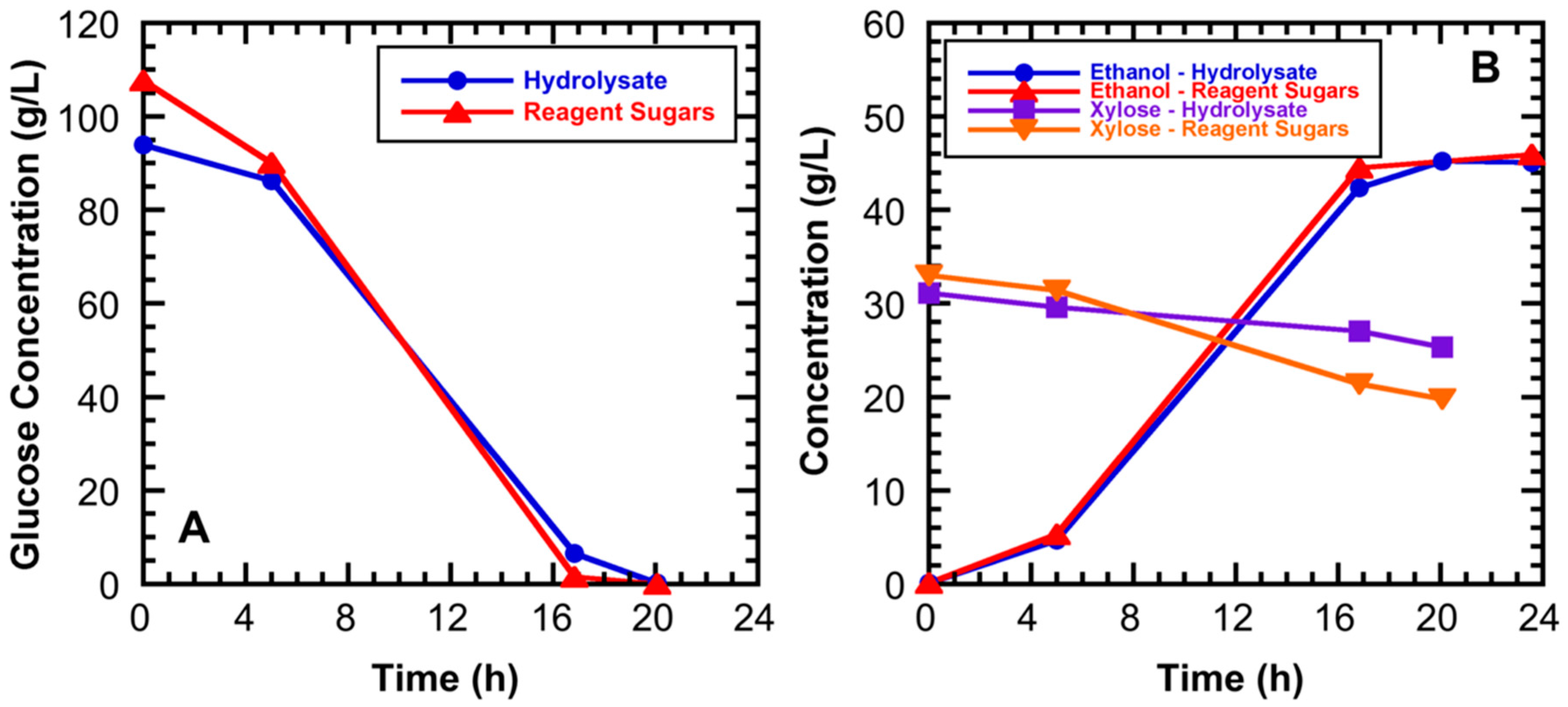
| Component | Composition (Weight %) | |
|---|---|---|
| Untreated Bagasse | Pretreated Bagasse | |
| Glucan | 41.34 ± 0.49 | 66.23 ± 0.33 |
| Xylan | 13.21 ± 0.17 | 17.19 ± 0.19 |
| Arabinan | 1.61 ± 0.14 | 2.34 ± 0.13 |
| Lignin | 20.66 ± 0.5 | 6.06 ± 0.17 |
| Ash | 6.76 ± 0.47 | 6.77 ± 0.48 |
| Extractive | 11.12 ± 0.34 | — |
| Solid residues (g) | 94.70 ± 2.12 | 98.58 ± 1.30 |
| Component | Amount in Shake Flask (25 mL) | |
|---|---|---|
| Hydrolysate | Reagent-Grade Sugar | |
| Hydrolysate (sugar concentration variable) | 21.7 mL | - |
| Water | - | 9.60 mL |
| Glucose solution (500 g/L) | - | 9.30 mL |
| Xylose solution (500 g/L) | - | 2.55 mL |
| Phosphate buffer, pH 5.8 (20×) | 1.25 mL | 1.25 mL |
| Trace metals solution (100×) | 0.25 mL | 0.25 mL |
| Iron(III) citrate solution (100×) | 0.25 mL | 0.25 mL |
| Magnesium sulfate solution (500×) | 0.05 mL | 0.05 mL |
| ECE (17 g dcw/L) | 1.50 mL | 1.50 mL |
| Xylose isomerase | 0.125 g | 0.125 g |
| YPD medium | - | 0.25 mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harcum, S.W.; Caldwell, T.P. High Gravity Fermentation of Sugarcane Bagasse Hydrolysate by Saccharomyces pastorianus to Produce Economically Distillable Ethanol Concentrations: Necessity of Medium Components Examined. Fermentation 2020, 6, 8. https://doi.org/10.3390/fermentation6010008
Harcum SW, Caldwell TP. High Gravity Fermentation of Sugarcane Bagasse Hydrolysate by Saccharomyces pastorianus to Produce Economically Distillable Ethanol Concentrations: Necessity of Medium Components Examined. Fermentation. 2020; 6(1):8. https://doi.org/10.3390/fermentation6010008
Chicago/Turabian StyleHarcum, Sarah W., and Thomas P. Caldwell. 2020. "High Gravity Fermentation of Sugarcane Bagasse Hydrolysate by Saccharomyces pastorianus to Produce Economically Distillable Ethanol Concentrations: Necessity of Medium Components Examined" Fermentation 6, no. 1: 8. https://doi.org/10.3390/fermentation6010008
APA StyleHarcum, S. W., & Caldwell, T. P. (2020). High Gravity Fermentation of Sugarcane Bagasse Hydrolysate by Saccharomyces pastorianus to Produce Economically Distillable Ethanol Concentrations: Necessity of Medium Components Examined. Fermentation, 6(1), 8. https://doi.org/10.3390/fermentation6010008






