Abstract
The degradation dynamics of lignin and cellulose were analyzed by means of a solid state biodegradation experiment, using residues from the essential oil extraction of the Palo Santo tree (Bursera graveolens). As such, two native Xylaria spp. and an exotic mushroom Trametes versicolor were incubated on the spent substrate (Residues of B. Graveolens, BGR’s). The relatively high lignin and cellulose contents of the BGRs (9.1% and 19%, respectively) indicated the potential of this resource for the production of methane (biogas) and ethanol. However, the degradation of the lignin and cellulose content could be traced back to the relatively high activity of the enzymes laccase, cellulase, and xylanase, produced by the fungi. The results showed that laccase (30.0 U/L and 26.6 U/L), cellulase (27.3 U/L and 35.8 U/L) and xylanase (189.7U/L and 128.3 U/L) activities of Xylaria feejeensis and Xylaria cf. microceras were generally higher than T. versicolor (9.0 U/L, 29.5 U/L, 99.5 U/L respectively). Furthermore, the total carbon (TC: 47.3%), total nitrogen (TN: 1.5%), total phosphorus (TP: 0.2%) and total potassium (TK: 1.2%) dynamics were analyzed during the experiment and their importance for the degradation process highlighted. The results of this work might serve as guidance for future studies in dry forest areas, while furthering the understanding of the potential use of native fungi as ecologic lignocellulosic decomposers and for industrial proposes.
1. Introduction
The Palo Santo (Bursera graveolens [Kunth] Triana & Planchon; Burseraceae) is a native, deciduous, dioecious, non-timber tree species of dry forest areas and is distributed from western Mexico to northwestern Peru. In Ecuador, B. graveolens is native in the western coastal plains and also on the Galapagos Islands [1]. Particularly, in the dry forest areas of southern Ecuador, the Palo Santo tree is the most dominant native tree species [2].
The Palo Santo is considered a vital resource for the local communities of the dry forest, as different parts of the tree are used in traditional medicine, as well as for the extraction of essential oil [3]. In addition, the wood and stalks are applied to prevent mosquito bites and to treat aches and pains of differing origins, such as fibrosarcoma, atherosclerosis, and arthritis [4]. The production of essential oil from different parts of the Palo Santo tree has grown during the last few decades due to the increase of the global demand within the cosmetic and pharmacological industry. Generally, the woody material of the tree is used for essential oil extraction. However, currently it is also extracted from the fruits, as is practiced in Ecuador.
The essential oil extraction process generates abundant organic waste, which is commonly discarded directly into the natural ecosystems or burned. The organic waste can cause environmental problems such as air pollution and/or water and soil contamination because of the low natural degradation capacity of these residues. The essential oil content of the different parts of B. graveolens is relatively high. The wood in the form of kindling contains up to 5.2% of essential oil and the shavings up to 3.4% [3]. Using the fruits, the distillation process is less efficient because the fresh fruits only contain up to 3% of essential oil. Therefore, a considerable amount of organic waste is concurrently produced (at least 95% of the fresh biomass), for which reason waste management is necessary.
One possible reuse of this waste is the production of vermicomposts for farming purposes. However, the waste must be mixed with other organic residues, like kitchen waste or animal manures, to make the product suitable for agricultural proposes [5]. Another reuse potential is enzyme production for industrial purposes by means of biodegradation of the wastes with specific fungi. Indeed, microbial enzymes are known to play a crucial role as metabolic catalysts, which is why they are frequently used in various industries among other applications. The use of enzymes is extremely wide-spread, especially in industries [6] where over 500 products are made of enzymes and about 150 industrial processes need enzymes as microbial cell catalysts [7]. Therefore, the demand for industrial enzymes is continuously rising, which leads to a growing need for sustainable solutions. Microbes are one of the largest and most useful sources of enzymes in nature [8], but research is still needed due to the immense biodiversity, particularly in tropical countries. Analyzing the diversity of potential substrates and microorganisms in different ecosystems, such as the dry forest of Ecuador, may expand the knowledge about enzyme production sources.
Some of the enzymes which are frequently used for industrial application are cellullase, laccase, and xylanase. These enzymes detoxify industrial effluents from the paper, pulp, textile, and petrochemical industries. Furthermore, they are used in medical diagnostic tools, as catalysts in drug manufacturing, and as cleaning agents for water purification systems. Additionally, these enzymes are needed as ingredients in the cosmetic industry, as well as for the bioremediation of herbicides and pesticides [9].
Cellulase is especially important as a detergent additive because it catalyzes the breakdown of chemical bonds and is used in the textile industry for cleaning processes and to reduce waste production. Besides this, cellulase contributes to the sustainable production of second generation biofuels and other chemical derivatives [10]. Laccase is used for decoloration of textile effluents and textile bleaching [11], as well as to oxidize phenolic and non-phenolic lignin-related compounds and other environmental pollutants [12]. Moreover, laccases are also used in the formulation of biofuels, biosensors and the synthesis of new hybrid molecules [13]. Xylanase is mainly needed in the pulp, paper, food, and beverage industries, as well as for the saccharification of pre-treated lignocellulosic biomass for the production of second generation biofuels [14].
In the tropics, a prominent fungus genus used for biodegradation and enzyme production is Xylaria Hill ex Schrank (Xylariaceae, Xylariales, Sordariomycetes, Ascomycota), which comprises more than 300 species [15]. Xylaria spp. are considered to be significant producers of different ligninolytic enzymes, including laccase, xylanase and cellulase [16], besides their ability to degrade lignin [17], hemicellulose [18], and cellulose from trees and agricultural residues [19,20,21,22]. Within the ecosystems, this type of fungus participates in the carbon and nitrogen cycles [23] and plays an important role in the biodegradation processes of wood and leaf litter due to its complex and diverse enzymatic system.
In other biotechnological applications, the spent substrates are analyzed, especially the amounts of lignin, hemicellulose and cellulose. Lignin and hemicellulose are used in bioconversion processes for the production of bioethanol, biogas, and other biofuel products, while cellulose is used in the manufacture of cosmetics as well as in the development of new renewable energy sources [24]. Besides these parameters, the C/N ratio of the spent substrate is important, because a high C/N ratio may cause the immobilization of nutrients, which limits some biological processes such as respiration rates and the development of microbial biomass [25]. Furthermore, inorganic nutrients (phosphorus and potassium) of spent substrates are essential during the biodegradation process, because these elements can restrict the degradation of the spent substrate and the development of the microbial cells [26].
As far as we know, residues of B. graveolens obtained during the essential oil extraction process have not been evaluated for their potential as a spent substrate for enzyme production, despite other tropical resources such as rice cane (Oryza sativa), banana stems (Musa paradisiaca), wheat straw (Triticum spp.), sugar cane (Saccharum officinarum), and olives (Olea europea) [27] being utilized. Furthermore, in Ecuador, only two fungi, namely Xylaria guianensis (Mont.) Fr. and Lentinula edodes (Berk.) Pegler were studied for enzyme production, in which the former didn’t show any enzymatic activity [28,29], which underlines the need for further biodegradation experiments with other Xylaria spp. to be carried out.
The overall objective of this study was to assess the possible biotechnological use of the Xylaria spp. using residues obtained from the Palo Santo essential oil extraction (BGR’s). For that, the degradation dynamics of lignin and cellulose of the BGR’s were analyzed by two native fungi (Xylaria spp.) of the dry forest areas of southern Ecuador, and compared to the behavior of Trametes versicolor (L.) Lloyd, due to its well-known degradation and enzyme production capacities. Furthermore, changes in the carbon, nitrogen, phosphorus, and potassium contents of the BGR’s during the degradation process were evaluated.
2. Materials and Methods
2.1. Identification of Fungi
The fruiting bodies of Xylaria spp. were collected on stumps of dead wood of B. graveolens trees in the tropical dry forest of southern Ecuador (Figure 1). The samples were analyzed and deposited in the Herbarium of the Technical University of Loja (HUTPL), Fungarium section, using taxonomic criteria [30,31]. The fungus used as control was Trametes versicolor (isolated on MEA: Malt Extract Agar), which was donated by the Spanish National Research Council (Consejo Superior de Investigaciones Científicas, CSIC). This fungus was selected as a model due to its high enzymatic activity, especially in laccase, xylanase, and cellulose, during the degradation process [27].
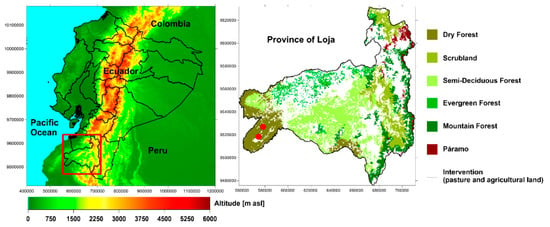
Figure 1.
Digital Elevation Model (DEM) of continental Ecuador (left) and natural ecosystems of the province of Loja (right). The map was adapted from the Ecuadorian Ministry of the Environment [32]. The red symbols indicate the sampling point of the Xylaria spp.
2.2. Isolation, DNA Extraction, PCR and Fungal Sequencing
After the taxonomic identification of the Xylaria spp. the samples were disinfected with 5% sodium hypochlorite solution for three minutes. To flush out the solution and to clean the samples, distilled water was used for three minutes. Then, a second disinfection was applied with a 70% ethanol solution for one minute. Finally, the samples were cleaned with distilled water for one minute again [20].
The disinfected ascomes of each fungus were placed separately into Petri dishes on MEA (malt extract Agar), where the cell cultures were incubated at 25 °C until the mycelia growth was completed (after seven days). After this, the individual fungi were extracted and placed into Petri dishes on MEA again to guarantee the purity of the fungal isolation.
To be sure that the individual fungal isolation only contained the required fungus species, a DNA extraction (PCR and sequencing test) was executed, following the protocol described by Iotti and Zambonelli [33] and Tamura et al. [34]. The phylogenetic location of the isolates was established by morphological observations and the DNA sequence of the ITS-5.8S region. As universal primers ITS1/LR5 or ITS1/NL4: ITS1 5′TCC GTA GGT GAA CCTGGG 3′ [35], LR5 5′TCC TGAGGG AAA CTT CG 3′ [36], NL4 5′GGT CCG TGT TTC AAG ACGG 3′ [35] were used to amplify the ITS-5.8S region. The DNA sequences of the Xylaria spp. were classified by means of the registered species in the GenBank database using BLAST searching (https://www.ncbi.nlm.nih.gov; see also (supplementary material).
2.3. Preparation and Chemical Analysis of the Spent Substrate
The spent substrate used for the degradation experiment were the fruit residues from the essential oil extraction of B. graveolens (BGR’s). The BGR’s mainly consists of the skin and seeds of the fruits, which contain fiber, water, and fatty acids [5]. The residues were obtained from the UTPL Natural Products Institute (Loja, Ecuador), where the majority of the country’s essential oil is produced.
The raw BGR’s were dried at 60 °C for 48 h to avoid contamination and biodeterioration [37]. Then, the substrate was sieved through a 2-mm mesh and the pH determined. The contents of acid detergent fiber (ADF), acid detergent lignin (ADL) and cellulose in the BGR’s were analyzed according to the method of Van Soest [38]. Briefly, the samples were digested with cetyl trimethyl ammonium bromide (CTAB) for 1 h at 150 °C and afterwards the residues washed with distilled water and then filtered (Whatman GF/C) using a vacuum pump. The retained residues in the filter were oven dried and weighed to calculate the ADF and the weight of the residues after the digestion were compared to the original weight and the difference determined. Then, a second digestion of the residues was applied using H2SO4 over 3 h at 25 °C. Afterwards, the residues were washed several times with distilled water to remove the excess acid, and then oven dried for one day at 105 °C and weighed again. The ADL was calculated by means of the percentage of the residues after the second digestion compared to the weight of the material after the first digestion. Finally, residues of the second digestion were incinerated in an oven for a period of 5 h at 500 °C to estimate the ash content. The percentage of cellulose was estimated by the difference between ADF and ADL [39].
The total carbon (TC) and total nitrogen (TN) contents of the BGR’s were measured using an auto-analyzer CHNS (Elemental Thermo Finnigan Flash EA1112 CHNS-O). The total phosphorus (TP) and total potassium (TK) contents were determined after the acid digestion following the methodology proposed by Sommer and Nelson [40]. Briefly, 200 mg of the crushed BGR’s samples were mixed with 5 mL of a perchloric acid solution (60%) and nitric acid (60%) in relation 3:5 (v/v), which was executed in a BD-40 digester block divided in two phases: 90 min at 130 °C and 75 min at 204 °C. Finally, TP and TK were measured by means of acid extractions in the Agilent 750 Series ICP-MS kit.
2.4. Solid State Fermentation
For the solid state fermentation experiment, 20 g of the BGR’s was added into 250 mL Erlenmeyer flasks and mixed with 80 mL of distilled water. To evaluate the biodegradation and enzymatic activities over time by means of the fermentation of the BGR’s, different test series of the two selected native fungi (Xylaria spp.) and the control fungus T. versicolor were prepared (20 Erlenmeyer flasks for each fungus type).
Before inoculating the fungi in their specific flask, the mycelium grew for 15 days on MEA, and all flasks containing the BGR’s were sterilized in a Gemmy SA-300VF autoclave. After cooling the flasks, the individual fungi were inoculated using 1 cm2 of each cell culture. Then, the flasks and their contents were incubated at a temperature of 25 °C, following the method proposed for Xylaria spp. by Liers et al. [41] and Rodrigues Negrão et al. [22].
The solid-state fermentation experiment lasted 60 days and samples were analyzed on day 7, 15, 30 and 60 after incubation. At each sampling day five recipients of each fungus, randomly chosen, were selected and examined, applying the quartering method [42]. Then, the five selected samples of each fungus were transferred to other recipients for lyophilization. Lyophilization was executed at −4 °C and 0.1 mm Hg using a LABCONCO equipment [43].
2.5. Chemical Analysis and Quantification of BGR’s Degradation
After lyophilization, the BGR’s were analyzed to quantify the degradation kinetics at each sampling day (7, 15, 30 and 60), applying the same methods related to the preparation and chemical analysis of the spent substrate. In the process, the degradation ratio of lignin and cellulose was calculated using the following equation [44]:
where Ri is the percentage of degradation for the sampling of the week; mo is the initial content of lignin and mi is the content of lignin sampling for each week of degradation.
Furthermore, the reduction in total carbon (TC), total nitrogen (TN), total phosphorus (TP), and total potassium (TK) of the BGR’s was determined.
2.6. Enzymatic Assay
The lyophilized samples were mixed with distilled-deionized water, filtered and centrifuged for 30 min at 7 °C and 8500 rpm, before the enzymatic analysis [45]. The laccase activity (E.C.1.10.3.2; p-diphenol: dioxygen oxidoreductase) was determined by the oxidation of syringaldazine (4-hidroxi- 3.5-dimetoxibenzaldehidacine) in a 0.22 mM methanolic solution [45,46]. The reaction was carried out at pH 6.5 to measure the corresponding quinone at a wave length of 530 nm (ε= 65,000 M−1 cm−1). The enzymatic activity of laccase was defined by the amount of enzyme that catalyzed the transformation of 1 µmol substrate per minute.
The xylanase activity was determined by measuring the reduction of glucose. Therefore, a 1% xylene solution in an acetate buffer (50 mM) was used [47,48]. The reaction was executed at a pH of 5.0 and measured at a wave length of 575 nm. To quantify the enzymatic activity, the amount of enzyme that reduced 1 µmol of xylose per minute was determined.
The cellulase activity (E.C.3.2.1.4; β-1,4-endoglucanase) was determined by measuring the formation of reduced sugar (glucose) [48,49] using carboxymethylcellulose (1%) in an acetate buffer (50 mM) as substrate. The reaction was executed at a pH of 5.0 and the product measured at a wave length 550 nm. The enzymatic activity was defined by the amount of enzyme that produced 1 µmol of glucose per minute.
In this study, the enzyme activity is expressed in terms of the volumetric activity in Units per Liter (U/L). To obtain the activity value for each enzyme (laccase, xylanase and cellulase) and for each fungus on the respective sampling day, the arithmetic mean of the five analyzed samples was calculated.
2.7. Statistical Analysis
The degradation capacity of the studied fungi inoculated on the BGR’s was evaluated by a one-way ANOVA using the SPSS Statistical Software package (v.15.0; SPSS Inc., Chicago, USA). The correlations between the measured variables were determined by the Pearson correlation coefficient; significance was accepted at p-value < 0.05 in all cases. The enzymatic activity of the fungi during the solid-state fermentation experiment was assessed through a repeated measures ANOVA. The differences between the means were evaluated through the multiple range Tukey Test (HSD) and accepted at a significance level of 5% (p-value < 0.05).
3. Results and Discussion
3.1. Identification of Fungi
The sequences of the utilized Xylaria sp. were compared to the species registered in the international GenBank (https://www.ncbi.nlm.nih.gov) to realize the phylogenetic analysis (Table 1).

Table 1.
Of accessions in the GenBank of species used for phylogenetic analyses (https://www.ncbi.nlm.nih.gov).
The results are shown in Figure 2, where two clades are presented, which indicate an accordance of over 70%, when analyzing the ITS-5.8S regions. By means of the GenBank sequences, one fungus used in this study was identified as X. feejeensis, whereas the other native fungus could not be classified precisely. The closest sequence was related to X. microceras (accession code GU300086), because the morphology was similar but the genetics different. Therefore, this fungus is probably a new species, which should be analyzed more systematically. Consequently, this fungus is named X. cf. microceras for the present study.
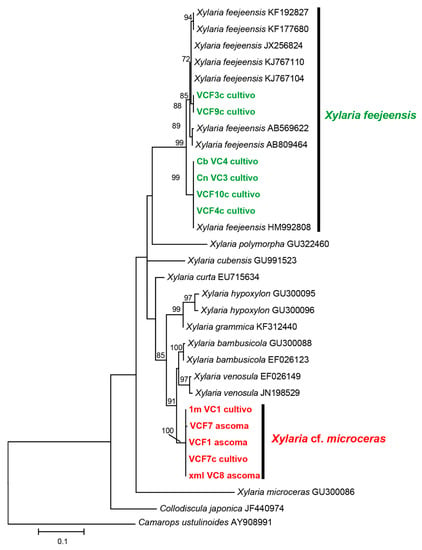
Figure 2.
Phylogenetic location of X. feejeensis (green) and X. cf. microceras (red) based on our ITS-5.8S sequences (in bold) and the most related sequences from the GenBank. Only values ≥ 70% are shown on the nodes. The sequence from Camarops ustulinoides with accession number AY908991 was used as out group.
3.2. Spent Substrate Characterization
Table 2 shows the chemical characterization of the BGR’s before the biodegradation by the three fungus species. The spent substrate had an average lignin and cellulose content of 9.1% and 19.5%, respectively. The mean TC and TN were 47.3% and 1.5 %, which resulted in an average C/N ratio of 30.4. The TP and TK contents were 0.2% and 1.2% respectively. Sulfur (S) was absent in the BGR’s, which indicates that the substrate is suitable for biodegradation because no SO2 (reactive gas) can be emitted [61]. The average pH value of the spent substrate was 7.0.

Table 2.
Biochemical properties of BGR’s in natural form, prior to inoculation with the strains (X. feejeensis, X. cf. microceras and T. versicolor) and their standard deviation based on four replicates.
The lignin content is important for methane (biogas) production [62], where garden waste (lignin: 10.5%), rice straw (10.8%), shells of Durio zibethinus (11.4%) and vinegar residues (lignin: 12.4%) are generally used [63]. The lignin content of the BGR’s (9.1%, Table 2) is slightly lower than these substrates, but higher compared to other wastes, which are also used for methane production, such as the leaves and seeds of Chenopodium album (lignin: 7.7%), its fruit and vegetable (7.9%), and seeds of Durio zibethinus (8.8%) [63,64], which makes the BGR’s suitable for methane (biogas) production.
The cellulose content is important for ethanol production [65], where generally banana peel and skin (13.2% and 9.2%, respectively) as well as rice bran are used. The BGR’s (19.5%, Table 2) had notably higher cellulose content, which indicates that ethanol can be potentially produced from the BGR’s. However, for the manufacture of cosmetics, higher cellulose contents are required [24], which can be found in fiber sorghum (Sorghum sp. 41.8%) and in rice husk (Oryza sativa, 33.0%) [66].
The TC (47.3%) and TN (1.5%) content establish the C/N ratio, which is important for biodegradation experiments, because substrates with high C/N ratios usually produce the immobilization of nutrients during the process [25]. The optimal range of the C/N ratio lies between 20 and 30, as Montingelli et al. [67] stated. For the BGR’s, a nearly optimal C/N ratio (30.4) was obtained, although the TC and TN contents were lower compared to other wastes used for oil extraction, like residues of olive fruits (Olea europaea; 58.5% TC, 1.8 % TN, C/N ratio: 31) or the seeds of the litchi (Litchi chinensis 56.1% TC, 1.1% TN; C/N ratio: 51) [68]. However, the C/N ratio of the BGR’s is much more appropriate than these spent substrates, as well as other spent substrates (e.g., rice straw, Oryza sativa; TC: 57.7, TN 0.5%, C/N ratio: 115.0) used in biodegradation experiments [69], which underlines the utility of the BGR’s. Furthermore, Motingelli et al. [67] found, that the maximum methane yield is produced when the C/N ratio is around 30, which additionally affirms the potential use of the BGR’s for biogas production.
The contents of TP and TK (Table 2) can restrict the degradation of the spent substrate, because these nutrients have an influence on the physiology and the growth of the fungi [26,70,71]. The BGR’s showed an average TP content of 0.2%, which is similar to olive residues and rice straw [68]. However, according to El-Haddad et al. [69], the optimal TP values range between 0.7% and 1.1%, which indicates a deficiency of this element in the BGR’s. The optimal range of TK lies between 1% and 3% [59], which indicates that the TK content of the BGR’s (1.2%, Table 2) is at the lower end of the optimal range, but still adequate for biodegradation experiments. Finally, the neutral pH of the BGR’s favors the development of the fungi, because pH values around 7 increase their growth, especially for Xylaria spp. [72].
3.3. Efficiency of Degradation of the Three Fungi in the BGR’s
Figure 3 shows the degradation of lignin (a) and cellulose (b) of the BGR’s in the presence of the fungi. The degradation increased for all fungi during the incubation period, reducing the original content of lignin between 15% and 34% and of cellulose between 28% and 56%.
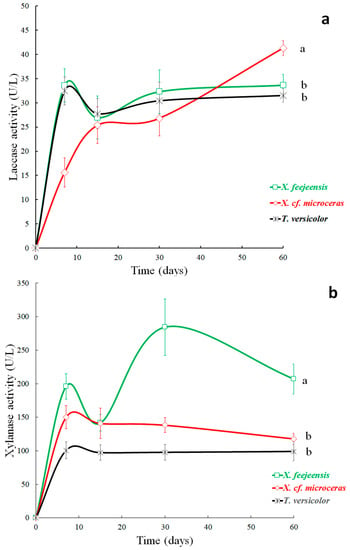
Figure 3.
Lignin (a) and cellulose (b) degradation of BGR’s by native fungi of Xylaria spp. and T. versicolor in solid state fermentation. The bars show the standard error of the mean measured activity of the four samples analyzed on each sampling day. Different letters (a,b) indicate significant difference (p ≤ 0.05%, HSD Tukey).
However, significant differences were found between the fungus types, at which T. versicolor was the most efficient fungus for lignin degradation (33.8%, Figure 3a), but the poorest for cellulose degradation (28.3%, Figure 3b). The two native fungi isolated from the Palo Santo wood, X. cf. microceras and X. feejeensis, were less effective in the degradation of lignin (Figure 3a), but demonstrated their capacity, which is also confirmed by Osono and Takeda [17] and Koide et al. [18]. These studies illustrated that Xylaria spp. have the potential to degrade lignin as well as holocellulose, because they produce selective delignification and therefore have a good ligninolytic capacity. Furthermore, the high degradation capacity of Xylaria spp. is reported by Pointing et al. [73], Chaparro et al. [20] and Rodrigues Negrão et al. [22], who consider Xylaria spp. within the group of white-rot fungi, which degrade lignin effectively.
In contrast, X. cf. microceras was the most effective fungus for cellulose degradation, reducing the original cellulose content of the BGR’s by about 56.6% during the incubation period (Figure 3b), followed by X. feejeensis (42.3%) and T. versicolor (28.3). The relatively high degradation rate of the two Xylaria spp. are consistent with findings of previous studies [73], where it was stated that all Xylariaceae taxa have high capacities to hydrolyze lignocellulosic resources.
3.4. Mineralization of TC, TN and Evaluation of C/N Ratio
During degradation of the organic matter, organic carbon is needed by the fungi as an energy and biomass source, converting it partially into CO2 and under certain circumstances also into methane (CH4). The organic nitrogen is mainly transformed into available N (nitrate and ammonium) by the fungi during the decomposition, and afterwards partially assimilated to build new biomass [74]. Therefore, after 60 days of incubation, the initial TC content of the BGR’s was strongly reduced, whereas the original TN content of the BGR’s (1.5%, Table 1) showed only small variations during the whole solid state experiment (Table 3).

Table 3.
Changes in biochemical constituents of Palo Santo waste (B. graveolens) during 60 days of solid-state fermentation, with native fungi Xylaria spp. and T. versicolor.
The TC mineralization was highest during the first seven days of incubation for all three fungi, when almost 50% of the initial TC content was degraded. However, TC content was most effectively degraded by T. versicolor (final content: 16.1%) followed by X. feejeensis (final content: 17.0%) and X. cf. microceras (final content: 19.1%), which can be traced back to the high secretion of cellulolytic enzymes of all fungi during the experiment, because the spent substrate is relatively rich in organic carbon.
The TC mineralization was positively correlated to the degradation of lignin (0.58, p < 0.01) and higher to the degradation of cellulose (0.82, p < 0.01; Table 4). In addition, the correlation between the reduction of lignin or cellulose and the decrease of the TC content in the BGR’s was significant, which was also found in other investigations [75].

Table 4.
Pearson correlation coefficient among BGR’s properties. Significant correlation is shown at p < 0.05 (*) and p < 0.01 (**).
TN content remained stable (~1.4%) during the incubation period for the three fungi (Table 3). The small variations in TN content during the mineralization process can be explained by the moderate TN content of the BGR’s. The fungi mainly used the carbon content as an energy source and to build biomass. This finding is confirmed by Rigby et al. [76], who stated that the TN content of the spent substrate is only degraded if it is needed for the metabolic requirements of the fungi (microbial cells). In this case, the TC content of the BGR’s is sufficient for the metabolic requirement and development of the fungi, while the TN content stayed more or less stable during the whole incubation period. Besides this, the moderate TN content of the BGR’s (initially; 1.5%) makes the substrate suitable as organic fertilizer, because, according to the European eco-label, an organic fertilizer should not exceed 3% of TN.
As expected, the C/N ratio decreased notably during the inoculation period (Table 3) because of the degradation of the TC content of the BGR’s [77]. The C/N ratio was positively correlated with the degradation of lignin (r = 0.56, p-value < 0.01) and cellulose (r = 0.84, p-value < 0.01), which underline the relation between TC degradation and lignin/cellulose reduction (Table 4).
3.5. Mineralization of Phosphorus and Potassium
The TP content of the three test series slightly increased from 0.2% to 0.3% during the solid state experiment (Table 3). This is caused by the transformation of the organic phosphorus (Po) into its inorganic form (Pi) [78]. The soluble Pi is afterwards incorporated into the OM of the fungi to build up biomass. These results coincide with those reported by Kuehn and Suberkropp [79], who also observed an increase of TP when inoculating different fungi in decaying litter of the Juncus effusus. The optimal range of TP content in organic fertilizer lies between 0.15% and 1.5%, which makes the BGR’s an adequate resource for soil improvers.
The initial TK content of the BGR’s (1.2%, Table 2) was reduced to 50% after the first 15 days of incubation by all three fungi, and afterwards the values increased again, reaching the final value of approximately 0.8% on the last sampling day (Table 3). Potassium is a very mobile and unstable nutrient, which is needed by the fungi particularly at the beginning of the degradation process [80]. However, the increase of TK at the end of the incubation period was probably due to the mineralization of the OM and the production of CO2 by the fungi. The typical range of potassium in organic fertilizers is 0.4-1.6%, which indicates that the BGR’s do not present a deficiency in this nutrient. Furthermore, the normal TK content of the BGR’s induces a good C/N balance, because TK plays an important role in carbon (C) and nitrogen metabolism (N) [81].
3.6. Enzyme Activities
The degradation of lignin and cellulose is a consequence of the enzymatic activity of the fungi, which is mainly caused by the production of the enzymes laccase, xylanase and cellulase [44,82]. Figure 4 shows the variation in laccase (a), xylanase (b) and cellulase (c) activities for the individual fungus types during the incubation period.
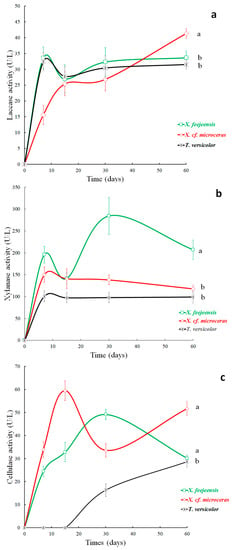
Figure 4.
Laccase (a), xylanase (b) and cellulase (c) activities in BGR’s with native species of Xylaria spp. and T. versicolor incubated at 25 °C. The bars show the standard error of the mean measured activity of the four samples analyzed on each sampling day. Different letters indicate significant difference (p ≤ 0.05%, HSD Tukey).
3.6.1. Laccase Activity
Laccase activity was detected for all three fungi on the first sampling day (Figure 4a), which was probably due to the absence of sulfur (S) and the moderate contents of TC and TN of the BGR’s (Table 2), facilitating the immediate decomposition of lignin [83]. The temporal variability of laccase activity of X. feejeensis and T. versicolor were similar, increasing notably during the first seven days of incubation (33.5 U/L and 32.4 U/L; respectively), and afterwards remaining more or less stable until the end of the incubation period (final values: of 33.7 U/L, X. feejeensis; 31.5 U/L, T. versicolor). X. cf. microseras showed a different behavior, especially during the first seven days of incubation, when the lowest laccase activity of all three fungi was detected (15.6 U/L), and during the end of the incubation period, when the laccase activity of X. cf. microceras increased notably, reaching 41.3 U/L, which was the highest value of all test series during the complete observation period.
However, the variability in the production of the laccase enzyme was expected, because, as Dong et al. [44] showed, laccase acts synergistically with other lignin-degrading enzymes such as polyphenol oxidase (PPO) and manganese peroxidase (MnP).
The observed laccase activity of the three studied fungi seems to be low in comparison to other studies [41], but these investigations applied liquid fermentation (liquid spent substrates) and used 2.5-xylidine or veratryl alcohol as an enzymatic inductor, which increases the laccase activity notably [84]. The solid-state experiment of this study did not utilize any enzyme inductor, which explains the relatively low laccase activity of T. versicolor and the Xylaria spp. However, investigations using solid substrates obtained similar activity values as observed here [20,85]. Generally, all Xylaria spp. have an unusually high ability to degrade lignin compared to other Ascomycota, as Liers et al. [23] and Rodrígues Negrão et al. [22] stated, because Xylaria spp. mineralize lignin almost as efficiently as the aggressive fungi of white-rot (e.g., T. versicolor).
Besides the solid fermentation applied, the moderate laccase enzyme activity might be due to the low lignin content of the BGR’s (9.1%; Table 2), which cause a reduction in laccase production. As Coronel et al. [85] indicated, the enzymatic activity is highly influenced by the chemical composition of the substrate where the fungus was incubated. Therefore, average laccase activity of all fungi was more or less similar (X. feejeensis: 30.0 U/L; T. versicolor: 29.5 U/L; X. cf. microceras: 26.6 U/L). However, the statistical analysis showed that a significant difference between X. cf. microceras and the other two fungi (X. feejeensis and T. versicolor) respective to laccase activity exists (p-value < 0.05; Figure 4a, different letters), whereas no difference was found between X. feejeensis and T. versicolor (Figure 4a, similar letters).
3.6.2. Xylanase Activity
Xylanase also act synergistically with other hydrolytic enzymes, such as esterase, to modify the structural configuration of lignocelluloses [44], which is necessary to make the cellulose accessible for degradation. For example, esterase, in combination with xylanase, cleaves covalent bonds between polysaccharides or hemicelluloses, and therefore plays a key role in the degradation of the hemicellulose matrix [82]. The preliminary degradation of the lignin-hemicellulose matrix is proven by the present study because xylanase activity was detected in all three fungi species on the first sampling day, reaching values between 100.7 U/L and 196.0 U/L (Figure 4b).
During the complete observation period, the xylanase activity of X. feejeensis was always highest compared to the other two fungi, except on day 15 when the activity of X. feejeensis decreased and similar values for both Xylaria spp. were measured (140.8 U/L and 141.6 U/L). Highest activity of X. feejeensis was observed on day 30 with 284.3 U/L, but afterwards activity decreased. The xylanase activity of X. cf. microceras and T. versicolor started with lower values (150.3 U/L and 100.7 U/L respectively), but their activity curves showed similar behaviors (Figure 4b), decreasing slightly between day 7 and day 15 after incubation and then remained almost stable during the rest of the observation period, reaching final values of 117.9 U/L (X. cf. microceras) and 99.0 U/L (T. versicolor). Statistically significant differences were found between X. feejeensis and the other two fungi species, but differences between X. cf. microceras and T. versicolor were not significant (Figure 4b).
In general, X. feejeensis and X. cf. microceras showed higher xylanase activity compared to T. versicolor, but also to other Xylaria spp. which were inoculated on solid-state experiments [23]. All Xylaria spp. can degrade hemicellulose effectively [18,19], but the two Xylaria spp. studied here showed even higher activity values than those reported in the literature and therefore might be suitable for commercial xylanase production required for industrial processes in the paper, food and wine industry. T. versicolor also showed high xylanase activity, although the xylanase production of this fungus species is typically low and an inducible mechanism (enzyme inductor) is needed to increase the activity. Irbe et al. [86] used glycerol alcohol as an inductor and observed a notable increase in xylanase activity and enzyme production. However, in this study no inductor was applied, but xylanase activity was high for all three fungi, which is probably due to the xylan content of the BGR’s. Xylan is the second most abundant hemicellulosic polysaccharide in nature and present in the cell walls of the plants [43]. The residues from the Palo Santo essential oil extraction consist mainly in parts of the fresh fruits [5] and therefore the xylan content was not reduced by other degradation processes, which might explain the high xylanase activity of all three fungi.
3.6.3. Cellulase Activity
As expected, cellulase activity was delayed (Figure 4c), because the lignin-hemicellulose matrix had to be degraded first to make the cellulose accessible [44], which indicates that cellulase is an inducible enzyme [87]. As Arantes and Sadler [88] stated, the cellulose regions are tightly packed with lignin and hemicellulose, which is the major contributing factor to cellulose resistance to degradation. Therefore, amorphogenesis, a process consisting of the degradation of lignin and hemicellulose, is needed to liberate the cellulose, which afterwards can be degraded by cellulase.
Cellulase activity of the two Xylaria spp. was detected on the first sampling day, in contrast to T. versicolor, where significant activity was not measured until day 30 (Figure 4c). The X. feejeensis fungus reached its maximum activity (49.2 U/L) on day 30, whereas X. cf. microceras peaked on day 15 (59.5 U/L), which was concurrently the highest value of all three fungi species during the entire observation period. T. versicolor did not show any cellulase activity until day 15 of incubation and afterwards displayed an almost linear increase to its maximum and final value of 28.7 U/L on the last sampling day.
In general, the cellulase enzyme system consists of three types of enzymes; endo-1,4-β-glucanase (cellulase), cellobiohydrolase or exoglucanases (avicelase), and β-glucosidase (cellobiase), which act in conjunction to degrade the cellulose content of the spent substrate [44]. As Du et al. [89] showed, T. versicolor first degrades cellulose through the enzyme cellobiase (β-glucosidase), and later in combination which the enzyme cellulase, for which reason a delay in cellulase activity of T. versicolor was observed. In contrast, the Xylaria spp. produced the enzyme cellulase immediately after incubation to degrade the cellulose content.
As is also shown in Figure 4c, maximum values of cellulase activity of the individual fungi were not reached simultaneously, due to the different metabolic requirements of each fungus. The difference may be due to the fungus type used (Basidiomycota and Ascomycota), because T. versicolor is a fungus of white-rot, which is specifically indicated in the degradation of lignin. Furthermore, the lower cellulase activity of T. versicolor might be a consequence of the substrate used (solid), because T. versicolor can produce up to 100 U/L of cellulase when inoculated in liquid mediums [90].
The differences in the cellulase activity are also depicted by the repeated measures ANOVA, in which no significant differences between X. feejeensis and X. cf. microceras were found, but differences between the Xylaria spp. and T. versicolor were significant (p-value < 0.05). In general, average cellulase production of the Xylaria spp. was notably higher (27.3 U/L to 35.8 U/L) compared to T. versicolor (9.0 U/L), especially during the first 30 days of incubation.
The high cellulase activity of the two Xylaria spp. is consistent with results from previous investigations comparing the enzymatic production of different fungi species [19,73]. These studies demonstrated that Xylaria spp. are potential producers of cellulolytic enzymes, due to their high cellulolytic activity, and therefore can be used in biotechnological applications as well as for industrial purposes. This is confirmed by Gutiérrez-Soto et al. [16], who measured the cellulase activity of Xylaria spp. up to 199 U/L. However, these studies incubated the fungi on liquid mediums; in solid spent substrates, the activity is generally lower [23]. The variation in cellulase enzyme activity of Xylaria spp. apparently depends on two factors: first, the species [73] and second, the type of substrate [19].
4. Conclusions
The content of lignin and cellulose in the BGR’s makes the substrate suitable for biotechnological applications, especially for the production of methane and ethanol. Furthermore, the contents of the macro nutrients were within the optimal range, and therefore the BGR’s can be applied to soils as fertilizers.
The BGR’s were also suitable for the production of enzymes for industrial purposes by means of fungal degradation. The native Xylaria spp. showed generally higher enzymatic activity than the control fungus and were especially practical for the production of xylanase and cellulase.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2311-5637/5/3/76/s1.
Author Contributions
Formal analysis, V.C.-P. and A.F.; Investigation, V.C.-P.; Methodology, V.C.-P., R.E.C. and R.G.-R.; Supervision, R.G.-R.; Validation, P.P.D.; Writing—original draft, V.C.-P., R.E.C. and R.G.-R.; Writing—review & editing, V.C.-P., A.F., P.P.D. and R.G.-R.
Funding
This research was funded by the Secretary of Science and Technology of Ecuador (SENESCYT—CEREPS—2007) and the Universidad Técnica Particular de Loja (UTPL).
Acknowledgments
We would like to thank the Secretary of Science and Technology of Ecuador (SENESCYT), the Ecuadorian Ministry of the Environment (MAE, research permits 015-IC-UPN-DRLEOZCH-MA and 027-2013-DPL-MA) and the Department of Biological Sciences of the Universidad Técnica Particular de Loja for their support. Special gratitude to the Spanish National Research Council (Consejo Superior de Investigaciones Científicas, CSIC) for their generous contribution. Finally, we would like to thank Gregory Gedeon for text revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weeks, A.; Tye, A. Phylogeography of palo santo trees (Bursera graveolens and Bursera malacophylla; Burseraceae) in the Galápagos archipelago. Bot. J. Linn. Soc. 2009, 161, 396–410. [Google Scholar] [CrossRef][Green Version]
- Carrión-Paladines, V.; García-Ruiz, R. Floristic composition and structure of a deciduous dry forest from southern Ecuador: Diversity and aboveground carbon accumulation. Int. J. Curr. Res. Acad. Rev. 2016, 4, 154–169. [Google Scholar] [CrossRef][Green Version]
- Yukawa, C.; Imayoshi, Y.; Iwabuchi, H.; Komemushi, S.; Sawabe, A. Chemical composition of three extracts of Bursera graveolens. Flavour Frag. J. 2006, 21, 234–238. [Google Scholar] [CrossRef]
- Alonso-Castro, A.; Villareal, M.; Salazar-Olivo, L.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Paladines, V.; Fries, A.; Gómez-Muñoz, B.; García-Ruiz, R. Agrochemical characterization of vermicomposts produced from residues of Palo Santo (Bursera graveolens) essential oil extraction. Waste Manag. 2016, 58, 135–143. [Google Scholar]
- Schmid, A.; Hollmann, F.; Park, J.B.; Bühler, B. The use of enzymes in the chemical industry in Europe. Curr. Opin. Biotechnol. 2002, 13, 359–366. [Google Scholar] [CrossRef]
- Johannes, T.; Zhao, H. Directed evolution of enzymes and biosynthetic pathways. Curr. Opin. Microbiol. 2006, 9, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech. 2016, 6, 3–15. [Google Scholar] [CrossRef]
- Demain, A.; Adrio, J. Contributions of microorganisms to industrial biology. Mol. Biotechnol. 2008, 38, 41–55. [Google Scholar] [CrossRef]
- Trivedi, N.; Reddy, C.R.K.; Radulovich, R.; Jha, B. Solid state fermentation (SSF)-derived cellulase for saccharification of the green seaweed Ulva for bioethanol production. Algal Res. 2015, 9, 48–54. [Google Scholar] [CrossRef]
- Araújo, R.; Casal, M.; Cavaco-Paulo, A. Application of enzymes for textile fibres processing. Biocatal. Biotransform. 2008, 26, 332–349. [Google Scholar]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, P.; Feitosa, V.; Brenelli de Paiva, L. Laccase production in biorreactor scale under saline condition by the marine-derived basidiomycete Peniophora sp. CBMAI 1063. Fungal Biol. 2018, 122, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; del Río, J.; Martínez, A. Microbial and enzymatic control of pitch in the pulp and paper industry. Appl. Microbiol. Biotechnol. 2009, 82, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Trierveiler-Pereira, L.; Romero, A.; Baltazar, J.; Loguercio-Leite, C. Addition to the knowledge of Xylaria (Xylariaceae, Ascomycota) in Santa Catarina, Southern Brazil. Mycotaxon 2008, 107, 139–156. [Google Scholar] [CrossRef]
- Gutiérrez-Soto, G.; Medina-González, G.; Treviño-Ramirez, J.; Hernández-Luna, C. Native macrofungi that produce lignin-modifyieng enzymes, cellulases, and xylanases with potential biotechnological applications. Bioresources 2015, 10, 6676–6689. [Google Scholar]
- Osono, T.; Takeda, H. Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 2002, 94, 421–427. [Google Scholar] [CrossRef]
- Koide, K.; Osono, T.; Takeda, H. Fungal succession and decomposition of Camellia japonica leaf litter. Ecol. Res. 2005, 20, 599–609. [Google Scholar] [CrossRef]
- Bezerra, J.; Santos, M.; Svedese, V.; Lima, D.; Fernandes, M.; Paiva, L.; Souza-Motta, C. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J. Microbiol. Biotechnol. 2012, 28, 1989–1995. [Google Scholar] [CrossRef]
- Chaparro, D.; Rosas, D.; Varela, A. Aislamiento y evaluación de la actividad enzimática de hongos descomponedores de madera (Quindío, Colombia). Rev. Iberoam. Micol. 2009, 26, 238–243. [Google Scholar] [CrossRef]
- Moissenko, K.; Vasina, D.; Farukshina, K.; Savinova, O.; Glazunova, O.; Fedorova, T.; Tyashelova, T. Orchestration of the expression of the laccase multigene family in white-rot basidiomycete Trametes hirsuta 972: Evidences of transcription level subfunctionalization. Fungal Biol. 2018, 122, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Negrão, D.; Fernandes da Silva J#xFA;nior, T.; de Souza Passos, J.; Angeli Sansílogo, C.; de Almeida Minhoni, M.; Furtado, E. Biodegradation of Eucalyptus urograndis wood by fungi. Int. Biodeterior. Biodegrad. 2014, 89, 95–102. [Google Scholar]
- Liers, C.; Ullrich, R.; Steffen, K.T.; Hatakka, A.; Hofrichllter, M. Mineralization of 14C-labelled synthetic lignin and extracellular enzyme activities of the wood-colonizing ascomycetes Xylaria hypoxylon and Xylaria polymorpha. Appl. Microbiol. Biotechnol. 2006, 69, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S. Cellulose: To depolymerize or not to? Biotechnol. Adv. 2017, 35, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, C.; Mazzarino, M.; Laos, F. Improving the quality of municipal organic waste compost. Bioresour. Thechnol. 2006, 98, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Dave, B.P. Effect of addition of nitrogen, phosphorus and potassium fertilizers on biodegradation of crude oil by marine bacteria. Indian J. Mar. Sci. 2010, 39, 143–150. [Google Scholar]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautováa, V.; Zervakis, G. Olive mill wastewater biodegradation potential of white-rot fungi—Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Camacho, R.; Evans, C.; Hedger, J. Production of ligninolytic enzymes by species assemblages of tropical higher fungi from Ecuador. In Tropical Mycology; Watling, R., Frankland, J.C., Ainsworth, A.M., Isac, S., Robinson, C.H., Eds.; CABI Publishing: Wallingford, UK, 2002; Volume 1, pp. 101–112. [Google Scholar]
- Vaca, M.; Izurieta, B.; Espín, N. Obtención de extractos enzimáticos con actividad celulolítica y ligninolítica a partir del hongo Pleurotus ostreatus 404 y 2171 en rastrojo de maíz. Revista Politec. 2014, 33, 2. [Google Scholar]
- Hladki, A.; Romero, A. A preliminary account of Xylaria in the Tucuman Province, Argentina, with a key to the known species from the northern provinces. Fungal Divers. 2010, 42, 79–96. [Google Scholar] [CrossRef]
- Medel, R.; Castillo, R.; Guzmán, G. Adiciones al conocimiento de Xylaria (Ascomycota, Xylariales) en México. Rev. Mex. Micol. 2010, 31, 9–18. [Google Scholar]
- Ministerio del Ambiente de Ecuador (MAE). Mapa de Cobertura y Uso de la Tierra. Available online: http://www.ambiente.gob.ec (accessed on 24 April 2017).
- Iotti, M.; Zambonelli, A. A quick and precise technique for identifying ectomycorrhizas by PCR. Mycol. Res. 2006, 110, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, N., Gelfand, D., Sninsky, J., White, J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Ruqayyah, T.; Jamal, P.; Alam, Z.; Mirghani, E. Biodegradation potential and ligninolytic enzyme of two locally isolated Panus tigrinus strain on selected agro-industrial wastes. J. Environ. Manag. 2013, 118, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds II: A rapid method for the determination of fibre and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage fiber analyses (apparatus, reagents, procedures, and some applications). In Agriculture Handbook No. 379; U.S. Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Sommer, L.; Nelson, D. Determination of total phosphorus in soils: A rapid perchloric acid digestion procedure. Soil Sci. Soc. Am. Proc. 1972, 53, 32–37. [Google Scholar] [CrossRef]
- Liers, C.; Ullrich, R.; Pecyna, M.; Schlosser, D.; Hofrichter, M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzyme Microb. Tech. 2007, 41, 785–793. [Google Scholar] [CrossRef]
- Roé-Sosa, A.; Estrada, M.; Calderas., F.; Sánchez-Arévalo, F.; Manero, O.; Orta, L.; de Velasquez, M.T. Degradation and biodegradation of polyethylene whit pro-oxidant additives under compost conditions establishing relationships between physicochemical and rheological parameters. J. Appl. Polym. Sci. 2015, 42721, 1–11. [Google Scholar]
- Wei, D.; Chou, H.; Cheng, M.; Chang, S. Purification and characterization of xylanase from Xylaria regalis. Fung. Sci. 2005, 20, 53–59. [Google Scholar]
- Dong, X.Q.; Yang, J.S.H.; Zhu, N.; Wang, E.T.; Yuan, H.L. Sugarcane bagasse degradation and characterization of three white-rot fungi. Bioresour. Technol. 2013, 131, 443–451. [Google Scholar] [CrossRef]
- Mata, G.; Savoie, J. Extracelullar enzyme activities in six Lentinula edodes strains during cultivation in wheat straw. World J. Microb. Biot. 1998, 14, 513–519. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Papadopoulou, K.; Lakhtar, H.; Roussos, S.; Parissopoulos, G.; Papanikolaou, S. Biomass, laccase and endoglucanase production by Lentinula edodes during solid state fermentation of reed grass, bean stalks and wheat straw residues. World J. Microb. Biot. 2011, 27, 285–297. [Google Scholar] [CrossRef]
- Sadaf, A.; Khare, S.K. Production of Sporotrichum thermophile xylanase by solid state fermentation utilizing deoiled Jatropha curcas seed cake and its application in xylooligosachharide synthesis. Bioresour. Technol. 2014, 153, 126–130. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosaIicyIic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Singhania, R.; Saini, J.K.; Saini, R.; Adsul, M.; Mathur, A.; Gupta, R.; Tuli, D.K. Bioethanol production from wheat straw via enzymatic route employing Penicillium janthinellum cellulases. Bioresour. Technol. 2014, 169, 490–495. [Google Scholar] [CrossRef]
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KF192827 (accessed on 24 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/JX256824 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KJ767110 and KJ767104 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/AB569622 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/AB809464 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/HM992808 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/GU322460, GU991523, GU300095, GU300088, EF026123, EF026149 and GU300086 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/EU715634 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/JN198529 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/JF440974 (accessed on 14 June 2016).
- The National Center for Biotechnology Information Advances Science and Health by Providing Access to Biomedical and Genomic Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KT250967, KT250968, KT250969, KT250970, KT250971, KT250972, KT250973, KT250974, KT250975, KT250976 and KT250977 (accessed on 14 June 2016).
- Zhang, H.; Schuchardt, F.; Li, G.; Yang, J.; Yang, Q. Emission of volatile sulfur compounds during composting of municipal solid waste (MSW). Waste Manag. 2013, 33, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, Y.; Li, Y. Fungal pretreatment of yard trimmings for enhancement of methane yield from solid-state anaerobic digestion. Bioresour. Technol. 2014, 156, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yan, H.; Liu, Y.; Huang, Y.; Zhang, R.; Chen, C.; Liu, G. Bio-energy conversion performance, biodegradability, and kinetic analysis of different fruit residues during discontinuous anaerobic digestion. Waste Manag. 2016, 52, 295–301. [Google Scholar] [CrossRef]
- Santos Michel, R.J., Jr.; Canabarro, N.I.; Alesio, C.; Maleski, T.; Laber, T.; Sfalcin, P.; Foletto, E.; Mayer, F.D.; Kuhn, R.C.; Mazutti, M. Enzymatic saccharification and fermentation of rice processing residue for ethanol production at constant temperature. Biosyst. Eng. 2016, 142, 110–116. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Tedesco, S.; Olabi, A.G. Biogas production from algal biomas: A review. Renew. Sustain. Energy Rev. 2015, 43, 961–972. [Google Scholar] [CrossRef]
- Sampedro, I.; Marinari, S.; D’Annibale, A.; Grego, S.; Ocampo, J.; García-Romera, I. Organic matter evolution and partial detoxification in two-phase olive mill waste colonized by white-rot fungi. Int. Biodeterior. Biodegrad. 2007, 60, 116–125. [Google Scholar] [CrossRef]
- El-Haddad, M.; Zayed, M.; El-Sayed, G.; Hassanein, M.; El-Satar, A. Evaluation of compost, vermicompost and their teas produced from rice straw as affected by addition of different supplements. Ann. Agric. Sci. 2014, 59, 243–251. [Google Scholar] [CrossRef]
- Bullerman, L. Effects of potassium sorbate on growth and aflatoxin production by Aspergillus parasiticus and Aspergillus flavus. J. Food Protect. 1983, 46, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Eze, J.M. Translocation of phosphate in mould mycelia. New Phytol. 1975, 75, 579–581. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, G.; Zhang, R.; Hu, D.; Wang, H.; Ng, T. A novel aspartic protease with HIV-1 reverse transcriptase inhibitory activity from fresh fruiting bodies of the wild mushroom Xylaria hypoxylon. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pointing, S.; Parungao, M.; Hyde, K. Production of wood-decay enzymes, mass loss and lignin solubilization in wood by tropical Xylariaceae. Mycol. Res. 2003, 107, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Digby, A.; Gleason, F.; Mcgee, P. Some fungi in the Chytridiomycota can assimilate both inorganic and organic sources of nitrogen. Fungal Ecol. 2010, 3, 261–266. [Google Scholar] [CrossRef]
- Fogarty, W.; Kelly, C. Microbial Enzymes and Biotechnology; Fogarty, W.M., Kelly, C., Eds.; Springer: Dordrecht, The Netherlands, 1990; pp. 71–472. [Google Scholar]
- Rigby, H.; Clarke, B.; Pritchard, D.; Meehan, B.; Beshah, F.; Smith, S.; Porter, N. A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment. Sci. Total Environ. 2016, 541, 1310–1338. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Ngo, P.; Rumpel, C.; Ngo, Q.; Alexis, M.; Velásquez Vargas, G.; Mora Gil, M.; Dang, D.; Jouquet, P. Biological and chemical reactivity and phosphorus forms of buffalo manure compost, vermicompost and their misture with biochar. Bioresour. Technol. 2013, 148, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, K.A.; Suberkropp, K. Decomposition of standing litter of the freshwater emergent macrophyte Juncus effuses. Freshw. Biol. 1998, 40, 717–727. [Google Scholar] [CrossRef]
- Sujatha, S.; Bhat, R. Impacts of vermicompost and nitrogen, phosphorus, and potassium application on soil fertility status in arecanut grown on a laterite soil. Commun. Soil Sci. Plant Anal. 2012, 43, 2400–2412. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.; Loka, D.; Oosterhuis, D.; Zhou, Z. Potassium deficiency effects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Dinis, M.J.; Bezerra, R.M.; Nunes, F.; Dias, A.; Guedes, C.; Ferreira, L.M.; Cone, J.W.; Marques, G.; Barros, A.; Rodrigues, M. Modification of wheat straw lignin by solid state fermentation with-rot fungi. Bioresour. Technol. 2009, 100, 4829–4835. [Google Scholar] [CrossRef]
- Osada, M.; Hiyoshi, N.; Sato, O.; Arai, K.; Shirai, M. Effect of sulfur on catalytic gasification of lignin in supercritical water. Energy Fuels 2007, 21, 1400–1405. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlant, P. Fungal laccase: Production, function, and applications in food processing. Enzyme Res. 2010. [Google Scholar] [CrossRef]
- Coronel, L.M.; Joson, L.M.; Mesina, O.G. Isolation and screening of thermophilic fungi for cellulose production. Philipp. J. Sci. 1991, 120, 379–389. [Google Scholar]
- Irbe, I.; Elisashvili, V.; Asatiani, M.; Janberga, A.; Andersone, I.; Andersons, B.; Biziks, V.; Grinins, J. Lignocellulolytic activity of Coniophora puteana and Trametes versicolor in fermentation of wheat bran and decay of hydrothermally modified hardwoods. Int. Biodeterior. Biodegrad. 2014, 86, 71–78. [Google Scholar] [CrossRef]
- Sukumaran, R.; Singhania, R.; Pandey, A. Microbial cellulases—Production, applications and challenges. J. Sci. Ind. Res. India 2005, 64, 832–844. [Google Scholar]
- Arantes, V.; Saddler, J. Access to cellulose limits the efficiency of enzymatic hydrolysis: The role of amorphogenesis. Biotechnol. Biofuels 2010, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Pu, G.; Shao, C.; Cheng, S.; Cai, J.; Zhou, L.; Jía, Y.; Tian, X. Potencial of extracellular enzymes from Trametes versicolor F21a in Mycrocystis spp. degradation. Mater. Sci. Eng. C 2015, 48, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.; Vega, M.; Lienqueo, M.; Garcia, A.; Carmona, R.; Salazar, O. Cloning of novel cellulases from cellulolytic fungi: Heterologous expression of a family 5 glycoside hidrolase from Trametes versicolor in Pichia pastoris. Enzyme Microb. Tech. 2011, 49, 485–491. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).