Development of A Low-Alcoholic Fermented Beverage Employing Cashew Apple Juice and Non-Conventional Yeasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Cashew Apple Juice
2.1.1. Plant Materials
2.1.2. Juice Extraction and Clarification
2.1.3. Sugar Determination
2.1.4. Total Polyphenol Determination
2.1.5. Ascorbic Acid Determination
2.2. Fermentation of Cashew Apple Juice
2.2.1. Yeast Strains
2.2.2. Yeast Screening
2.2.3. Growth Kinetics
2.2.4. Ethanol Determination
2.2.5. Aroma Analysis
2.2.6. Other Analytical Determinations
2.3. Statistical Analysis
3. Results and Discussion
3.1. Composition of Clarified Cashew Apple Juice
3.1.1. Sugar Content
3.1.2. Total Polyphenol
3.1.3. Ascorbic Acid
3.2. Yeast Screening
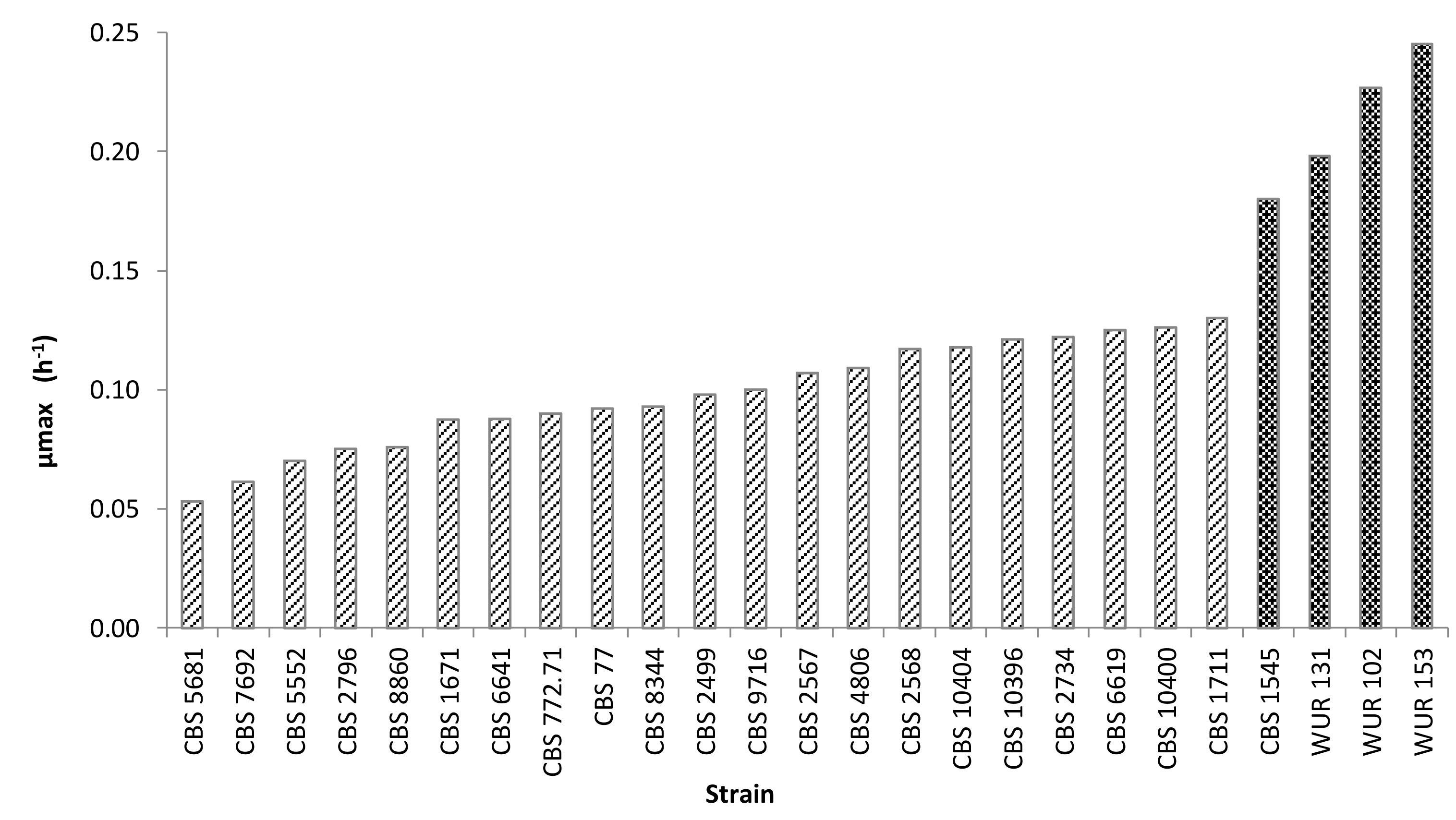
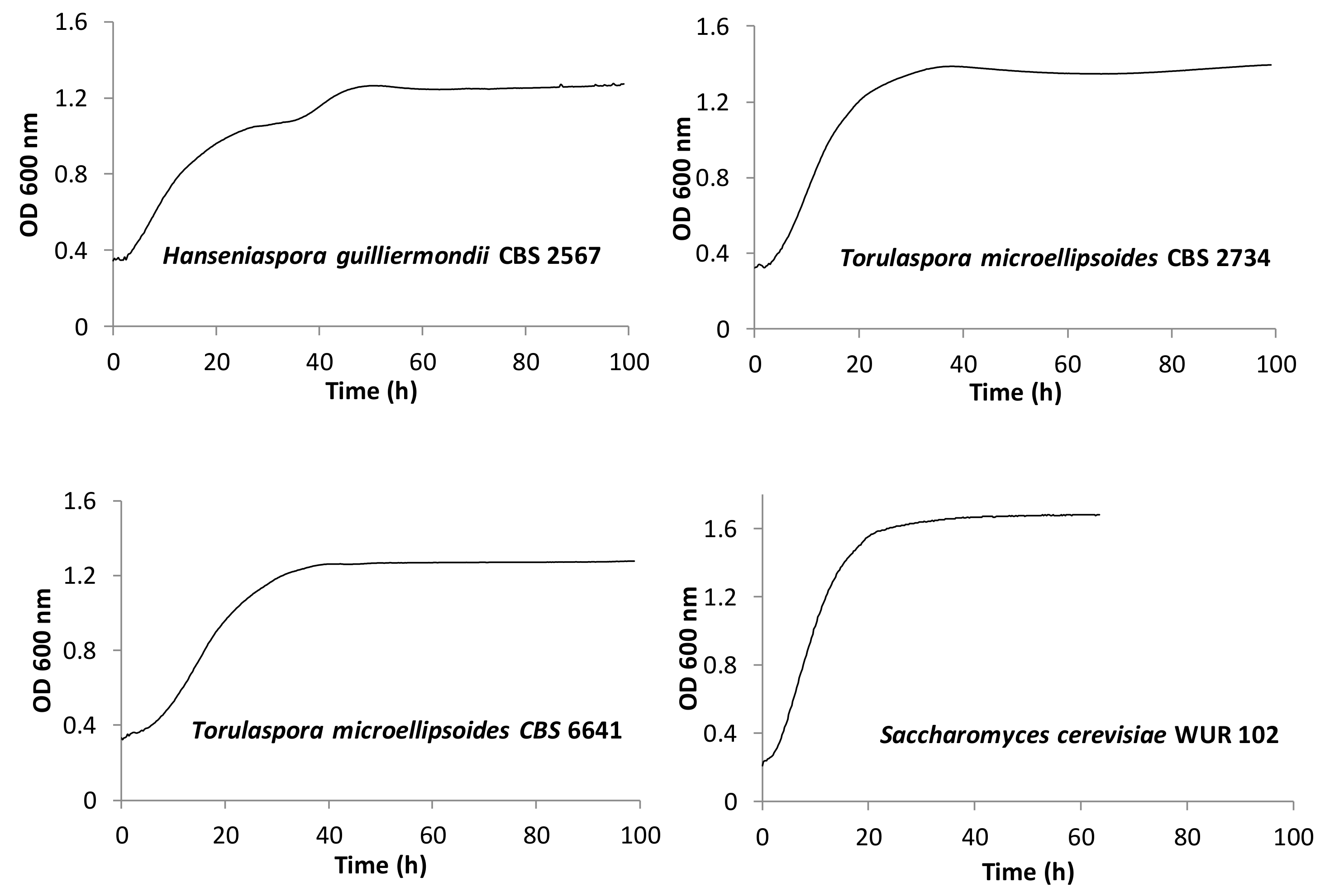
3.2.1. Growth Kinetics
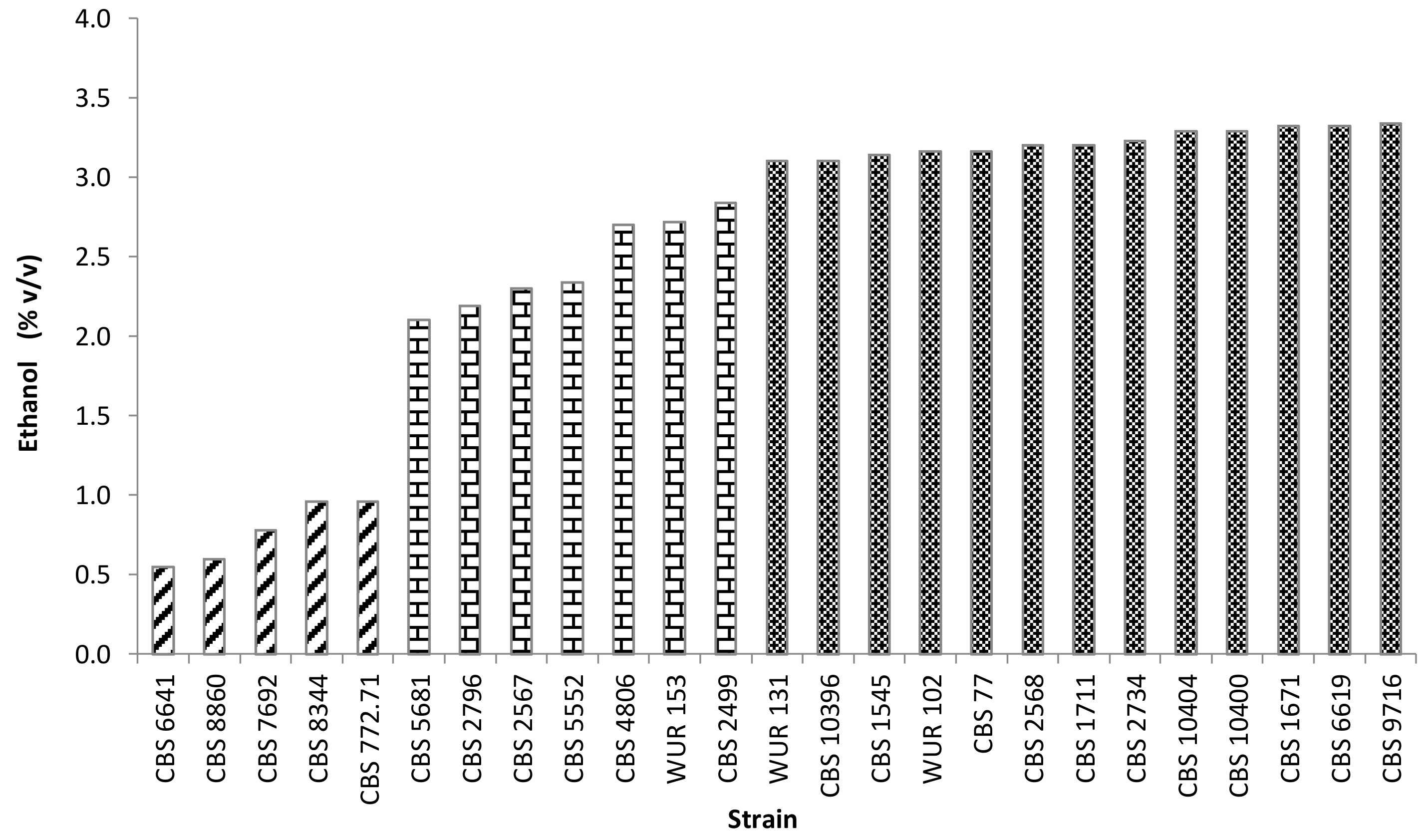
3.2.2. Sugar Conversion to Ethanol
3.2.3. Yeast Selection
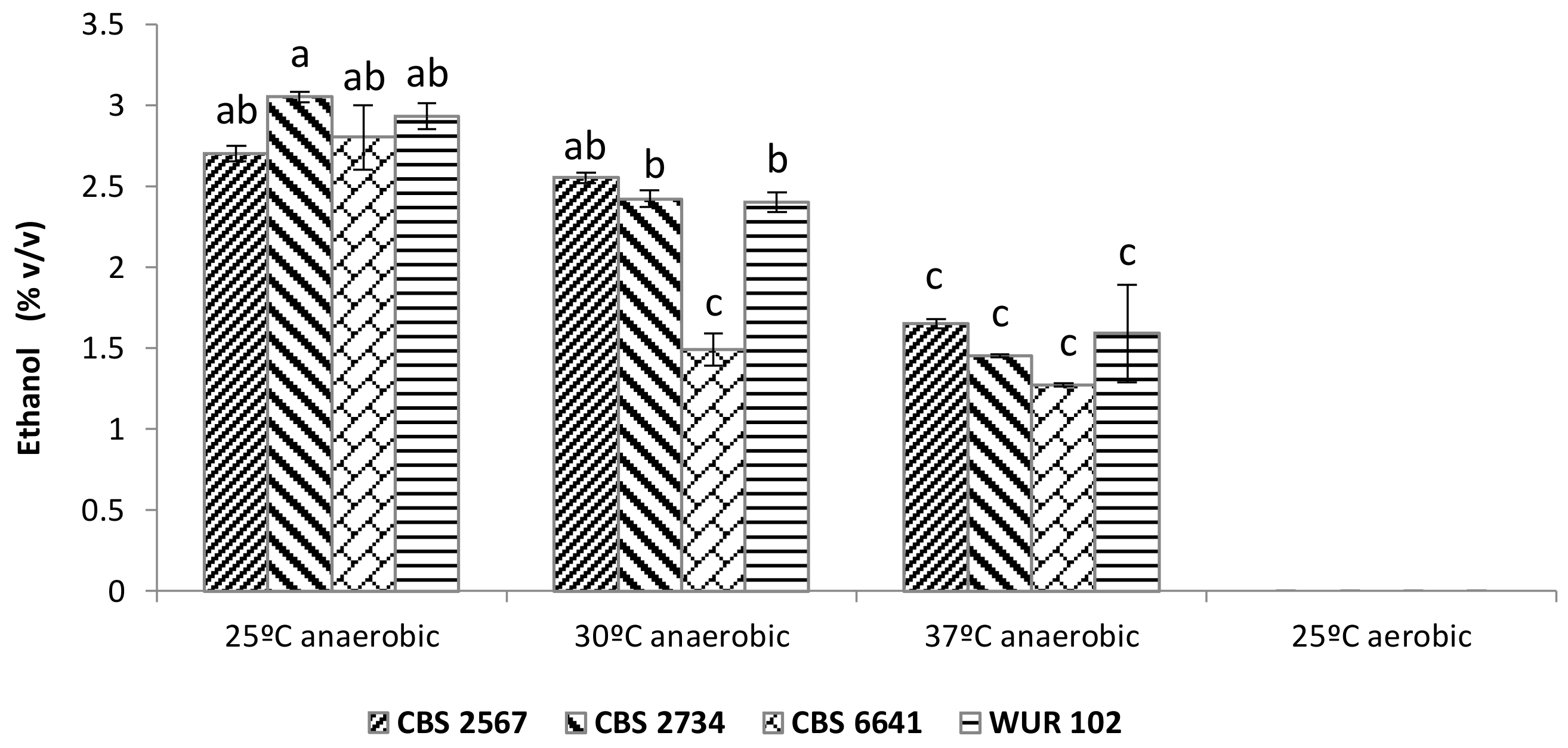
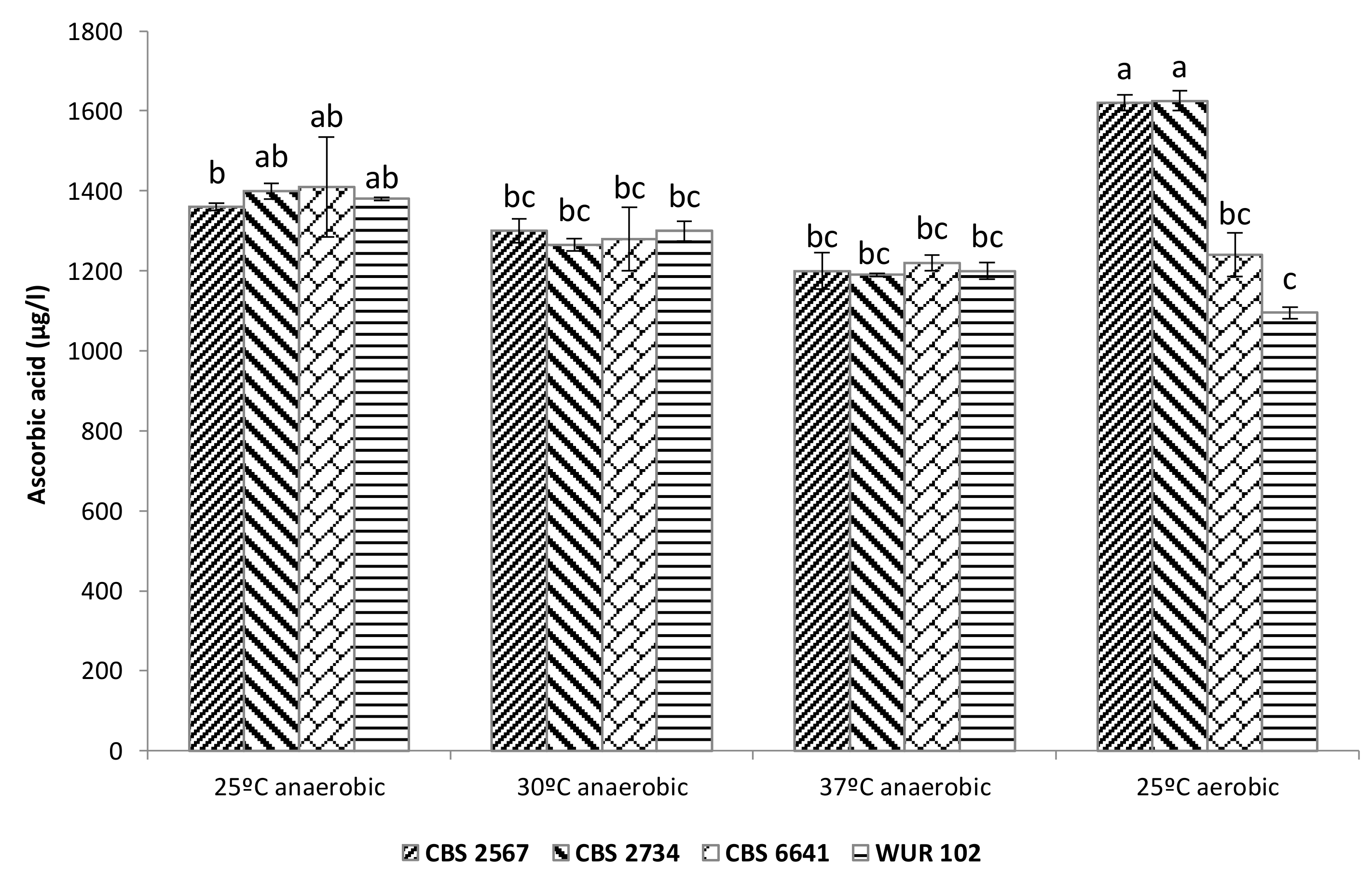
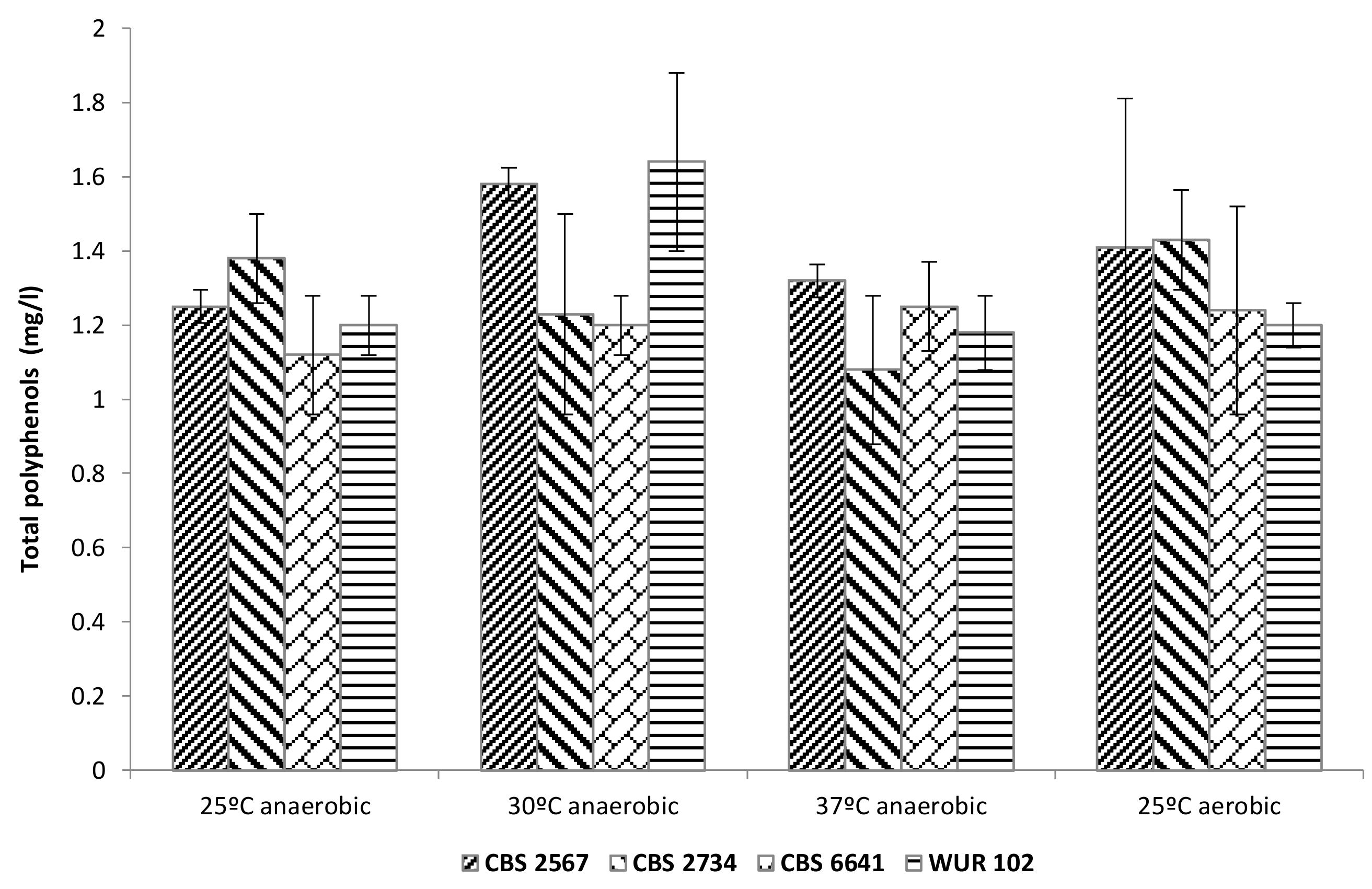
3.3. Cashew Apple Juice Fermentation with the Selected Strains
3.3.1. Sugar Conversion to Ethanol
3.3.2. Ascorbic Acid Content in Fermented Cashew Apple Juice
3.3.3. Total Polyphenol Content in the Fermented Cashew Apple Juice
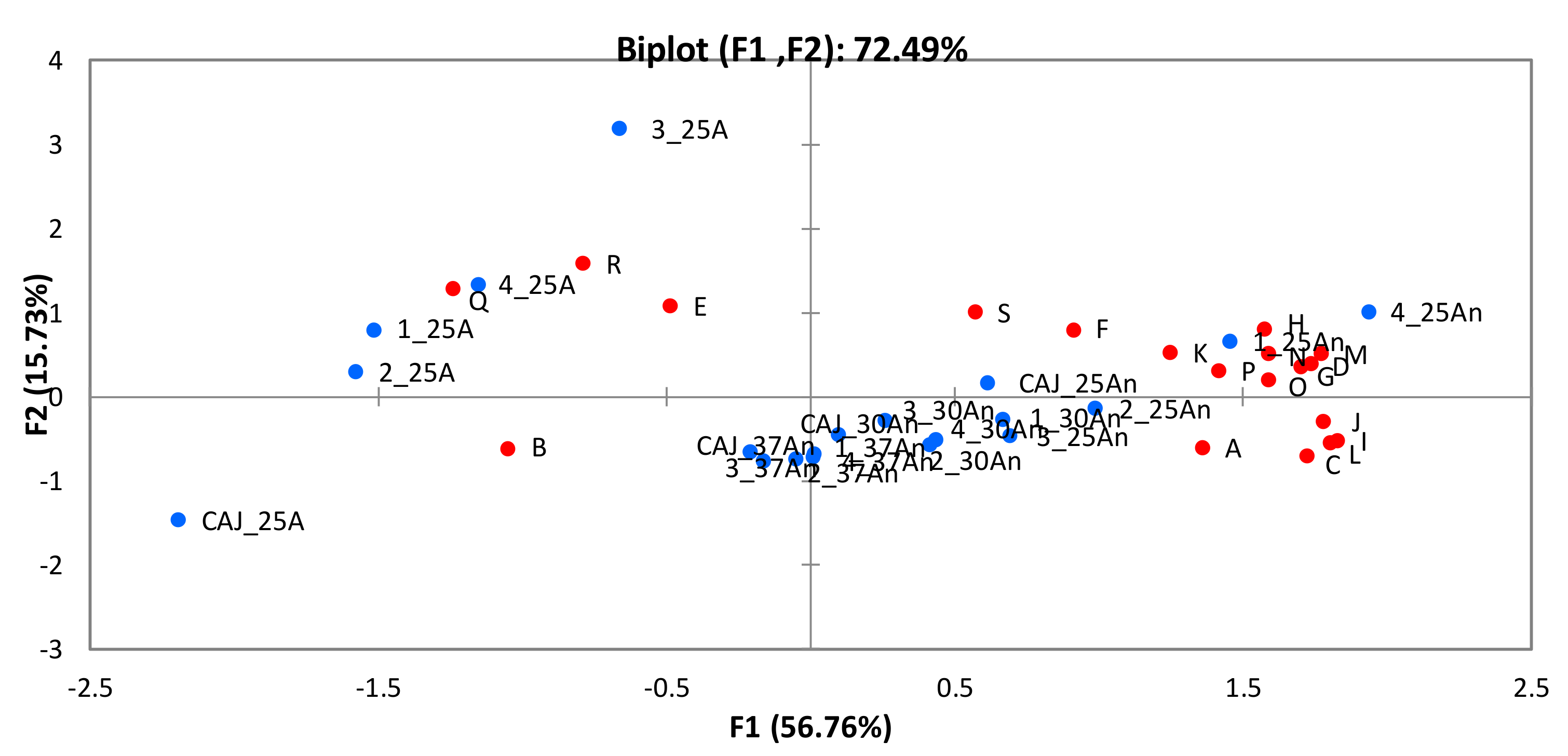
3.3.4. Aroma Profiles of the Fermented Cashew Apple Juice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, I.; Arora, A. Post-harvest processing technology for cashew apple—A review. J. Food Eng. 2017, 194, 87–98. [Google Scholar] [CrossRef]
- Garruti, D.S.; Franco, M.R.B.; da Silva, M.A.A.P.; Janzantti, N.S.; Alves, G.L. Assessment of aroma impact compounds in a cashew apple-based alcoholic beverage by GC-MS and GC-olfactometry. LWT Food Sci. Technol. 2006, 39, 373–378. [Google Scholar] [CrossRef]
- Lopes, T.; Rabelo, M.C.; Barros, L.R.; Saavedra, G.A.; Rodrigues, S. Fermentation of cashew apple juice to produce high added value products. World J. Microbiol. Biotechnol. 2007, 23, 1409–1415. [Google Scholar]
- Michodjehoun-Mestres, L.; Souquet, J.M.; Fulcrand, H.; Bouchut, C.; Reynes, M.; Brillouet, J.M. Monomeric phenols of cashew apple (Anacardium occidentale L.). Food Chem. 2009, 112, 851–857. [Google Scholar] [CrossRef]
- Michodjehoun-Mestres, L.; Souquet, J.M.; Fulcrand, H.; Bouchut, C.; Reynes, M.; Brillouet, J.M. Characterisation of highly polymerised prodelphinidins from skin and flesh of four cashew apple (Anacardium occidentale L.) genotypes. Food Chem. 2009, 114, 989–995. [Google Scholar] [CrossRef]
- Cormier, R. Clarification of Cashew Apple Juice and Commercial Applications; Oxfam: Quebec, Benin, 2008; pp. 1–9. [Google Scholar]
- Jayalekshmy, V.G.; John, P.S. ‘Sago’—A natural product for cashew apple juice clarification. J. Trop. Agric. 2004, 42, 67–68. [Google Scholar]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Hainal, A.R.; Ignat, I.; Volf, I.; Popa, V.I. Transformation of polyphenols from biomass by some yeast species. Cellul. Chem. Technol. 2011, 45, 211–219. [Google Scholar]
- Ettayebi, K.; Errachidi, F.; Jamai, L.; Tahri-Jouti, M.A.; Sendide, K.; Ettayebi, M. Biodegradation of polyphenols with immobilized Candida tropicalis under metabolic induction. FEMS Microbiol. Lett. 2003, 223, 215–219. [Google Scholar] [CrossRef]
- Silveira, M.S.; Fontes, C.P.M.L.; Guilherme, A.A.; Fernandes, F.A.N.; Rodrigues, S. Cashew apple juice as substrate for lactic acid production. Food Bioproc. 2012, 5, 947–953. [Google Scholar] [CrossRef]
- Kubo, I.; Ochi, M.; Vieira, P.C.; Komatsu, S. Antitumor agents from the cashew (Anacardium occidentale) apple juice. Food Chem. 1993, 41, 1012–1015. [Google Scholar] [CrossRef]
- Melo, A.A.; Rubensam, G.; Picada, J.N.; Gomes, E.; Fonseca, J.C.; Henriques, J.A. Mutagenicity, antioxidant potential, and antimutagenic activity against hydrogen peroxide of cashew (Anacardium occidentale) apple juice and cajuina. Environ. Mol. Mutagen. 2003, 41, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.A.; Rübensam, G.; Erdtmann, B.; Brendel, M.; Henriques, J.A.P. Cashew (Anacardium occidentale) apple juice lowers mutagenicity of aflatoxin B1 in S. typhimurium TA102. Genet. Mol. Biol. 2005, 28, 328–333. [Google Scholar]
- Nogueira, A.K.; Martins, J.J.L.; Lima, J.G.; Giroa, M.E.A.; Franco, K.; Maciel, V.M.; Loiola, O.D.; Valderez, M.; Barros, L.R.; de Santiago, R.S. Purification and characterization of a biosurfactant produced by Bacillus subtilis in cashew apple juice and its application in the remediation of oil-contaminated soil. Colloids Surf. B Biointerfaces 2019, 175, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Betiku, E.; Emeko, H.A.; Solomon, B.O. Fermentation parameter optimization of microbial oxalic acid production from cashew apple juice. Heliyon 2016, 2, e00082. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C.M.; Honorato, T.L.; Pinto, G.A.; Maia, G.A.; Rodrigues, S. Dextran sucrase production using cashew apple juice as substrate: Effect of phosphate and yeast extract addition. Bioprocess Biosyst. Eng. 2007, 30, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.V.; Souza, M.C.; Benedicto, S.C.; Bezerra, M.S.; Macedo, G.R.; Pinto, G.A.; Gonçalves, L.R. Production of biosurfactant by Pseudomonas aeruginosa grown on cashew apple juice. Appl. Biochem. Biotechnol. 2007, 137–140, 185–194. [Google Scholar]
- Barros, E.M.; Rodrigues, T.H.S.; Pinheiro, A.D.T.; Angelim, A.L.; Melo, V.M.M.; Rocha, M.V.P.; Gonçalves, L.R.B. A yeast isolated from cashew apple juice and its ability to produce first- and second-generation ethanol. Appl. Biochem. Biotechnol. 2014, 174, 2762–2776. [Google Scholar] [CrossRef]
- Talasila, U.; Vechalapu, R.R. Optimization of medium constituents for the production of bioethanol from cashew apple juice using Doehlert experimental design International. J. Fruit Sci. 2015, 15, 161–172. [Google Scholar] [CrossRef]
- Teles, A.D.; da Silva, A.; Meneses, E.; Ceccato, S.R.; Marques, S.J.; Valderez, M.; Rocha, L. Mathematical modeling of the ethanol fermentation of cashew apple juice by a flocculent yeast: The effect of initial substrate concentration and temperature. Bioprocess Biosyst. Eng. 2017, 40, 1221–1235. [Google Scholar]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Thumthanaruk, B.; Shetty, K. Changes in physico-chemical, astringency, volatile compounds and antioxidant activity of fresh and concentrated cashew apple juice fermented with Lactobacillus plantarum. J. Food Sci. Technol. 2018, 55, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Somboonpanyakul, P.B. Vitamins and prebiotic fructooligosaccharides of cashew apple fermented with probiotic strains Lactobacillus spp., Leuconostoc mesenteroides and Bifidobacterium longum. Process. Biochem. 2018, 70, 9–19. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process. Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Dantas, F.N.; Bezerra, J.; da Costa, M.; dos Santos, M.; Bertoldo, M.T.; Estevez, M.M.; de Souza, J.; Leite, E. Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro-industrial by product on Lactobacillus species. J. Sci. Food Agric. 2017, 97, 3712–3719. [Google Scholar]
- de Godoy, E.; Soares, T.H.; Narciso, F.A.; Fernandes, A.L.; Narain, N.; Sousa, E.; Rodrigues, S. Chemometric evaluation of the volatile profile of probiotic melon and probiotic cashew juice. Food Res. Int. 2017, 99, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.M.; Silva, C.F.; Moreira, J.J.S.; Narain, N.; Souza, R.R. Biotechnological process for obtaining new fermented products from cashew apple fruit by Saccharomyces cerevisiae strains. J. Ind. Microbiol. Biotechnol. 2011, 38, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Ray, P.; Swain, M.R.; Ray, R.C. Fermentation of cashew (Anacardium occidentale L.) ‘apple’ into wine. J. Food Process. Pres. 2005, 30, 314–322. [Google Scholar] [CrossRef]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Dashko, S.; Zhou, N.; Tinta, T.; Sivilotti, P.; Lemut, M.S.; Trost, K.; Gamero, A.; Boekhout, T.; Butinar, L.; Vhrovsek, U.; et al. Use of non-conventional yeast improves the wine aroma profile of Ribolla Gialla. J. Ind. Microbiol. Biotechnol. 2015, 42, 997–1010. [Google Scholar] [CrossRef]
- Zhou, N.; Schifferdecker, A.J.; Gamero, A.; Compagno, C.; Boekhout, T.; Piskur, J.; Knecht, W. Kazachstania gamospora and Wickerhamomyces subpelliculosus: Two alternative baker´s yeasts in the modern bakery. Int. J. Food Microbiol. 2017, 250, 45–58. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomycesyeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Van Rijswijck, I.M.H.; Wolkers-Rooijackers, J.C.M.; Abee, T.; Smid, E.J. Performance of non-conventional yeasts in co-culture with brewers’ yeast for steering ethanol and aroma production. Microb. Biotechnol. 2017, 10, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Adou, M.; Kouassi, D.A.; Tetchi, F.A.; Amani, G. Phenolic profile of cashew apple juice (Anacardium occidentale L.) from Yamoussoukro and Korhogo (Côte d’Ivoire). J. Appl. Biosci. 2012, 49, 3331–3338. [Google Scholar]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Hernández, Y.; Lobo, M.G.; González, M. Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem. 2006, 96, 654–664. [Google Scholar] [CrossRef]
- Azevedo, D.C.S.; Rodrigues, A. Obtainment of high-fructose solutions from cashew (Anacardium occidentale) apple juice by simulated moving-bed chromatography. Sep. Sci. Technol. 2000, 35, 2561–2581. [Google Scholar] [CrossRef]
- Assunção, R.B.; Mercadante, A.Z. Carotenoids and ascorbic acid from cashew apple (Anacardium occidentale L.): Variety and geographic effects. Food Chem. 2003, 81, 495–502. [Google Scholar] [CrossRef]
- George, S.E.; Costenbader, C.J.; Melton, T. Diauxic growth in Azotobacter vinelandii. J. Bacteriol. 1985, 164, 866–871. [Google Scholar]
- De Deken, R.H. The Crabtree effect: A regulatory system in yeast. J. Gen. Microbiol. 1966, 44, 149–156. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Erasmus, D.J. Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of icewine. Am. J. Enol. Vitic. 2004, 4, 371–378. [Google Scholar]
- Finogenova, T.V.; Morgunov, I.G.; Kamzolova, S.V.; Chernyavskaya, O.G. Organic acid production by the yeast Yarrowia lipolytica: A review of prospects. Appl. Biochem. Microbiol. 2005, 41, 418–425. [Google Scholar] [CrossRef]
- Petrescu, S.; Hulea, S.A.; Stan, R.; Avram, D.; Herlea, V. A yeast strain that uses D-galacturonic acid as a substrate for L-ascorbic acid biosynthesis. Biotechnol. Lett. 1992, 14, 6. [Google Scholar] [CrossRef]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.M.; Fornairon-Bonnefond, C.; Mazauric, J.P. Interactions between wine lees and polyphenols: Influence on oxygen consumption capacity during simulation of wine aging. J. Food Sci. 2002, 67, 1604–1609. [Google Scholar] [CrossRef]
- Nisbet, M.A.; Tobias, H.J.; Brenna, J.T.; Sacks, G.L.; Mansfield, A.K. Quantifying the contribution of grape hexoses to wine volatiles by high-precision [U13C]-glucose tracer studies. J. Agric. Food Chem. 2014, 62, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Leffingwell & Associates. Flavor-base 10th Edition. Available online: https://www.leffingwell.com/flavbase.htm (accessed on 27 June 2019).
| Strain | Genus | Isolation Source/Origin |
|---|---|---|
| CBS 77 | Dekkera anomala | Stout beer, England |
| CBS 772.71 | Galactomyces geotrichum | Soil, Puerto Rico |
| CBS 1545 | Saccharomyces bayanus | Beer, The Netherlands |
| CBS 1671 | Zygosaccharomyces rouxii | Urine, The Netherlands |
| CBS 1711 | Wickerhamomyces subpelliculosus | Fermenting cucumber brine, USA |
| CBS 2499 | Dekkera bruxellensis | Wine, France |
| CBS 2567 | Hanseniaspora guilliermondii | Grape must, Israel |
| CBS 2568 | Hanseniaspora vineae | Fruit fly |
| CBS 2734 | Torulaspora microellipsoides | Black currants, Denmark |
| CBS 2796 | Dekkera bruxellensis | Sparkling Mosselle wine, Germany |
| CBS 4806 | Brettanomyces custersianus | Bantu-beer brewery, South Africa |
| CBS 5552 | Wickerhamomyces subpelliculosus | Molasses |
| CBS 5681 | Zygosaccharomyces bailii var. bailii | Moselle wine |
| CBS 6619 | Hanseniaspora guilliermondii | Unknown |
| CBS 6625 | Zygosaccharomyces bailii var. bailii | Myoporum sp., Japan |
| CBS 6641 | Torulaspora microellipsoides | Sandalwood tree, Hawaii (USA) |
| CBS 7692 | Starmera caribaea | Pricklypear cactus, Bahamas |
| CBS 8344 | Wickerhamomyces subpelliculosus | Unknown |
| CBS 8849 | Zygosaccharomyces kombuchaensis | Kombucha tea, Russia |
| CBS 8860 | Barnettozyma californica | Berries, Russia |
| CBS 9716 | Zygosaccharomyces rouxii | Honey pot, Germany |
| CBS 10396 | Kazachstania zonata | Japan |
| CBS 10399 | Kazachstania zonata | Japan |
| CBS 10400 | Kazachstania gamospora | Japan |
| CBS 10404 | Kazachstania gamospora | Japan |
| WUR 102 | Saccharomyces cerevisiae | Masau fruits, Muzarabani (Zimbabwe) |
| WUR 131 | Saccharomyces cerevisiae | Masau fruits, Muzarabani (Zimbabwe) |
| WUR 153 | Saccharomyces cerevisiae | Masau fruits, Muzarabani (Zimbabwe) |
| Compound | Yellow CAJ | Red CAJ |
|---|---|---|
| Glucose (g/L) | 60 ± 3 | 52 ± 2.5 |
| Fructose (g/L) | 52 ± 3 | 48 ± 1 |
| Total polyphenols (mg/mL) | 1.6 ± 0.13 | 2.3 ± 0.25 |
| Ascorbic acid (mg/mL) | 1.1 ± 0.4 | 0.45 ± 0.2 |
| Strain | Species | Ethanol (%) | Optical Density (ODmax) | μmax | Diauxic Growth |
|---|---|---|---|---|---|
| CBS 2567 | Hanseniaspora guilliermondii | 2.3 | 1.1 | 0.11 | yes |
| CBS 2734 | Torulaspora microellipsoides | 3.2 | 1.3 | 0.12 | no |
| CBS 6641 | Torulaspora microellipsoides | 0.6 | 1.1 | 0.09 | no |
| WUR 102 | Saccharomycescerevisiae | 3.2 | 1.7 | 0.23 | no |
| Compound | Odour Description | |
|---|---|---|
| A | Acetaldehyde | Pungent, fruity |
| B | 2-Butanol | Sweet, cashew, fermented, oily, wine, alcoholic |
| C | 3-Hydroxybutanal | Pungent |
| D | Isobutanol | Sweet, sweaty-chemical, whiskey, fermented, stinky |
| E | 3-Methylbutanal | Fruity, almond, toasted, malty |
| F | Acetic acid | Vinegar, fermented fruit |
| G | 3-Methyl-1-butanol | Smoky, overripe cashew |
| H | 2-Methyl-1-butanol | Sour |
| I | 1-Pentanol | Alcoholic, burnt |
| J | Ethyl butyrate | Ethereal, fruity, buttery, ripe fruit |
| K | Ethyl lactate | Ethereal, rum-buttery, milky, acid, plastic |
| L | 4-Methyl-1-pentanol | Oily green-fruity, herbaceous, yeasty-fermented |
| M | 3-Methyl-1-pentanol | Mild alcoholic |
| N | Isoamyl acetate | Sweet, fruity, banana, pear |
| O | n-Amyl acetate | Banana, ethereal, fruity |
| P | Ethyl caproate | Fruity, wine, apple, banana, pineapple |
| Q | Benzeneacetaldehyde | Bitter almonds, wild cherry, vanilla |
| R | Phenylethyl alcohol | Sweet, dried fruit, tea, tobacco |
| S | β-Phenylethyl acetate | Sweet, fresh, floral, rose, hyacinth |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamero, A.; Ren, X.; Lamboni, Y.; de Jong, C.; Smid, E.J.; Linnemann, A.R. Development of A Low-Alcoholic Fermented Beverage Employing Cashew Apple Juice and Non-Conventional Yeasts. Fermentation 2019, 5, 71. https://doi.org/10.3390/fermentation5030071
Gamero A, Ren X, Lamboni Y, de Jong C, Smid EJ, Linnemann AR. Development of A Low-Alcoholic Fermented Beverage Employing Cashew Apple Juice and Non-Conventional Yeasts. Fermentation. 2019; 5(3):71. https://doi.org/10.3390/fermentation5030071
Chicago/Turabian StyleGamero, Amparo, Xiao Ren, Yendouban Lamboni, Catrienus de Jong, Eddy J. Smid, and Anita R. Linnemann. 2019. "Development of A Low-Alcoholic Fermented Beverage Employing Cashew Apple Juice and Non-Conventional Yeasts" Fermentation 5, no. 3: 71. https://doi.org/10.3390/fermentation5030071
APA StyleGamero, A., Ren, X., Lamboni, Y., de Jong, C., Smid, E. J., & Linnemann, A. R. (2019). Development of A Low-Alcoholic Fermented Beverage Employing Cashew Apple Juice and Non-Conventional Yeasts. Fermentation, 5(3), 71. https://doi.org/10.3390/fermentation5030071