Production of d-Lactate from Avocado Seed Hydrolysates by Metabolically Engineered Escherichia coli JU15
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Growth Conditions
2.2. Avocado Seed Hydrolysate Medium (ASH) Preparation
2.3. Bioreactor Cultivation
2.4. Chemicals
2.5. Analytical Methods
3. Results
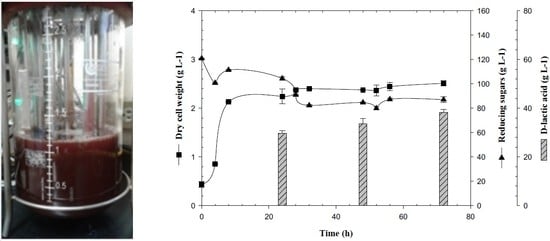
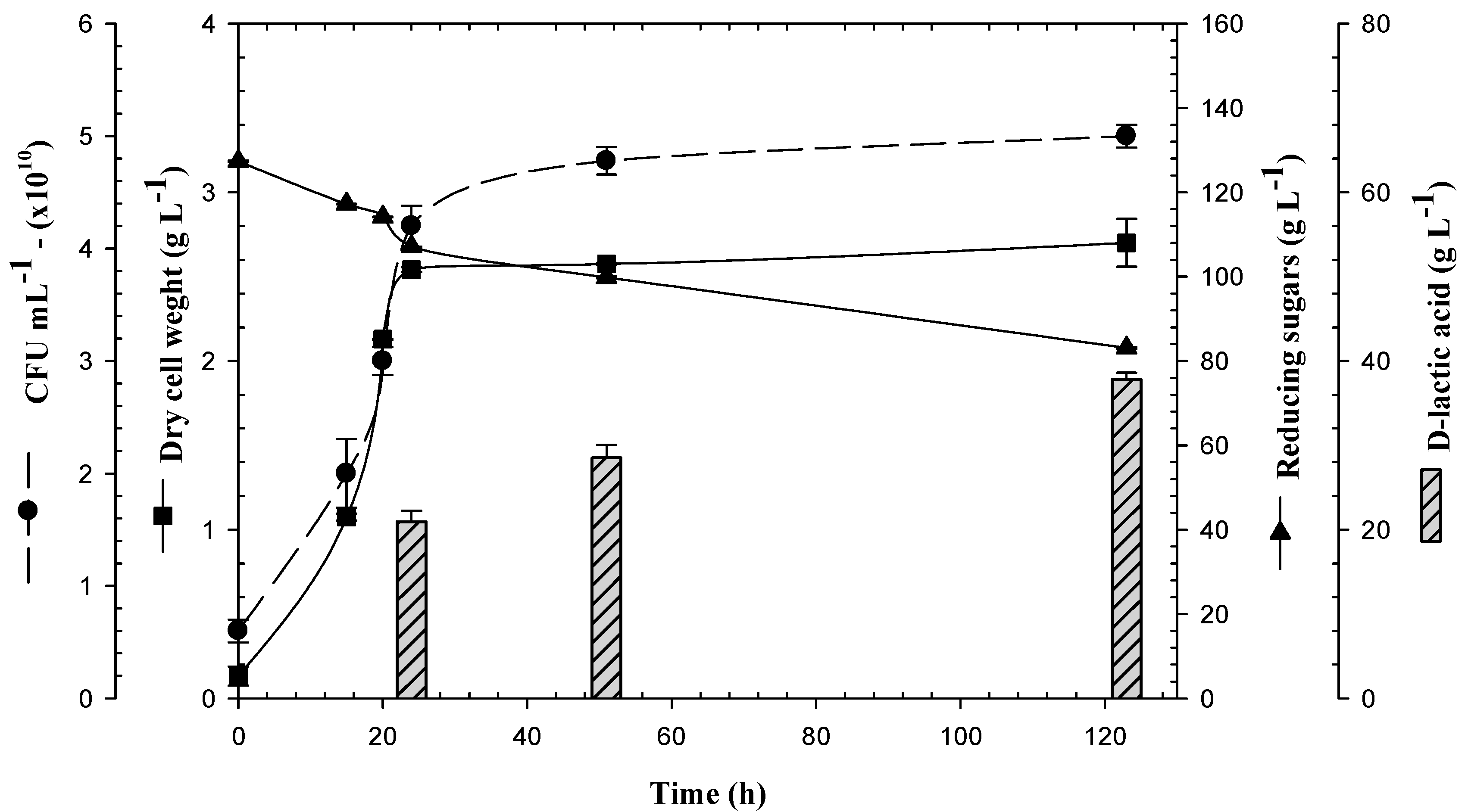
3.1. Biomass Production of Lactogenic E. coli JU15 as a Function of the Quantity of Initial Reducing Sugars in 3-L Bioreactor
3.2. Production of D-LA and Consumption of Reducing Sugars by the Lactogenic E. coli JU15 in Batch Fermentations Using the ASH Medium
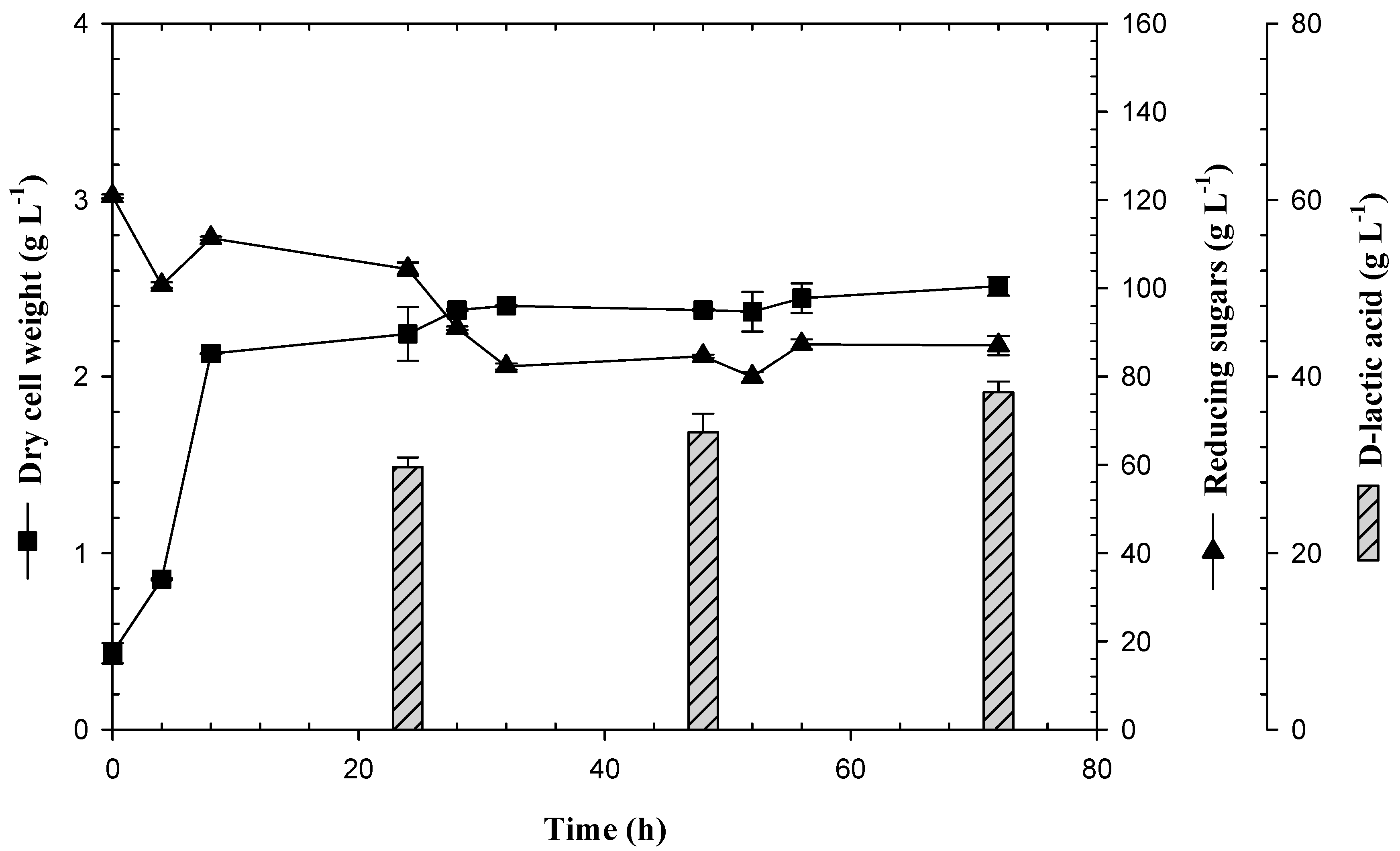
3.3. Feedback Fermentation Slightly Improves the Productivity of Lactic Acid by E. coli JU15 in the ASH Medium
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S.A. Department of Agriculture. Available online: https://gain.fas.usda.gov/RecentGAIN20Publications/AvocadoAnnual_MexicoCity_Mexico_11-27-2018.pdf (accessed on 13 March 2019).
- Domínguez, M.P.; Araus, K.; Bonert, P.; Sánchez, F.; San Miguel, G.; Toledo, M. The avocado and its waste: An approach of fuel potential/application. In Environment, Energy and Climate Change II; Springer International Publishing: Berlin, Germany, 2014; pp. 199–223. [Google Scholar]
- Nwaokobia, K.; Oguntokun, M.O.; Okolie, P.L.; Ogboru, R.O.; Iduboe, O.D. Evaluation of the chemical composition of Persea Americana (Mill) pulp and seed. J. Biosci. Biotechnol. Discov. 2018, 3, 83–89. [Google Scholar]
- Sánchez, F.; Araus, K.; Domínguez, M.P.; San Miguel, G. Thermochemical transformation of residual avocado seeds: Torrefaction and carbonization. Waste Biomass Valoriz. 2017, 8, 2495–2510. [Google Scholar] [CrossRef]
- Kahn, V. Characterization of Starch Isolated from Avocado Seeds. J. Food Sci. 1987, 52, 1646–1648. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Martinez, A.; Rodríguez-Alegría, M.E.; Fernandes, M.C.; Gosset, G.; Vargas-Tah, A. Metabolic engineering of Escherichia coli for lactic acid Production from renewable resources. In Engineering of Microorganisms for the Production of Chemicals and Biofuels from Renewable Resources; Gosset, G., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 125–145. [Google Scholar]
- Gorini, C. Studies on the biology of lactic acid bacteria: A summary of personal investigations. J. Bacterial. 1922, 7, 271–276. [Google Scholar]
- Katagiri, H.; Kitahara, K. The lactic dehydrogenase of lactic acid bacteria. Biochem. J. 1938, 32, 1654. [Google Scholar] [CrossRef]
- Chang, D.E.; Jung, H.C.; Rhee, J.S.; Pan, J.G. Homofermentative production of d- or l-lactate in metabolically engineered Escherichia coli RR1. Appl. Environ. Microbiol. 1999, 65, 1384–1389. [Google Scholar]
- Nakasaki, K.; Adachi, T. Effects of intermittent addition of cellulase for production of L-lactic acid from wastewater sludge by simultaneous saccharification and fermentation. Biotechnol. Bioeng. 2003, 82, 263–270. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn-Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- Venus, J.; Fiore, S.; Demichelis, F.; Pleissner, D. Centralized and decentralized utilization of organic residues for lactic acid production. J. Clean. Prod. 2018, 172, 778–785. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef]
- Espinel-Rios, S.; Palmerin-Carreño, D.M.; Hernandez-Orihuela, A.L.; Martinez-Antonio, A. A plackett-burman design for substituting MRS medium components with avocado seed hydrolysate for growth and lactic acid production by Lactobacillus sp. Rev. Mex. Ing. Quim. 2019, 18, 131–141. [Google Scholar] [CrossRef]
- Gao, C.; Ma, C.; Xu, P. Biotechnological routes based on lactic acid production from biomass. Biotechnol. Adv. 2011, 29, 930–939. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Zhang, Y.; Bates, D.M.; Keating, D.H.; Sato, T.K.; Ong, I.M.; Landick, R. Death by a thousand cuts: The challenges and diverse landscape of lignocellulosic hydrolysate inhibitors. Front. Microbiol. 2014, 5, 90. [Google Scholar] [CrossRef]
- Vargas-Tah, A.; Moss-Acosta, C.L.; Trujillo-Martinez, B.; Tiessen, A.; Lozoya-Gloria, E.; Orencio-Trejo, M.; Gosset, G.; Martinez, A. Non-severe thermochemical hydrolysis of stover from white corn and sequential enzymatic saccharification and fermentation to ethanol. Bioresour. Technol. 2015, 198, 611–618. [Google Scholar] [CrossRef]
- Zhou, L.; Cui, W.J.; Liu, Z.M.; Zhou, Z.M. Metabolic engineering strategies for d-lactate overproduction in Escherichia coli. J. Chem. Technol. Biotechnol. 2016, 91, 576–584. [Google Scholar] [CrossRef]
- Eiteman, M.A.; Ramalingam, S. Microbial production of lactic acid. Biotechnol. Lett. 2015, 37, 955–972. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Huang, F.; Wang, J.; Zhao, J.; Zhao, X.; Garza, E.; Manow, R.; Grayburn, S.; Zhou, S. Engineering and adaptive evolution of Escherichia coli W for L-lactic acid fermentation from molasses and corn steep liquor without additional nutrients. Bioresour. Technol. 2013, 148, 394–400. [Google Scholar] [CrossRef]
- Utrilla, J.; Licona-Cassani, C.; Marcellin, E.; Gosset, G.; Nielsen, L.K.; Martinez, A. Engineering and adaptive evolution of Escherichia coli for d-lactate fermentation reveals GatC as a xylose transporter. Metab. Eng. 2012, 14, 469–476. [Google Scholar] [CrossRef]
- Utrilla, J.; Vargas, A.; Trujillo, B.; Gosset, G.; Martínez, A. Production of d-lactate from sugarcane bagasse and corn stover hydrolysates using metabolic engineered Escherichia coli strains. Bioresour. Technol. 2016, 220, 208–214. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau, D.; D´Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 23, 8746–8749. [Google Scholar] [CrossRef]
- Tzintzun-Camacho, O.; Sánchez-Segura, L.; Minchaca-Acosta, A.Z.; Rosales-Colunga, L.M.; Hernández-Orihuela, A.L.; Martinez-Antonio, A. Development of bacterial culture medium from avocado seed. Rev. Mex. Ing. Quim. 2016, 15, 831–842. [Google Scholar]
- Martinez-Antonio, A.; Minchaca-Acosta, A.Z.; Hernández-Orihuela, A.L.; Rosales-Colunga, L.M.; Estévez-Palmas, J.M.; Espinel-Ríos, S. Culture Medium Derived from Avocado Seed Material. WO2016079568A1, 2016. [Google Scholar]
- Chauhan, K.; Trivedi, U.; Patel, K. Statistical screening of medium components by Plackett-Burman design for lactic acid production by Lactobacillus sp. KCP01 using date juice. Bioresour. Technol. 2007, 98, 98–103. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Saqib, A.A.N.; Whitney, P.J. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono-and di-saccharide sugars. Biomass Bioenergy 2011, 35, 4748–4750. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Kim, Y.; Ingram, L.O.; Shanmugam, K.T. Construction of an Escherichia coli K-12 mutant for homoethanologenic fermentation of glucose or xylose without foreign genes. App. Environ. Microbiol. 2007, 73, 1766–1771. [Google Scholar] [CrossRef]
- Dien, B.; Nichols, N.; Bothast, R. Recombinant Escherichia coli engineered for production of L-lactic acid from hexose and pentose sugars. J. Ind. Microb. Biotechnol. 2001, 27, 259–264. [Google Scholar] [CrossRef]
- Neeman, I.; Lifshitz, A.; Kashman, Y. New antibacterial agent isolated from the avocado pear. Appl. Environ. Microbiol. 1970, 19, 470–473. [Google Scholar]
- Rodríguez-Sánchez, D.G.; Pacheco, A.; García-Cruz, M.I.; Gutiérrez-Uribe, J.A.; Benavides-Lozano, J.A.; Hernández-Brenes, C. Isolation and structure elucidation of avocado seed (Persea americana) lipid derivatives that inhibit Clostridium sporogenes endospore germination. J. Agric. Food Chem. 2013, 61, 7403–7411. [Google Scholar] [CrossRef]
| System | Initial Reducing Sugars (g L−1) | Residual Reducing Sugars (g L−1) | Lactic Acid Production (g L−1) † | Final Biomass (g L−1) | YD-LA (gD-LA gsugars−1) | Overall Volumetric Productivity (mg L−1 h−1) |
|---|---|---|---|---|---|---|
| Batch | 40 ± 1.8 | 21.9 ± 0.8 | 17.9 ± 0.5 | 2.22 ± 0.15 | 0.42 ± 0.05 | 0.25 ± 0.06 |
| Batch | 70 ± 0.4 | 50.7 ± 1.3 | 18.9 ± 0.2 | 2.57 ± 0.07 | 0.26 ± 0.10 | 0.26 ± 0.01 |
| Batch | 120 ± 3.3 | 83.1 ± 0.2 | 37.8 ± 0.3 | 2.73 ± 0.18 | 0.31 ± 0.08 | 0.52 ± 0.07 |
| Fed-Batch | 110 ± 4.1 (initial) | 21.4 ± 0.7 | 37.6 ± 0.4 | 2.52 ± 0.05 | 0.33 ± 0.07 | 0.48 ± 0.05 |
| Parameter | Bioreactor Results |
|---|---|
| Xmax (CFU mL−1) | 5.0 × 1010 |
| Specific growth rate, (h−1) | 0.63 (R-sq = 0.97) |
| Doubling time, td (h) | 1.1 |
| Q D-LA, (gD-LA L−1 h−1) | 0.31 ± 0.08 |
| YD-LA, (gD-LA g−1 reducing sugars) | 0.31 ± 0.08 |
| Reducing sugar consumption (%) | 34.7 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmerín-Carreño, D.M.; Hernández-Orihuela, A.L.; Martínez-Antonio, A. Production of d-Lactate from Avocado Seed Hydrolysates by Metabolically Engineered Escherichia coli JU15. Fermentation 2019, 5, 26. https://doi.org/10.3390/fermentation5010026
Palmerín-Carreño DM, Hernández-Orihuela AL, Martínez-Antonio A. Production of d-Lactate from Avocado Seed Hydrolysates by Metabolically Engineered Escherichia coli JU15. Fermentation. 2019; 5(1):26. https://doi.org/10.3390/fermentation5010026
Chicago/Turabian StylePalmerín-Carreño, Dulce María, Ana Lilia Hernández-Orihuela, and Agustino Martínez-Antonio. 2019. "Production of d-Lactate from Avocado Seed Hydrolysates by Metabolically Engineered Escherichia coli JU15" Fermentation 5, no. 1: 26. https://doi.org/10.3390/fermentation5010026
APA StylePalmerín-Carreño, D. M., Hernández-Orihuela, A. L., & Martínez-Antonio, A. (2019). Production of d-Lactate from Avocado Seed Hydrolysates by Metabolically Engineered Escherichia coli JU15. Fermentation, 5(1), 26. https://doi.org/10.3390/fermentation5010026