Abstract
Arthrospira platensis (spirulina), a filamentous fresh-water planktonic cyanobacterium, possesses diverse biological activities and a unique nutritional profile, due to its high content of valuable nutrients. This study aimed to further improve the bioactive profile of spirulina, by fermenting it with the lactic acid bacterium Lactobacillus plantarum. In vitro comparison of the total phenolic content (TPC), C-phycocyanin, free methionine, DPPH radical scavenging capacity, ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC) and protein fragmentation via SDS-PAGE in untreated versus 12 to 72 h fermented spirulina is reported here. After 36 h fermentation, TPC was enhanced by 112%, FRAP by 85% and ORAC by 36%. After 24 h, the DPPH radical scavenging capacity increased 60%, while the free methionine content increased by 94%, after 72 h. Past 36 h of fermentation, the total antioxidant capacity (TAC) diminished, possibly due to deterioration of the heat-sensitive antioxidants. However, protein fragmentation and free methionine content increased, linearly, with the fermentation time. Cyanobacterial peptides and other bioactive compounds trapped within the spirulina cell wall are released during fermentation and have a significant potential as a functional ingredient in nutraceuticals and pharmaceuticals, in addition to their nutritive value.
1. Introduction
Cyanobacteria, the most archaic group of oxygenic phototrophs, were first named in the 8th edition of Bergey’s Manual of Determinative Bacteriology in 1974. Prior to that, cyanobacteria were termed blue-green algae, as they are found in water and contain the photosynthetic pigments—chlorophyll (green) and phycocyanin (blue) [1]. Although cyanobacteria have been consumed as a food for centuries, their commercial production began only in recent years in Japan, later spreading to America, Australia, and certain European and Asian countries [2]. The Arthrospira species falls under the prokaryotic cyanobacteria category. Arthrospira platensis, hereafter referred to as spirulina, is a planktonic filamentous cyanobacterium of the Phormidiaceae family and is part of the phytoplankton biomass found in alkaline water [3]. Approximately 3000 tones (dw) are produced per year by commercial brands, for the purpose of dietary supplements, cosmetics, food dyes, and aquaculture. The utilisation of cyanobacteria in the healthy food industry is fast growing, as they are a relatively easy-to-produce, cost-effective source of valuable biomolecules [4]. In particular, spirulina has an enhanced nutritional profile with high bioavailability of essential amino acids (64 to 74% protein content), biliproteins, and other pigments, such as allophycocyanin, C-phycocyanin, a-chlorophyll, B and E vitamins, mineral substances and trace elements, glycolipids, sulpholipids, and essential polyunsaturated fatty acids, including γ-linoleic acid [5,6]. They are readily absorbed in the body and help to bring the nutrient status up to normal levels [7]. They also provide therapeutic properties in the treatment and prevention of a variety of disorders, including hypercholesterolaemia [8], diabetes [9], various types of cancer [10], and atherosclerosis [11]. Recently, spirulina has been studied for the prevention and treatment of diabetes, malnutrition, as an antiviral agent, immune-stimulator, anti-inflammatory and anticancer supplementation, improved digestive capacity, as well as the growth of Lactobacilli in the gut [12,13,14].
Spirulina is extensively grown for nutraceutical compounds, functional food development and other purposes, including food additives, such as natural pigments, thickening and gelling agents, animal feed, and medicinal bioassays. Spirulina is indicated as a nontoxic supplement and has been declared to be a Humanitarian Instrument in fighting severe malnutrition, by the WHO [15]. Spirulina has significantly enhanced biomarkers of mammalian health in vivo, when incorporated as 0.1–1.0% of daily feed. Reported enhancements include probiotic, antioxidant, analgesic, anti-allergic, growth, antiviral, antidiuretic, hypocholesterolaemic, anti-carcinogenic, and cardiovascular protective effects [16,17].
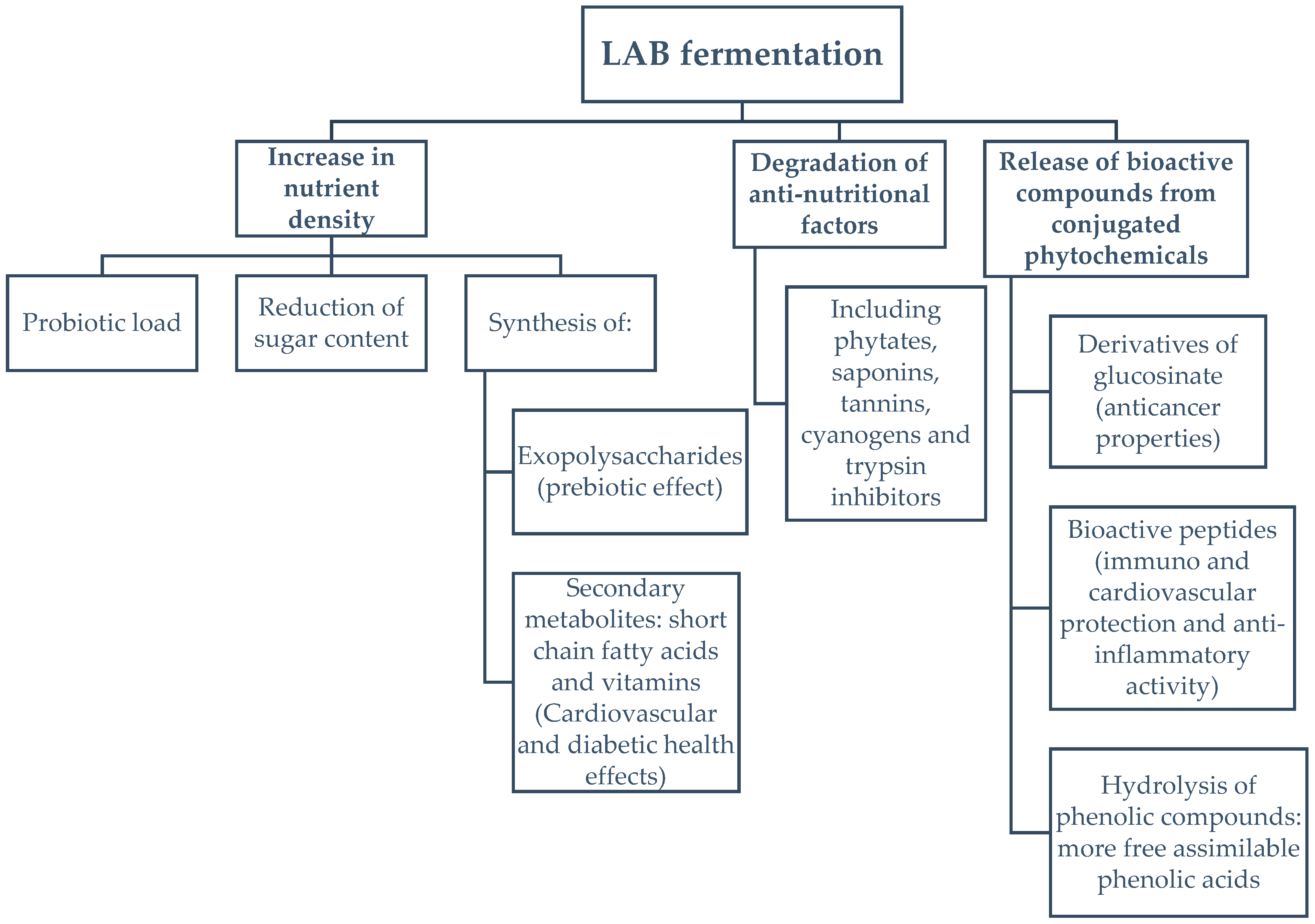
Fermentation is widely used as a food preservation method. However, the use of lactic acid bacteria (LAB) to improve the nutraceutical profile of food (Figure 1) is a novel area of study. LAB have the ability to degrade plant and cyanobacterial cell walls, via hydrolysis, resulting in the conversion of complex organic compounds, such as polysaccharides, lipids and proteins, within the cell, into smaller molecules with enhanced antioxidant, anti-inflammatory, and immunomodulatory activity [18,19,20,21]. Among LAB, L. plantarum has been studied as a suitable strain for the fermentation of food, due to its endogenous enzymes, which are capable of producing antioxidants such as, hydroxytyrosol and pyrogallol, or approved flavouring agents, like 4-vinyl phenol [22]. Furthermore, via peptide bond hydrolysis of inactive parent proteins, LAB proteases yield bioactive peptides with multiple health benefits, such as ACE-inhibition, modulation of the immune system and antioxidant activity [23]. Fermented foods are a new trend in the nutrition-health sector for the increasing number of consumers seeking natural sources of bioavailable nutraceuticals, in nutrient-dense foods, while enjoying new flavours, textures and aromas with no, or reduced, requirement for synthetic additives [24,25].
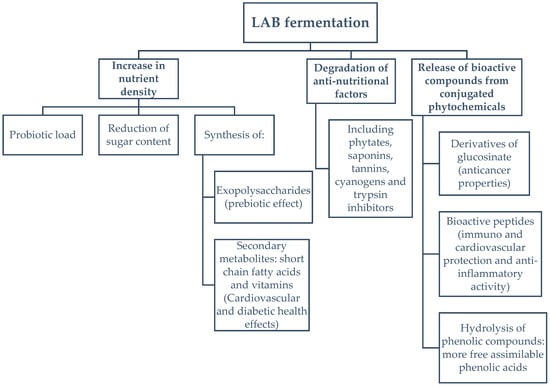
Figure 1.
Enhancements of the nutritional quality of foods during lactic acid fermentation.
This study aimed to quantify and compare the total antioxidant activity, total phenolic, C-phycocyanin and the free methionine content of LAB fermented spirulina to untreated spirulina; to analyse the protein fragmentation pattern of spirulina before and after fermentation using SDS-PAGE; and overall, to determine the optimum fermentation time for maximum enhancement of nutraceutical properties in spirulina.
Cyanobacterial peptides and other bioactive compounds trapped within the spirulina cell wall were released during fermentation, as shown by the increased antioxidant capacity and protein fragmentation of the fermented samples. Thus, fermented spirulina is a promising functional ingredient in nutraceuticals and pharmaceuticals.
2. Materials and Methods
2.1. Preparation of the Lactobacillus plantarum Stock
L. plantarum ATCC 8014 was purchased as a lyophilised powder from Microbiologics®, St Cloud, MN, USA, in the form of a KWIK-STIK™. Stock cultures were prepared by growing the strain aerobically on de Man, Rogosa and Sharpe (MRS) agar (72 h at 37 °C), after which ~2 colonies of L. plantarum were transferred into a 2 mL Eppendorf, containing 1 mL of sterile 20% glycerol.
2.2. Spirulina Fermentation
Spirulina fermentation with L. plantarum was conducted as per Gupta et al. [26], with one modification; autoclaved ddH2O was used in place of the MRS broth. Five grams of untreated wet biomass and 1 mL of L. plantarum stock (log 6 to 7 CFU/mL) were added to a 500 mL Erlenmeyer flask containing 24 mL of ddH2O. This mixture was fermented in a shaker incubator (37 °C) and samples were taken every 12 h, for 72 h. The pH was measured at each time point. Samples were frozen, lyophilised for 72 h, subjected to mortar and pestle treatment, and stored at −80 °C. At the time of analysis, samples were diluted in ddH2O or MeOH (according to the solubility of the nutraceutical compounds of interest) and centrifuged (5 min, 13,000× g). The supernatant was used for testing the spirulina properties as a fermented nutraceutical product.
2.3. Total Phenolic Content (TPC) Determination
Spirulina’s TPC was determined as per Jaiswal et al. [27]. Briefly, 100 µL of the sample was poured into individual test tubes and the blank was prepared with 100 µL of ddH2O. Then, 2 mL of 2% sodium carbonate was added to each tube and left in darkness, at 25 °C. After 2 min, 100 µL of 50% Folin Ciocalteu’s phenol reagent was added to each tube and incubated in darkness, at 25 °C, for 30 min, where the reaction mixture changed from yellow to blue. A total of 200 µL from each test tube was transferred to a 96-well microplate and the absorbance was read at 720 nm, using a UV-Vis spectrophotometric microplate reader. The results were compared to the calibration curve of gallic acid (0 to 500 µg/mL) and expressed as mg of gallic acid equivalents, per gram (dw) of spirulina (mg GAE/g).
2.4. C-phycocyanin Determination
Total C-phycocyanin content (PC) was determined, according to the method by Bennett and Bogorad [28], by measuring the absorbance of 200 µL of spirulina sample in a 96-well microplate, using a UV-Vis spectrophotometric microplate reader at 615 and 652 nm (Equation (1)). The equation for calculating PC content was derived by combining the extinction coefficients with three simultaneous equations that considered the wavelength corresponding to the maximum absorbance of this pigment [28]. Results were compared to a standard curve of pure C-phycocyanin (50 to 1000 µg/mL) and expressed as µg of C-phycocyanin per mg (dw).
2.5. DPPH Assay
The measurement of the DPPH radical scavenging activity was performed according to the method described by Jaiswal and Abu-Ghannam [29]. In brief, 100 µL of sample was added to six wells of a 96-well microplate. 100 µL of freshly prepared DPPH radical solution (165 µM, in methanol) was added to the experimental-wells. In the blank-wells, 100 µL of ddH2O was added instead. As a control, 100 µL of ddH2O was used, in place of spirulina sample. The reaction mixture was incubated at 25 °C, for 30 min, in the dark, before reading the absorbance at 517 nm, in a UV-Vis spectrophotometric microplate reader. Results were obtained as a percentage decrease, with respect to the control values, using Equation (2) and compared to an ascorbic acid standard curve (1 to 12.5 µg/mL) and expressed as mg ascorbic acid equivalents, per gram (dw) of spirulina (mg AAE/g).
2.6. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay was performed according to Benzie and Strain [30]. Concisely, the FRAP reagent was freshly prepared by mixing in a 10:1:1 (v/v/v) ratio: 300 mM sodium acetate buffer, pH 3.6 with 20 mM FeCl3•6H2O, and with 10 mM TPTZ in 40 mM HCl and incubated at 37 °C, until use. The reaction was performed in a 96-well microplate, where 100 µL of the preheated FRAP reagent was dispensed into each well, already containing 50 µL of the spirulina sample. The reagent blank wells contained 50 µL of ddH2O in place of the sample. The absorbance was read at 593 nm in a UV-Vis spectrophotometric microplate reader, after 10 min of incubation in darkness, at 25 °C. Results were compared to a Trolox standard curve (0.75 to 12.5 µg/mL) and expressed as micrograms of the Trolox equivalents per gram (dw) of spirulina (µg TE/g).
2.7. Oxygen Radical Absorbance Capacity (ORAC) Assay
The ORAC assay was carried out, as per Huang et al. [31]. A total of 75 mM phosphate buffered saline pH 7.4 was prepared, using potassium phosphate monobasic and dibasic. Fluorescein sodium salt was used to prepare sodium fluorescein stock solution, which was diluted to a concentration of 4 × 10−3 mM. 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) (153 mM) was prepared and diluted to a final concentration of 4 × 10−6 mM. Both fluorescein and AAPH were prepared and diluted, using 75 mM phosphate buffered saline (PBS). The outer wells of a 96-well microplate were filled with 200 µL of ddH2O. The reaction mixture in the interior wells was prepared by adding 25 µL of each sample to the 150 µL of fluorescein. The blank wells received 25 µL of PBS in place of the spirulina sample. The microplate was inserted into the preheated UV-Vis spectrophotometric microplate reader and incubated at 37 °C, for 30 min. Then, 25 µL of diluted AAPH was added to every well and the fluorescence was monitored, kinetically, for 90 min. Two fluorescence readings were carried out—an excitation wavelength (485 nm) and an emission wavelength (528 nm). Results were obtained, as indicated in Equation (3) and expressed as the µM Trolox per gram (dw) of spirulina, equivalent to ORAC units, using the Trolox calibration curve (1.25 to 60 µM/mL). Each ORAC unit equalled the net protection produced by 1 µM of Trolox [32].
2.8. Protein Fragmentation Using SDS-PAGE
Following the Bio-Rad Electrophoresis (2015) protocol, an 8% SDS-PAGE solution was prepared and each 12 h interval-fermented sample was loaded against a protein ladder, control (untreated + L. plantarum), and an untreated sample. Running buffer was made by diluting 100 mL of the 10× running buffer stock into ddH2O (900 mL). The electrophoresis cell was assembled and filled with a running buffer, prior to the sample loading. Samples were mixed with the sample buffer, Laemmli 2× Concentrate (1:1 ratio) in 2 mL Eppendorf tubes, then denatured by incubation in a boiling water bath, for 5 min. A total of 10 µL of each sample and control were loaded into different wells, and the gel was run for 65 min at 140 V. A Bradford protein assay was run prior to sample preparation, to ensure an equal protein concentration loading (20 μg of protein). Later, the gel was stained by submerging it into the staining solution of ddH2O, methanol and glacial acetic acid 50/40/10 (v/v/v), and 2 g of the Coomassie Blue (40 min at 55 rpm), followed by distaining, using a solution of ddH2O, methanol and glacial acetic acid 50/40/10 (v/v/v) (24 h at 55× g). An UV chamber was used for the gel visualisation and the protein fragmentation was determined through a comparison with the molecular weight protein marker ladder.
2.9. Free Methionine Content Determination
Reversed phase high performance liquid chromatography (RP-HPLC) analysis of spirulina was carried out, according to the method developed by Varzaru et al. [33], with the following modifications—a flow rate of 0.8 mL/min and detection at 220 nm, were used. In brief, the solvents used were disodium phosphate (3.85 g/L, pH 7.8) (A) and water, acetonitrile, and methanol (20:20:60 v/v/v) (B). The HPLC programme was set as follows—3 injections per sample, 20 μL volume injection, 0.8 mg/mL flow rate, 45 °C column temperature, 20 min/sample run time and detection at 220 nm. DL-Methionine (0.75, 1.0 and 1.75 mg/mL) was used as a standard (elution time 3.3 min). The solvent gradient programme was set as follows. Solvent A: 2 min at 100%, 23 min transition to 25%, 1 min transition to 0% and hold for 3 min, 1 min transition to 100% and hold for 5 min. Solvent B: 2 min at 0%, 23 min transition to 75%, 1 min transition to 100% and hold for 3 min, 1 min transition to 0% and hold for 5 min.
2.10. Statistical Analyses
All data were collected from seven independent assays performed in triplicates (n = 3) and replicated, at least twice. Results were expressed as the mean ± standard deviation (SD) of the three measurements. Statistically significant differences were obtained using STATGRAPHICS Centurion XV software. Statistical comparisons were performed via analysis of variance and Fisher’s least significant difference. Differences at P ≤ 0.05 were considered significant. Correlations between the evaluated parameters were obtained using Pearson’s correlation coefficient (r). Means within each column denoted with different letters differed significantly (P ≤ 0.05).
3. Results
3.1. pH and Colour Variation during Fermentation
Untreated spirulina was found to have the highest pH value (6.0 ± 0), with a 63.5% drop after a 36 h fermentation (pH 3.8), increasing to a pH 4.6, by the end of the fermentation treatment (72 h). Table 1 illustrates the colour change during fermentation. This was significant since colour is highly variable within biological samples, especially foods, possibly interfering with the absorbance reading [34].

Table 1.
Diluted spirulina samples (2 mg/mL) in ddH2O.
3.2. Total Phenolic Content (TPC)
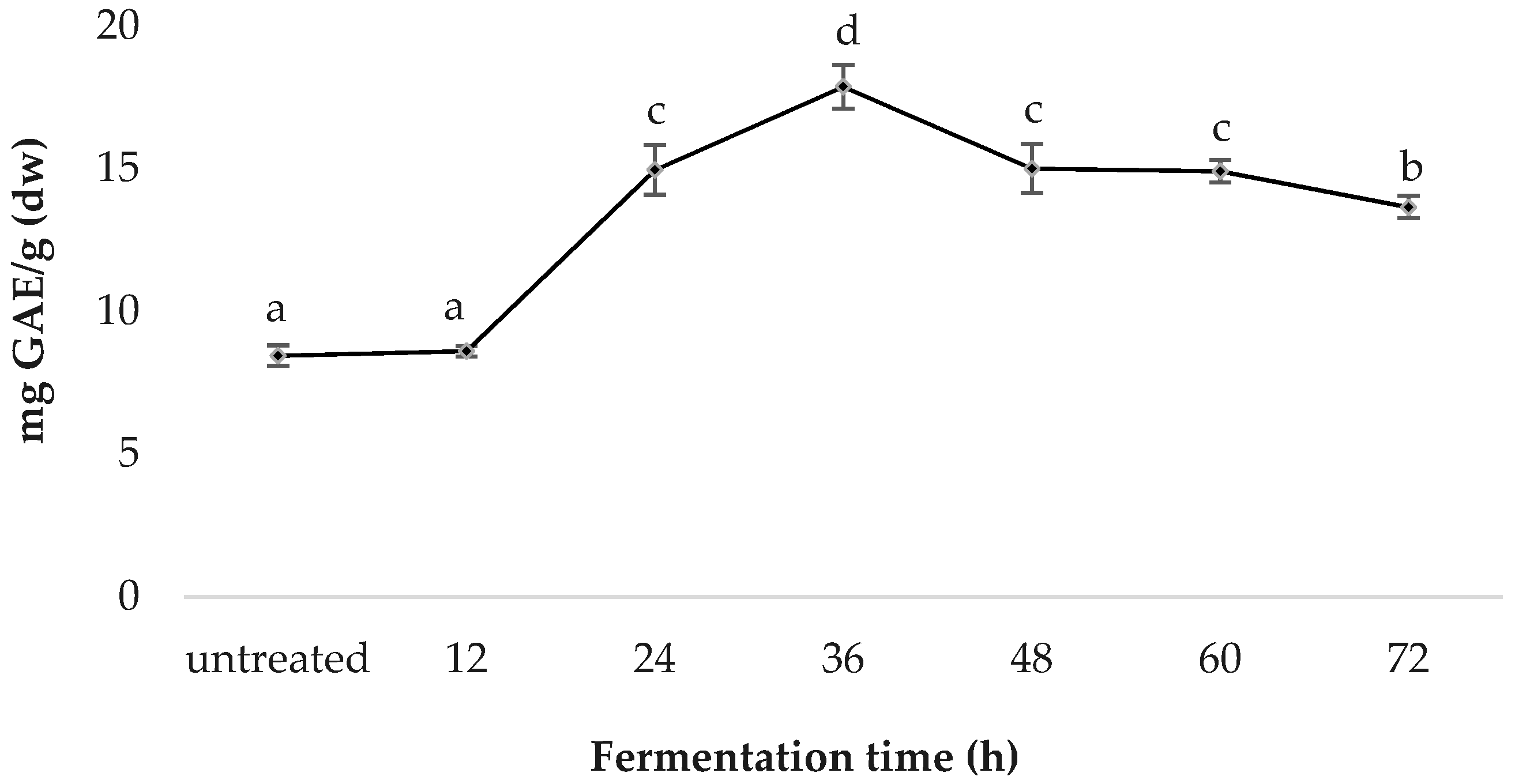
Spirulina fermented for 36 h was determined to have the highest TPC (17.87 ± 0.77); followed by those treated for 48 h (15.00 ± 0.86), 24 h (14.95 ± 0.87), 60 h (14.91 ± 0.40), 72 h (13.65 ± 0.40), 12 h (8.60 ± 0.36) and the untreated sample (8.44 ± 0.39); TPC values are in mg GAE/g (dw) (Figure 2).
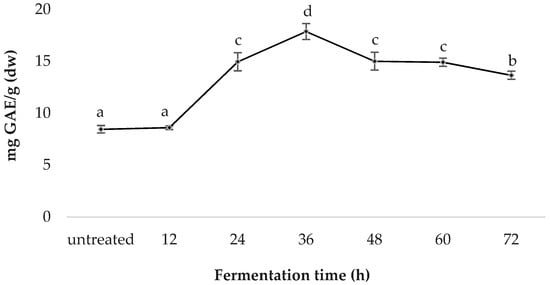
Figure 2.
Comparative total phenolic content of fermented spirulina.
3.3. C-phycocyanin Content
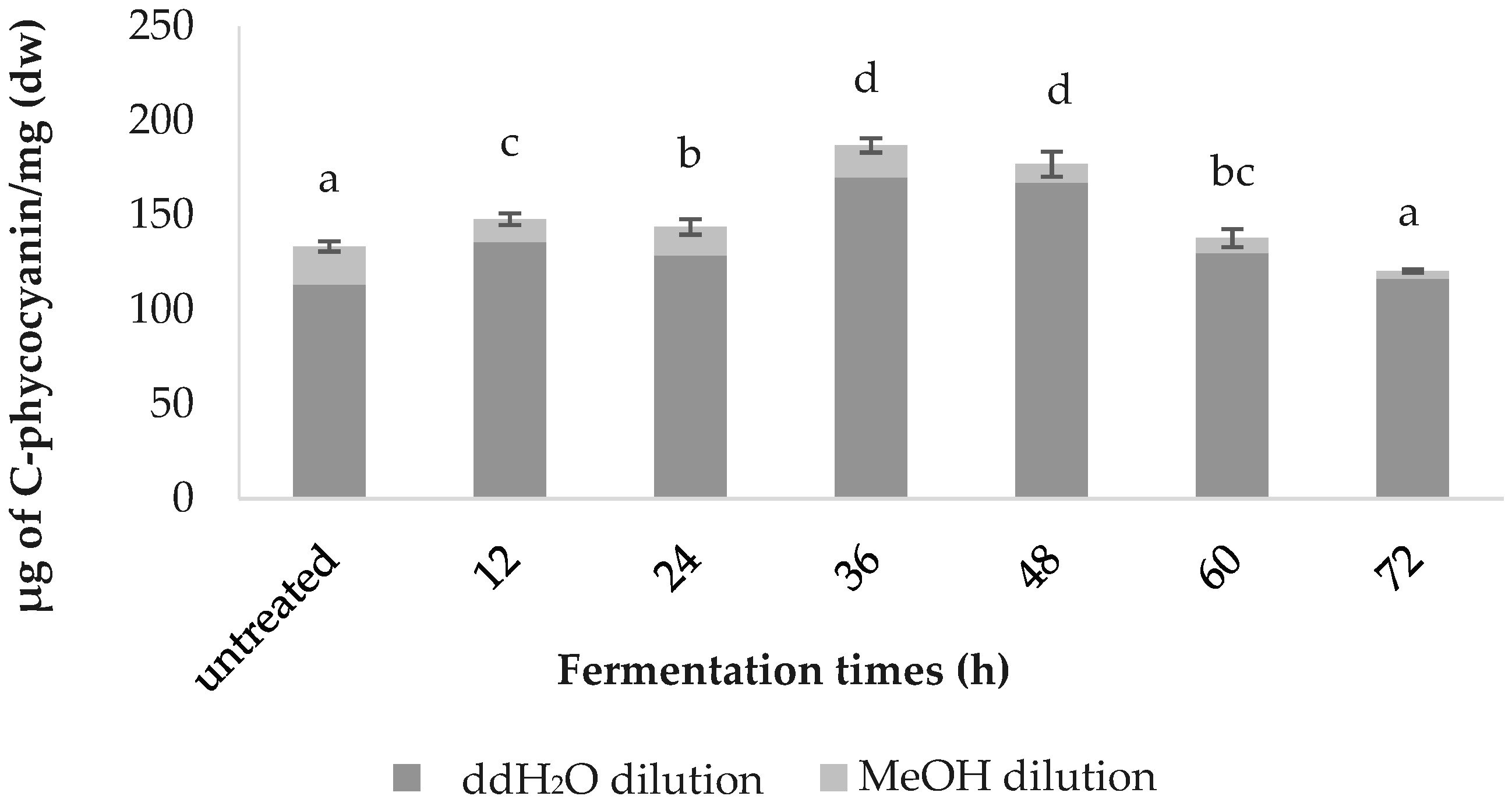
The C-phycocyanin content was highest in the 36-h-fermented samples (187.0 ± 3.79), followed by the samples fermented for 48 h (177.17 ± 6.60), 12 h (148.07 ± 3.04), 24 h (143.83 ± 4.07), 60 h (138.01 ± 4.82), the untreated (133.57 ± 2.64) and 72 h (120.61 ± 0.86); values are in µg of C-phycocyanin/mg (Figure 3).
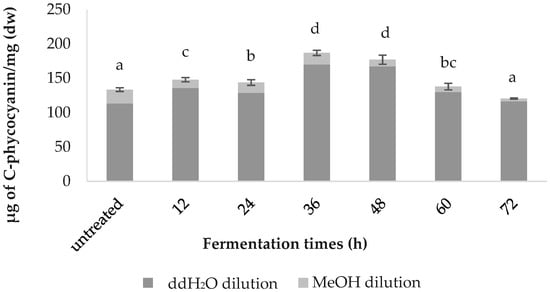
Figure 3.
Comparative C-phycocyanin content of the fermented spirulina.
3.4. DPPH Radical Scavenging Capacity
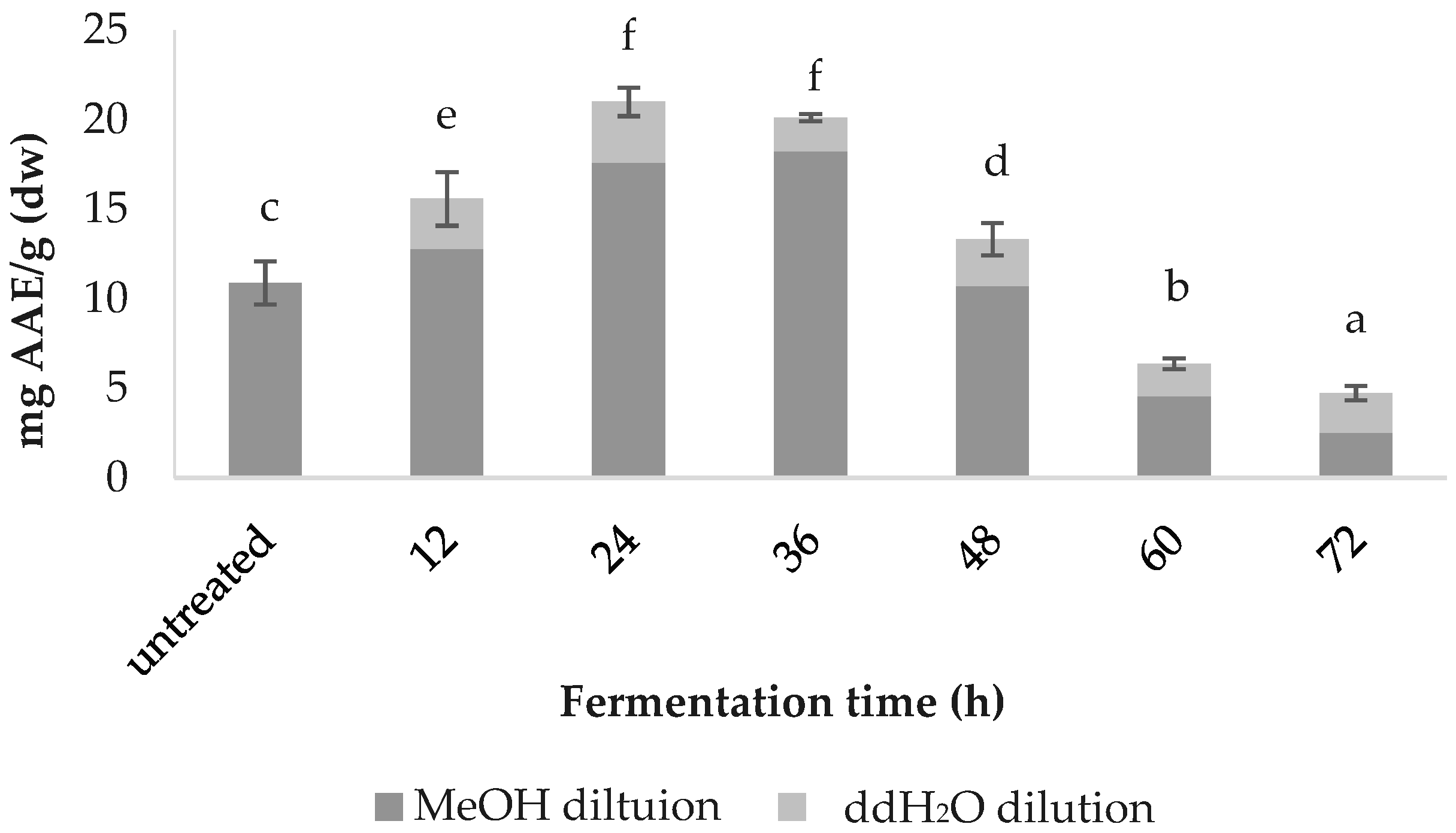
DPPH radical scavenging capacity was greatest in 24 h fermented spirulina at 21.00 ± 0.8, followed by that fermented for 36 h (20.12 ± 0.2), 12 h (15.58 ± 1.5), 48 h (13.32 ± 0.9), the untreated (12.58 ± 1.2), 60 h (6.36 ± 0.3) and 72 h (4.73 ± 0.4); values are in mg AAE/g (dw) (Figure 4).
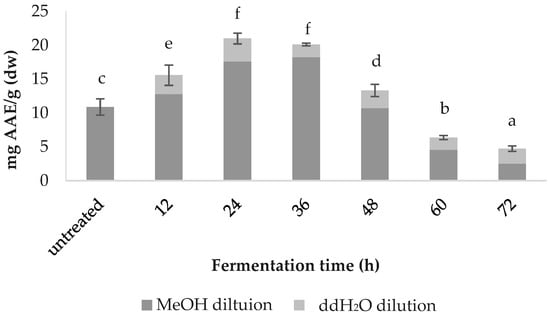
Figure 4.
Comparative DPPH values of fermented spirulina.
3.5. FRAP Values
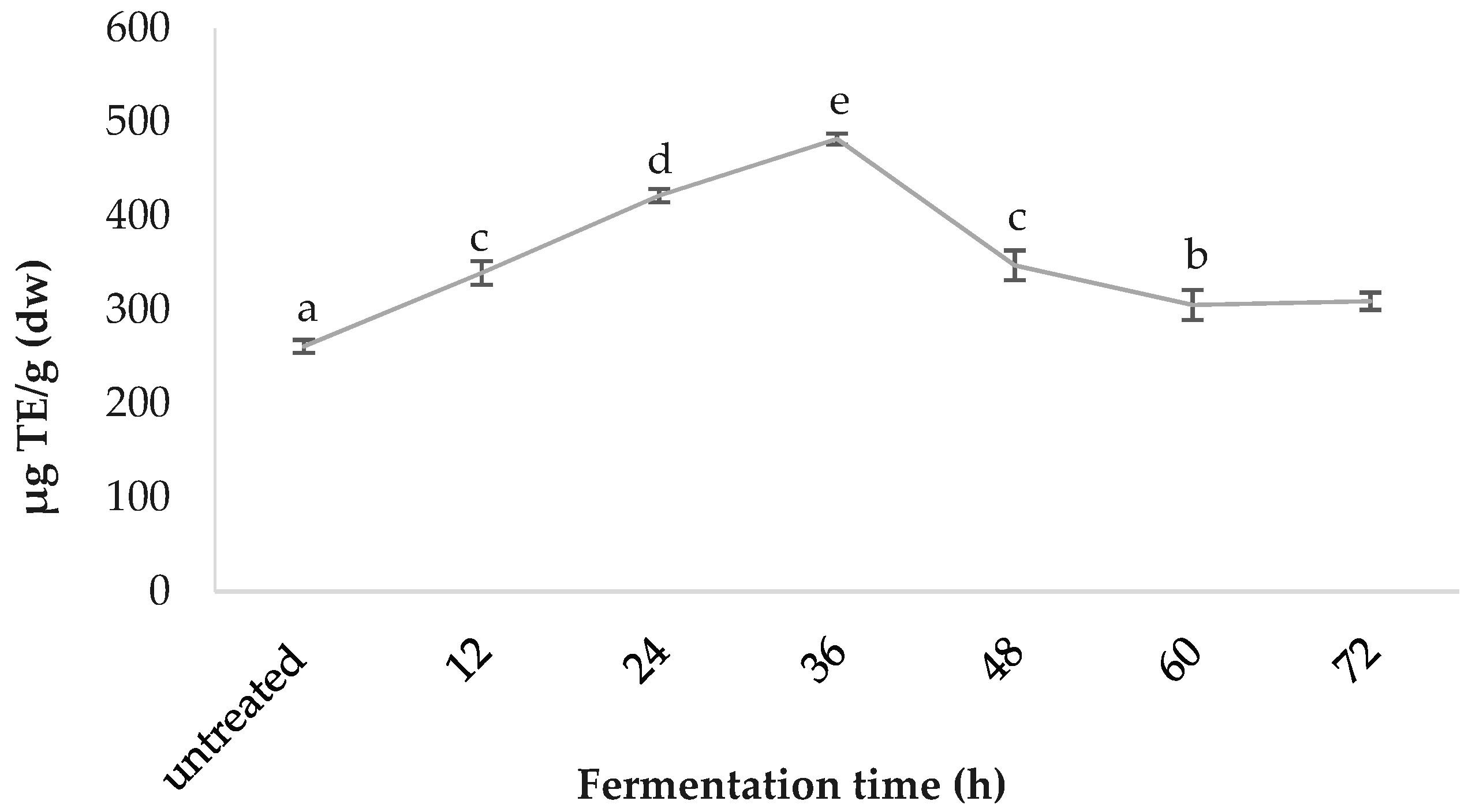
Comparative FRAP values are presented in Figure 5. The 36-h-fermented samples demonstrated the highest ferric reducing antioxidant power of 482.00 ± 6.02, followed by 24 h (421.76 ± 6.96), 48 h (347.47 ± 15.94), 12 h (339.44 ± 12.54), 72 h (309.32 ± 9.20), 60 h (305.30 ± 15.94) and the untreated (261.12 ± 6.95); values are in µg TE/g (dw).
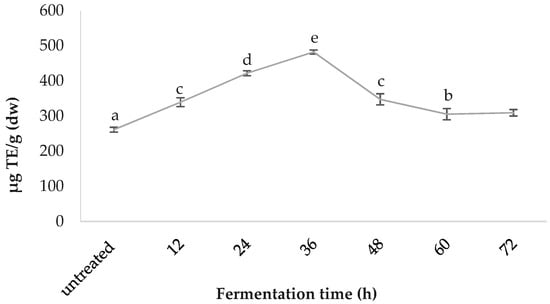
Figure 5.
Comparative ferric reducing antioxidant power (FRAP) values of the fermented spirulina.
3.6. ORAC
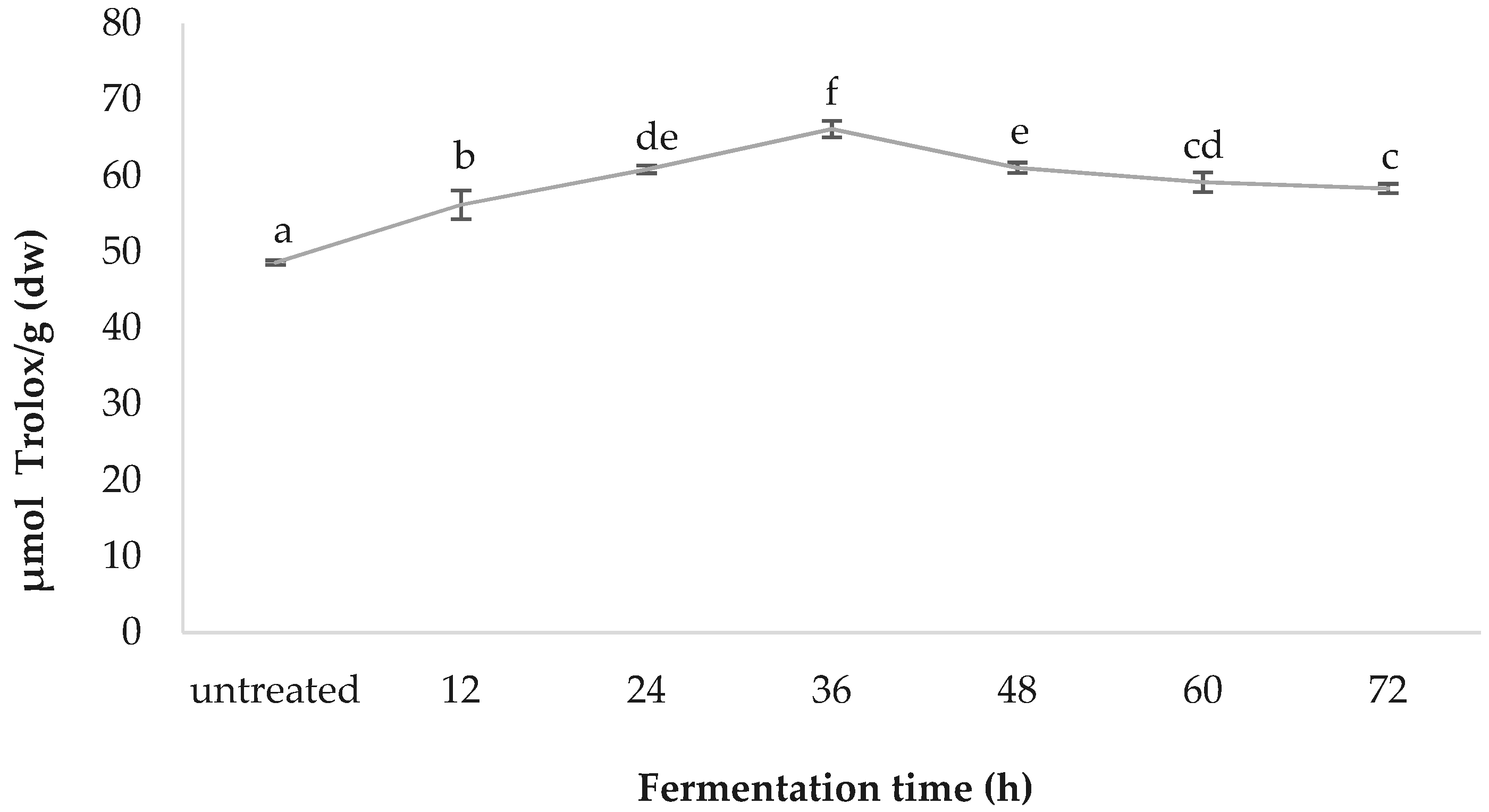
The greatest oxygen radical antioxidant capacity was observed in the 36-h-fermented samples, with a value of 66.14 ± 1.09, followed by those fermented for 48 h (61.06 ± 0.69), 24 h (60.85 ± 0.50), 60 h (59.17 ± 1.27), 72 h (58.33 ± 0.63), 12 h (56.20 ± 1.88), and the untreated (48.61 ± 0.29); values are in µmol Trolox/g (dw) (Figure 6).
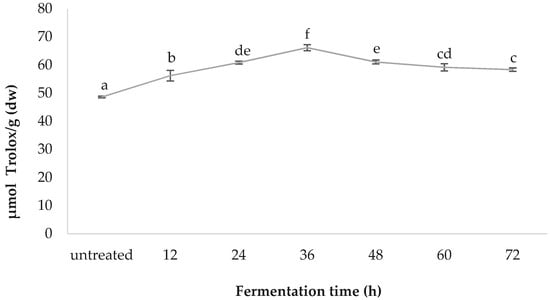
Figure 6.
Comparative oxygen radical absorbance capacity (ORAC) values of fermented spirulina.
3.7. Protein Fragmentation
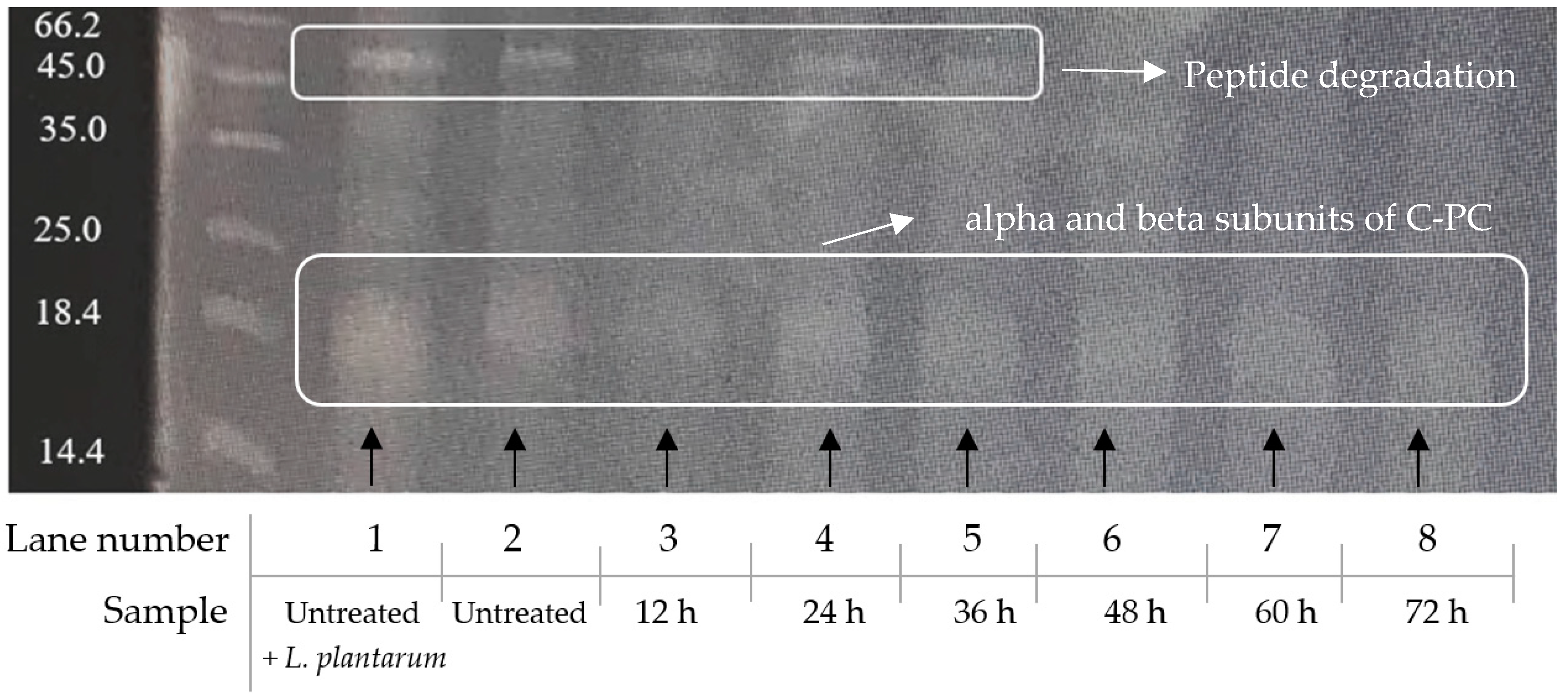
SDS-PAGE analysis of the spirulina protein content is shown in Figure 7. The main band at ~50 kDa visible in lanes 1 to 5 (untreated to 36 h) is no longer present in lanes 6, 7 and 8 (48–72 h). This suggests protein fragmentation via lactic acid bacterial hydrolysis of the peptide bonds, during the fermentation process. The strong band at 16–17 kDa, just below the 18.4 kDa marker, are the C-phycocyanin alpha and beta subunits, and remain un-fragmented.
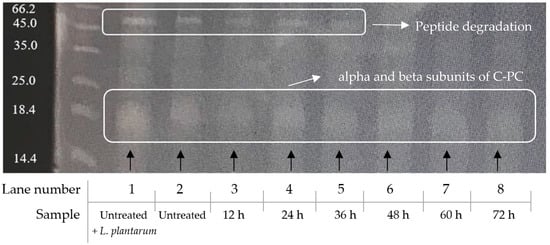
Figure 7.
SDS-PAGE analysis of the total protein in spirulina.
3.8. Free Methionine Content
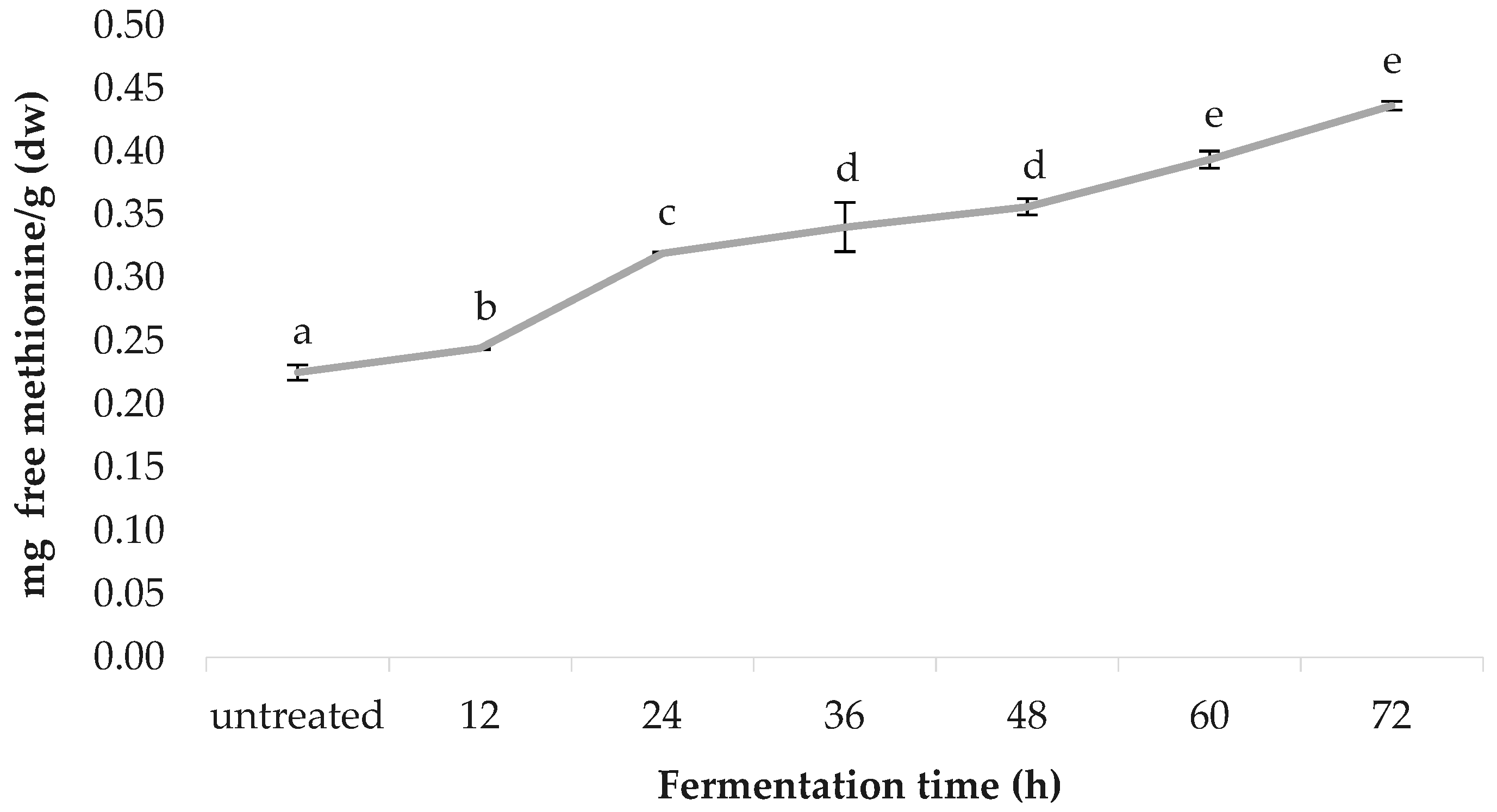
Figure 8 shows the free methionine content of the spirulina samples, before and after fermentation, as quantified by RP-HPLC. A linear correlation was observed between the fermentation time and the free methionine content. Untreated spirulina had a free methionine content of 0.225 ± 0.001, followed by 12 h (0.245 ± 0.001), 24 h (0.320 ± 0.019), 36 h (0.340 ± 0.006), 48 h (0.357 ± 0.007), 60 h (0.394 ± 0.003) and 72 h (0.437 ± 0.012); values are in mg-free methionine/g (dw).
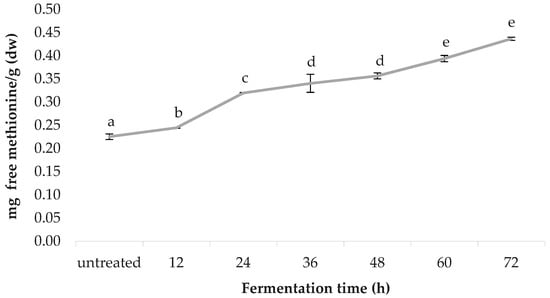
Figure 8.
Free methionine content of the untreated and the fermented spirulina.
4. Discussion
4.1. Effect of Fermentation on Spirulina
This study shows that fermentation enhances the nutraceutical profile of spirulina. It is important to note that the observed variability of results in the literature might be due to the botanical source, genetic background, environmental effects during growth, processing techniques, and storage conditions of each species [34].
When examining the spirulina’s nutraceutical composition, the total phenolic compound concentration present in the samples was the first parameter assessed. Phenolic compounds serve as essential antioxidants because of their ability to stabilise radicals, by donating a hydrogen atom or an electron. TPC values increased by 111.73%, during the first 36 h of fermentation, due to the release of the phenolic compounds, upon bacterial enzymatic hydrolysis of the spirulina cell walls. The modest TPC decrease, after the initial fermentation period, might be due to the temperature damage of the unstable phenolic substances [35]. TPC values in-line with those determined in the present study have been reported for Arthrospira [36,37,38] and other similar aquatic microorganisms, such as microalgae [39]. In all cases, the TPC was higher than those found in terrestrial plant species [40]. Different studies suggest that TPC is a major contributor to the total antioxidant capacity (TAC) [41,42,43], whereas others contradict this claim [44,45]. Due to the highly oxidising nature of the Folin Ciocalteu reagent and the sample–colour interference, the results vary between studies [34,37,46,47]. In the present study, TPC showed a high correlation with the ORAC results (r = 0.90), suggesting that phenolic compounds did contribute significantly to spirulina’s TAC. There is no overall consensus on the role that TPC plays in TAC, and further research is necessary.
Phycobiliproteins characterisation and cultivation enhancement has been explored for nutraceutical and cosmeceutical applications. The C-phycocyanin (C-PC) content in fermented spirulina increased by 32.64%. In this context, an increase in C-phycocyanin does not refer to the net C-PC content in spirulina, which remains constant. Instead, it reflects a release of this protein-bound pigment, through the fermentation process, via enzyme hydrolysis. It is distinguishable from Table 1 that C-PC was released since the fermented samples appeared to be bluer than the untreated sample. However, C-PC is a temperature-sensitive pigment [48], which, under the fermentation conditions set for this project (37 °C), might have been degraded after 36 h. Moreover, Boussiba and Richmond [49] suggested that a decrease in the C-phycocyanin content is evident during nitrogen starvation conditions, which parallels the increased pH during the second half of the fermentation period, due to L. plantarum digestion of the available nitrogen. The results obtained in the present study are in-line with those from the literature; slightly below average for the untreated samples, and above average for the fermented samples [50,51]. The variability in results could be due to the factors laid out previously, highlighting the cultivation conditions, since environmental stress, together with selection of the growth medium, are key variables for the concentration of photo-pigments like C-PC [52].
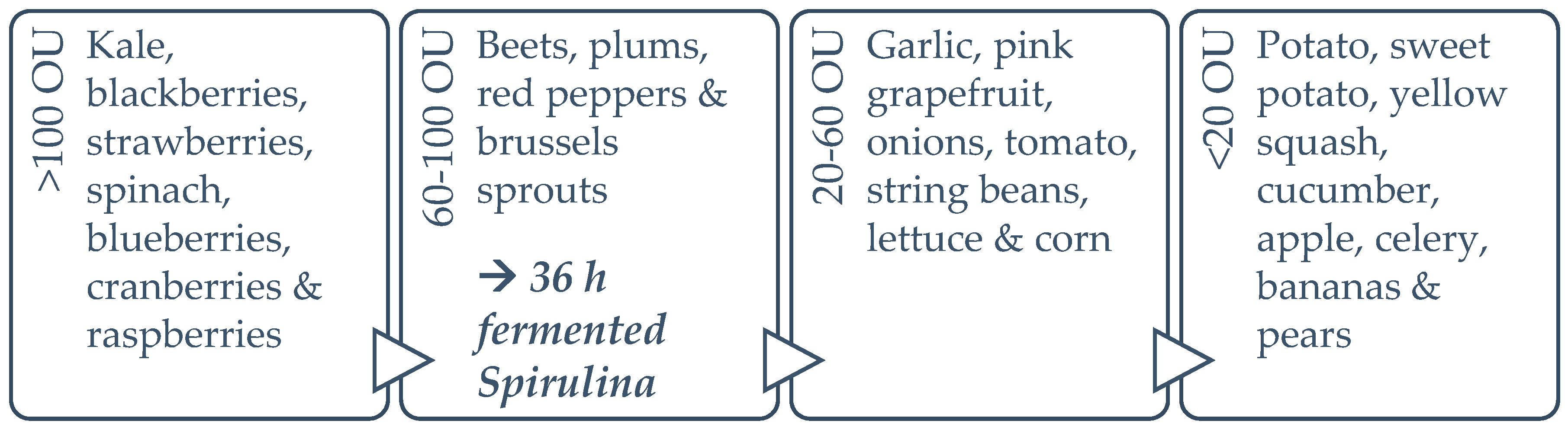
As for spirulina’s in vitro bioactivity, the DPPH radical scavenging capacities reported in the present study fall within previously described ranges for spirulina and increase significantly (by 66.93%) with fermentation treatment [37,53,54]. To obtain an overall perspective, synthetic antioxidant thiobarbituric acid (TBA) and butylated hydroxytoluene (BHT) can inhibit DPPH radicals, by up to 93.0% and 95.6% respectively; while α-tocopherol inhibits it by 91.5% [37]. The maximum percentage inhibition achieved by spirulina in the present study was 56.0%, after 24 h fermentation, which is more than half that of the synthetic antioxidants. This is significant, considering spirulina is a natural source, and has an enhanced nutrient profile, in addition to the increased antioxidant capacity. The ferric reducing antioxidant power (FRAP) increased by 84.59% during the fermentation process, reaching a maximum at 36 h and diminishing afterwards, possibly due to the temperature damage of thermo-sensitive antioxidant compounds. The FRAP results reported for this study fall within the average values of the literature [38]. It has been suggested that C-PC and carotene are key contributors to FRAP [55,56]. Figure 9 illustrates the published ORAC values for the edible portion of various fruits and vegetables, in order to facilitate the understanding of where spirulina TAC as per ORAC stands, compared to other foods. Increased consumption of plant-based foods rated with a high ORAC value can enhance plasma antioxidant capacity in humans [57].
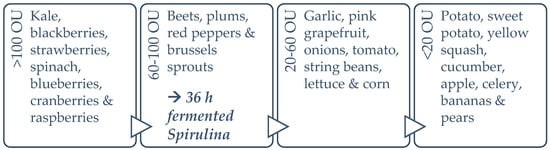
Figure 9.
ORAC values (in ORAC Units (OU)) for the dry weight of various foods [58,59,60,61].
Regarding the protein fragmentation patterns, visible bands represent subunits of C-PC, one of the main contributors to spirulina’s bioactivity. As shown in Figure 7, the bundle of monomers at ~50 kDa is only present for the first 36 h of the fermentation treatment. Afterwards, the band vanishes, suggesting a peptide bond cleavage by the action of LAB. According to Boussiba and Richmond [5], C-PC has a molecular weight of 44 kDa. However, lower molecular weight trimers of this peptide-bond pigment have been identified in previous research, to be between 14.4 and 30 kDa [47], which explains the appearance of a broad band at ~16–17 kDa, just below the 18.4 kDa marker. These have been identified in previous studies as alpha and beta subunits of C-PC, with molecular masses of 16 and 17 kDa [51,62]. The present study cannot claim bioactivity of specific peptides, since protein purification and identification was not performed. However, SDS-PAGE, together with RP-HPLC for free methionine quantification, show protein fragmentation, which demonstrates that smaller peptides were released from the parent proteins, during the fermentation process. Gibbs et al. [63] observed a similar fragmentation of proteins, due to the lactic acid bacterial protease activity in fermented soy samples.
The nutritional value of spirulina is well-recognised, due to its unusually high protein content (60–70% of its dry weight, greater w/w than red meat), which can be hydrolysed into bioactive peptides (BPs) with potent in vitro and ex vivo bioactivity [64]. Recent studies have provided evidence that BPs derived from marine microorganisms, which share great similarities with cyanobacteria, such as spirulina, play an important role in human health and nutrition and, therefore, have a high potential as active ingredients for preparation of functional foods and nutraceutical products [65,66,67]. So far, the efficacy of these BPs have been studied in vitro, in animal models and in human digestive simulation systems. Therefore, detailed human studies are needed in order to confirm the bioactivity of these peptides [68,69]. The findings of the present study, via the RP-HPLC revealed a 94.22% increase of free methionine content, from baseline to the end of the fermentation period. However, further fermentation periods are recommended to understand to what extent free methionine content increases in spirulina upon peptide hydrolysis. Published data for free methionine content in thirty-seven varieties of spirulina reported by Al-Dhabi and Valan Arasu [36] ranges from 0.0035 to 0.0098 mg/g (fresh/wet weight). After taking into consideration that spirulina biomass is ~95% water [70], this is in accordance with the values reported in the present study. The variation in the literature could be due to the Arthrospira species selected, and the extent of native protein cleaved by fermentation.
4.2. Correlation between pH as an Indicator of Fermentation Status and Results Trend
A decrease in pH during fermentation is a result of the bacterial production of organic acids, primarily lactic acid. In this study, untreated spirulina showed the highest, most basic pH value of 6.0, with a continuous decrease during the first half of the fermentation treatment (pH 3.8 at 36 h), followed by a less accentuated increase to pH 4.6, after 72 h fermentation. Once the substrate concentration for bacterial fermentation is too low, L. plantarum can no longer survive. Therefore, the breakdown of spirulina compounds and release of lactic acid slows down, reaching its maximum at 36 h and coinciding with the minimum pH and highest TAC. The increase in pH during the last 36 h of the fermentation process might be due to the release of basic ammonia, cleaved-off from the high amino acid content found in spirulina. L. plantarum has a greater demand for carbon than for nitrogen, leading to a net release of ammonia in the medium, which attracts protons to form ammonium in aqueous solution, thus, increasing the pH [71].
4.3. Benefits of the ORAC Assay Versus other Traditional TAC Assays
Since DPPH, FRAP, TPC, Bradford and C-phycocyanin content assays rely on a colorimetric-based assessment, the powerful green/blue hue of the spirulina has the potential to interfere with the absorbance readings, leading to inconsistent results. The DPPH and FRAP assays are technically simple and quick to perform; however, it has been argued that they are not a chemically sound method for assaying TAC [72,73]. Both assays undergo a Hydrogen Atom Transfer (HAT)-based mechanism, when reacting with the substrate(s) present in the sample, which bears little resemblance to the reaction in vivo. Additionally, DPPH as a radical is highly stable, compared to other reactive radical species present in most food matrices [73], and the reversibility between DPPH and ortho-methoxyphenol compounds reaction, together with its low tolerance for pH changes, could lead to false low-absorbance readings. Furthermore, the FRAP assay enhances an unnatural redox propagation [46], releases Fe(II), and fails to detect thiol- or carotenoid-containing antioxidants [74]. ORAC has multiple advantages over other TAC methods [31,32,34,61,75], and it was the preferred assay for this study, offering the highest correlation with pH change, during fermentation (r = 0.95).
4.4. Potential Application of Spirulina Fermentation
Cyanobacteria are the oldest form of autotrophic life on Earth and compose the main part of the biomass of the planet. It is believed that the key to their success lies in their unique metabolism and cytoplasmic composition, which also makes them excellent nutraceutical candidates for the highly demanding modern food industry constantly seeking to create healthier, cheaper, more convenient novel foods [76]. In addition, health-pursuing consumers of this century are trending towards functional foods and nutraceutical products [77].
Spirulina protein’s quality is highly superior to that of almost any other ’green’ source, since it offers all essential amino acids (AA), with no limiting factor for assimilation [78], which is rare for non-animal foods. In fact, non-essential AA represent almost half of the protein content in spirulina [16]. Moreover, in terms of its digestibility, its biological value and net protein utilisation are in the same range as most common standard protein sources. When evaluating spirulina protein by an animal feeding test, its protein efficiency ratio scored much higher than most plant proteins [79]. Therefore, spirulina could be used as a supplement to increase the content of the limited AA in certain foods, such as cereals [80].
Further, AA production is a multi-billion-dollar, multi-national industry. Glutamic acid, lysine and methionine account for the majority, by weight, of AAs sold. AAs are consumed in a variety of markets, the largest by volume being the food-flavouring industry, followed by the animal feed industry [81]. Methionine is an essential AA for humans and livestock. Its production is achieved via enzymatic synthesis (bioconversion of precursors), or by submerged fermentation, using microorganisms. The lack of knowledge regarding feedback regulation of methionine biosynthesis is a major impediment for methionine extraction, via fermentation [82]. Therefore, most commercially pure L-methionine products are synthesised chemically, from aspartate [81]. Plant proteins are normally deficient in methionine, consequently, plant-based diets might need methionine supplementation to avoid dietary deficiency. Therefore, methionine, together with other essential AAs, is of significant interest and its demand has increased greatly, over the last few decades [82].
A limited number of studies have examined the properties of fermented spirulina. Spirulina has been confirmed to be a suitable substrate for L. plantarum, thus, holding a potential for the production of probiotic-based products [83]. Additionally, spirulina fermentation with L. plantarum increased digestibility by 4.4%, and the antioxidant activity and total phenolic content by 79% and 320%, respectively [83]. Fermentation with L. plantarum and Bacillus subtilis improved deodorisation of off-flavour and protein hydrolysis, also yielding an improved ratio of essential-to-total AAs, compared to the unfermented spirulina and consequently enhancing the sensory and antioxidant capacity, during product development [84]. Further, LAB-fermented spirulina was examined for its antioxidant effects and UVB protective activity in skin-care models. Results showed higher levels of free polyphenols and phycocyanobilin in fermented versus untreated spirulina [85]. Lastly, Choi et al. [86], likewise, demonstrated an enhanced TAC and beta-carotene profile, upon L. plantarum fermentation and ultrasonic extraction of Arthrospira maxima, which was thought to contribute to the apparent higher brain-derived neuroprotective factor of fermented Arthrospira maxima, compared to its untreated control. All of these data agree with the previously laid-out ‘cell wall biodegradation’ hypothesis upon LAB fermentation of the Arthrospira species.
5. Conclusions
Fermentation increased the nutraceutical value of spirulina, significantly (P ≤ 0.05), for all evaluated parameters. The 36-h-fermented spirulina had the highest TPC, C-phycocyanin content, FRAP and ORAC values of all fermentation treatment periods, except for the DPPH scavenging activity, which peaked at 24 h. Additionally, ORAC, the only fluorescence-based method, had the highest correlation with pH drop, during fermentation (r = 0.95), and is recommended as a sole measure of antioxidant capacity for coloured-food matrices. Total antioxidant capacity diminished after the first half of the fermentation treatment, probably due to the damage of air-, and heat-sensitive antioxidants, while free methionine content and protein fragmentation were reported to be highest in the 72-h-fermented spirulina samples. Thus, while total antioxidant capacity diminished after 36 h (expected for thermolabile antioxidants), the thermostable proteins and bioactive peptides increased in a linear manner, as a direct function of fermentation time. Further research is needed to explore the bioactivity of fermented spirulina in vivo.
Author Contributions
E.d.M.C. and E.S. performed the experiments, analysed the data and drafted this paper. E.S. and N.A.-G. developed the concept, designed the experiments and revised the manuscript for publication.
Funding
This research received no external funding.
Acknowledgments
Arthrospira platensis F&M-C256 (Fotosintetica & Microbiologica) biomass was provided by Archimede Ricerche S.r.l. (Imperia, Italy).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sapp, J. The prokaryote-eukaryote dichotomy: Meanings and mythology. J. Mol. Biol. 2005, 69, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Miranda, M.; Cintra, R.; Barros, S.; Mancini-Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Bishop, W.M.; Zubeck, H.M. Evaluation of microalgae for use as nutraceuticals and nutritional supplements. J. Nutr. Food Sci. 2012, 2, 147–153. [Google Scholar] [CrossRef]
- Boussiba, S.; Richmond, A.E. Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Premakumari, S. Effect of supplementation of Spirulina on hypercholesterolemic patients. J. Food Sci. Technol. 1996, 33, 124–128. [Google Scholar]
- Layam, A.; Reddy, C. Antidiabetic property of spirulina. Diabetol. Croat. 2006, 35, 29–33. [Google Scholar]
- Konícková, R.; Vanková, K.; Vaníková, J.; Vánová, K.; Muchová, L.; Subhanová, I.; Zadinová, J.; Zelenka, D.A.; Kolár, M.; Strnad, H.; et al. Anti-cancer effects of blue-green alga Spirulina platensis, a natural source of bilirubin-like tetrapyrrolic compounds. Ann. Hepatol. 2014, 13, 273–283. [Google Scholar]
- Cheong, S.; Kim, M.; Sok, D.; Hwang, S.; Kim, J.; Kim, H.; Lee, J.; Kim, Y.; Kim, M. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J. Nutr. Sci. Vitaminol. 2010, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. Microalgae nutraceuticals. Foods 2016, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Bandarra, N.; Raymundo, A.; Gouveia, L. Food Chemistry Research Development, Chapter 2; Nova Science Publishers, Inc.: New York, NY, USA, 2008; pp. 1–36. [Google Scholar]
- Intergovernmental Institution for the Use of Micro Algae Spirulina against Malnutrition (IIMSAM). Available online: https://www.who.int/pmnch/about/members/database/iimsam/en/ (accessed on 11 November 2018).
- Holman, B.; Malau-Aduli, A. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2012, 97, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Chow, T. Hypolipidemic, antioxidant, and anti-inflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, 33–45. [Google Scholar] [CrossRef]
- Sanjukta, S.; Rai, A. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Rai, A.K.; Jeyaram, K. Health Benefits of Functional Proteins in Fermented Foods, 1st ed.; Tamang, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 2015; Volume 1, pp. 455–474. [Google Scholar]
- Limón, R.; Peñas, E.; Torino, M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef]
- Hur, S.; Lee, S.; Kim, Y.; Choi, I.; Kim, G. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez- Cordovés, C. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein peptides with angiotensin I- converting enzyme inhibitory (ACEI) activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2017, 51, 128–136. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; Van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N.; Scannell, A.G. Growth and kinetics of Lactobacillus plantarum in the fermentation of edible Irish brown seaweeds. Food Bioprod. Process. 2011, 89, 346–355. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of different solvents on polyphenolic content, antioxidant capacity and antibacterial activity of Irish York cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complimentary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Abu-Ghannam, N. Degradation kinetic modelling of color, texture, polyphenols and antioxidant capacity of York cabbage after microwave processing. Food Res. Int. 2013, 53, 125–133. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Prior, R. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Cao, G.H.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Martura, T.; Olteanu, M.O.; Panaite, T.D.; Schitea, M.S.; Van, I. Development and validation of an RP-HPLC method for methionine, cystine and lysine separation and determination in corn samples. Rev. Chim. Buchar. 2013, 64, 673–679. [Google Scholar]
- Prior, R.L.; Cao, G. Analysis of botanicals and dietary supplements for antioxidant capacity: A review. AOAC Int. 2000, 83, 950–956. [Google Scholar]
- Lawton, W.R. Heat Sensitive Recording Composition Containing a Complexed Phenolics and a Spiropyran or Leuco Lactone. U.S. Patent 4,097,288, 27 June 1978. [Google Scholar]
- Al-Dhabi, N.; Valan Arasu, M. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evid.-Based Complement. Altern. Med. 2016, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, H.H.A.; El Baz, F.K.; El-Baroty, G.S. Characterization of nutraceutical compounds in blue green alga Spirulina maxima. J. Med. Plants Res. 2008, 2, 292–300. [Google Scholar]
- Shukla, V.; Vashistha, M.; Singh, S.N. Evaluation of antioxidant profile and activity of amalaki (Emblica officinalis), spirulina and wheat grass. Indian J. Clin. Biochem. 2009, 24, 70–75. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Faramarzi, M.A.; Mohammadi, N.; Soltani, N.; Oveisi, M.R.; Nafissi-Varcheh, N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J. Phycol. 2010, 22, 43–50. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Jimenez-Escrig, A.; Jimenez-Jimenez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.A.J.; Perera, C.O.; Hemar, Y. Production of bioactive proteins and peptides from the diatom Nitzschia laevis and comparison of their in vitro antioxidant activities with those from Spirulina platensis and Chlorella vulgaris. Int. J. Food Sci. Techonol. 2017, 29, 128–130. [Google Scholar] [CrossRef]
- Sarada, R.M.G.P.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Proc. Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Boussiba, S.; Richmond, A.E. C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Arch. Microbiol. 1980, 125, 143–147. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Stukenberg, M. Blue-Green Algae (Cyanobacteria). In Encyclopedia of Dietary Supplements, 1st ed.; Informa Healthcare: London, UK, 2006; Volume 1. [Google Scholar]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Express. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A.; Kobus, M. Influence of growth medium composition on synthesis of bioactive compounds and antioxidant properties of selected strains of Arthrospira cyanobacteria. Czech J. Food Sci. 2012, 30, 258–267. [Google Scholar] [CrossRef]
- Zaid, A.; Hammad, D.; Sharaf, E. Antioxidant and anticancer activity of Spirulina platensis water extracts. Int. J. Pharm. 2015, 11, 846–851. [Google Scholar] [CrossRef]
- Chu, W.; Lim, Y.; Radhakrishnan, A.; Lim, P. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement. Altern. Med. 2010, 10, 112–119. [Google Scholar] [CrossRef]
- Madhyastha, H.; Vatsala, T.M. Cysteine rich cyanopeptide β2 from Spirulina fussiformis exhibits plasmid DNA pBR322 scission prevention and cellular antioxidant activity. Indian J. Exp. Biol. 2010, 48, 486–493. [Google Scholar]
- Madhyastha, H.K.; Sivashankari, S.; Vatsala, T.M. C-phycocyanin from Spirulina fussiformis exposed to blue light demonstrates higher efficacy of in vitro antioxidant activity. Biochem. Eng. J. 2009, 43, 221–224. [Google Scholar] [CrossRef]
- Cao, G.; Booth, S.L.; Sadowski, J.A.; Prior, R.L. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am. J. Clin. Nutr. 1998, 68, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Total antioxidant capacity of fruits. J. Agric. Food Chem. 1996, 44, 701–705. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.A.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Vo, T.S.; Ryu, B.; Kim, S.K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. A 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Tokuşoglu, Ö.; Üunal, M.K. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ropp, J.K.; Zaman, S.; Melgar, A. Effects of essential oils on in vitro ruminal fermentation and ammonia release. Anim. Feed Sci. Technol. 2008, 144, 55–64. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R. The chemistry behind antioxidant capacity assays. J. Agric. Food Sci. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic anti- oxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.; Darzins, A.; Posewitz, M. Genetic engineering of algae for enhanced biofuel production. J. Eukaryot Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Siro, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. J. Appet. 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [PubMed]
- Richmond, A. Microalgal biotechnology. J. Chem. Technol. Biochem. 1988, 47, 181–182. [Google Scholar]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Ikeda, M. Amino acid production processes. In Microbial Production of L-Amino Acids; Robert, F., Jugen, T., Bathe, V.G., Debabov, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–35. [Google Scholar]
- Kumar, D.; Gomes, J. Methionine production by fermentation. Biotechnol. Adv. 2005, 23, 41–47. [Google Scholar] [CrossRef]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2018, 1–7. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.H.; Ren, D.F.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Liu, J.G.; Hou, C.W.; Lee, S.Y.; Chuang, Y.; Lin, C.C. Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Proc. Biochem. 2011, 46, 1405–1410. [Google Scholar] [CrossRef]
- Choi, W.; Kang, D.; Heo, S.J.; Lee, H. Enhancement of the neuroprotective effect of fermented Spirulina maxima associated with antioxidant activities by ultrasonic extraction. Appl. Sci. 2018, 8, 2469. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).