Abstract
d-lactic acid is a building block for heat resistant polylactic acid, a biobased polymer with a high potential. Nevertheless, an economically efficient industrial process for d-lactic acid production still needs to be implemented. Yeast extract is an expensive nutrient source, which is used to fulfill the complex nutritional requirements in lactic acid fermentations. The substitution of yeast extract by cheap alternative nutrient sources is a challenge in many fermentation processes. In this study, chemical and enzymatic hydrolysis techniques for protein rich agricultural residues and their effectiveness are compared, as well as their impact on the d-lactic acid production of Sporolactobacillus inulinus. An efficient substitution of yeast extract could be achieved by a variety of agricultural residues, hydrolysed with 3M H2SO4, demonstrating the much higher versatility and effectiveness of this method compared to enzymatic methods. In a fed-batch experiment with chemically hydrolyzed rapeseed meal and minimal supplementation, a lactic acid titer of 221 g L−1 and an overall productivity of 1.55 g (L h)−1 (96% yield) were obtained.
1. Introduction
The utilization of alternative nutrient sources to yeast extract is a challenge in many fermentation processes. The nutrient source not only needs to deliver organic nitrogen like amino acids, but also vitamins, salts, trace elements, and nucleic acids to compensate auxotrophies, prevent limitations, and save metabolic energy [1,2]. In lots of processes, yeast extract is still the nutrient source of choice, leading to positive results due to its low molecular mass distribution and high B-vitamin content [3,4]. However, with a price of 9–10 €/kg, it is a major factor in the production costs [5]. Cheap protein-rich agricultural residues can be used as alternative nutrient sources, but a prior disintegration of high molecular cell components is necessary. Therefore, enzymatic and chemical hydrolysis methods can be applied. The predominant method is enzymatic hydrolysis using proteases in a separate or simultaneous hydrolysis and fermentation processes [6,7]. This method benefits from a low salt content in the hydrolysate since a neutralization step is not required [8]. However, the cleavages of the peptide bonds depend on the selectivity of the enzyme and inhibition can occur. Moreover, in a simultaneous process the enzyme incubation conditions often do not correspond to the cultivation conditions. On the other hand, chemical hydrolysis is rarely used for disintegration of protein-rich agricultural residues, because the hydrolysate must be neutralized for fermentation and it often results in lower productivities and final concentrations [9,10]. However, the advantages like a high flexibility caused by the non-selective cleavage of peptide bonds and the possibility to adapt the reaction conditions to the requirements, it provides broad application opportunities. In addition to the hydrolysis method, an optimized supplementation strategy can help to fulfill the nutritional requirements.
An example for a process with a high demand for complex nutrients is lactic acid fermentation [11]. The yearly worldwide industrial production of lactic acid amounts about 750 ktons and is achieved by fermentation of lactic acid bacteria, which are known to be auxotroph for distinct amino acids and vitamins [12,13,14,15]. The predominantly produced enantiomer is L-lactic acid, which is identical with the lactic acid naturally is produced in the body and is thus the preferred isomer in food and pharmaceutical applications [16]. Furthermore, it is an important building block for polylactic acid (PLA). PLA is one of the most promising bio-based and biodegradable plastics with an estimated annual production of more than 500 ktons in 2015 [17]. However, due to its low thermal stability its applications are limited, examples are packaging materials, medical implants, and 3D-printing [18,19]. In order to synthesize heat-resistant PLA, d-lactic acid, respectively, poly-d-lactic acid (PDLA) is needed in a high enantiomeric purity. By formation of an isotactic stereocomplex of poly-L-lactic acid (PLLA) and PDLA, in which the L- and D-polymer chains are stabilized via hydrogen bonds and van-der-Waals interactions, a melting point of 230 °C is attained, which is 50 °C higher than pure PLLA and PDLA [20,21]. This opens up a broad spectrum of new application opportunities. However, to reach these goals, an efficient and cost-effective process for industrial D-lactic acid production still needs to be developed and implemented.
Sporolactobacillus inulinus is able to produce enantiomerically pure d-lactic acid and shows high titers and productivities [22,23]. Furthermore, it is characterized by a high substrate and product tolerance [24]. In a recent study, the nutritional requirements of S. inulinus were analyzed in detail, showing an influence of vitamins B1–B5, phosphate and nucleosides, and exhibiting a limitation by an insufficient uptake of peptides, so that high amounts of free amino acids are needed [22]. This information provides a basis for a successful substitution of yeast extract fulfilling all requirements.
This study compares various chemical and enzymatic hydrolysis methods for disintegration of protein-rich agricultural residues. The impact of the hydrolysis method and the type of agricultural residue on the fermentation of Sporolactobacillus inulinus is investigated and the supplementation strategy was adapted to this process with the aim of developing a cost-efficient process for d-lactic acid production.
2. Materials and Methods
2.1. Chemicals
Fine chemicals were either purchased from Merck KGaA (Darmstadt, Germany), Carl Roth GmbH and Co. KG (Karlsruhe, Germany) or from Sigma Aldrich (St. Louis, MO, USA) in an appropriate purity for biochemistry. In all cultivation experiments Fermtech® yeast extract from Merck KGaA was used as a reference. Pulverized CaCO3 (99%) from Carl Roth served as buffer agent. The agricultural residues were purchased as follows: Corn gluten from Cargill Inc., Wayzata, USA; DDGS (Distillers’ Dried Grains with Solubles) from CropEnergies AG, Mannheim, Germany; sunflower meal from Cargill Inc., Wayzata, MN, USA; rape cake JKI, Braunschweig, Germany; rapeseed meal from Archer Daniels Midland AG, Chicago, IL, USA; wheat gluten from Cargill Inc., Wayzata, MN, USA.
2.2. Microorganisms and Culture Conditions
Sporolactobacillus inulinus DSM 20,348 was purchased from the DSMZ (German collection of microorganisms and cell cultures GmbH, Braunschweig, Germany). The preculture medium contained (g·L−1): glucose, 20; Merck Fermtech® yeast extract, 10; tryptone (Carl Roth), 10; MgSO4·7H2O, 0.2; FeSO4·7H2O, MnSO4·H2O and NaCl 10 mg L−1 each. FeSO4·7H2O, MnSO4·H2O, and NaCl were added by sterile filtration from a 1 g L−1 stock solution. The pH was adjusted to 6.2 with 1M HCl. The preculture medium was inoculated with cryo stock cultures of S. inulinus in 50 vol% glycerol (stored at −80 °C) and incubated at 38 °C, 0 rpm for about 17 h until an optical density (λ = 605 nm) of 0.2–0.4 was reached. For cultivation of S. inulinus GY-medium was used (g L−1): glucose, 120; Merck Fermtech® yeast extract, 10; sodium acetate·3H2O, 2; MgSO4·7H2O, 0.2; FeSO4·7H2O, MnSO4·H2O and NaCl 10 mg L−1 each (sterile filtration); CaCO3, 60. The inoculum volume was 4 vol%. The cultivations were performed in 100 mL culture volume in 100 mL shaking flasks at 38 °C, 0 rpm. All experiments were carried out in duplicate to confirm the results. The mean values are presented without error bars, because the deviations were negligibly small.
2.3. Mild Chemical Hydrolysis
The agricultural residues were ground and sieved to a particle size of 700 µm. 150 g of this powder were suspended in 500 mL water and the pH was adjusted to 1 with 3M H2SO4. This mixture was hydrolyzed in an autoclave for 20 min at 121 °C. After cooling down to room temperature, the suspension was centrifuged at 10,000× g for 15 min and pH of the supernatant was adjusted with Ca(OH)2 to 6.2. The hydrolysate was centrifuged again (10,000× g, 15 min) and filtered. The filtrate was freeze dried and utilized as a nutrient source in the concentrations given below. The compositions of the nutrient sources of the mildly chemically produced raw material hydrolysates are presented in the Supplementary Materials.
2.4. Chemical Hydrolysis
The agricultural residues were ground, sieved to a particle size of 700 µm and according to the total nitrogen concentration, the following amounts were weight into a 20 mL crimp neck vial (g): corn gluten, 2.3; DDGS (Distillers’ Dried Grains with Solubles), 4.9; sunflower meal, 4.0; rape cake, 4.3; rapeseed meal, 4.4; wheat gluten, 1.8. After adding 10 mL sulfuric acid (3M, 2M, 1.5M or 1M), the vial was sealed with a septum and heated to 110 °C for 24 h. After cooling down to room temperature, the hydrolysate was transferred into a beaker and pH was adjusted to 5 by adding Ca(OH)2. The slurry was centrifuged (10 000× g, 15 min) and the pellet was washed with 20 mL water 2-times. The supernatants were collected, 10 mL phosphate solution (12.1 g L−1 K2HPO4/10.0 g L−1 KH2PO4) and 668 µL NH3 10 vol% were added, pH was adjusted to 6.2 and the hydrolysate was diluted to 125 mL. The mixture was sterilized for 20 min at 121 °C and 50 mL of this hydrolysate was used for each cultivation with a culture volume of 100 mL.
2.5. Enzymatic Hydrolysis
Rapeseed meal was ground and sieved to a particle size of 700 µm. 4.4 g rapeseed meal were weight into a 250 mL bottle and suspended in 70 mL water, pH was adjusted according to the following enzyme incubation conditions: Alcalase® (Novozymes, Copenhagen, Denmark), pH 9.0, 60 °C; Fermgen™ (DuPont™ Genencor, Rochester, NY, USA), pH 4.0, 30 °C; Flavourzyme® (Novozymes, Copenhagen, Denmark), pH 7.0, 50 °C; Neutrase® (Novozymes, Copenhagen, Denmark), pH 6.0, 50 °C; Protamex™ (Novozymes, Copenhagen, Denmark), pH 8.0, 60 °C; Protex™ 6L (DuPont™ Genencor, Rochester, NY, USA), pH 9.5, 60 °C. 1% enzyme solution referred to the protein concentration (15 µL) was added to the suspended rapeseed meal. The incubation was performed at the given temperatures with 100 rpm for 48 h. Afterwards, 10 mL phosphate solution (12.1 g L−1 K2HPO4/10.0 g L−1 KH2PO4) and 668 µL NH3 10 vol% were added, pH was adjusted to 6.2 and the hydrolysate was diluted to 125 mL. For sterilization and inactivation of the enzyme, the hydrolysate was autoclaved for 20 min at 121 °C. For each cultivation with a culture volume of 100 mL, 50 mL of the hydrolysate were used. Simultaneous hydrolysis was carried out by preparing GY-medium with untreated rapeseed meal and adding 15 µL enzyme solution.
2.6. Variation of the Nutrient Source
The fermentation of S. inulinus with different nutrient sources was carried out in GY-medium. The total nitrogen concentration of Merck Fermtech® yeast extract is 95.4 mg/g and the phosphate concentration is 55.1 mg/g. The Merck Fermtech® yeast extract was substituted by the nutrient sources keeping a constant total nitrogen amount of 0.095% and a minimal phosphate concentration of 0.55 g·L−1 in the medium which corresponds to 10 g·L−1 Merck Fermtech® yeast extract. The following mildly hydrolyzed agricultural residues were tested as nutrient sources in the given concentrations (g·L−1): Corn gluten, 16.4 (455 mg·L−1 PO43−); DDGS (Distillers’ Dried Grains with Solubles), 35.8 (310 mg L−1 PO43−); sunflower meal, 19.2 (414 mg L−1 PO43−); rape cake, 15.5 (448 mg L−1 PO43−); rapeseed meal, 19.9 (411 mg L−1 PO43−); and wheat gluten, 8.4 (489 mg L−1 PO43−). Additional phosphate was added as NaH2PO4.
The preparation of enzymatic and chemically hydrolyzed (1M–3M H2SO4) agricultural residues is described above. The given weights correspond to the following concentrations in the medium (g L−1): Corn gluten 9.2, DDGS 19.5, sunflower meal 15.9, rape cake 17.2, rapeseed meal 17.7, wheat gluten 7.4, 555 mg L−1 PO43− were added as K2HPO4/KH2PO4. Furthermore, the hydrolysates were supplemented with the following vitamins (µg L−1): thiamine·HCl (B1), 1 070; riboflavin (B2), 495; niacin (B3), 9 840; Ca-pantothenate (B5), 2 370; pyridoxine·HCl (B6), 939; biotin (B7), 18.3; folic acid (B9), 583; cyanocobalamin (B12), 0.93; inositol, 58.4; and 4.5 g L−1 tryptophan.
2.7. Fed-Batch Experiments
The fed-batch experiments were carried out in 300 mL shaking flasks containing 100 mL GY-medium with either 10 g L−1 yeast extract as reference or 17.7 g L−1 hydrolyzed rapeseed meal. In the reference experiment, the following components were added after 45 h and 76 h: 80 g L−1 glucose, 60 g L−1 CaCO3, amino acids (mg L−1): Glycine, 15; valine, 175; leucine, 295; isoleucine, 120; threonine, 140; serine, 105; asparagine, 50; methionine, 60; glutamic acid, 285; phenylalanine, 165; tyrosine, 70; tryptophan, 30 and the following vitamins (µg L−1): thiamine·HCl (B1), 1 070; riboflavin (B2), 495; niacin (B3), 9840; Ca-pantothenate (B5), 2 370; pyridoxine·HCl (B6), 939; biotin (B7), 18.3; folic acid (B9), 583; and cyanocobalamin (B12), 0.93; inositol, 58.4.
In the fed-batch experiments containing hydrolyzed rapeseed meal as the nutrient source, the following components were added after 40 h and 66 h: 80 g L−1 glucose, 60 g L−1 CaCO3, 8.8 g L−1 rapeseed meal hydrolysate, tryptophan 4.5 g L−1 and the vitamins in the same concentrations given above. For minimal supplementation no tryptophan and only thiamine·HCl (B1), riboflavin (B2), niacin (B3), and Ca-pantothenate (B5) were added.
2.8. Analytical Methods
Samples with lactic acid concentrations above the limit of solubility of Ca-lactate were first hydrolyzed with 5M HCl to dissolve the Ca-lactate. Therefore, the pH was adjusted to <1. The samples were centrifuged at 20,800× g for 10 min and the supernatants were diluted with 50mM sulfuric acid.
The lactic acid and glucose concentrations were measured by high-performance liquid chromatography (HPLC) on a Dionexᵀᴹ ICS-5000 system from Thermo scientific (Sunnyvale, CA, USA) equipped with a Aminex® HPX-87H column (300 × 7.8 mm) from Bio-Rad (Hercules, CA, USA), and a refraction index detector RI-101 from Shodex (Tokyo, Japan). The mobile phase was 5 mM sulfuric acid with a flow rate of 0.7 mL/min (60 °C).
The enantiomeric excess of d-lactic acid was measured on an HPLC-system from Knauer (Berlin, Germany) equipped with a Chirex® 3126 D-penicillamin column (150 × 4.6 mm) from Phenomenex (Torrance, CA, USA) and a refraction index detector K-2301 from Knauer (Berlin, Germany). The mobile phase was 2mM CuSO4 with a flow rate of 1 mL/min (RT).
The free amino nitrogen was determined with ninhydrin. Therefore, 2 mL hydrolysate (diluted 100-fold) are mixed with 1 mL ninhydrin solution (5.0 g Na2HPO4·2H2O, 6.0 g KH2PO4, 500 mg ninhydrin and 300 mg fructose are dissolved in water, pH is adjusted to 6.6–6.8, and the volume is adjusted to 100 mL) in a sealable reaction tube and heated in a boiling water bath for 16 min. Afterwards, the mixture is cooled down in a 20 °C tempered water bath for 20 min and then 5 mL potassium iodate solution are added (1.0 g KIO3 dissolved in 300 mL water and 200 mL ethanol 96%). After a further reaction time of 3 min at room temperature, the absorption at 570 nm is measured. The blank was subtracted from the measured value and the method was calibrated with glycine standards.
Further analytical methods for the analysis of the nutrient sources are described in the Supplementary Materials.
3. Results and Discussion
The widely used method for chemical hydrolysis, in order to valorize agricultural residues as nutrient sources, is a treatment under acidic conditions (pH 1) by autoclave at 121 °C, followed by neutralization and filtration [9,10]. This mild chemical hydrolysis method was tested for various residues but resulted in lower lactic acid titers and low productivities of S. inulinus, compared to yeast extract (Figure 1). In the reference experiment with yeast extract, S. inulinus reached an average D-lactic acid concentration of 97 g L−1 (>99% ee) with a maximal productivity of 3.10 g (L h)−1. Since it is known that high amounts of free amino acids are required for an efficient lactic acid production by S. inulinus, it was concluded that the hydrolysis degree achieved with this method is insufficient [22]. Using the example of DDGS, a modified hydrolysis method, in which the raw material is directly suspended in diluted sulfuric acid, was conducted and the concentration of sulfuric acid was sequentially increased from 1M to 3M (Figure 2).
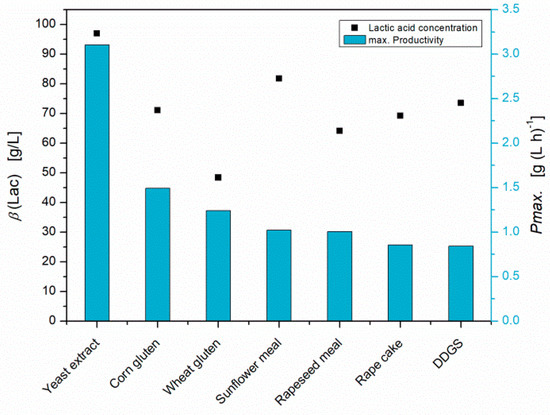
Figure 1.
Lactic acid concentrations and maximal productivities of S. inulinus obtained with mildly hydrolysed agricultural residues as nutrient sources in GY-medium compared to the reference with yeast extract, incubated at 38 °C, 0 rpm.
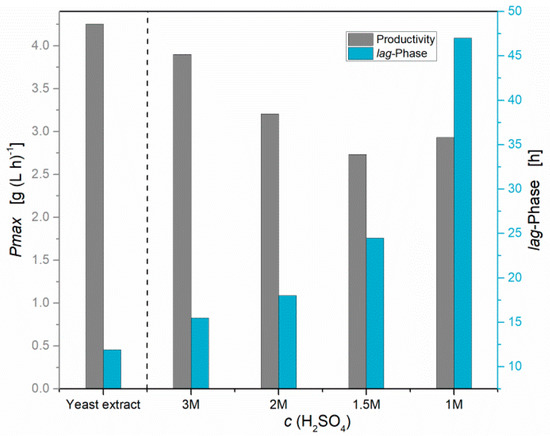
Figure 2.
Influence of sulfuric acid concentration variation during chemical hydrolysis of DDGS on the maximal productivity and lag-phase of S. inulinus cultivation at 38 °C, 0 rpm.
Figure 2 shows the effect of the H2SO4 concentration on the lag-phase and the maximal productivity of the fermentation. The lag-phase was graphically determined from the lactic acid production curve. The end of the lag-phase has been set as start of the lactic acid production with a lactic acid concentration > 1g/L, and at the end of these fermentations similar lactic acid concentrations between 92 and 103 g L−1 were obtained. It is clearly observable that a sequential increase in acid concentration leads to shorter lag-phases and higher productivities. Hydrolysis with 3M H2SO4 even nearly reached identical results compared to yeast extract. Taking these results into correlation with the free amino nitrogen concentration, which in this case describes the hydrolysis degree, a strong dependence between free amino nitrogen and the acid concentration, respectively, productivity, and duration of the lag-phase can be demonstrated (Figure 3).
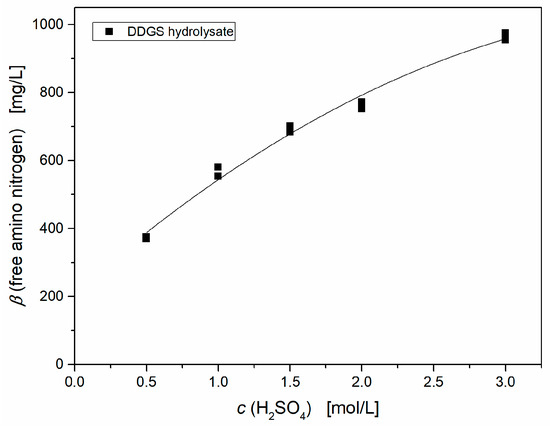
Figure 3.
Correlation between sulfuric acid concentration and free amino nitrogen amount after chemical hydrolysis of DDGS.
A huge advantage of chemical hydrolysis methods is their high versatility, because every peptide bonds breaks under certain reaction conditions, no matter how the whole protein is configured. In contrast to the effectiveness of enzymatic hydrolysis methods, it is strongly dependent on the protein composition and the selectivity of the used protease. In order to prove this effectiveness and versatility of hydrolysis with 3M H2SO4, a variety of agricultural residues, like wheat gluten, rapeseed meal, and sunflower meal was hydrolyzed and deployed as nutrient sources for S. inulinus. Therefore, a constant total nitrogen concentration according to 10 g L−1 yeast extract was kept in the medium. After a cultivation time of 88 h, the yields (produced lactic acid per consumed glucose) of the hydrolysates obtained by chemical hydrolysis with 3M H2SO4 of distinct agricultural residues and applied as nutrient sources in GY-medium and that of the reference with yeast extract were as follows: Yeast extract 0.84 (w/w), rapeseed meal 0.81 (w/w), DDGS 0.86 (w/w), sunflower meal 0.85 (w/w), rape cake 0.89 (w/w), wheat gluten 0.86 (w/w), and corn gluten 0.84 (w/w). The yields of all nitrogen sources are comparable to the reference with yeast extract. With exception of corn gluten hydrolysate, all nitrogen sources resulted not only in high lactic acid concentrations, but also in high productivities and short lag-phases comparable to the results obtained with yeast extract (Figure 4). The early interruption of the exponential phase during fermentation with corn gluten hydrolysate is possibly due to a decline of the pH value during this fermentation stage so that only a lower lactic acid concentration of 81 g L−1 was reached. The best results were achieved with rapeseed meal with a maximal productivity of 3.85 g (L h)−1 and 103 g L−1 lactic acid and DDGS with a maximal productivity of 3.44 g (L h)−1 and 107 g L−1 lactic acid, even higher than yeast extract with a maximal productivity of 3.27 g (L h)−1 and a lactic acid concentration of 101 g·L−1. This important result proves that nearly every nitrogen rich residue can be hydrolyzed and applied as an efficient nitrogen source for lactic acid production with S. inulinus. Consequently, an industrial production would not be depending on the availability of one single raw material, but could substitute it in case of a shortage or long transportation ways lowering the production costs.
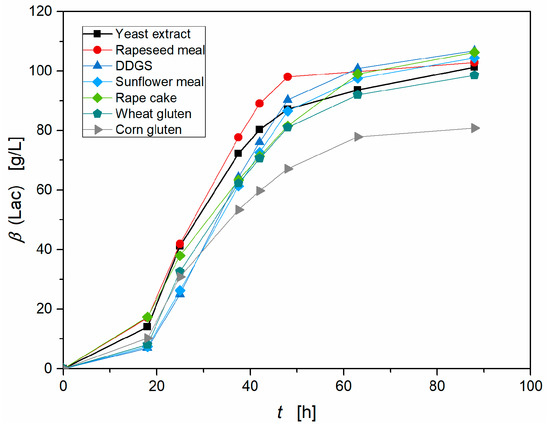
Figure 4.
Comparison of the lactic acid production by S. inulinus with hydrolysates obtained by chemical hydrolysis with 3M H2SO4 of distinct agricultural residues and applied as nutrient sources in GY-medium compared to the reference with yeast extract, incubated at 38 °C, 0 rpm.
This versatility and flexibility cannot be achieved with enzymatic hydrolysis methods. Nevertheless, a comparison of chemical hydrolysis and enzymatic hydrolysis with different proteases regarding their hydrolyzing efficiency for the example of rapeseed meal seemed very interesting, because in literature most attempts for substitution of yeast extract were conducted using enzymatically hydrolyzed nutrient sources [7,23]. After enzymatic conversion with different proteases for 48 h, the enzymatically hydrolyzed rapeseed meals were applied as nutrient sources in fermentation leading to a strong variation in productivity of S. inulinus depending on the used protease (Figure 5). The according concentrations of free amino nitrogen, displayed in Table 1, are 10-fold lower compared to chemical hydrolysis. However, good maximal productivities were achieved with Protex™ 6L (Pmax = 2.71 g (L h)−1) and Alcalase® (Pmax = 2.59 g (L h)−1), but they could not compete with the high productivity reached with chemically hydrolyzed rapeseed meal (Pmax = 3.85 g (L h)−1). The used proteases possess endo-selectivity, except Flavourzyme®, which possesses exo-selectivity. Comparing the results from the endo-proteases, again a correlation between free amino nitrogen and the productivity of S. inulinus can be assumed. Due to its exo-selectivity, hydrolysis with Flavourzyme® leads to a different profile of free amino acids, which is the reason for its poor productivity, although it delivered the highest concentration of free amino nitrogen. Wang et al. improved the lactic acid production of S. inulinus CASD in a simultaneous hydrolysis and fermentation experiment using Neutrase® [10]. Thus, in addition to the separate hydrolysis, a simultaneous hydrolysis and fermentation experiment was performed with Neutrase®, which is the only protease in this selection with matching incubation conditions, but only a slightly increase in productivity was observable (Figure 5). Altogether, it can be concluded that chemical hydrolysis is the much more efficient hydrolysis method for disintegration of nitrogen rich agricultural residues.
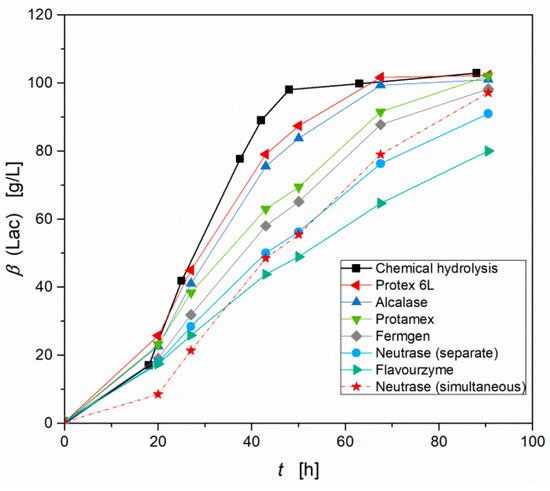
Figure 5.
Effect of different proteases for enzymatic hydrolysis of rapeseed meal on lactic acid production by S. inulinus compared to the chemical hydrolysis, incubated at 38 °C, 0 rpm.

Table 1.
Hydrolysis of rapeseed meal with different proteases, resulting free amino nitrogen content and the effect on the cultivation of S. inulinus at 38 °C, 0 rpm.
A previous work showed that the lactic acid production of S. inulinus is limited by the availability of certain free amino acids and that this limitation can be overcome in a fed-batch experiment feeding these amino acids and vitamins, resulting in high lactic acid titers of up to 222 g·L−1. Furthermore, the nutritional requirements were elucidated and it pointed out that only the vitamins B1–B5 have an influence on the lactic acid production of S. inulinus [22]. In order to investigate the limits of the application of chemically hydrolyzed rapeseed meal (hydrolyzed with 3M H2SO4), a similar 2-step fed-batch experiment with hydrolyzed rapeseed meal as nutrient source and supplementation with all B-vitamins, and on the other hand, minimal supplementation of the vitamins B1 - B5 was performed. After 40 h and 60 h 8.84 g L−1 hydrolyzed rapeseed meal, vitamins, glucose, and CaCO3 were fed. Figure 6 demonstrates that all three fed-batch fermentations show the same course with nearly identical overall productivities and lactic acid concentrations. In this fermentation 221 g L−1 lactic acid were produced by S. inulinus with chemically hydrolyzed rapeseed meal and minimal supplementation with an overall productivity of 1.55 g (L h)−1 and a yield of 96%, while the fermentation based on yeast extract resulted in 222 g·L−1 lactic acid with an overall productivity of 1.57 g (L h)−1. This confirms that all nutritional requirements are fulfilled by minimal supplementation and proves that chemically hydrolyzed rapeseed meal can efficiently substitute yeast extract without any limitations. By monitoring the utilization of the free amino acids during fermentation and optimizing the feeding profile, the amount of fed hydrolysate could probably be reduced, which would make this process even more cost-effective.
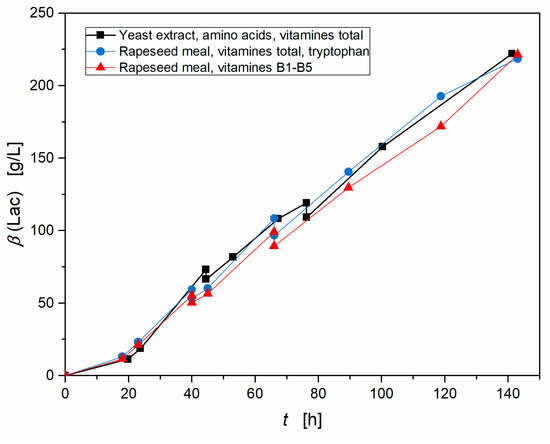
Figure 6.
Lactic acid production by S. inulinus in a 2-step fed-batch with chemically hydrolyzed rapeseed meal as nutrient source (hydrolyzed with 3M H2SO4) and comparison between supplementation with the complete B-vitamins, minimal supplementation with vitamins B1–B5 and a similar attempt based on yeast extract, incubated at 38 °C, 0 rpm.
4. Conclusions
Regarding the utilization of nitrogen rich agricultural residues as nutrient sources, a major problem is the insufficient hydrolysis degree, respectively, the availability of free amino acids and small peptides. While enzymatic hydrolysis is still the predominant hydrolysis method, this study demonstrates that this problem can be overcome by chemical hydrolysis with 3M H2SO4. It was further shown that not the type of agricultural residue, but only its hydrolysis degree is decisive for the fermentation efficiency of S. inulinus, so that in this process, yeast extract could be successfully substituted by a variety of hydrolyzed agricultural residues. In a fed-batch fermentation with hydrolyzed rapeseed meal and the vitamins B1–B5, the lactic acid titer was increased to 221 g L−1 with 96% yield and an overall productivity of 1.55 g (L h)−1. The demonstrated versatility, flexibility, and effectiveness of the presented chemical hydrolysis method are huge advantages compared to enzymatic pretreatment, especially for an industrial implementation of this process. Nevertheless, further research should be done in the optimization of chemical protein hydrolysis in order to reduce side products like CaSO4 and make this process even more efficient.
Supplementary Materials
The following are available online at http://www.mdpi.com/2311-5637/5/1/12/s1, Supplementary Method: Analytical methods (Dry matter and ash content, total nitrogen, colorimetric assays (amino nitrogen, total carbohydrates, reducing sugars, polyphenols and flavonoids), fatty acids, carbohydrates, inhibitors, soluble ions, trace elements); Table S1: Temperature gradient applied for thermal degradation; Table S2: Solvent gradient for HPAEC-PAD measurement of carbohydrates; Table S3: Parameters for anion and cation analysis by IC; Table S4: Plasma and measurement parameters applied for trace element analysis by ICP-OES. Composition of mildly chemically hydrolyzed nutrient sources: corn gluten, DDGS (Distillers’ Dried Grains with Solubles), sunflower meal, rape cake, rapeseed meal, wheat gluten.
Author Contributions
S.B. planned the study, designed and performed hydrolysis and cultivations and wrote the manuscript. A.K. and U.P. designed and supervised the experiments and revised the manuscript. A.K. had the project administration and performed the needed changes during the revision process. All authors read and agree to the final revised version of the article.
Funding
This research was funded by the German Federal Ministry of Food and Agriculture, following a decision of the German Bundestag, via the Agency of Renewable Resources (Grant No. 22037711) and the Südzucker AG.
Acknowledgments
We thank Klaus-Dieter Vorlop for his excellent support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Thomsen, M.H. Complex media from processing of agricultural crops for microbial fermentation. Appl. Microbiol. Biotechnol. 2005, 68, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R. Yeast extracts: Production, properties and components. Food Aust. 1998, 50, 181–183. [Google Scholar]
- Bai, Z.; Gao, Z.; Sun, J.; Wu, B.; He, B. D-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresourc. Technol. 2016, 207, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.-K.; Chang, H.N.; Lee, E.G.; Chang, Y.K.; Moon, S.-H. Effect of B vitamin supplementation on lactic acid production by Lactobacillus casei. J. Ferment. Bioeng. 1997, 84, 172–175. [Google Scholar] [CrossRef]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Motamedzadegan, A.; Ovissipour, M.; Regenstein, J.M.; Gildberg, A.; Rasco, B. Use of hydrolysates from Yellowfin Tuna (Thunnus albacares) heads as a complex nitrogen source for lactic acid bacteria. Food Bioprocess Technol. 2012, 5, 73–79. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Ju, J.; Yu, B.; Ma, Y. Efficient production of polymer-grade D-lactate by Sporolactobacillus laevolacticus DSM442 with agricultural waste cottonseed as the sole nitrogen source. Bioresourc. Technol. 2013, 142, 186–191. [Google Scholar] [CrossRef]
- Gildberg, A.; Batista, I.; Strøm, E. Preparation and characterization of peptones obtained by a two-step enzymatic hydrolysis of whole fish. Biotechnol. Appl. Biochem. 1989, 11, 413–423. [Google Scholar]
- Gao, M.-T.; Hirata, M.; Toorisaka, E.; Hano, T. Study on acid-hydrolysis of spent cells for lactic acid fermentation. Biochem. Eng. J. 2006, 28, 87–91. [Google Scholar] [CrossRef]
- Wang, L.M.; Zhao, B.; Li, F.S.; Xu, K.; Ma, C.Q.; Tao, F.; Li, Q.G.; Xu, P. Highly efficient production of D-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl. Microbiol. Biotechnol. 2011, 89, 1009–1017. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Guyot, J.P.; Kiel, P. Batch fermentations on synthetic mixed sugar and starch medium with amylolytic lactic acid bacteria. Appl. Microbiol. Biotechnol. 2007, 74, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Available online: https://www.researchandmarkets.com/research/3n8b8s/ global_lactic (accessed on 5 April 2018).
- Chopin, A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 21–37. [Google Scholar] [CrossRef]
- Deguchi, Y.; Morishita, T. Nutritional requirements in multiple auxotrophic lactic acid bacteria: Genetic lesions affecting amino acid biosynthetic pathways in Lactococcus lactis, Enterococcus faecium, and Pediococcus acidilactici. Biosci. Biotechnol. Biochem. 1992, 56, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Kaufmann, N.; Kuenz, A.; Pruesse, U. Biotechnological production of enantiomerically pure D-lactic acid. Appl. Microbiol. Biotechnol. 2016, 100, 9423–9437. [Google Scholar] [CrossRef] [PubMed]
- Komesu, A.; Oliveira, J.A.R.d.; Martins, L.H.d.S.; Wolf Maciel, M.R.; Maciel Filho, R. Lactic acid production to purification: A review. BioRES 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Global Market Insights. Available online: https://www.gminsights.com/industry-analysis/lactic-acid-and-polylactic-acid-market (accessed on 5 April 2018).
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bose, S.; Das, S. 3D printing of biomaterials. MRS Bull. 2015, 40, 108–115. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Klotz, S.; Kuenz, A.; Pruesse, U. Nutritional requirements and the impact of yeast extract on the D-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Reddy Tadi, S.R.; Evr, A.; Limaye, A.M.; Sivaprakasam, S. Enhanced production of optically pure D (–) lactic acid from nutritionally rich Borassus flabellifer sugar and whey protein hydrolysate based–fermentation medium. Biotechnol. Appl. Biochem. 2017, 64, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, K.; Suzuki, J. Sporolactobacillus nov. subgen. J. Gen. Appl. Microbiol. 1963, 9, 59–71. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).