Improved Raoultella planticola Strains for the Production of 2,3-Butanediol from Glycerol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Medium Composition and Culture Conditions
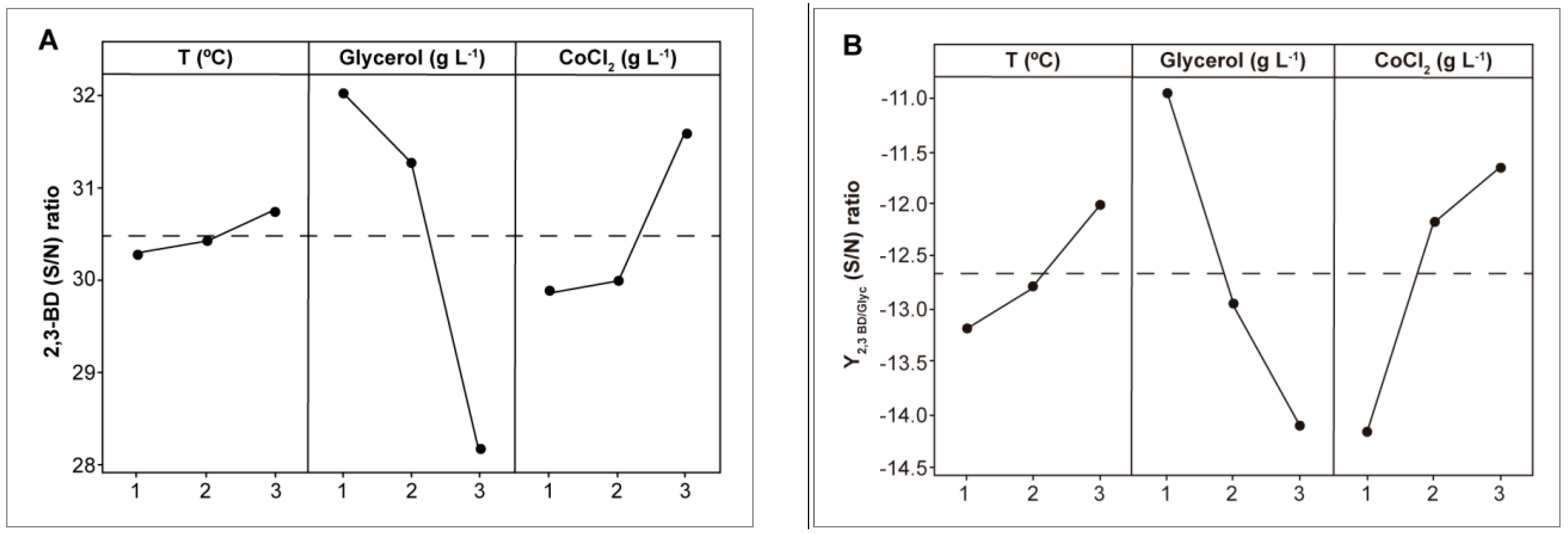
2.2. Design of Experiments and Data Interpretation
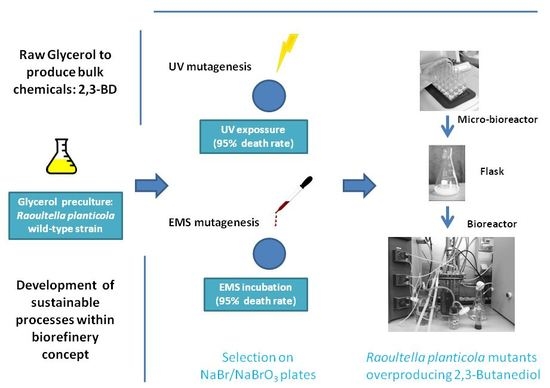
2.3. Random Mutagenesis and Screening Protocols
2.3.1. Ethyl Methane Sulfonate (EMS) Mutagenesis
2.3.2. UV Mutagenesis
2.3.3. Mutant Screening
2.4. Analytical Methods
3. Results and Discussion
3.1. Optimization of Fermentation Conditions for the Wild-Type Strain
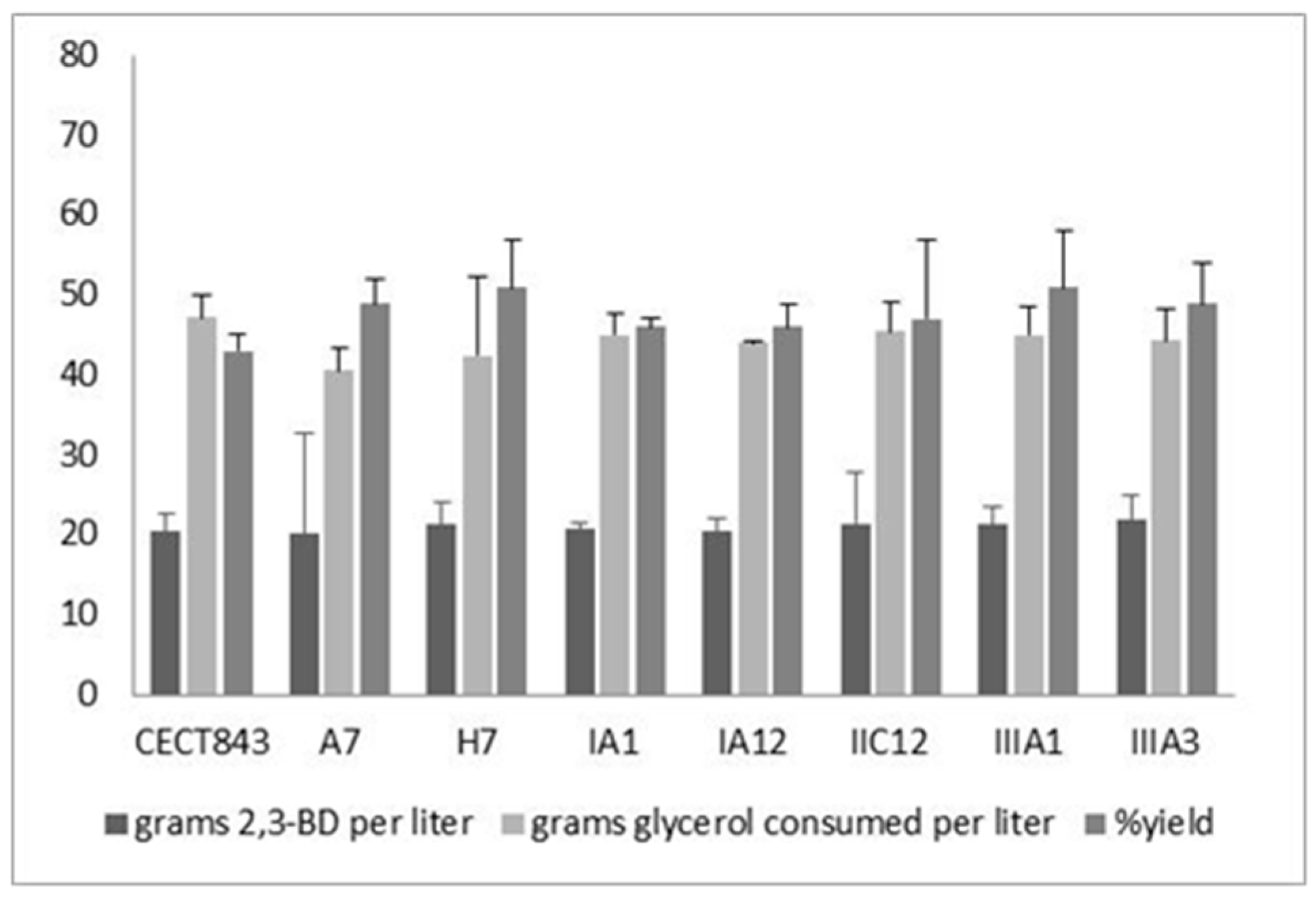
3.2. Mutation of Raoultella Planticola CECT843 and Selection of the 2,3-BD Overproducing Strains
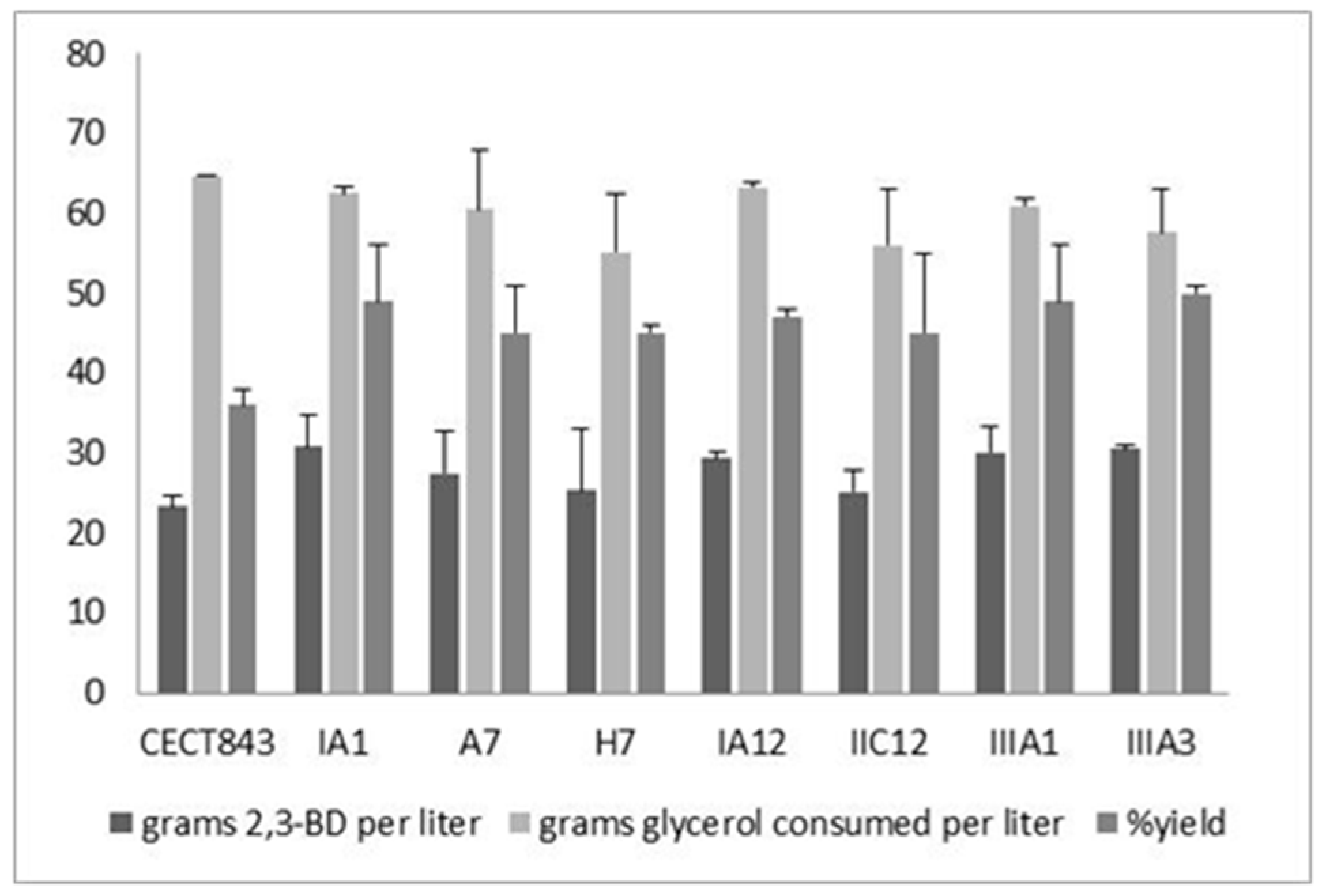
3.3. Characterization of Fermentation Capacity of the Mutant Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Białkowska, A.M. Strategies for efficient and economical 2,3-butanediol production: New trends in this field. World J. Microbiol. Biotechnol. 2016, 32, 200. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Merriman, W.W.; Quintana, R.L. Renewable Gasoline, Solvents, and Fuel Additives from 2,3-Butanediol. ChemSusChem 2016, 9, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.T.; Matei-Rutkovska, F.; Huchede, M.; Jaillardon, K.; Qingyi, G.; Michel, C.; Millet, J.M.M. Production of 1,3-butadiene in one step catalytic dehydration of 2,3-butanediol. Catal. Today 2018. [Google Scholar] [CrossRef]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbial 2,3-butanediol production: A state-of-the-art review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Rao, Z.; Zhang, X.; Xu, M.; Xu, Z.; Yang, S. Improved production of 2,3-butanediol in Bacillus amyloliquefaciens by over-expression of glyceraldehyde-3-phosphate dehydrogenase and 2,3-butanediol dehydrogenase. PLoS ONE 2013, 8, e76149. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gao, S.; Yu, Y.; Yang, H. Microbial production of 2,3-butanediol by a newly-isolated strain of Serratia marcescens. Biotechnol. Lett. 2014, 36, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, V.; de Vicente, G.; Morán, B.; Rojas, A.; Segarra, S.; Montesinos, A.; Tortajada, M.; Ramón, D.; Ladero, M.; Santos, V.E. Novel biocatalysts for glycerol conversion into 2,3-butanediol. Process. Biochem. 2016, 51, 740–748. [Google Scholar] [CrossRef]
- Mallonee, D.H.; Speckman, R.A. Development of mutant strain of Bacillys polymyxa showing enhanced production of 2,3-butanediol. Appl. Environm. Microbiol. 1988, 54, 168–171. [Google Scholar]
- Parate, R.D.; Rode, C.V.; Dharne, M.S. 2,3-Butanediol production from biodiesel derived glycerol. Curr. Environ. Eng. 2018, 5, 4–12. [Google Scholar] [CrossRef]
- Petrov, K.; Petrova, P. High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl. Microbiol. Biotechnol. 2009, 84, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.; Petrova, P. Enhanced production of 2,3-butanediol from glycerol by forced pH fluctuations. Appl. Microbiol. Biotechnol. 2010, 87, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Bielb, H.; Zeng, A.-P.; Menzel, K.; Deckwer, W.-D. Deckwer, Fermentation of glycerol to 1,3-propanediol and 2,3-butanediol by Klebsiella pneumonia. Appl. Microbiol. Biotechnol. 1998, 50, 24–29. [Google Scholar]
- Kumar, V.; Park, S. Potential and limitations of Klebsiella pneumoniae as a microbial cell factory utilizing glycerol as the carbon source. Biotechnol. Adv. 2018, 36, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, T.; Woo, H.M.; Kim, Y.; Lee, J.; Um, Y. High production of 2,3-butanediol from biodiesel-derived crude glycerol by metabolically engineered Klebsiella oxytoca M1. Biotechnol. Biofuels 2015, 8, 146. [Google Scholar] [CrossRef]
- Rathnasingh, C.; Park, J.M.; Kim, D.; Song, H.; Chang, Y.K. Metabolic engineering of Klebsiella pneumoniae and in silico investigation for enhanced 2,3-butanediol production. Biotechnol. Lett. 2016, 38, 975–982. [Google Scholar] [CrossRef]
- Kim, T.; Cho, S.; Woo, H.M.; Lee, S.; Lee, J.; Um, Y.; Seo, J. High production of 2,3-butanediol from glycerol without 1,3-propanediol formation by Raoultella ornithinolytica B6. Appl. Microbiol. Biotechnol. 2017, 101, 2821–2830. [Google Scholar] [CrossRef]
- Rodriguez, A.; Ripoll, V.; Santos, V.E.; Gomez, E.; García-Ochoa, F. Effect of fluid dynamic conditions on 2,3-butanediol production by Raoultella terrigena in SBTR: Oxygen transfer and uptake rates. J. Chem. Technol. Biotechnol. 2017, 92, 1266–1275. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Yepez, B.; Kopsahelis, N.; Freire, D.M.; de Castro, A.M.; Papanikolaou, S.; Kookos, I.K. Techno-economic evaluation of a complete bioprocess for 2,3-butanediol production from renewable resources. Bioresour. Technol. 2016, 204, 55–64. [Google Scholar] [CrossRef]
- Nakashimada, Y.; Kanai, K.; Nishio, N. Optimization of dilution rate, pH and oxygen supply on optical purity of 2,3-butanediol produced by Paenibacillus polymyxa in chemostat culture. Biotechnol. Lett. 1998, 20, 1133–1138. [Google Scholar] [CrossRef]
- Wojtusik, M.; Rodríguez, A.; Ripoll, V.; Santos, V.E.; García, J.L.; García-Ochoa, F. 1,3-Propanediol production by Klebsiella oxytoca NRRL-B199 from glycerol, Medium composition and operational conditions. Biotechnol. Rep. 2015, 6, 100–107. [Google Scholar] [CrossRef]
- Cueto, P.H.; Méndez, B.S. Direct selection of Clostridium acetobutylicum fermentation mutants by a proton suicide method. App. Environ. Microbiol. 1990, 56, 578–580. [Google Scholar]
- Desnuelle, P.; Naudet, M. Colorimetric determination of acetaldehyde formed on periodate oxidation of 2,3-BD. Bull. Soc. Chim. Fr. 1945, 12, 871–878. [Google Scholar]
- Benjaminson, M.A.; de Guzmán, B.C.; Weil, A.J. Voges-Proskauer test: Expeditious techniques for routine use. J. Bacteriol. 1963, 87, 234–235. [Google Scholar]
- Speckman, R.A.; Collins, E.B. Specificity of the Westerfeld adaptation of the Voges Proskauer test. Appl. Environ. Microbiol. 1982, 44, 40–43. [Google Scholar] [PubMed]
- Dai, J.Y.; Zhang, L.F.; Xiu, Z.L. AI-2 Key enzyme S-Ribosylhomocysteinase from strain Klebsiella pneumoniae CICC 10011 producing 2,3-Butanediol. Chem. Res. Chin. Univ. 2011, 27, 273–276. [Google Scholar]
- Shrivastav, A.; Lee, J.; Kim, H.Y.; Kim, Y.R. Recent insights in the removal of Klebsiella pathogenicity factors for the industrial production of 2,3-butanediol. J. Microbiol. Biotechnol. 2013, 23, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Jiang, Y.F.; Guo, G.L.; Hwang, W.-S. Method of 2,3-butanediol production from glycerol and acid-pretreated rice straw hydrolysate by newly isolated strains: Pre-evaluation as an integrated biorefinery process. Biores. Technol. 2013, 135, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Fulgence, S.; Yan, X.; Cong, R. Current advances in the production of 2,3-butanediol by microbial fermentation. J. Acad. Ind. Res. 2018, 6, 188–197. [Google Scholar]
| Exp. | Factors | Results | |||||
|---|---|---|---|---|---|---|---|
| T (°C) | [Glyc] (g L−1) | [CoCl2] (g L−1) | CDW (g L−1) | [2,3-BD]max (g L−1) | [Glyc]cons (g L−1) | Y2,3-BD/Glyc (g g−1) | |
| E1 | 28 (1) | 75 (2) | 0.036 (3) | 6.0 ± 1.5 | 18.0 ± 2.1 | 37.9 ± 2.7 | 0.24 ± 0.02 |
| E2 | 30 (2) | 60 (1) | 0.012 (1) | 6.5 ± 0.5 | 14.2 ± 3.4 | 34.6 ± 1.6 | 0.24 ± 0.04 |
| E3 | 33 (3) | 90 (3) | 0.024 (2) | 3.8 ± 1.0 | 20.4 ± 1.7 | 23.3 ± 3.6 | 0.23 ± 0.03 |
| E4 | 28 (1) | 90 (3) | 0.012 (1) | 7.7 ± 0.7 | 15.2 ± 1.6 | 27.0 ± 2.4 | 0.17 ± 0.01 |
| E5 | 30 (2) | 75 (2) | 0.024 (2) | 8.4 ± 0.3 | 19.1 ± 3.3 | 40.0 ± 3.3 | 0.25 ± 0.02 |
| E6 | 33 (3) | 60 (1) | 0.036 (3) | 10.6 ± 1.3 | 22.3 ± 4.5 | 54.4 ± 4.1 | 0.37 ± 0.05 |
| E7 | 28 (1) | 60 (1) | 0.024 (2) | 6.0 ± 0.8 | 15.5 ± 2.4 | 33.9 ± 1.2 | 0.26 ± 0.03 |
| E8 | 30 (2) | 90 (3) | 0.036 (3) | 8.3 ± 0.7 | 18.0 ± 3.7 | 26.6 ± 0.9 | 0.20 ± 0.01 |
| E9 | 33 (3) | 75 (2) | 0.012 (1) | 8.4 ± 0.2 | 14.0 ± 2.6 | 32.3 ± 1.8 | 0.19 ± 0.03 |
| Strain | Device | [2,3-BD]max (g L−1) | [Glyc]cons (g L−1) | Y2,3-BD/Glyc (g g−1) | [Acet]max (g L−1) | [EtOH]max (g L−1) |
|---|---|---|---|---|---|---|
| CECT 843 | Flask | 15.0 ± 1.2 | 60.2 ± 4.3 | 0.25 ± 0.02 | 6.6 ± 0.5 | 2.4 ± 0.1 |
| Bioreactor | 22.0 ± 4.1 | 64.2 ± 3.5 | 0.34 ± 0.05 | 6.8 ± 0.3 | 0.9 ± 0.1 | |
| CECT 8158 | Flask | 23.4 ± 2.3 | 61.5 ± 6.4 | 0.38 ± 0.01 | 3.7 ± 0.3 | 1.9 ± 0.2 |
| Bioreactor | 33.6 ± 1.5 | 61.8 ± 1.6 | 0.54 ± 0.06 | 4.3 ± 0.2 | 0.5 ± 0.1 | |
| CECT 8159 | Flask | 18.5 ± 2.1 | 60.8 ± 2.3 | 0.30 ± 0.04 | 3.8 ± 0.7 | 1.2 ± 0.3 |
| Bioreactor | 30.7 ± 3.5 | 61.3 ± 4.6 | 0.50 ± 0.02 | 3.5 ± 0.8 | 0.8 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustamante, D.; Segarra, S.; Montesinos, A.; Tortajada, M.; Ramón, D.; Rojas, A. Improved Raoultella planticola Strains for the Production of 2,3-Butanediol from Glycerol. Fermentation 2019, 5, 11. https://doi.org/10.3390/fermentation5010011
Bustamante D, Segarra S, Montesinos A, Tortajada M, Ramón D, Rojas A. Improved Raoultella planticola Strains for the Production of 2,3-Butanediol from Glycerol. Fermentation. 2019; 5(1):11. https://doi.org/10.3390/fermentation5010011
Chicago/Turabian StyleBustamante, Daniel, Silvia Segarra, Alejandro Montesinos, Marta Tortajada, Daniel Ramón, and Antonia Rojas. 2019. "Improved Raoultella planticola Strains for the Production of 2,3-Butanediol from Glycerol" Fermentation 5, no. 1: 11. https://doi.org/10.3390/fermentation5010011
APA StyleBustamante, D., Segarra, S., Montesinos, A., Tortajada, M., Ramón, D., & Rojas, A. (2019). Improved Raoultella planticola Strains for the Production of 2,3-Butanediol from Glycerol. Fermentation, 5(1), 11. https://doi.org/10.3390/fermentation5010011