The Microbial Diversity of Sherry Wines
Abstract
1. Introduction: The Origin of Sherry Wines
2. Biodiversity of Flor Biofilm
3. Genetic and Biochemical Characteristics of the Flor Yeasts
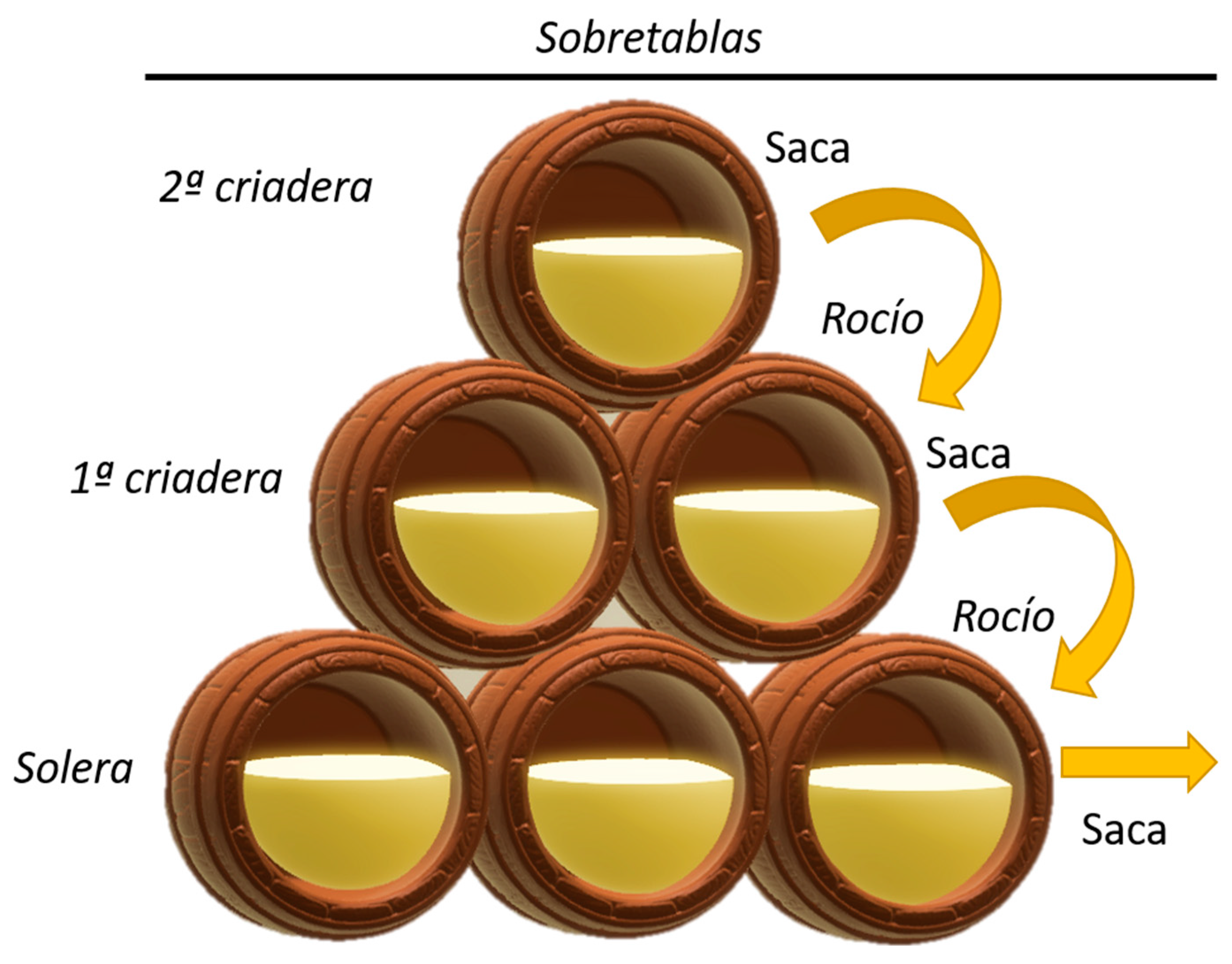
4. Factors Which Affect Veil Formation
5. Chemistry and Biochemistry of the Biological Ageing
6. Why Are Fino Wines Different from Manzanilla Wines?
7. The Outlook for the Future of the Veil-Forming Yeasts
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Legras, J.L.; Moreno-Garcia, J.; Zara, S.; Zara, G.; Garcia-Martinez, T.; Mauricio, J.C.; Mannazzu, I.; Coi, A.L.; Zeidan, M.B.; Dequin, S.; et al. Flor yeast: New perspectives beyond wine aging. Front. Microbiol. 2016, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Mauricio, J.C. Biologically Aged Wines. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 82–96. [Google Scholar]
- González Gordon, M.M. Jerez-Xeres “Scherish”: Apuntes Sobre el Origen de la Ciudad, Sobre su Historia y su vino; Gráficas del Exportador: Jerez de la Frontera, Spain, 1935; ISBN CABP 0045594. [Google Scholar]
- Esteve-Zarzoso, B.; Peris-Torán, M.J.; García-Maiquez, E.; Uruburu, F.; Querol, A. Yeast Population Dynamics during the Fermentation and Biological Aging of Sherry Wines. Appl. Environ. Microbiol. 2001, 67, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Zarzoso, B.; Fernández-Espinar, M.T.; Querol, A. Authentication and identification of Saccharomyces cerevisiae “flor” yeast races involved in sherry ageing. Antonie Leeuwenhoek 2004, 85, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Muñoz, M.; Bernal-Grande, M.; Cordero-Bueso, G.; González, M.; Hughes-Herrera, D.; Cantoral, J. A Microtiter Plate Assay as a Reliable Method to Assure the Identification and Classification of the Veil-Forming Yeasts during Sherry Wines Ageing. Fermentation 2017, 3, 58. [Google Scholar] [CrossRef]
- Berlanga, M.T.; Peinado, R.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Influence of blending on the content of different compounds in the biological aging of sherry dry wines. J. Agric. Food Chem. 2004, 52, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Infante, J.J.; Mesa, J.J.; Rebordinos, L.; Cantoral, J.M. Enological behavior of biofilms formed by genetically-characterized strains of sherry flor yeast. Open Biotechnol. J. 2013, 7, 23–29. [Google Scholar] [CrossRef][Green Version]
- Marin-Menguiano, M.; Romero-Sanchez, S.; Barrales, R.R.; Ibeas, J.I. Population analysis of biofilm yeasts during Fino sherry wine aging in the Montilla-Moriles D.O. region. Int. J. Food Microbiol. 2017, 244, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Espinazo-Romeu, M.; Cantoral, J.M.; Matallana, E.; Aranda, A. Btn2p is involved in ethanol tolerance and biofilm formation in flor yeast. FEMS Yeast Res. 2008, 8, 1127–1136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mesa, J.J.; Infante, J.J.; Rebordinos, L.; Cantoral, J.M. Characterisation of yeasts involved in the biological ageing of sherry wines. LWT Food Sci. Technol. 1999, 32, 114–120. [Google Scholar] [CrossRef]
- Roldán, A.; Lasanta, C.; Caro, I.; Palacios, V. Effect of lysozyme on “flor” velum yeasts in the biological aging of sherry wines. Food Microbiol. 2012, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Lasanta, C.; Roldán, A.; Caro, I.; Pérez, L.; Palacios, V. Use of lysozyme for the prevention and treatment of heterolactic fermentation in the biological aging of sherry wines. Food Control 2010, 21, 1442–1447. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Zara, S.; Bakalinsky, A.T.; Zara, G.; Demontis, M.A.; Budroni, M.; Pirino, G. FLO11-Based Model for Air-Liquid Interfacial Biofilm Formation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; Gross, M.K.; Zara, G.; Budroni, M.; Bakalinsky, A.T. Ethanol-independent biofilm formation by a flor wine yeast strain of Saccharomyces cerevisiae. Appl. Environ. Microbial. 2010, 76, 4089–4091. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: London, UK, 2011; p. 2363. ISBN 9780080542690. [Google Scholar]
- Suárez Lepe, J.A. Levaduras Vínicas: Funcionalidad y uso en Bodega; Grupo Mundi-Prensa: Madrid, Spain, 1997; ISBN 8471146851. [Google Scholar]
- Zara, G.; Angelozzi, D.; Belviso, S.; Bardi, L.; Goffrini, P.; Lodi, T.; Budroni, M.; Mannazzu, I. Oxigen is required to restore flor strain viability and lipid biosynthesis under fermentative conditions. FEMS Yeast Res. 2009, 9, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Marcilla, J.; Alas, G.; Feduchy, E. Contribución al Estudio de las Levaduras que Forman velo sobre Ciertos vinos de Elevado Grado Alcohólico; Anales Centro Inv. Vitivinicolas: Madrid, Spain, 1939; Volume 1, p. 1. [Google Scholar]
- Moreno-Arribas, M.V.; Carmen Polo, M. Occurrence of lactic acid bacteria and biogenic amines in biologically aged wines. Food Microbiol. 2008, 25, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Valcarcel, M.J.; González, P.; Domecq, B. Influence of Botrytis infection of the grapes on the biological aging process of Fino Sherry. Am. J. Enol. Vitic. 1991, 42, 58–62. [Google Scholar]
- Fernández-Espinar, M.T.; Esteve-Zarzoso, B.; Querol, A.; Barrio, E. RFLP analysis of the ribosomal internal transcribed spacers and the 5.8S rRNA gene region of the genus Saccharomyces: A fast method for species identification and the differentiation of flor yeasts. Antonie Leeuwenhoek 2000, 78, 87–97. [Google Scholar] [CrossRef]
- Blandino, A.; Caro, I.; Cantero, D. Comparative study of alcohol dehydrogenase activity in flor yeast extracts. Biotechnology 1997, 19, 651–654. [Google Scholar] [CrossRef]
- Infante, J.J.; Dombek, K.M.; Rebordinos, L.; Cantoral, J.M.; Young, E.T. Genome-Wide Amplifications Caused by Chromosomal Rearrangements Play a Major Role in the Adaptive Evolution of Natural Yeast. Genetics 2003, 165, 1745–1759. [Google Scholar] [PubMed]
- Mauricio, J.C.; Moreno, J.J.; Ortega, J.M. In Vitro Specific Activities of Alcohol and Aldehyde Dehydrogenases from Two Flor Yeasts during Controlled Wine Aging. J. Agric. Food Chem. 1997, 45, 1967–1971. [Google Scholar] [CrossRef]
- Budroni, M.; Giordano, G.; Pinna, G.; Farris, G.A. A genetic study of natural flor strains of Saccharomyces cerevisiae isolated during biological ageing from Sardinian wines. J. Appl. Microbiol. 2000, 89, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Ibeas, J.I.; Jiménez, J. Mitochondrial DNA loss caused by ethanol in Saccharomyces flor yeasts. Appl. Environ. Microbiol. 1997, 63, 7–12. [Google Scholar] [PubMed]
- Coi, A.L.; Bigey, F.; Mallet, S.; Marsit, S.; Zara, G.; Gladieux, P.; Galeote, V.; Budroni, M.; Dequin, S.; Legras, J.L. Genomic signatures of adaptation to wine biological ageing conditions in biofilm-forming flor yeasts. Mol. Ecol. 2017, 26, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Kvitek, D.J.; Will, J.L.; Gasch, A.P. Variations in stress sensitivity and genomic expression in diverse S. cerevisiae isolates. PLoS Genet. 2008, 4, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, T.M.; Atanasio, C.; Mauricio, J.C.; Ortega, J.M. Influence of aeration on the physiological activity of flor yeasts. J. Agric. Food Chem. 2001, 49, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, J.C.; Valero, E.; Millán, C.; Ortega, J.M. Changes in nitrogen compounds in must and wine during fermentation and biological aging by flor yeasts. J. Agric. Food Chem. 2001, 49, 3310–3315. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.; Budroni, M.; Mannazzu, I.; Zara, S. Air–liquid biofilm formation is dependent on ammonium depletion in a Saccharomyces cerevisiae flor strain. Yeast 2011, 28, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Ibeas, J.I.; Jimenez, J. Genomic complexity and chromosomal rearrangements in wine-laboratory yeast hybrids. Curr. Genet. 1996, 30, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Legras, J.L.; Erny, C.; Charpentier, C. Population structure and comparative genome hybridization of European flor yeast reveal a unique group of Saccharomyces cerevisiae strains with few gene duplications in their genome. PLoS ONE 2014, 9, e108089. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Feuillat, M. Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 225–242. ISBN 0-415-27850-31. [Google Scholar]
- Suárez-Lepe, J.A.; Leal, I. Microbiología Enológica: Fundamentos de Vinificación; Mundi-Prensa: Madrid, Spain, 2004; pp. 673–716. ISBN 84-8476-184-3. [Google Scholar]
- Martinez-Rodriguez, A.J.; Polo, M.C. Characterization of the nitrogen compounds released during yeast autolysis in a model wine system. J. Agric. Food Chem. 2000, 48, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Valcarcel, M.J.; Gonzalez, P.; Benítez, T.; Pérez, L. Consumo de Etanol, Glicerina y Aminoácidos Totales en vinos Finos Durante la Crianza Biológica Bajo “Velo de Flor”; Alimentación, Equipos y Tecnología; Reed Business Information S.A.: Bilbao, Spain, 1993; Volume 12, pp. 61–65. ISSN 0212-1689. [Google Scholar]
- Mart́ınez, P.; Valcárcel, M.J.; Pérez, L.; Benítez, T. Metabolism of Saccharomyces cerevisiae flor yeasts during fermentation and biological aging of Fino sherry: By-products and aroma compounds. Am. J. Enol. Vitic. 1998, 49, 240–250. [Google Scholar]
- Cortes, M.B.; Moreno, J.; Zea, L.; Moyano, L.; Medina, M. Changes in Aroma Compounds of Sherry Wines during Their Biological Aging Carried out by Saccharomyces cerevisiae Races bayanus and capensis. J. Agric. Food Chem. 1998, 46, 2389–2394. [Google Scholar] [CrossRef]
- Medina, M.; Mérida García, J.; Zea, L.; Moyano, L.; Moreno, J.; Mayén Riego, M.; Sherry Wines, J. Food Composition and Analysis. 2017. Available online: http://helvia.uco.es/xmlui/bitstream/handle/10396/14301/Sherry.pdf?sequence=1 (accessed on 3 February 2018).
- Martínez de la Ossa, E.; Caro, I.; Bonat, M.; Pérez, L.; Domecq, B. Dry extract in sherry and its evolution in the aging of sherry. Am. J. Enol. Vitic. 1987, 38, 321–325. [Google Scholar]
- Mauricio, J.C.; Ortega, J.M. Nitrogen compounds in wine during its biological aging by two flor film yeasts: An approach to accelerated biological aging of dry sherry-type wines. Biotechnol. Bioeng. 1997, 53, 159–167. [Google Scholar] [CrossRef]
- Botella, M.A.; Perez-Rodriguez, L.; Domecq, B.; Valpuesta, V. Amino acid content of Fino and oloroso sherry wines. Am. J. Enol. Vitic. 1990, 41, 12–15. [Google Scholar]
- Ingledew, W.M.; Magnus, C.A.; Sosulski, F.W. Influence of oxygen on proline utilization during the wine fermentation. Am. J. Enol. Vitic. 1987, 38, 246–248. [Google Scholar]
- Peinado, R.A.; Moreno, J.J.; Ortega, J.M.; Mauricio, J.C. Effect of gluconic acid consumption during simulation of biological aging of sherry wines by a flor yeast strain on the final volatile compounds. J. Agric. Food Chem. 2003, 51, 6198–6203. [Google Scholar] [CrossRef] [PubMed]
- Robledo, M.J.L. La Palma y los Vinos del Condado. eDAP Documentos de Arquitectura y Patrimonio;. 2010, pp. 11–24. Available online: http://arquitecturaypatrimonio.com/edap02_articulos/2_La_palma_y_los_vinos_del_condado.pdf (accessed on 4 February 2018).
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Belloch, C.; Orlic, S.; Barrio, E.; Querol, A. Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int. J. Food Microbiol. 2008, 122, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Fierro-Risco, J.; Rincón, A.M.; Benítez, T.; Codón, A.C. Overexpression of stress-related genes enhances cell viability and velum formation in Sherry wine yeasts. Appl. Microbiol. Biotechnol. 2013, 97, 6867–6881. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Biological aging of sherry wines using pure cultures of two flor yeast strain under controlled microaeration. J. Agric. Food Chem. 2005, 53, 5258–5264. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, J.C.; Moreno, J.; Ortega, J.M. Aceleración de la crianza biológica mediante aireaciones periódicas. In VII Jornadas Científicas de los Grupos de Investigación Enológica; Gobierno de la Rioja: Logroño, Spain, 2003; pp. 117–119. [Google Scholar]
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Biological aging of sherry wines under periodic and controlled microaerations with Saccharomyces cerevisiae var. capensis: Effect on odorant series. Food Chem. 2007, 100, 1188–1195. [Google Scholar] [CrossRef]
- Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Differential proteome analysis of a flor yeast strain under biofilm formation. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Moreno-García, J.; López-Muñoz, B.; Mauricio, J.C.; García-Martínez, T. Use of a flor velum yeast for modulating colour, ethanol and major aroma compound contents in red wine. Food Chem. 2016, 213, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, F.; Peinado, R.A.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int. J. Food Microbiol. 2006, 110, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Roldán, A.M.; Lloret, I.; Palacios, V. Use of a submerged yeast culture and lysozyme for the treatment of bacterial contamination during biological aging of sherry wines. Food Control 2017, 71, 42–49. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Arai, Y.; Toda, Y.; Yamamura, H.; Okuda, T.; Hayakawa, M.; Iimura, Y. Glucose repression of FLO11 gene expression regulates pellicle formation by a wild pellicle-forming yeast strain isolated from contaminated wine. Biotechnol. Biotechnol. Equip. 2017, 31, 120–127. [Google Scholar] [CrossRef]
- García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R. Natural sweet wine production by repeated use of yeast cells immobilized on penicillium chrysogenum. LWT Food Sci. Technol. 2015, 61, 503–509. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.J.; Villalba, J.M.; González-Reyes, J.A.; Ortega, J.M.; Mauricio, J.C. Yeast biocapsules: A new immobilization method and their applications. Enzym. Microb. Technol. 2006, 40, 79–84. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero-Bueso, G.; Ruiz-Muñoz, M.; González-Moreno, M.; Chirino, S.; Bernal-Grande, M.D.C.; Cantoral, J.M. The Microbial Diversity of Sherry Wines. Fermentation 2018, 4, 19. https://doi.org/10.3390/fermentation4010019
Cordero-Bueso G, Ruiz-Muñoz M, González-Moreno M, Chirino S, Bernal-Grande MDC, Cantoral JM. The Microbial Diversity of Sherry Wines. Fermentation. 2018; 4(1):19. https://doi.org/10.3390/fermentation4010019
Chicago/Turabian StyleCordero-Bueso, Gustavo, Marina Ruiz-Muñoz, Mónica González-Moreno, Salvador Chirino, María Del Carmen Bernal-Grande, and Jesús Manuel Cantoral. 2018. "The Microbial Diversity of Sherry Wines" Fermentation 4, no. 1: 19. https://doi.org/10.3390/fermentation4010019
APA StyleCordero-Bueso, G., Ruiz-Muñoz, M., González-Moreno, M., Chirino, S., Bernal-Grande, M. D. C., & Cantoral, J. M. (2018). The Microbial Diversity of Sherry Wines. Fermentation, 4(1), 19. https://doi.org/10.3390/fermentation4010019





