Production and Characterization of a Novel Glycolipid Biosurfactant from Bradyrhizobium sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Kinetics of Biosurfactant Production
2.2.1. Cell Growth and pH
2.2.2. Determination of Sunflower Oil
2.2.3. Biosurfactant Concentration
2.2.4. Biosurfactant Mass
2.3. Recovery and Purification of the Biosurfactant
2.4. Physicochemical Characterization
2.4.1. Emulsification Index (E24)
2.4.2. Critical Micelle Concentration (CMC)
2.4.3. Interfacial Tension (IFT)
2.5. Biosurfactant Stability
2.6. Structural Characterization
2.6.1. Thin-Layer Chromatography (TLC) Analysis
2.6.2. Fatty Acid Methyl Ester Analysis (FAME) Analysis
2.6.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6.4. Mass Spectrometry (MALDI-TOF) Analysis
2.7. Statistical Analysis
3. Results
3.1. Biosurfactant Production
3.2. Physicochemical Properties
3.3. Stability of Biosurfactant
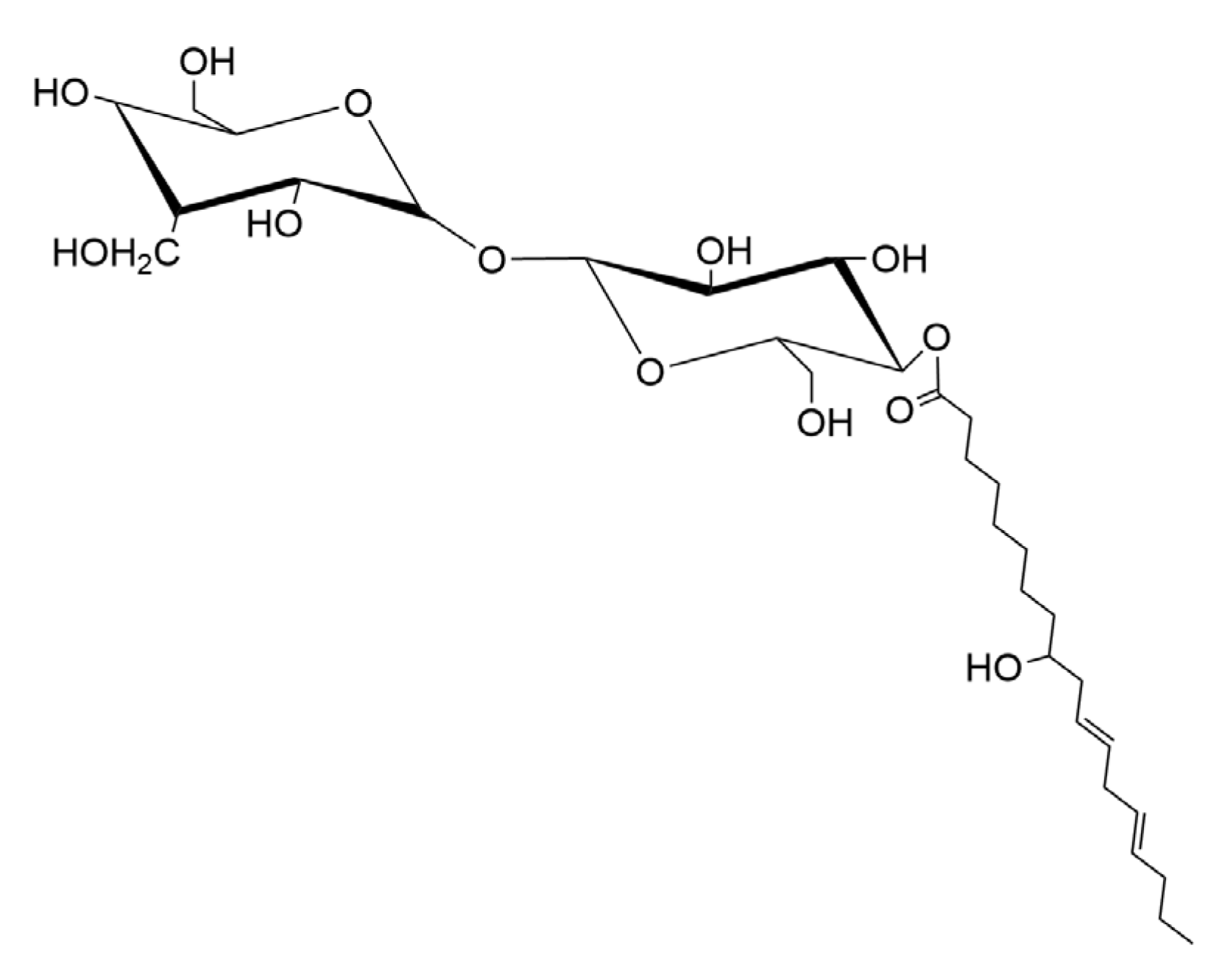
3.4. Characterization of Biosurfactant Structure
3.4.1. Thin-Layer Chromatography (TLC)
3.4.2. Fatty Acid Methyl Ester Analysis (FAME)
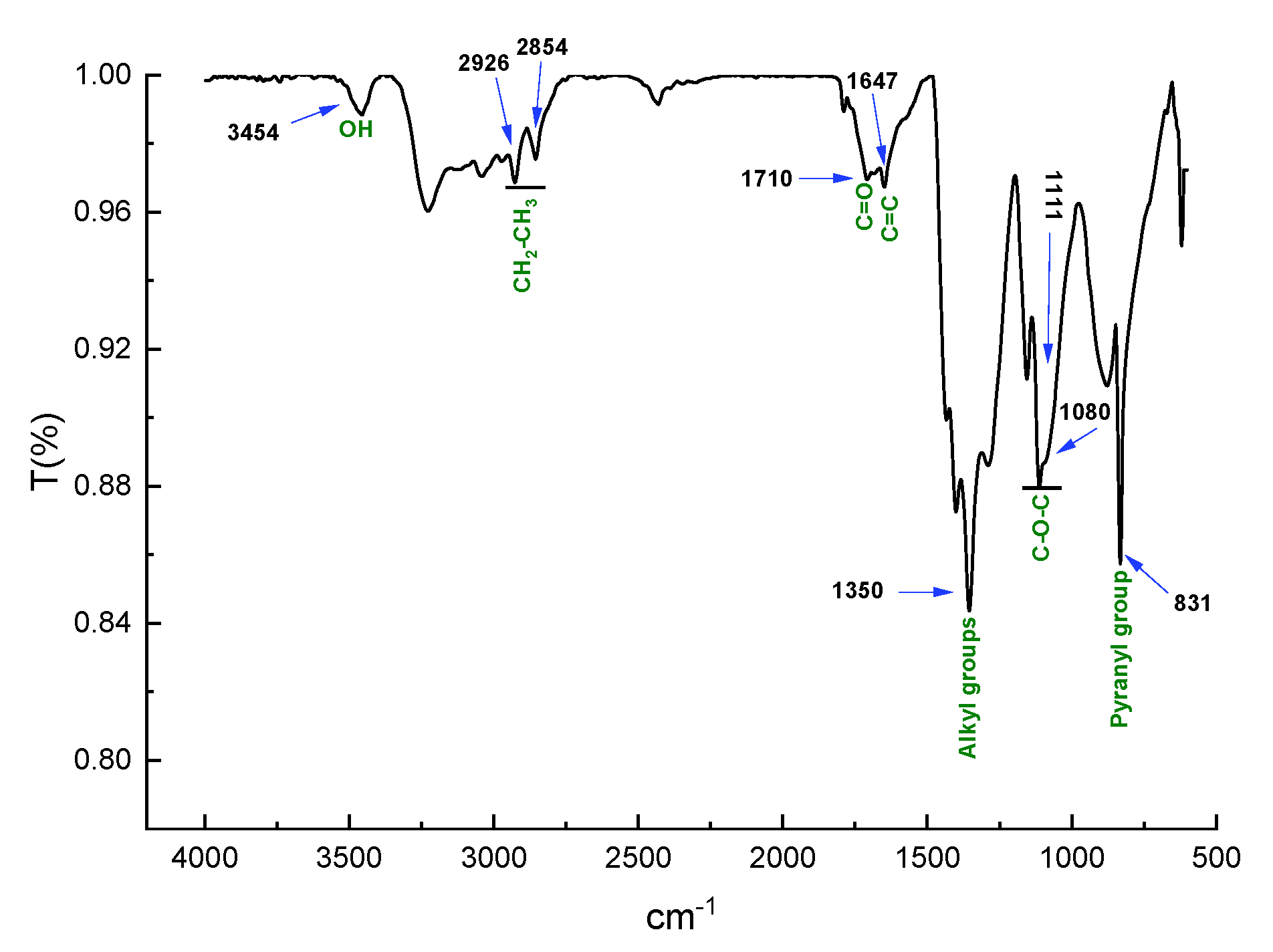
3.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
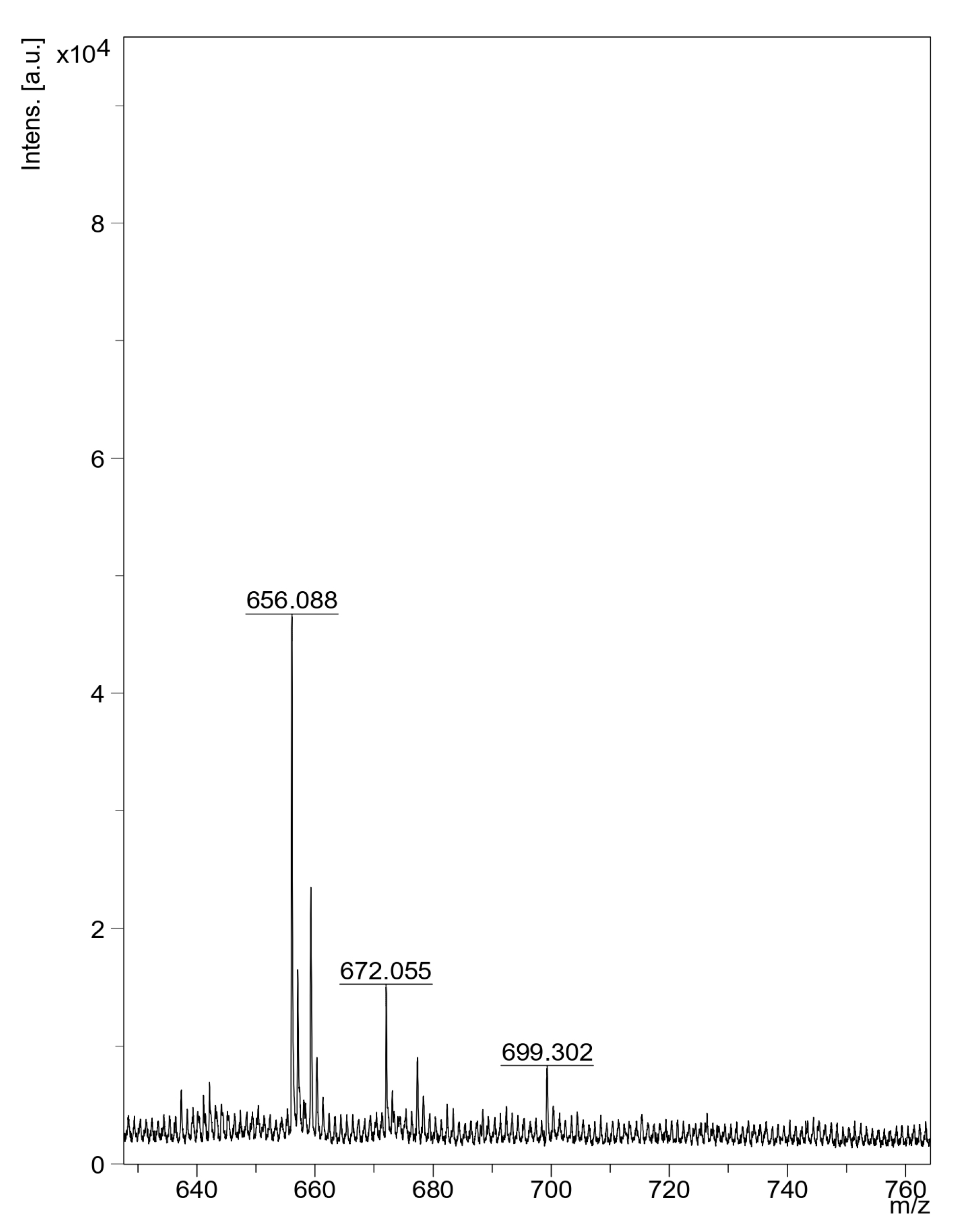
3.4.4. Mass Spectrometry (MALDI-TOF)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BS | Biosurfactant |
| YM | Yeast Malt |
| OD | Optical Density |
| CFU | Colony-Forming Unit |
| pH | Potential of Hydrogen |
| CMD | Critical Micelle Dilution |
| CMC | Critical Micelle Concentration |
| IFT | Interfacial Tension |
| TLC | Thin-Layer Chromatography |
| FAME | Fatty Acid Methyl Ester Analysis |
| FTIR | Fourier Transform Infrared Spectroscopy |
| MALDI- TOF | Matrix-Assisted Laser Desorption/Ionization—Time Of Flight |
| ST | Surface Tension |
| EI | Emulsification Index |
| TL | Trehalolipid |
References
- Janek, T.; Gudiña, E.J.; Połomska, X.; Biniarz, P.; Jama, D.; Rodrigues, L.R.; Rymowicz, W.; Lazar, Z. Sustainable Surfactin Production by Bacillus subtilis Using Crude Glycerol from Different Wastes. Molecules 2021, 26, 3488. [Google Scholar] [CrossRef]
- Dierickx, S.; Castelein, M.; Remmery, J.; De Clercq, V.; Lodens, S.; Baccile, N.; De Maeseneire, S.L.; Roelants, S.L.K.W.; Soetaert, W.K. From Bumblebee to Bioeconomy: Recent Developments and Perspectives for Sophorolipid Biosynthesis. Biotechnol. Adv. 2022, 54, 107788. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Rajendran, D.S.; Kumar, P.S.; Vo, D.-V.N.; Vaidyanathan, V.K. Extraction, Purification and Applications of Biosurfactants Based on Microbial-Derived Glycolipids and Lipopeptides: A Review. Environ. Chem. Lett. 2022, 20, 949–970. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A. Bacillus licheniformis: The Unexplored Alternative for the Anaerobic Production of Lipopeptide Biosurfactants? Biotechnol. Adv. 2022, 60, 108013. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Moosavi-Nasab, M.; Tiwari, B.K. Stimulation of Biosurfactant Production by Lactobacillus plantarum Using Ultrasound. Ultrason. Sonochem. 2019, 59, 104724. [Google Scholar] [CrossRef]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of Synthetic Surfactants on the Environment and the Potential for Substitution by Biosurfactants. Adv. Colloid. Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Dias, M.A.M.; Nitschke, M. Bacterial-Derived Surfactants: An Update on General Aspects and Forthcoming Applications. Braz. J. Microbiol. 2023, 54, 103–123. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; do Amaral, F.P.; Ané, J.-M.; Stacey, G. Diazotrophic Bacteria and Their Mechanisms to Interact and Benefit Cereals. Mol. Plant Microbe Interact. 2021, 34, 491–498. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R. Bioprospecting of Endophytic Microorganisms for Bioactive Compounds of Therapeutic Importance. Arch. Microbiol. 2021, 203, 1917–1942. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Lopes, É.; Castellane, T.C.; Moretto, C.; Lemos, E.; De Souza, J.A. Emulsification Properties of Bioemulsifiers Produced by Wild-Type and Mutant Bradyrhizobium elkanii Strains. J. Bioremediat Biodegrad. 2014, 5, 245. [Google Scholar] [CrossRef]
- Sun, R.; Belcher, R.W.; Liang, J.; Wang, L.; Thater, B.; Crowley, D.E.; Wei, G. Effects of Cowpea (Vigna unguiculata) Root Mucilage on Microbial Community Response and Capacity for Phenanthrene Remediation. J. Environ. Sci. 2015, 33, 45–59. [Google Scholar] [CrossRef]
- Ashitha, A.; Radhakrishnan, E.K.; Mathew, J. Characterization of Biosurfactant Produced by the Endophyte Burkholderia sp. WYAT7 and Evaluation of Its Antibacterial and Antibiofilm Potentials. J. Biotechnol. 2020, 313, 1–10. [Google Scholar] [CrossRef]
- Bodour, A.A.; Miller-Maier, R.M. Application of a Modified Drop-Collapse Technique for Surfactant Quantitation and Screening of Biosurfactant-Producing Microorganisms. J. Microbiol. Methods. 1998, 32, 273–280. [Google Scholar] [CrossRef]
- dos Santos, J.W.M.; da Silva, J.F.; dos Santos Ferreira, T.D.; Dias, M.A.M.; Fraiz, A.C.R.; Escobar, I.E.C.; dos Santos, R.C.; de Lima, L.M.; Morgante, C.V.; Fernandes-Júnior, P.I. Molecular and Symbiotic Characterization of Peanut Bradyrhizobia from the Semi-Arid Region of Brazil. Appl. Soil. Ecol. 2017, 121, 177–184. [Google Scholar] [CrossRef]
- Schwartz, W.J.M.; Vincent, A. Manual for the Practical Study of the Root-Nodule Bacteria (IBP Handbuch No. 15 Des International Biology Program, London). XI u. 164 S., 10 Abb., 17 Tab., 7 Taf. Oxford-Edinburgh 1970: Blackwell Scientific Publ., 45 s. Z. Allg. Mikrobiol. 1972, 12, 440. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Unás, J.H.; de Alexandria Santos, D.; Azevedo, E.B.; Nitschke, M. Brevibacterium luteolum Biosurfactant: Production and Structural Characterization. Biocatal. Agric. Biotechnol. 2018, 13, 160–167. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Chou, C.-L.; Chang, J.-S. Rhamnolipid Production by Indigenous Pseudomonas aeruginosa J4 Originating from Petrochemical Wastewater. Biochem. Eng. J. 2005, 27, 146–154. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhou, H.; Zeng, F.; Jiang, L.; Atakpa, E.O.; Chen, G.; Zhang, C.; Xie, Q. A Biosurfactant-Producing Yeast Rhodotorula sp.CC01 Utilizing Landfill Leachate as Nitrogen Source and Its Broad Degradation Spectra of Petroleum Hydrocarbons. World J. Microbiol. Biotechnol. 2022, 38, 68. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Gupta, S.; Jain, A. Production, Characterization, and Kinetic Modeling of Biosurfactant Synthesis by Pseudomonas aeruginosa Gi |KP 163922|: A Mechanism Perspective. World J. Microbiol. Biotechnol. 2023, 39, 178. [Google Scholar] [CrossRef]
- Ghazi Faisal, Z.; Sallal Mahdi, M.; Alobaidi, K.H. Optimization and Chemical Characterization of Biosurfactant Produced from a Novel Pseudomonas guguanensis Strain Iraqi ZG.K.M. Int. J. Microbiol. 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Ekprasert, J.; Kanakai, S.; Yosprasong, S. Improved Biosurfactant Production by Enterobacter cloacae B14, Stability Studies, and Its Antimicrobial Activity. Pol. J. Microbiol. 2020, 69, 273–282. [Google Scholar] [CrossRef]
- Joy, S.; Rahman, P.K.S.M.; Khare, S.K.; Sharma, S. Production and Characterization of Glycolipid Biosurfactant from Achromobacter sp. (PS1) Isolate Using One-Factor-at-a-Time (OFAT) Approach with Feasible Utilization of Ammonia-Soaked Lignocellulosic Pretreated Residues. Bioprocess. Biosyst. Eng. 2019, 42, 1301–1315. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial Lipases and Their Industrial Applications: A Comprehensive Review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Elkhawaga, M.A. Optimization and Characterization of Biosurfactant from Streptomyces griseoplanus NRRL-ISP5009 (MS1). J. Appl. Microbiol. 2018, 124, 691–707. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Xu, R.; Jia, Y. Screening and Evaluation of Biosurfactant-Producing Strains Isolated from Oilfield Wastewater. Indian. J. Microbiol. 2013, 53, 168–174. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, Isolation and Characterization of Biosurfactant Producing Bacillus subtilis Strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef]
- Zargar, A.N.; Mishra, S.; Kumar, M.; Srivastava, P. Isolation and Chemical Characterization of the Biosurfactant Produced by Gordonia sp. IITR100. PLoS ONE 2022, 17, e0264202. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F.; et al. Inhibition of Bacterial Adhesion and Antibiofilm Activities of a Glycolipid Biosurfactant from Lactobacillus rhamnosus with Its Physicochemical and Functional Properties. Antibiotics 2021, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Zargar, A.N.; Lymperatou, A.; Skiadas, I.; Kumar, M.; Srivastava, P. Structural and Functional Characterization of a Novel Biosurfactant from Bacillus sp. IITD106. J. Hazard. Mater. 2022, 423, 127201. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Felix, A.K.; Martins, J.J.L.; Lima Almeida, J.G.; Giro, M.E.A.; Cavalcante, K.F.; Maciel Melo, V.M.; Loiola Pessoa, O.D.; Ponte Rocha, M.V.; Rocha Barros Gonçalves, L.; Saraiva de Santiago Aguiar, R. Purification and Characterization of a Biosurfactant Produced by Bacillus subtilis in Cashew Apple Juice and Its Application in the Remediation of Oil-Contaminated Soil. Colloids Surf. B Biointerfaces 2019, 175, 256–263. [Google Scholar] [CrossRef]
- Behzadnia, A.; Moosavi-Nasab, M.; Tiwari, B.K.; Setoodeh, P. Lactobacillus plantarum-Derived Biosurfactant: Ultrasound-Induced Production and Characterization. Ultrason. Sonochem. 2020, 65, 105037. [Google Scholar] [CrossRef]
- Ahmadi, M.; Niazi, F.; Jaafarzadeh, N.; Ghafari, S.; Jorfi, S. Characterization of the Biosurfactant Produced by Pesudomonas areuginosa Strain R4 and Its Application for Remediation Pyrene-Contaminated Soils. J. Environ. Health Sci. Eng. 2021, 19, 445–456. [Google Scholar] [CrossRef]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and Characterization of Biosurfactant Production from Kitchen Waste Oil Using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2018, 25, 14934–14943. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, R.; Ma, F.; Han, S.; Zhang, Y. Oxygen Effects on Rhamnolipids Production by Pseudomonas aeruginosa. Microb. Cell Fact. 2018, 17, 39. [Google Scholar] [CrossRef]
- Sharma, J.; Kapley, A.; Sundar, D.; Srivastava, P. Characterization of a Potent Biosurfactant Produced from Franconibacter sp. IITDAS19 and Its Application in Enhanced Oil Recovery. Colloids Surf. B Biointerfaces 2022, 214, 112453. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, M.; Diwu, Z.; Lei, Y.; Li, H.; Bai, X. Characterization of Trehalose Lipids Produced by a Unique Environmental Isolate Bacterium Rhodococcus qingshengii Strain FF. J. Appl. Microbiol. 2019, 127, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Adiandri, R.S.; Purwadi, R.; Hoerudin, H.; Setiadi, T. Evaluation of Biosurfactant Production by Bacillus Species Using Glucose and Xylose as Carbon Sources. Curr. Microbiol. 2023, 80, 250. [Google Scholar] [CrossRef] [PubMed]
- Argentin, M.N.; Martins, L.F.; Sousa, M.P.; Bossolan, N.R.S. Biosurfactant from a Thermo-Halophilic Strain of Bacillus alveayuensis Isolated from a Brazilian Oil Reservoir: Production, Chemical Characterization, Antimicrobial Activity, and Efficiency in Wettability Reversal and Oil Removal from Oil-Soaked Sand. Geoenergy Sci. Eng. 2023, 231, 212324. [Google Scholar] [CrossRef]
- Hollenbach, R.; Völp, A.R.; Höfert, L.; Rudat, J.; Ochsenreither, K.; Willenbacher, N.; Syldatk, C. Interfacial and Foaming Properties of Tailor-Made Glycolipids—Influence of the Hydrophilic Head Group and Functional Groups in the Hydrophobic Tail. Molecules 2020, 25, 3797. [Google Scholar] [CrossRef]
- Guo, P.; Xu, W.; Tang, S.; Cao, B.; Wei, D.; Zhang, M.; Lin, J.; Li, W. Isolation and Characterization of a Biosurfactant Producing Strain Planococcus sp. XW-1 from the Cold Marine Environment. Int. J. Environ. Res. Public Health 2022, 19, 782. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Yu, Z.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Liang, H.; Qazi, M.A. Production and Characterization of Surfactin-like Biosurfactant Produced by Novel Strain Bacillus Nealsonii S2MT and It’s Potential for Oil Contaminated Soil Remediation. Microb. Cell Fact. 2020, 19, 145. [Google Scholar] [CrossRef]
- Kathiravan, N.; Rajesh, A.; Kim, J.-W.; Davoodbasha, M. Isolation and Characterization of Biosurfactant-Producing Soil Fungus Penicillium sp. Appl. Biochem. Biotechnol. 2024, 196, 3234–3245. [Google Scholar] [CrossRef]
- Khademolhosseini, R.; Jafari, A.; Mousavi, S.M.; Hajfarajollah, H.; Noghabi, K.A.; Manteghian, M. Physicochemical Characterization and Optimization of Glycolipid Biosurfactant Production by a Native Strain of Pseudomonas aeruginosa HAK01 and Its Performance Evaluation for the MEOR Process. RSC Adv. 2019, 9, 7932–7947. [Google Scholar] [CrossRef]
- Kügler, J.H.; Muhle-Goll, C.; Kühl, B.; Kraft, A.; Heinzler, R.; Kirschhöfer, F.; Henkel, M.; Wray, V.; Luy, B.; Brenner-Weiss, G.; et al. Trehalose Lipid Biosurfactants Produced by the Actinomycetes Tsukamurella spumae and T. pseudospumae. Appl. Microbiol. Biotechnol. 2014, 98, 8905–8915. [Google Scholar] [CrossRef]
- Tuleva, B.; Christova, N.; Cohen, R.; Stoev, G.; Stoineva, I. Production and Structural Elucidation of Trehalose Tetraesters (Biosurfactants) from a Novel Alkanothrophic Rhodococcus wratislaviensis Strain. J. Appl. Microbiol. 2008, 104, 1703–1710. [Google Scholar] [CrossRef]
- Andreolli, M.; Villanova, V.; Zanzoni, S.; D’Onofrio, M.; Vallini, G.; Secchi, N.; Lampis, S. Characterization of Trehalolipid Biosurfactant Produced by the Novel Marine Strain Rhodococcus sp. SP1d and Its Potential for Environmental Applications. Microb. Cell Fact. 2023, 22, 126. [Google Scholar] [CrossRef]
- Luong, T.M.; Ponamoreva, O.N.; Nechaeva, I.A.; Petrikov, K.V.; Delegan, Y.A.; Surin, A.K.; Linklater, D.; Filonov, A.E. Characterization of Biosurfactants Produced by the Oil-Degrading Bacterium Rhodococcus erythropolis S67 at Low Temperature. World J. Microbiol. Biotechnol. 2018, 34, 20. [Google Scholar] [CrossRef]
- Christova, N.; Lang, S.; Wray, V.; Kaloyanov, K.; Konstantinov, S.; Stoineva, I. Production, Structural Elucidation, and In Vitro Antitumor Activity of Trehalose Lipid Biosurfactant from Nocardia farcinica Strain. J. Microbiol. Biotechnol. 2015, 25, 439–447. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Baeva, T.A.; Kochina, O.A.; Gein, S.V.; Chereshnev, V.A. Trehalolipid Biosurfactants from Nonpathogenic Rhodococcus Actinobacteria with Diverse Immunomodulatory Activities. New Biotechnol. 2015, 32, 559–568. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, M.A.M.; Rossini, E.L.; de Britto, D.; Nitschke, M. Production and Characterization of a Novel Glycolipid Biosurfactant from Bradyrhizobium sp. Fermentation 2025, 11, 471. https://doi.org/10.3390/fermentation11080471
Dias MAM, Rossini EL, de Britto D, Nitschke M. Production and Characterization of a Novel Glycolipid Biosurfactant from Bradyrhizobium sp. Fermentation. 2025; 11(8):471. https://doi.org/10.3390/fermentation11080471
Chicago/Turabian StyleDias, Marcos André Moura, Eduardo Luiz Rossini, Douglas de Britto, and Marcia Nitschke. 2025. "Production and Characterization of a Novel Glycolipid Biosurfactant from Bradyrhizobium sp." Fermentation 11, no. 8: 471. https://doi.org/10.3390/fermentation11080471
APA StyleDias, M. A. M., Rossini, E. L., de Britto, D., & Nitschke, M. (2025). Production and Characterization of a Novel Glycolipid Biosurfactant from Bradyrhizobium sp. Fermentation, 11(8), 471. https://doi.org/10.3390/fermentation11080471









