Abstract
Bacteriocins are ribosomal synthesized antimicrobial peptides produced by bacteria, but their low yields limit industrial applications as food preservatives. This study aimed to optimize the culture conditions of Pediococcus acidilactici CCFM18 and investigate the biological properties of the bacteriocin. The culture temperature, initial pH, and culture time significantly affected the growth of P. acidilactici CCFM18 and bacteriocin production. The optimal culture conditions determined through response surface methodology (RSM) were a culture temperature of 35 °C, an initial pH of 7.0, and a growth time of 16 h. Under these conditions, bacteriocin production reached 1454.61 AU/mL, representing a 1.8-fold increase compared to pre-optimization levels. Biological characterization revealed that the bacteriocin exhibited strong thermal stability (up to 100 °C for 30 min) and pH stability (pH 2–9), but was sensitive to proteolytic enzymes, including pepsin, trypsin, papain, and protease K. The bacteriocin demonstrated antimicrobial activity against both Gram-positive and Gram-negative bacteria, including Enterococcus faecalis and Escherichia coli. These findings provide a theoretical basis for the industrial production and application of the bacteriocin.
1. Introduction
Microbial contamination has raised significant concerns for food safety, making the search for safe and reliable food preservatives an essential research focus in food engineering [1]. There is increasing interest in developing and applying natural preservatives, compared with synthetic chemical alternatives [2,3]. Bacteriocins are ribosomal synthesized antimicrobial peptides produced by bacteria and have gained attention due to their non-toxic nature, susceptibility to degradation by proteases in the human digestive tract, and the relatively low incidence of resistance development compared to conventional antibiotics [4,5,6]. Since the 1980s, numerous bacteriocins have been identified and characterized, of which nisin is the only bacteriocin approved by both the World Health Organization (WHO) and the U.S. Food and Drug Administration (FDA) as a food preservative [7,8]. In addition to nisin, the FDA has also approved pediocin for use in food preservation. Bacteriocins can not only inhibit the growth of spoilage bacteria in food preservation but also offer benefits such as regulating gut microbiota and inducing apoptosis in tumor cells, making them highly promising for applications in food, medicine, and animal husbandry [9,10].
Pediocin-like bacteriocins have attracted significant research due to their favorable physicochemical properties, including superior thermal and pH stability, as well as strong activity against Listeria monocytogenes. However, their low yield limits industrial applications [11,12]. Therefore, enhancing the production of pediocin is crucial, and various strategies are being explored. Factors affecting bacteriocin synthesis and secretion by lactic acid bacteria (LAB) include fermentation conditions, genetic factors, and quorum sensing [13,14,15]. Optimizing cultivation conditions is a widely studied and fundamental method to improve bacteriocin production. Trinetta et al. [16] successfully optimized the culture conditions of Lactobacillus sakei through a one variable at a time approach, increasing the yield of bacteriocin sakacin A from 180 AU/mL to 480 AU/mL. It was one of the first examples of optimizing pediocin-like bacteriocin production conditions using the design of experiment approach. For Bacillus subtilis ZY05, optimizing the culture parameters such as carbon source, NaCl concentration, incubation time, and pH increased the bacteriocin activity from 2041 AU/mL to 4403.85 AU/mL through a combination of single-factor and response surface methodology (RSM) experiments [17]. Similarly, RSM optimization of the culture conditions (pH: 7.19, temperature: 33.3 °C, and incubation time: 22.2 h) resulted in a 2-fold increase in antibacterial activity to 4652.15 AU/mL [18]. Jawan et al. [19] assessed the effects of various factors, including initial pH, inoculum age, inoculum size, and nitrogen and carbon sources, on the production of bacteriocin by Lactococcus lactis Gh1, achieving a 0.9-fold increase in bacteriocin production to 715.36 AU/mL. In another study, the bacteriocin yield increased 2-fold under optimal conditions (temperature: 37 °C, initial pH: 6.0, and culture time: 18 h) compared with pre-optimization levels [20]. It is evident that culture temperature, pH, and culture time play a crucial role in bacteriocin production by LAB. Pediococcus acidilactici CCFM18 is a pediocin PA-1-producing bacterium derived from pickles and has been shown to inhibit the growth of L. monocytogenes [21]. However, the conditions for bacteriocin production have not yet been optimized, and its basic biological properties remain unknown.
This study aimed to address these knowledge gaps. The effects of culture temperature, initial pH, and culture time on the growth of P. acidilactici CCFM18 and bacteriocin production were investigated, and the optimal conditions were determined using RSM. Moreover, the stability of the bacteriocin against different temperatures, pH, and enzymes, as well as the antimicrobial spectrum, was evaluated. The results would guide the effective synthesis of bacteriocin from P. acidilactici CCFM18 and provide a promising natural alternative for food preservation.
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
The LAB P. acidilactici CCFM18, originally isolated from Chinese pickles, was provided by Jiangnan University (Wuxi, China). Indicator strains, including Lactobacillus helveticus ATCC10797, Streptococcus thermophilus ATCC19258, and Bacillus cereus ATCC11778, were obtained from the China Center of Industrial Culture Collection, while other strains were sourced from the laboratory stock. Culture media (MRS, LB, and YPD) were purchased from Hopebio (Qingdao, China) and sterilized at 121 °C for 20 min. All Lactobacillus, Pediococcus, and Streptococcus strains were grown in MRS broth at 37 °C. Bacillus strains, Micrococcus luteus SNSS174, and Escherichia coli CMCC44102 were grown in LB broth at 37 °C. Saccharomyces cerevisiae SNSS176 was propagated in YPD medium. All strains were maintained in MRS, LB, or YPD containing 30% glycerol at −80 °C. Prior to use, 500 µL of the glycerol stock was inoculated into 10 mL of the corresponding medium and statically incubated at 37 °C for revival, followed by two successive subcultures with 2% (v/v) inoculum.
2.2. Detection of Antibacterial Activity of Bacteriocin
The cell-free fermentation supernatant was obtained by centrifugation (6000× g, 4 °C, 15 min) and neutralized to pH 6.5 with NaOH to exclude the influence of organic acids. The supernatant was filtered through a sterile 0.22 µm cellulose acetate membrane and subjected to a serial double-dilution in sterile saline. The antibacterial activity was assayed using the agar well diffusion method. For this assay, 200 µL of a 24 h culture of the indicator strain of Enterococcus faecalis was mixed with 20 mL of MRS agar and then poured into petri dishes. After the agar had solidified, wells were punched and filled with 20 µL of supernatants from each dilution gradient. Plates were treated at 4 °C for approximately 12 h to facilitate radial diffusion, followed by incubation at 37 °C for 48 h. The agar well diffusion test was performed in triplicate, and the diameters of the inhibition zones were recorded using a vernier caliper to calculate the average value. The antibacterial activity was quantified in arbitrary units (AU), defined as the reciprocal of the highest dilution exhibiting a clear inhibition zone [22,23]. The calculation formula is as follows:
where n represents the highest dilution showing a clear antibacterial zone and x represents the volume of the sample added to each well (µL).
The standard curve was plotted using serial geometric dilutions of the fermentation broth, with the horizontal axis representing the inhibition zone diameters of the diluted supernatant and the vertical axis representing the logarithm of the bacteriocin titer.
2.3. Optimization of Culture Conditions for Bacteriocin Production
2.3.1. Optimization of Initial pH for Bacteriocin Production
The initial pH of 20 mL MRS medium in 50 mL flasks was adjusted to 5.5, 6.0, 6.5, 7.0, and 7.5 using HCl or NaOH, respectively. Each flask was then inoculated with 2% (v/v) of a 12-h culture of P. acidilactici, followed by static incubation at 37 °C for 24 h. Bacterial growth was monitored by measuring optical density at 600 nm using a spectrophotometer, with MRS broth as the blank control. The diameter of the inhibition zones in the supernatant was determined by agar well diffusion tests. The antimicrobial activity (AU/mL) was quantified using the standard curve.
2.3.2. Optimization of Culture Time for Bacteriocin Production
The 12 h culture of P. acidilactici CCFM18 was inoculated (2%, v/v) into 20 mL of MRS broth in a 50 mL flask and statically incubated at 37 °C for 24 h. Samples were taken at 2 h intervals to measure changes in optical density at 600 nm and assess the inhibitory effect of the supernatant.
2.3.3. Optimization of Culture Temperature for Bacteriocin Production
The effect of different temperatures on bacterial growth and bacteriocin production was evaluated by incubating a 12 h culture of P. acidilactici CCFM18 (2%, v/v) into MRS broth and culturing it at 27, 32, 37, 42, and 47 °C for 24 h, respectively.
2.3.4. Experimental Design of RSM
Based on the results of the single-factor experiments, a Box–Behnken response surface design was applied using Design-Expert 10.0 software (Stat-Ease Inc., Minneapolis, MN, USA) to optimize the production of bacteriocin. Three variables (A: culture temperature, B: initial pH, and C: culture time) were used as independent variables, with bacteriocin activity as the response variable. Each factor was set at three levels, and the specific factor levels are shown in Table S1.
2.3.5. Model Validation
The optimization results and the developed second-order quadratic model were validated by measuring the antimicrobial activity of the supernatant in actual experiments.
2.4. Biochemical Characterization of Bacteriocin
2.4.1. Protease Sensitivity
P. acidilactici CCFM18 was inoculated into a 50 mL flask containing 20 mL of MRS broth and incubated under the optimal conditions described above to obtain fermentation supernatant. Then, the pH of the fermentation supernatant was adjusted to the optimal pH for each enzyme, and pepsin, trypsin, protease K, and papain were added to a final concentration of 2 mg/mL. The samples were treated in a 37 °C water bath for 2 h, followed by enzyme inactivation in boiling water for 5 min. The pH of the samples was adjusted back to 6.5 according to the aforementioned antibacterial activity detection method. Then, the inhibition assay was performed to calculate the retention of bacteriocin activity [24].
2.4.2. pH Stability
The fermentation supernatant was adjusted to a pH ranging from 2 to 12 using HCl or NaOH and incubated in a 37 °C water bath for 2 h. Then, to standardize the detection conditions for antibacterial activity and facilitate comparison of differences in antibacterial activity among different samples, the pH of each group was adjusted back to 6.5 before their antimicrobial activities were evaluated. The changes in antimicrobial activity after treatment at different pH levels were observed.
2.4.3. Temperature Stability
To assess the thermal stability of the bacteriocin produced by P. acidilactici CCFM18, the fermentation supernatant was treated at 60, 80, 100, and 121 °C for 15 and 30 min, respectively. After cooling to room temperature, the antibacterial assay was conducted, with untreated supernatant serving as the control.
2.4.4. Antibacterial Spectrum Analysis
Twelve laboratory strains, including Gram-positive bacteria, Gram-negative bacteria, and yeast, were selected as indicator bacteria. Among them, four Bacillus strains were vegetative cells rather than spores. The bacteriostatic effects of the cell-free fermentation supernatant were evaluated using the agar well diffusion method to determine the antibacterial spectrum of P. acidilactici CCFM18 bacteriocin.
2.5. Statistical Analysis
All experiments were performed in triplicate, with data presented as mean ± standard deviation. Graphing was performed using Origin 9.0 software, and analysis of variance (ANOVA) was conducted using SPSS 22.0 software, with significance defined at p < 0.05.
3. Results and Discussion
3.1. Standard Curve for Bacteriocin Titer
Using the two-fold dilution method, the minimum diameter of the inhibition zone for P. acidilactici CCFM18 bacteriocin was observed at a 32-fold dilution with 20 µL of fermentation supernatant. As shown in Figure S1, the standard curve for bacteriocin titer was obtained through geometric serial dilution. The equation of the standard curve was y = 0.1746x + 0.5568, with a correlation coefficient of 0.9956 (p < 0.05), indicating a high degree of linearity.
3.2. Optimization of Culture Conditions
3.2.1. Effect of pH on Bacteriocin Production
As shown in Figure 1, the bacteriocin production of P. acidilactici CCFM18 showed an increasing trend with initial pH ranging from 5.5 to 7.0, reaching maximum activity of 536.36 AU/mL at pH 7.0. This bacteriocin production was significantly higher than the culture at other pH values (p < 0.05). The bacterial density peaked at pH 6.5, though no significant differences in density were observed between pH 6 and 7 (p > 0.05). This suggested differential trends in the growth of bacteria and their bacteriocin production. Previous studies have shown that the optimal conditions for bacteriocin production by LAB may differ from those for bacterial growth [25,26]. Although bacteriocin production is linked to bacterial density, it does not entirely depend on growth. Considering both bacteriocin production and bacterial growth, an initial pH of 7 was selected as optimal.
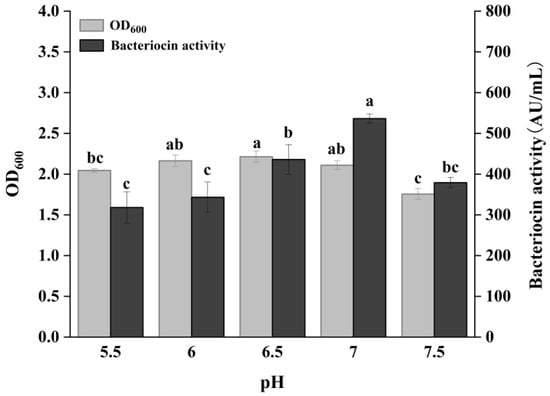
Figure 1.
Effect of pH on bacterial growth and bacteriocin production. Different letters denote significant differences between groups (p < 0.05).
3.2.2. Effect of Culture Time on Bacteriocin Production
Bacteriocin production is closely related to culture time. The growth and bacteriocin production patterns differ among strains, but most LAB begin bacteriocin synthesis during the exponential phase and reach maximum production at the end of the exponential phase or the stationary phase [27,28,29]. As shown in Figure 2, P. acidilactici CCFM18 entered the exponential phase after 4 h of incubation, with a rapid increase in bacterial density. Bacteriocin synthesis began during the mid-exponential phase (8 h), reaching its peak antimicrobial activity of 604.93 AU/mL at 16 h, coinciding with the stationary phase. These results were consistent with previous findings for Staphylococcus epidermidis [30]. Beyond 16 h, the bacteria enter the decline phase, where bacteriocin activity decreased slightly, though the reduction was not statistically significant (p > 0.05). This may be due to nutrient depletion or hydrolysis of the bacteriocin by proteolytic enzymes in the medium, or adsorption of the bacteriocin to the bacterial surface. Based on bacteriocin production and bacterial growth, 16 h was selected as the optimal cultivation time for P. acidilactici CCFM18.
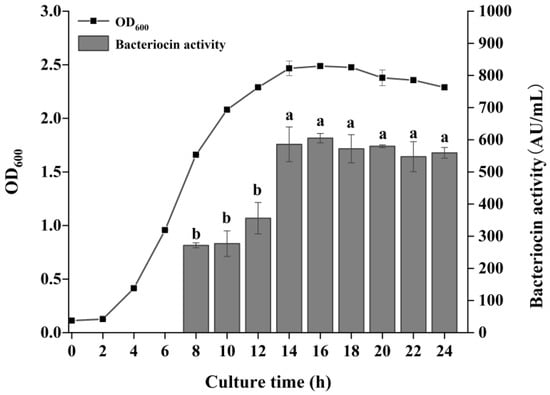
Figure 2.
Effect of culture time on bacterial growth and bacteriocin production. Different letters denote significant differences between groups (p < 0.05).
3.2.3. Effect of Culture Temperature on Bacteriocin Production
Figure 3 showed the effect of culture temperature on bacteriocin production by P. acidilactici CCFM18 in MRS broth. As the culture temperature increased from 27 °C to 32 °C, both bacterial growth and bacteriocin production exhibited an upward trend, reaching a maximum at 32 °C. The maximum bacteriocin activity was 1350.56 AU/mL, identifying 32 °C as the optimal culture temperature for P. acidilactici CCFM18. Further increasing culture temperatures resulted in a gradual decrease in both bacteriocin production and bacterial density. The optimal temperature for growth and metabolism of P. acidilactici strains is typically around 37 °C [31]. However, the optimal temperature for bacteriocin production of P. acidilactici NCDC is 28.2 °C [32], while P. acidilactici MM33 produces the highest bacteriocin yield at 45 °C [33]. In the case of P. acidilactici CCFM18, temperature stress at 32 °C may stimulate the production of shock proteins and related metabolic pathways, leading to higher bacteriocin output [34].
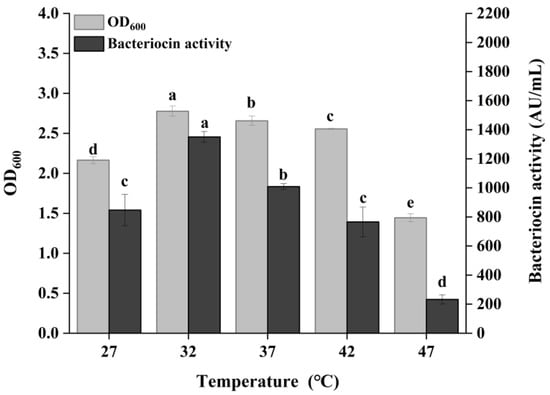
Figure 3.
Effect of culture temperature on bacterial growth and bacteriocin production. Different letters denote significant differences between groups (p < 0.05).
3.2.4. Establishment and Analysis of the RSM Model
Table S2 shows the Box–Behnken experimental design and response values for bacteriocin production. There is a total of 17 sets of independent variables, with the bacteriocin activities ranging from 499.04 to 1722.89 AU/mL. Multiple regression analysis was conducted on the experimental data using Design Expert 8.0.6 software to assess the effect of culture temperature (A), initial pH (B), and culture time (C) on bacteriocin activity (Y). The relationship between the response variable and the independent variables was represented by the following second-order polynomial equation:
Y = 1641.58 + 128.29A − 19.48B − 3.01C + 7.63AB − 51.85AC − 34.53BC − 578.45A2 − 242.59B2 − 393.22C
Analysis of variance (ANOVA) was performed to evaluate the adequacy of the regression model and to determine the significance and degree of influence of each factor on bacteriocin activity. As shown in Table 1, the model was statistically significant (p < 0.0001), with a high coefficient of determination (R2 = 0.9914) and an insignificant lack of fit test (p > 0.05). This suggested that the equation adequately explained the relationship between variables and bacteriocin activity and had reliability and suitability for predicting bacteriocin production under varying culture conditions. Among the independent variables, culture temperature (A) exhibited the most substantial linear effect on bacteriocin activity, followed by initial pH (B) and culture time (C). The p-values for the culture temperature (A) on bacteriocin production were less than 0.05, indicating a significant contribution to the model. The quadratic terms for all three variables (A2, B2, and C2) on bacteriocin activity were highly significant, with p-values less than 0.0001. In contrast, interaction effects (AB, AC, and BC) were less pronounced, with AC showing the strongest influence among them.

Table 1.
Analysis of variance of the experimental results and regression coefficients in the Box–Behnken experimental design.
3.2.5. Curve Analysis of RSM
Figure 4 illustrates the contour plots and 3D surface graphs, providing a visual representation of the interaction effects among culture temperature, initial pH, and culture time on bacteriocin activity. The steep response surface and dense contours indicated that culture temperature exerted the most significant effect on bacteriocin activity. Furthermore, there were slight interactions between culture temperature and culture time, as well as between initial pH and culture time, according to elliptical contours. In contrast, culture temperature hardly interacted with initial pH.
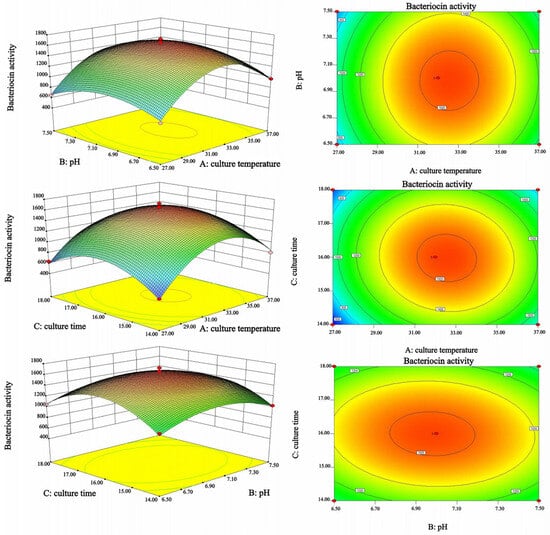
Figure 4.
Three-dimensional response surfaces and contour plots of the interaction effects of three different reaction parameters (A: culture temperature, B: initial pH, and C: culture time) on bacteriocin activity.
3.2.6. Validation of the Optimal Processes
According to the regression model and three-dimensional contour plots, the optimal culture conditions for bacteriocin production by P. acidilactici CCFM18 were determined to be as follows: a culture temperature of 35.18 °C, pH of 6.99, and culture time of 15.91 h. The predicted bacteriocin activity under these conditions was 1489.88 AU/mL, which was higher than the bacteriocin activity of 1350.56 AU/mL obtained after the single-factor experiment mentioned above. For practical implementation, the parameters were adjusted to a culture temperature of 35 °C, an initial pH of 7.0, and a culture time of 16 h. Validation experiments conducted under these adjusted conditions showed an average bacteriocin activity of 1454.61 ± 70.36 AU/mL, closely matching the predicted value. This alignment demonstrated the reliability of the RSM-optimized culture process, which can be effectively applied to bacteriocin production. As shown in Figure S2, the antimicrobial activity of bacteriocin under optimized conditions was 1.8 times higher than the activity of 517.85 AU/mL under the original culture conditions.
3.3. Biochemical Characterization of Bacteriocin
3.3.1. Sensitivity to Enzymes
After treatment with pepsin, trypsin, protease K, and papain, the antibacterial activity of the fermentation supernatant was completely lost, indicating that the bacteriocin produced by P. acidilactici CCFM18 was sensitive to these proteases (Table 2). This property ensures that the bacteriocin is digested and degraded by human proteases when used as a food preservative, minimizing residue and ensuring safety. However, this enzymatic sensitivity limits its ability to reach the gut intact for modulating intestinal flora. To overcome this limitation, strategies such as incorporating bacteriocin-producing strains or employing microencapsulation techniques can be used to shield the bacteriocin from protease degradation and enable targeted delivery for probiotic effects [35,36].

Table 2.
Effect of proteases on the antibacterial activity of bacteriocin produced by P. acidilactici CCFM18.
3.3.2. pH Stability of Bacteriocin
As illustrated in Figure 5, the bacteriocin produced by P. acidilactici CCFM18 exhibited antibacterial activity across a broad pH range of 2 to 9. The maximum antibacterial activity appeared at pH 2 and 3, suggesting that a strongly acidic environment helps preserve bacteriocin activity. As the pH increased, a gradual decline in bacteriocin activity was observed. In neutral to slightly alkaline conditions (pH 7–8), bacteriocin activity decreased by about 42%. Remarkably, the bacteriocin retained an activity level of 649.27 AU/mL at pH 9, but its activity was completely lost at pH 10. These results demonstrated the high tolerance of bacteriocin to acidic and neutral environments. This is consistent with previous studies, which showed that most pediocins retain high activity within the pH range of 2–10 but may suffer partial or total loss of activity under alkaline conditions [37,38,39].
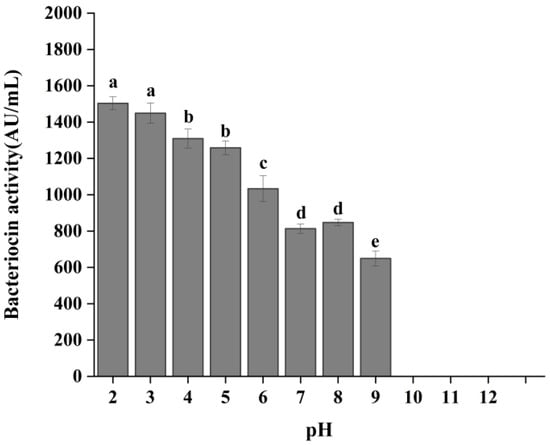
Figure 5.
Effect of pH on the antibacterial activity of bacteriocin produced by P. acidilactici CCFM18. Different letters denote significant differences between groups (p < 0.05).
3.3.3. Thermal Resistance of Bacteriocin
As shown in Figure 6, the antibacterial activity of the bacteriocin was not significantly affected by treatment at 60 and 80 °C for 15 min (p > 0.05). Although treatment at 100 °C for 15 min resulted in a significant decrease in bacteriocin activity, residual activity remained at 92.32%, indicating that the bacteriocin could withstand short-term high-temperature treatment. However, the antibacterial activity dropped sharply when the supernatant was treated at 80 °C and 100 °C for 30 min (p < 0.05). The antimicrobial activity continuously declined with increasing temperature and treatment time, and it was completely lost after high-pressure treatment at 121 °C. In general, the antibacterial activity of bacteriocin from P. acidilactici CCFM18 showed a decreasing trend with increasing heating temperature and time. Notably, the antibacterial activity of bacteriocin heated at 100 °C for 15 min was slightly higher than that heated at 80 °C for 30 min, though the difference was not significant. This trend was roughly consistent with previous findings on the thermal stability of pediocin [40]. The reason for this stability might lie in the presence of stable structures, such as disulfide bonds, in the bacteriocin, which help it withstand short-term high-temperature treatment. However, prolonged heating can damage its protein structure and thus reduce its antibacterial activity [41]. Bacteriocins with thermal and pH stability are crucial for their commercial applications. The pasteurization temperatures typically used in the food industry range from 65 to 80 °C. Therefore, the bacteriocin retained significant antibacterial activity within this temperature range, highlighting its potential for application as a food preservative.
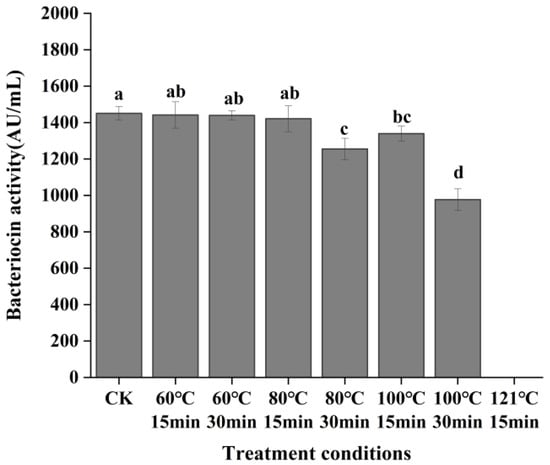
Figure 6.
Effect of temperature on the antibacterial activity of bacteriocin produced by P. acidilactici CCFM18. Different letters denote significant differences between groups (p < 0.05).
3.3.4. Antimicrobial Spectrum of Bacteriocin
Table 3 indicates that the bacteriocin produced by P. acidilactici CCFM18 could inhibit Gram-positive bacteria (such as S. thermophilus ATCC 19258, B. cereus ATCC 11778, and Enterococcus faecalis SNSS1133) and Gram-negative bacteria (such as Escherichia coli CMCC44102), demonstrating broad-spectrum antimicrobial activity. Among the tested strains, the most sensitive was the opportunistic pathogen E. faecalis SNSS1133. Pediocins are known for their antibacterial activity against Gram-positive bacteria. However, the results of this study demonstrated that pediocin also exhibited antibacterial activity against certain Gram-negative bacteria, such as E. coli CMCC44102. In addition, previous studies have shown that the pediocin produced by P. acidilactici CCFM18 can effectively inhibit L. monocytogenes, a common foodborne pathogen [42]. The inhibitory mechanism involves the N-terminal transmembrane domain of pediocin interacting with the target cell membrane via electrostatic interactions and recognizing the membrane receptor of the mannose phosphotransferase system (man-PTS). This facilitates insertion into the membrane, where the C-terminal forms pores by interacting with the hydrophobic core and man-PTS subunits, leading to solute leakage, membrane potential dissipation, and eventual cell death. Since man-PTS is not present in all bacterial strains, this specificity explains the selective bacteriostatic effect of pediocin [43,44]. The bacteriocin produced by P. acidilactici CCFM18 exhibited promising potential as a food preservative to effectively combat pathogenic bacteria.

Table 3.
Antimicrobial spectrum of the bacteriocin produced by P. acidilactici CCFM18.
4. Conclusions
In this study, the production conditions for bacteriocin by P. acidilactici CCFM18 were optimized, and its biological properties were examined. The culture temperature, initial pH, and culture time significantly influenced the growth of P. acidilactici CCFM18 and the production of the bacteriocin. Through single-factor and Box–Behnken RSM experiments, a quadratic multiple regression model correlating bacteriocin activity with temperature, pH, and culture time was established. The optimal culture conditions for bacteriocin production by P. acidilactici CCFM18 were determined to be static incubation at 35 °C for 16 h in MRS medium with an initial pH of 7.0. Under these conditions, bacteriocin activity increased from 517.85 AU/mL to 1454.61 AU/mL, representing a 1.8-fold increase, which closely matched the predicted value and confirmed the authenticity and reliability of the model. Biological characterization revealed that the bacteriocin exhibited strong thermal stability (up to 100 °C for 30 min) and pH stability (pH 2–9), but was sensitive to proteolytic enzymes, including pepsin, trypsin, papain, and protease K. The bacteriocin demonstrated antimicrobial activity against both Gram-positive and Gram-negative bacteria. These excellent biological properties may make it highly suitable for inhibiting E. faecalis, L. monocytogenes, and E. coli in meat and dairy products, as well as stubborn Bacillus in foods such as fruit juice, extending the shelf life of food products. This study successfully increased the yield of P. acidilactici CCFM18 bacteriocin, which was beneficial for the industrial production optimization of pediocin and enhanced its potential application in food preservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11080470/s1, Figure S1: Standard curve for bacteriocin titer; Figure S2: Antibacterial effect of bacteriocin produced by P. acidilactici CCFM18 before (a) and after (b) optimization using response surface methodology; Table S1: Three independent variables and coded levels in the Box–Behnken experimental design; Table S2: Box–Behnken experimental design and response values for bacteriocin production.
Author Contributions
Conceptualization, X.G. and Z.Z.; methodology, X.G., X.B. and Z.Q.; validation, X.G., Z.Z. and X.Q.; formal analysis, X.B. and Z.Q.; investigation, X.G., X.B. and Z.Q.; resources, Y.Q.; data curation, X.B. and Z.Q.; writing—original draft preparation, X.G.; writing—review and editing, X.G., X.B., Z.Q. and Y.Q.; visualization, Z.Z. and X.Q.; supervision, Y.Q.; project administration, Y.Q.; funding acquisition, Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [No.32202008].
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
We gratefully acknowledge all of the people who have contributed to this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhuo, Q.; Shi, C.; Geng, Q.; Wang, S.; Wang, B.; Zhang, N.; Yang, K.; Tian, J. Role of mitochondrial farnesyltransferase gene in the prevention of the food spoilage fungi Aspergillus flavus by the antimicrobial natural preservative perillaldehyde. Food Microbiol. 2024, 118, 104422. [Google Scholar] [CrossRef] [PubMed]
- Gokoglu, N. Novel natural food preservatives and applications in seafood preservation: A review. J. Sci. Food Agric. 2019, 99, 2068–2077. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jung, D.Y.G.; Jin, D.Y.Y.; Jayabalan, D.R.; Yang, D.S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 2018, 58, 2743–2767. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Multiple bacteriocin production in lactic acid bacteria. J. Biosci. Bioeng. 2022, 134, 277–287. [Google Scholar] [CrossRef]
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lü, X. Current status and potentiality of class II bacteriocins from lactic acid bacteria: Structure, mode of action and applications in the food industry. Trends Food Sci. Technol. 2022, 120, 387–401. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lu, N.; Wang, J.; Chen, Z.; Chen, C.; Mac Regenstein, J.; Zhou, P. Effect of N-terminal modification on the antimicrobial activity of nisin. Food Control 2020, 114, 107227. [Google Scholar] [CrossRef]
- López-Cuellar, M.D.R.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N. LAB bacteriocin applications in the last decade. Biotechnol. Biotechnol. Equip. 2016, 30, 1039–1050. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current applications of bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.S.R.; Chalón, M.C.; Navarro, S.A.; Bellomio, A. Pediocin-like bacteriocins: New perspectives on mechanism of action and immunity. Curr. Genet. 2018, 64, 345–351. [Google Scholar] [CrossRef]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.O.S.; Vitolo, M.; Oliveira, R.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Bashokouh, F.; Ramakrishnan, N.R.; Mustafa, S.; Ariff, A.B. Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: A review. RSC Adv. 2017, 7, 29395–29420. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Quorum sensing circuits in the communicating mechanisms of bacteria and its implication in the biosynthesis of bacteriocins by lactic acid bacteria: A review. Probiotics Antimicrob. Proteins 2020, 12, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Sidooski, T.; Brandelli, A.; Bertoli, S.L.; Souza, C.K.D.; Carvalho, L.F.D. Physical and nutritional conditions for optimized production of bacteriocins by lactic acid bacteria—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Trinetta, V.; Rollini, M.; Manzoni, M. Development of a low cost culture medium for sakacin A production by L. sakei. Process Biochem. 2008, 43, 1275–1280. [Google Scholar] [CrossRef]
- Chandrika, K.; Sachan, A. Enhanced production of bacteriocin by Bacillus subtilis ZY05. 3 Biotech 2024, 14, 37. [Google Scholar] [CrossRef]
- Kumar, M.; Jain, A.K.; Ghosh, M.; Ganguli, A. Statistical optimization of physical parameters for enhanced bacteriocin production by L. casei. Biotechnol. Bioprocess Eng. 2012, 17, 606–616. [Google Scholar] [CrossRef]
- Jawan, R.; Abbasiliasi, S.; Tan, J.S.; Mustafa, S.; Halim, M.; Ariff, A.B. Influence of culture conditions and medium compositions on the production of bacteriocin-like inhibitory substances by Lactococcus lactis Gh1. Microorganisms 2020, 8, 1454. [Google Scholar] [CrossRef]
- Altuntas, E.G.; Cosansu, S.; Ayhan, K. Some growth parameters and antimicrobial activity of a bacteriocin-producing strain Pediococcus acidilactici 13. Int. J. Food Microbiol. 2010, 141, 28–31. [Google Scholar] [CrossRef]
- Qiao, Y.; Tian, F.; Yu, L.; Zhao, J.; Zhai, Q.; Chen, W. Imaging mass spectrometry and genome mining reveal antimicrobial peptides of novel Pediococcus acidilactici CCFM18. Foods 2024, 13, 2213. [Google Scholar] [CrossRef]
- Aktaş, H.M. Bacteriocin characterization of Enterococcus faecium isolates and evaluation of their in situ anti-Listerial activity in Beyaz cheese. Food Biosci. 2024, 61, 104741. [Google Scholar]
- Todorov, D.S.; Dicks, M.L. Bacteriocin production by Lactobacillus pentosus ST712BZ isolated from boza. Braz. J. Microbiol. 2007, 38, 166–172. [Google Scholar] [CrossRef]
- Qiao, Z.; Sun, H.; Zhou, Q.; Yi, L.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lu, X. Characterization and antibacterial action mode of bacteriocin BMP32r and its application as antimicrobial agent for the therapy of multidrug-resistant bacterial infection. Int. J. Biol. Macromol. 2020, 164, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.P.M.; Bizani, D.; Cladera-Olivera, F.; Brandelli, A. Cerein 8A production in soybean protein using response surface methodology. Biochem. Eng. J. 2007, 35, 238–243. [Google Scholar] [CrossRef]
- Mataragas, M.; Metaxopoulos, J.; Galiotou, M.; Drosinos, E.H. Influence of pH and temperature on growth and bacteriocin production by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442. Meat Sci. 2003, 64, 265–271. [Google Scholar] [CrossRef]
- Gautam, N.; Sharma, N.; Kumar, S.; Sharma, N. Optimization of Growth conditions for enhanced bacteriocin production from Lactobacillus brevis UN by one variable at a time (OVAT) and response surface methodology (RSM). Indian J. Ecol. 2022, 49, 2167–2173. [Google Scholar]
- Omachi, H.; Terahara, T.; Futami, K.; Kawato, S.; Imada, C.; Kamei, K.; Waku, T.; Kondo, A.; Naganuma, T.; Kobayashi, T. Distribution of class IId bacteriocin-producing Virgibacillus salexigens in various environments. World J. Microbiol. Biotechnol. 2021, 37, 121. [Google Scholar] [CrossRef]
- Samani, M.K.; Noormohammadi, Z.; Fazeli, M.R.; Samadi, N. Bacteriocin activity of various iranian honey-associated bacteria and development of a simple medium for enhanced bacteriocin activity. J. Environ. Health Sci. Eng. 2021, 19, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Ruiz, M.; Daille, L.K.; Machuca, P.; Bittner, M. Antibacterial activity of a complex bacteriocin secreted by Staphylococcus epidermidis against Porphyromonas gingivalis. Arch. Oral Biol. 2023, 152, 105730. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; Mendonça, C.M.N.; Vieira, V.B.; Vieira, V.B.; Robl, D.; Franco, B.D.G.D.; Todorov, S.D.; Tome, E.; O’Connor, P.M.; Converti, A.; et al. Pediocin PA-1 production by Pediococcus pentosaceus ET34 using non-detoxified hemicellulose hydrolysate obtained from hydrothermal pretreatment of sugarcane bagasse. Bioresour. Technol. 2021, 338, 125565. [Google Scholar] [CrossRef]
- Garsa, A.K.; Kumariya, R.; Kumar, A.; Lather, P.; Kapila, S.; Sood, S.K. Industrial cheese whey utilization for enhanced production of purified pediocin PA-1. LWT-Food Sci. Technol. 2014, 59, 656–665. [Google Scholar] [CrossRef]
- Turgis, M.; Vu, K.D.; Millette, M.; Dupont, C.; Lacroix, M. Influence of environmental factors on bacteriocin production by human isolates of Lactococcus lactis MM19 and Pediococcus acidilactici MM33. Probiotics Antimicrob. Proteins 2016, 8, 53–59. [Google Scholar] [CrossRef]
- El-Sharoud, W.M.; Zalma, S.A.; Yousef, A.E. Inducing the production of the bacteriocin paenibacillin by Paenibacillus polymyxa through application of environmental stresses with relevance to milk bio-preservation. Int. J. Food Microbiol. 2022, 371, 109637. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiu, Z.; Tian, F.; Yu, L.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Pediococcus acidilactici strains improve constipation symptoms and regulate intestinal flora in mice. Front. Cell. Infect. Microbiol. 2021, 11, 655258. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Dong, S.; Zhang, Y.; Ismael, M.; Wang, S.; Shi, C.; Yang, J.; Wang, X.; Lü, X. Interaction of Companilactobacillus crustorum MN047-derived bacteriocins with gut microbiota. Food Chem. 2022, 396, 133730. [Google Scholar] [CrossRef] [PubMed]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Ramanan, R.N.; Kadkhodaei, S.; Mustafa, S.; Ariff, A.B. Kinetic modeling of bacteriocin-like inhibitory substance secretion by Pediococcus acidilactici Kp10 and its stability in food manufacturing conditions. J. Food Sci. Technol. 2018, 55, 1270–1284. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial activity of pediocin and pediocin-producing bacteria against Listeria monocytogenes in meat products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Wachsman, M.; Tome, E.; Vaz-Velho, M.; Ivanova, I.V. Plasmid-associated bacteriocin produced by Pediococcus pentosaceus isolated from smoked salmon: Partial characterization and some aspects of his mode of action. Probiotics Antimicrob. Proteins 2024, 16, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Thu, N.P.A.; Nghia, N.H.; Thao, D.T.P.; Trinh, N.T.M. Heterologous expression of pediocin PA-1 in Pichia pastoris: Cloning, expression, characterization, and application in pork bologna preservation. Braz. J. Microbiol. 2024, 55, 2169–2177. [Google Scholar] [CrossRef]
- Fimland, G.; Johnsen, L.; Axelsson, L.; Brurberg, M.B.; Nes, I.F.; Eijsink, V.G.; Nissen-Meyer, J. A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J. Bacteriol. 2000, 182, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Qiu, Z.; Tian, F.; Yu, L.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice. Food Sci. Hum. Wellness 2022, 11, 238–246. [Google Scholar] [CrossRef]
- Gravesen, A.; Ramnath, M.; Rechinger, K.B.; Andersen, N.; Jänsch, L.; Héchard, Y.; Knøchel, S. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology 2002, 148, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ju, X.; Du, L.; Wang, L.; He, R.; Chen, Z. The Man-PTS subunit IIC is responsible for the sensitivity of Listeria monocytogenes to durancin GL. Food Sci. Nutr. 2020, 8, 150–161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).