Abstract
The cosmetics industry has been seeking to develop products with renewable natural ingredients to reduce the use of or even replace synthetic substances. Biosurfactants can help meet this demand. These natural compounds are renewable, biodegradable, and non-toxic or have low toxicity, offering minimal risk to humans and the environment, which has attracted the interest of an emerging consumer market and, consequently, the cosmetics industry. The aim of the present study was to produce a biosurfactant from the yeast Starmerella bombicola ATCC 22214 cultivated in a mineral medium containing 10% soybean oil and 5% glucose. The biosurfactant reduced the surface tension of water from 72.0 ± 0.1 mN/m to 33.0 ± 0.3 mN/m after eight days of fermentation. The yield was 53.35 ± 0.39 g/L and the critical micelle concentration was 1000 mg/L. The biosurfactant proved to be a good emulsifier of oils used in cosmetic formulations, with emulsification indices ranging from 45.90 ± 1.69% to 68.50 ± 1.10%. The hydrophilic–lipophilic balance index demonstrated the wetting capacity of the biosurfactant and its tendency to form oil-in-water (O/W) emulsions, with 50.0 ± 0.20% foaming capacity. The biosurfactant did not exhibit cytotoxicity in the MTT assay or irritant potential. Additionally, an antioxidant activity of 58.25 ± 0.32% was observed at a concentration of 40 mg/mL. The compound also exhibited antimicrobial activity against various pathogenic microorganisms. The characterisation of the biosurfactant using magnetic nuclear resonance and Fourier transform infrared spectroscopy revealed that the biomolecule is a glycolipid with an anionic nature. The results demonstrate that biosurfactant produced in this work has potential as an active biotechnological ingredient for innovative, eco-friendly cosmetic formulations.
1. Introduction
The cosmetics sector experienced significant growth in the 20th Century, especially in developed nations. The expansion of means of communication contributed significantly to the promotion and marketing of these products, in addition to driving technological innovations. By the end of the century, the cosmetics industry had established itself as a considerably important segment of the economic landscape [1].
New technologies and concerns emerged with the advance of the cosmetics market. The sector began to invest in improving raw materials, equipment, packaging, and formulation methods, such as microemulsions, liposomes, nanotechnology, biotechnology, and sustainable practices. Attention to the quality, safety, stability, and efficacy of products also intensified, which are fundamental aspects for innovation in the sector [1,2].
Large cosmetics companies can produce as much as ten thousand different cosmetic products, with as much as 30% of these products reformulated every year. Approximately 10% of reformulated products employ novel active ingredients, with around 80 new ingredients introduced into the product portfolio of these companies annually [3,4]. Moreover, the growing concern for sustainability has led industries to prioritise practices directed at the preservation of natural resources and the assessment of the environmental impacts of cosmetics. Thus, sustainable cosmetics—also known as green cosmetics—have been gaining ground, reflecting the consumer trend towards natural organic products [1,5].
Shampoos, which are the most widely consumed cosmetic products, contain surfactants with emulsifying properties and other additives that have benefits for the skin, while also making the consistency and look of the product appealing to consumers [6].
Surfactants have hydrophobic and hydrophilic portions that preferentially partition between fluid phases with different degrees of polarity, forming an ordered molecular film that results from the reduction in surface/interfacial tension [7]. Hydrocarbon chains or aglycones generally compose the hydrophobic portion, whereas a hydroxyl, ester, phosphate, sugar, or carboxyl group generally composes the hydrophilic portion [8,9].
The main properties of surfactants include the ability to form foams, emulsions, and suspensions as well as to provide surface detergency, wetting, and the formation of liquid films. These properties give surfactants a range of applications that go beyond the cleaning and cosmetics industries, with uses also in the petrochemical/oil, textile, agricultural, paint, pharmaceutical, leather, and paper industries [10].
An effective surfactant has the ability to lower surface and interfacial tensions, thus enabling greater interaction between molecules of different polarities [9]. The lowest concentration of a surfactant required to achieve the lowest surface tension is known as the critical micelle concentration (CMC). When the CMC is reached, the amphipathic molecules are aggregated, with the hydrophilic portions positioned towards the external part of the molecule and the hydrophobic portions positioned towards the internal part [11], and no additional amount of surfactant will further reduce the surface tension. Thus, surfactants with a low CMC are preferable over those with a higher CMC [12].
Most commercially available surfactants are not biodegradable and are toxic to both humans and the environment [13]. Chemical surfactants are derived from by-products of the petroleum industry and account for the vast majority of surfactants on the market [14]. As these surfactants are produced on a large scale, the prices are much more competitive. However, synthetic surfactants are toxic, and degradation takes a long time [15].
Synthetic surfactants of sulphate origin, such as sodium lauryl sulphate, sodium lauryl ether sulphate, and ammonium lauryl sulphate, are among the most widely used in shampoo formulations. With the spread of information that “sulphated” products can be harmful to health, consumers have been searching for formulations without these surfactants, and it is currently possible to find the term sulphate-free on some labels, referring to the absence of sulphated surfactants in the composition [16].
Therefore, the search for natural surfactants as an alternative to synthetic compounds is necessary, as natural surfactants have a high rate of biodegradability, low toxicity, environmental compatibility, and stability in a broad range of temperatures, pH values, and salt concentrations. The increase in environmental concerns among consumers, combined with new environmental legislation, are other factors that encourage the development of products with natural components, such as green surfactants [9].
A diversity of living organisms is able to synthesise green surfactants, such as microorganisms and plants, as well as bile salts produced by animals [14]. Microorganisms, in particular, are able to produce surfactants with a variety of molecular structures and surfactant activities when grown on different substrates that serve as carbon sources, such as hydrocarbons, carbohydrates, oils, and fats. These green surfactants are better known scientifically as biosurfactants and are some of the most promising biomolecules of this century [10,17].
Traditional surfactants pose significant environmental and health concerns due to their toxicity, persistence in ecosystems, and potential to cause skin and eye irritation or allergic reactions in humans. In contrast, biosurfactants offer a safer and more sustainable alternative. These characteristics make biosurfactants especially attractive for use in personal care, pharmaceutical, and environmental applications, where minimising ecological impact and ensuring consumer safety are increasingly critical priorities [9,13,14,15,16,17].
Lifetime CO2 emissions would be reduced by 8% if synthetic surfactants were replaced with biosurfactant, avoiding the release of approximately 1.5 million tons of CO2 into the atmosphere. Biosurfactants currently account for approximately one-tenth of the global production of surfactants which corresponds to roughly 10,000,000 tons every year [9,18]. Investments in research applied to these biomolecules have a good chance of being applicable in innovative cosmetic reformulations that ensure greater safety [19].
The wetting, foaming, solubilising, and dispersing properties of biosurfactants are essential to cosmetics. Foaming is considered a desirable property in shaving creams, soaps, and shampoos. Wetting ability facilitates the penetration of water-in-oil creams into skin. Solubilising and dispersing enable the incorporation of pigments into nail polish and hair dyes [20].
The classification of surfactants of a microbial origin is performed based on chemical composition and the type of producing microorganism. The largest classes are lipopeptides, glycolipids, particulate surfactants, fatty acids, polymeric surfactants, and phospholipids [10]. Vecino et al. [21] state that microbial biosurfactants exhibit surface properties suitable for cosmetic applications, especially when combined with their biological activities. The development of formulations using biosurfactants as active ingredients is a promising possibility and investment in research for the development of these biomolecules can result in innovative, safe formulations in the market [22].
Glycolipids are promising for use in cosmetic formulations, offering the advantages of high selectivity, low toxicity, ecological acceptance, and high biodegradability. Sophorolipids are produced by the yeast Candida (Starmerella) bombicola and have no cytotoxic effects. This type of glycolipid has received approval from the US Food and Drug Administration for use in different industries [23,24,25]. The hydrophilic portion of sophorolipids is formed by a sophorose molecule linked to a long chain of fatty acids (hydrophobic portion) through a β-glycosidic bond. Sophorose is a glucose disaccharide with an unusual β-1,2 bond that can be acetylated at the 6′ and/or 6′′ positions. The fatty acid chain generally contains 16 or 18 carbon atoms, such that the carboxylic extremity of the fatty acid can be free (acidic form, which is anionic under alkaline conditions) or internally esterified in the 4′′ position or 6′/6′′ position (lactone form, which is neutral) (Figure 1) [25,26].

Figure 1.
Molecular representation of two forms of sopholipids: (A) lactone form; (B) acidic form.
Sophorolipids have been used as active ingredients in personal care products, mainly as detergents, foaming agents, wetting agents, stabilisers, and emulsifiers [27]. Sophorolipids have low cytotoxicity to human fibroblasts and keratinocytes and can enhance collagen synthesis in the dermis of the skin, thus serving as an anti-ageing agent [28].
The use of sustainable ingredients, which is increasingly valued in the international market, can constitute a prosperous investment for the cosmetics industry. Thus, the aim of the present study was to produce a biosurfactant with emulsifying properties for application in green formulations. The methodologies employed were designed to demonstrate the suitability of the biosurfactant for the cosmetic industry.
2. Materials and Methods
2.1. Production of Biosurfactant
The yeast Starmerella bombicola ATCC 22214 was tested as a biosurfactant producer and was maintained in a yeast mould agar (YMA) medium with the following composition: yeast extract (0.3%), peptone (0.5%), D-glucose (1%), agar (2%), and distilled water q.s.p. (100 mL). For sterilisation, the solubilised components were placed in an autoclave for 20 min at 121 °C. The growth medium was yeast mould broth (YMB) (exclusion of agar). The culture was transferred to a tube containing YMB medium to obtain a young culture and standardise the inoculum, followed by transference of the sample was to flasks containing 250 mL of YMB and incubation under orbital stirring at 200 rpm and 28 °C for 48 h. Dilutions were then obtained, achieving a final cell concentration of 10% (v/v).
For biosurfactant production, the yeast was placed in a medium consisting of 10% soybean oil, 5% glucose, 0.5% yeast extract, 0.1% KH2PO4, 0.07% peptone, 0.05% MgSO4·7H2O, and 0.01% sodium chloride. Production was performed in 2 L Erlenmeyer flasks with orbital stirring at 200 rpm and 28 °C for a period of eight days (192 h).
2.2. Isolation of Biosurfactant
The biosurfactant contained in the metabolic broth obtained after fermentation was extracted twice with ethyl acetate at a ratio of 1:2 (v/v). The extract collected in the solvent was centrifuged for 15 min at 4500 rpm to remove the microorganism and filtered through Whatman No. 1 paper and set to dry on a hot plate in the exhaust hood. After drying, 80% ethyl alcohol was added for dissolution and the solution was transferred to a separatory funnel. Hexane was added at a ratio of 1:3 (v/v) and the contents were shaken vigorously. The alcoholic portion was collected and placed to evaporate in a laboratory oven at 60 °C until dry [29].
2.3. Determination of Surface Tension and Critical Micelle Concentration of Biosurfactant
A Sigma 700 tensiometer (KSV Ltd., Helsinki, Finland) and du Noüy ring were used to determine surface tension. For such, the platinum ring was immersed in the liquid and the force needed to pull the ring through the liquid–air interface was recorded.
2.4. Determination of Ionic Charge of Biosurfactant
The modified double diffusion in agar method was used to determine the ionic charge of the biosurfactant [30]. Two test tubes were prepared with low-viscosity agar (1% solution). The solution of the isolated biosurfactant was added to one of the tubes, whereas the second tube contained a pure compound whose ionic charge was known. Sodium dodecyl sulphate (SDS) (concentration: 0.02 M) was used as the anionic substance and barium chloride (concentration: 0.05 M) was the cationic substance. Monitoring was performed at room temperature for 48 h to observe the emergence of precipitation, indicating the anionic or cationic nature of the biosurfactant.
2.5. Determination of Emulsification Activity of Biosurfactant
To determine emulsification activity, a solution of the isolated biosurfactant was prepared at its previously determined CMC (1 g/L) and analysed following the method described by Cooper and Goldenberg [31]. An oily substrate (3.0 mL of grape seed oil, coconut oil, almond oil, neem oil, and avocado oil), was placed in a graduated tube with the biosurfactant solution (3.0 mL). The tube was vortexed for two minutes. After 24 h, the height of the emulsion was divided by the total height of the mixture and the quotient as multiplied by 100 to provide the percentage of the emulsion formed in the tube, thus determining the emulsification index.
2.6. Determination of Hydrophilic–Lipophilic Balance of Biosurfactant
The hydrophilic–lipophilic balance (HLB) was determined for the biosurfactant through the rapid determination of emulsion stability [32]. The oil phase was mineral oil, whose HBL was 10.5. Tween 20 (HLB: 16.7) and Tween 80 (HLB: 15.0) were the co-emulsifiers used along with the biosurfactant. After three to five min of centrifugation at 10,000 rpm, the emulsions formed using different mixtures of emulsifiers were analysed for stability. Griffin’s equation (Equation (1)) was used to determine the HLB value of the biosurfactant based on the most stable emulsions:
in which W is the weight (quantity) of the emulsifier and the HLB is the value for the emulsifier, where the HLB of the mineral oil is the value required for the type of emulsion desired. Water solubility and the HLB are classified as follows: 1–4 = no ability to disperse in water; 3–6 = little dispersibility in water; 6–8 = unstable milky dispersion after emulsification; 8–10 = stable milky dispersion; 10–13 = translucent to clear solution; and >13 = clear solution. The application of surfactants according to the HLB range is classified as follows: 4–6 = water-in-oil (W/O) emulsifiers; 7–9 = wetting agents; 8–18 = oil-in-water (O/W) emulsifiers; 13–15 = detergents; and 10–18 = solubilizers.
HLB of mineral oil = (WTween × HLBTween + Wbiosurfactante × HLBbiosurfactant)/(WTween + Wbiosurfactant)
2.7. Foam Formation and Dirt Dispersion Capacity of Biosurfactant
To determine the foaming capacity of the biosurfactant, 50 mL of the biosurfactant solution at the CMC (1 g/L) was placed in a 250 mL graduated cylinder, which was capped for subsequent shaking 10 times at one-minute intervals. The volumes of foam after one minute of shaking was recorded. Foam height was then divided by total height of the solution and the quotient was multiplied by 100 to provide the percentage of foam formed in the tube [33].
To determine the dispersion capacity of the biosurfactant, a drop of India ink was added to a test tube containing 10 mL of 1% biosurfactant solution. The tube was then capped and shaken manually ten times. The anionic surfactant sodium cocoyl isethionate was used for comparison purposes. The categorisation of the amount of ink in the foam was as follows: none, light, moderate, or heavy [33].
2.8. Structural Characterisation of Biosurfactant
After dissolving the isolated biosurfactant in methanol, FTIR analysis was performed in a Bruker IFS66 spectrometer, Karlsruhe, Germany) considering the region from 4000 to 400 cm−1, with precision in the wavenumber range from −0.1 to +0.1 cm−1 [34].
Nuclear magnetic resonance (NMR) was used for the chemical characterisation of the isolated biosurfactant dissolved in deuterated chloroform (CDCl3). A Bruker AVANCE III 400 NMR spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) operating at 9.4 T was used to acquire NMR data at 298 K in DMSO-d6 in, observing 1H and 13C at 400 and 100 MHz, respectively. The spectrometer had a 5 mm direct detection probe with a z-gradient. All 1H and 13C NMR chemical shifts (δ) were given in ppm related to the TMS signal at 0.00 as the internal reference. The spectra were processed in the TOPSPIN software 4.5.0 version.
2.9. Determination of Antimicrobial Activity of Biosurfactant
A 96-well microplate (flat bottom) was used for the determination of the minimum inhibitory concentration (MIC), employing the microdilution test. The biosurfactant was diluted to a concentration of 100 μg/mL in sterile distilled water. The test microorganisms were Streptococcus mutans ATCC 25175, Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATCC 25922, and Candida albicans ATCC 1106. All microorganisms were inoculated (5 mL of brain heart infusion broth for bacteria and Sabouraud broth for the yeast) for 18 h. Spectrophotometry was performed for the standardisation of the inoculum and preparation of the pre-inoculum, with readings at 530 nm in the range of 0.140 to 0.150, corresponding to a suspension of approximately 1.5 × 108 colony-forming units/mL [24].
Microplates with different proportions of the test substance and culture medium were prepared to obtain different concentrations of the test products (0 to 60 μg/mL for the biosurfactant) and determine the minimum inhibitory concentration (MIC). Each well had a final volume of 100 μL. An amount of 80 μL of the medium was added for the control of microbial viability and 100 μL was used for the control of sterility. With the exception of the sterility control well, all wells then received 20 μL of the microbial inoculum. The test was conducted in triplicate. After the preparation of the wells (biosurfactant, culture medium, and microbial inoculum), the microorganisms were incubated for 24 h at 37 °C for the cultivation of the bacteria and for 48 h at 28 °C for the cultivation of the yeast. After the incubation period, 30 μL of resazurin were added to the wells to determine cell viability of the microorganisms. One hour after the addition of resazurin, the colour reading was performed. A blue–violet colour indicated the absence of microbial growth, whereas pink–red variations indicated the presence of viable cells [24].
2.10. Eye Irritation Potential of Biosurfactant
The HET-CAM test was performed to determine the eye irritation potential of the biosurfactant at a concentration of 1.0% in the chorioallantoic membrane (CAM) of chicken eggs, as described by Wilson and Steck [35]. A sodium lauryl sulphate (SLS) solution (concentration: 1.0%) dissolved in phosphate–buffered saline (PBS) was the positive control and PBS alone was the negative control. The volume was 0.2 mL. Changes in the membrane were observed for 300 s and the time of onset of any lesions was recorded. Irritation was indicated by the occurrence of haemorrhage, vascular lysis, and coagulation (intra- and extravascular protein denaturation), as described by the Interagency Coordinating Committee on the Validation of Alternative Methods [36]. The irritation index (II) was calculated using Equation (2), in which H corresponds to the time, in seconds, required for haemorrhage to begin, L corresponds to the time for lysis to occur, and C corresponds to the time of the onset of coagulation [37].
The analyses were repeated three times. The irritation potential was defined as follows:
0.0–0.9 = non-irritant
1.0–4.9 = mildly irritant
5.0–8.9 = moderately irritant
9.0–21.0 = irritant
2.11. Assessment of 2,2-Diphenyl-1-picrylhydrazyl Radical Sequestering Activity of Biosurfactant
The free radical scavenging method was used to determine the antioxidant activity of the biosurfactant [38]. A stock solution of methanolic 2,2-diphenyl-1-picrylhydrazyl (DPPH) (200 mM) was further diluted in methanol until it reached UV–VIS absorbance between 0.6 and 0.7 at 517 nm. Solutions (40 mL) of the biosurfactant at concentrations of 1.25, 2.5, 5.0, 10.0, 20.0, and 40.0 mg/mL were mixed with a 250 mL solution of DPPH. After incubation for 30 min in the absence of light, absorbance was read at the wavelength described above. Inhibition activity was calculated from measurements made in triplicate based on the percentage of DPPH eliminated (percentage of inhibition [I%]) calculated using the following equation:
in which Abs0 is the control absorbance and Abs1 is absorbance in the presence of the biosurfactant. The calibration curve was established using a standard solution of Trolox (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid) (synthetic antioxidant) at concentrations of 10 to 200 mM.
I% = [(Abs0 − Abs1)/Abs0]100
2.12. Assessment of Cytotoxicity of Biosurfactant
Cytotoxicity of the biosurfactant was assessed using the colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The L929 (mouse fibroblast) cell line was maintained in Dulbecco’s modified Eagle’s medium. Supplementation of all media was performed with foetal bovine serum (10%) and a 1% solution of penicillin/streptomycin solution. The cells were subjected to a CO2-enriched humid atmosphere (5%) and kept at 37 °C [39,40]. Cell suspensions with a cell density of 2 × 105 cells/mL were plated in 96-well plates and incubated for 24 h, followed by the addition of 100 µL of the biosurfactant solution to the wells at concentrations of 1000 mg/L (CMC) and 2000 mg/L (twice the CMC). Twenty-five µL of MTT (5 mg/mL) were added after 72 h, followed by incubation for three hours. The culture media with MTT were aspirated, followed by the addition of 100 µL of dimethyl sulfoxide to each well. Absorbance was read at 560 nm in a microplate reader (DR-200BN-BI, Kasuaki, Campinas, Brazil). Mean cell viability was determined from experiments performed in triplicate.
2.13. Statistical Analysis
All tests were conducted in triplicate, with the determination of mean and standard deviation values. Analysis of variance (ANOVA) was employed for the statistical analysis. All p-values less than 0.05 indicated statistical significance.
3. Results and Discussion
3.1. Biosurfactant Production
Despite being a widely explored topic in recent decades, the production of biosurfactants for industrial use is affected by multiple factors, such as the producing strain, cultivation conditions, combination of substrates, production methods, and even the equipment used. Thus, obtaining satisfactory results that combine efficiency (low surface tension and CMC) and economic viability, which is achieved by obtaining a high production yield, remains a challenge.
In the present study, a biosurfactant was produced by S. bombicola ATCC 22214 for use as an active ingredient in a green cosmetic formulation. The biosurfactant had a liquid appearance with little viscosity, dark brown colour, and a characteristic odour, lowering the surface tension of water from 72.0 ± 0.1 mN/m to 33.0± 0.3 mN/m after eight days of fermentation. The yield was 53.35 ± 0.39 g/L.
Biosurfactants can be considered effective when exhibiting the ability to lower the surface tension of water from 72 to ≤35 mN/m [41]. As biosurfactant production was performed in flasks, the surface tension and yield obtained were satisfactory in comparison to data in previous studies described for yeast-produced surfactants, as will be discussed below.
A biosurfactant produced by the same yeast in a medium composed of glucose and oleic acid obtained in a 1 L fermenter lowered surface tension to 31.56 ± 1.0 mN/m, with a yield of 10 ± 0.5 g/L [42]. In the study conducted by Silva et al. [43], S. bombicola ATCC 22214 grown in flasks in a low-cost medium containing canola vegetable oil, corn starch, and sucrose produced a biosurfactant that reduced the surface tension to 32.76 N/m, the yield of which was 23.0 g/L. The biosurfactant also produced by S. bombicola ATCC 22214 in a medium containing olive oil and glucose lowered the surface tension to 32.30 mN/m [29]. Shah et al. [44] used S. bombicola ATCC 22214 for the production of sophorolipids using different hydrophobic substrates. The yields obtained using Tapis oils, Melita oil, and Ratawi oil were 26, 21, and 19 g/L, respectively, with surface tension lowered to 36.38, 37.84, and 38.92 mN/m, respectively. The biosurfactant produced by S. bombicola in a corncob hydrolysate medium in a fermenter yielded 49.2 g/L [45]. Jadhav et al. [46] produced 41.6 g/L of a sophorolipid in flasks using S. bombicola grown in residual acid sunflower oil obtained from a vegetable oil refinery and glucose. The biosurfactant was able to reduce the surface tension to 35.5 mN/m after fermentation.
A biosurfactant produced by the yeast Candida lipolytica UCP 0988 grown in a medium containing sugarcane molasses, corn steep liquor, and soybean oil fry waste lowered the surface tension to 25 mN/m, with a yield of 12 g/L [47]. The yield of a biosurfactant from C. utilis UFPEDA1009 grown in flasks in a medium supplemented with canola oil fry waste and glucose was 24.22 g/L [48]. A biosurfactant from Saccharomyces cerevisiae URM 6670 grown in a medium containing soybean oil fry waste and corn steep liquor lowered the surface tension of the medium to 26 mN/m, although the yield was only 5.8 g/L [49]. C. mogii UFPEDA 3968 was also used for the production of a biosurfactant in a medium containing licuri oil and glucose, lowering the surface tension from 71.04 mN/m to 28.66 mN/m [50]. Gaur et al. [51] investigated the growth of C. albicans and C. glabrata in media composed of glucose and a nitrogenous yeast base in distilled water, respectively, lowering the surface tension from 72 mN/m to 42 mN/m and 55 mN/m. The biosurfactant yield was 1.320 g/L for C. albicans and 1.6 g/L for C. glabrata.
The reduction in surface tension is dependent on the surfactant concentration. The CMC is defined as the lowest concentration of surfactant able to achieve the maximum reduction in the surface tension of water, initiating the formation of micelles, which are necessary for emulsification. Thus, an efficient surfactant will have a low CMC [9]. The CMC of the isolated biosurfactant from S. bombocola ATCC 22214 was 1000 mg/L, achieving a surface tension of 33.0 ± 0.5 mN/m, as shown in the graph illustrated in Figure 2.
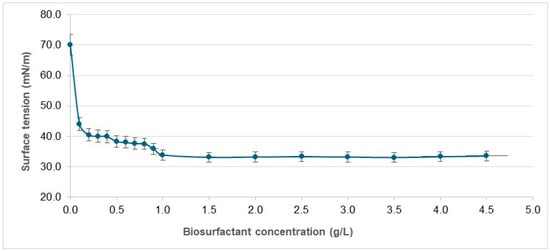
Figure 2.
Critical micelle concentration of biosurfactant produced by Starmerella bombicola ATCC 22214 in mineral medium containing canola oil and glucose.
Other biosurfactants produced by the same yeast in media composed of hydrophobic and hydrophilic carbon sources had CMCs of 366 mg/L [42] and 600 mg/L [43], respectively. However, biosurfactants produced by S. bombicola ATCC 22214 using Tapis, Melita, and Ratawi oils had much lower CMCs (54.39, 55.68, and 58.34 mg/L, respectively) [44]. Such differences may be related to the purification levels obtained, among other factors. Biosurfactants produced by other yeasts of the genus Candida have similar CMCs, such as 500 mg/L for the biosurfactant produced by C. lipolytica UCP 0988 [46] and 800 mg/L for the biosurfactant produced by C. mogii UFPEDA 3968 [50].
Studies have demonstrated that sophorolipids possess surface-active properties comparable to or superior to those of synthetic surfactants, including low critical micelle concentration (CMC), making them effective at lower doses [26,27]. Synthetic surfactants, such as sodium lauryl sulphate (SLS), sodium laureth sulphate (SLES), and linear alkylbenzene sulfonates (LASs), are known to exhibit moderate to high aquatic toxicity, poor biodegradability under anaerobic conditions, and a potential for skin irritation and disruption of skin barrier function [52,53,54,55]. Moreover, synthetic surfactants, although cost-effective and widely used, are often associated with ecological risks due to their resistance to biodegradation and potential to bioaccumulate, which can lead to aquatic toxicity and endocrine disruption [54,55,56]. Thus, biosurfactants from S. bombicola not only offer functional equivalence to synthetic surfactants but also align with growing regulatory and consumer demands for green and health-conscious ingredients.
3.2. Emulsification, Foaming, and Dirt Dispersing Capacities and Hydrophilic–Lipophilic Balance (HLB) of Biosurfactant
To be a good emulsifying agent, a biosurfactant must have the ability to form stable emulsions (above 50%) for at least 24 h [57]. The results of the emulsification index test after 24 h (displayed in Table 1) show that the biosurfactant exhibited greater affinity for the neem and coconut oils, as illustrated in Figure 3, although the percentages found for other vegetable oils commonly used in cosmetic formulations were also within the satisfactory emulsification range.

Table 1.
Emulsification of hydrophobic substrates by biosurfactant produced by Starmerella bombicola ATCC 22214 in mineral medium containing soybean oil and glucose. Results expressed as means ± SD (n = 3); p < 0.05 indicative of significant difference.

Figure 3.
Emulsification of coconut oil (a) and neem oil (b) by biosurfactant produced from S. bombicola ATCC 22214.
The hydrophilic–lipophilic balance (HLB) is used to determine what emulsifier works best with the oil phase of an emulsified product. All emulsifiers have a hydrophilic head and a lipophilic tail, as shown above. The ratio between these portions in a surfactant molecule in terms of the weight percentages is an indication of the behaviour that can be expected from a product. A more lipophilic emulsifier has a low HLB value, and a more hydrophilic emulsifier receives a high value [32].
In contact with water, the biosurfactant from S. bombicola ATCC 22214 exhibited stable milky dispersion, characterising an HLB in the range of 8–10, which is typical of biosurfactants with humectant (wetting) capacity and ability to form oil-in-water (O/W) emulsions (Figure 4). In cosmetic applications, lipophilic biosurfactants (lower HLB values) range between 1 and 4 and are preferable due to the nature of the skin, which contains a lipid film and favours oil-soluble active ingredients. However, consumers prefer oil-in-water (O/W) emulsions with HLB values between 8 and 16 due to the less oily effect and higher absorption rate. Furthermore, O/W cosmetic emulsions are generally found in semi-solid or liquid formulations [28].

Figure 4.
Appearance of dispersion of biosurfactant produced by Starmerella bombicola ATCC 22214 in water.
Although foam generation has little effect on the cleansing capacity of shampoos, this feature is important to consumers and therefore an aspect to consider when assessing cosmetic formulations. The biosurfactant from S. bombicola ATCC 22214 exhibited foaming capacity of 50.0 ± 0.20%.
Surfactants with detergency characteristics should not enable dirt to concentrate in the foam; dirt should remain in the water [58]. However, surfactants with wetting characteristics do not have this capacity, as observed for the biosurfactant from S. bombicola ATCC 22214, which had a large amount of ink in its foam, demonstrating its wetting nature, as observed for the HLB value. The surfactant sodium cocoyl isethionate used for comparison purposes was able to disperse a greater amount of ink, as observed by the concentration of ink on the wall of the tube, demonstrating its detergency capacity, which was compatible with its HLB (~16) (Figure 5).

Figure 5.
Dispersion of India ink by foam of sodium cocoyl isethionate (a) and foam of biosurfactant produced by Starmerella bombicola ATCC 22214 (b).
3.3. Characterisation of Biosurfactant
Ionic behaviour is an important factor of biosurfactants in cosmetics. According to the polar charge, most biosurfactants are anionic, such as sophorolipids from S. bombicola [26]. Anionic surfactants are more effective in terms of foaming, wetting, and emulsification, but are also more irritating to the skin and eyes, followed by non-ionic and amphoteric surfactants. However, such effects can be eliminated by using lower concentrations of these compounds to ensure compatibility with mucous membranes and skin while maintaining effectiveness [28]. The ionic charge test revealed precipitation when in contact with barium chloride (cationic compound). In contrast, no precipitate was formed in contact with SDS (anionic compound), demonstrating the anionic nature of the biomolecule (Figure 6).
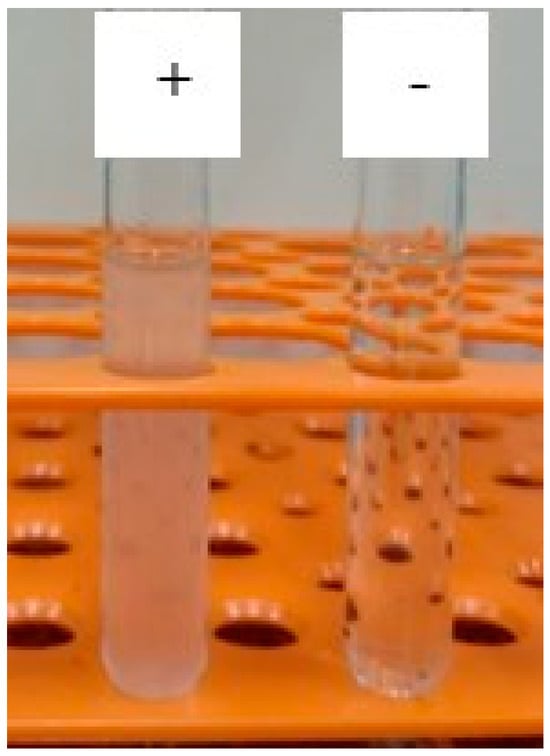
Figure 6.
Interaction between biosurfactant produced by Starmerella bombicola ATCC 22214 and barium chloride (+) and sodium dodecyl sulphate (−). Biosurfactant reacted with barium chloride, indicating anionic nature.
The purified, isolated biosurfactant was subjected to Fourier transform infrared (FTIR) and NMR analyses (Figure 7, Figure 8 and Figure 9). In the FTIR spectrum, C–H stretching characteristic of alkanes was evident by the bands at 2924.18 cm−1 and 2853.81 cm−1, suggesting a hydrophobic structure. The band at 1743.60 cm−1 is characteristic of the C=O stretching common in carbonyl groups, such as esters, aldehydes, ketones, or carboxylic acids. The FTIR spectrum of the biosurfactant produced by C. mogii UFPEDA 3968 grown in licuri oil and glucose exhibited identical peaks [50]. The band at 1454.91 cm−1 may be related to C–H deformation vibrations in alkanes. The peak at 1160.48 cm−1 may indicate the presence of C–O stretching in ethers, alcohols, or esters. The band at 720.99 cm−1 may be associated with deformation vibrations of long alkane chains. The literature shows a spectra of glycolipids with typical absorption bands of aliphatic carbon and carbonyl in the same regions, corresponding to the fatty acid portion of the biosurfactant [59].
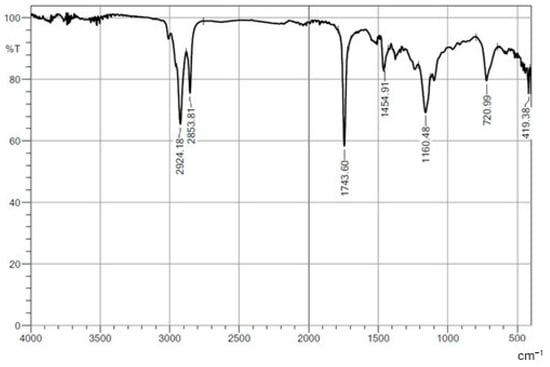
Figure 7.
FTIR spectrum of biosurfactant produced by Starmerella bombicola ATCC 22214.
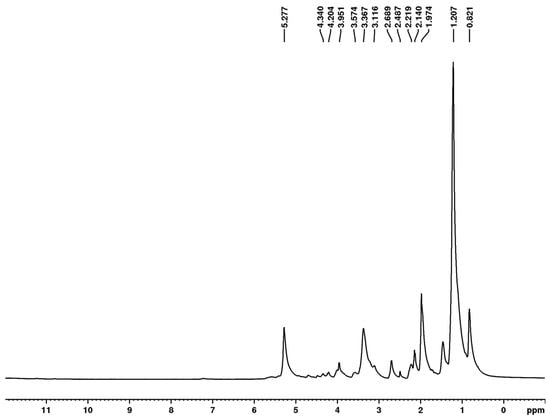
Figure 8.
1H NMR spectrum of biosurfactant produced by Starmerella bombicola ATCC 22214 (DMSO-d6, 400 MHz).
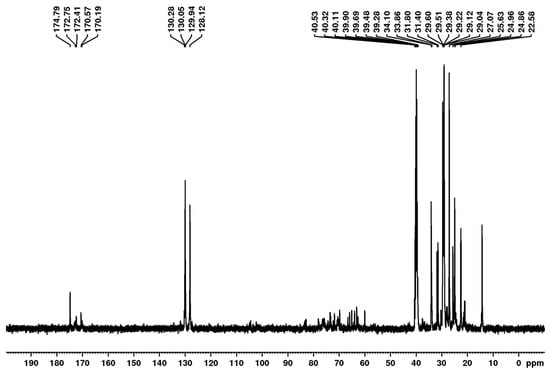
Figure 9.
13C NMR spectrum of biosurfactant produced by Starmerella bombicola ATCC 22214 (DMSO-d6, 100 MHz).
The 1H NMR analysis revealed signals between 0 and 3 ppm, corresponding to the aliphatic chain in the structure of the molecule, which constitutes the nonpolar portion of the biosurfactant (Figure 8). The signals between 3 and 5 ppm suggest the hydroxyl functional group. The signal at 5.277 ppm shows hydrogen bonded to carbon containing double bonds, suggesting unsaturation in the molecule.
In the 13C NMR spectrum shown in Figure 9, the region from 0 to 50 ppm suggests the presence of long aliphatic chains, which are characteristic of fatty acids, confirming the presence of hydrocarbon chains in the structure. Peaks between 128.12 and 130.28 ppm suggest the presence of sp2 carbons, likely associated with unsaturated double bonds (C=C). The region from 150 to 200 ppm corresponds to carbons in carbonyl groups, such as ketones, esters, and carboxylic acids, as demonstrated by the signals between 170.19 and 174.79. Taken together, these results suggest that the biosurfactant from S. bombicola ATCC 22214 is a glycolipid.
3.4. Antimicrobial Activity of Biosurfactant
Biosurfactants have recognised antimicrobial activity, making these compounds potential candidates for the development of cosmetic products with hair and skin applications. The antimicrobial activity of glycolipids is related to the synergistic effect of the sugar and lipid moieties, which cause changes in or the rupture of the cell membrane, inducing lysis and extravasation of the cytoplasmic contents of microorganisms. Due to their amphiphilic characteristics, glycolipids alter the properties of the plasma membranes of pathogens, thus demonstrating germicidal characteristics against Gram-positive and Gram-negative bacteria [25,60,61]. According to Diaz de Rienzo et al. [62], an alteration in the charge–charge properties is hypothesised as the mechanism of action of biosurfactants, which may reduce the likelihood of bacteria acquiring antibiotic resistance.
While interactions between carbohydrates and bacterial membranes have been investigated for years, studies have only recently sought to demonstrate the effect of monosaccharides and disaccharides on the membrane structure, such as the disaccharide sophorose in the sophorolipid molecule, which is an effective bactericidal agent able to induce the death of planktonic cells as well as the biofilms of Gram-positive and Gram-negative bacteria [63,64].
The minimum inhibitory concentrations (MICs) of the biosurfactant from S. bombicola ATCC 22214 against typical microorganisms are displayed in Table 2. The biosurfactant was effective against all microorganisms tested. The best results were found for Streptococcus mutans and Escherichia coli.

Table 2.
Minimum inhibitory concentrations (MICs) of biosurfactant produced by Starmerella bombicola ATCC 22214 (μg/mL) for inhibition of pathogenic microorganisms.
3.5. Irritation Potential of Biosurfactant
Although the biocompatibility and low toxicity of biosurfactants make these natural compounds strong candidates for the development of novel cosmetic products, it is necessary to investigate toxicity before proposing applications in cosmetic formulations [9,10,22]. The HET-CAM test is a measure of the acute effects of a given compound through the changes in the blood vessels and proteins found in the chorioallantoic membrane of embryonic eggs with gestational age between seven and ten days [36]. The determination of the irritant potential of biosurfactants is particularly important to the pharmaceutical and cosmetic fields by enabling the prediction of possible toxic effects. The HET-CAM test is accepted as an official in vitro assay in some European countries as a fast, non-animal test for the determination of the irritant potential of a given compound [65], offering the advantages of sensitivity, simplicity, and low cost [66,67,68].
In the analysis of changes in the chorioallantoic membrane after 300 s, the positive control—1% sodium lauryl sulphate (SLS), which is widely used as a surfactant in cosmetics—caused vasoconstriction (55 s), haemorrhage (14 s), and coagulation (39 s), which were detected by the reduction in vessel diameter, bleeding, and clot formation, respectively, proving to be severely irritating (irritation potential = 18.38). Considering the irritation scale, the positive control (SLS) is classified as extremely irritating, whereas the negative control (PBS) had no irritating effect. The biosurfactant from S. bombicola ATCC 22214 was classified as non-irritating (irritation potential = 0).
Ferreira et al. [23] investigated the emulsifying capacity of a biosurfactant synthesised from a strain of Lactobacillus paracasei for application in a cosmetic formulation. The biomolecule was added to oil-in-water emulsions containing a natural antioxidant extract and essential oils, and the formulation was compared to oil-in-water emulsions containing SDS (synthetic surfactant). Cytotoxicity was determined using a mouse fibroblast cell line. The solutions with 5.0 g/L of biosurfactant exhibited cell proliferation rates higher than 97%, differing significantly from the solutions with SDS.
3.6. Antioxidant Activity of Biosurfactant
The antioxidant properties of natural compounds are of considerable interest to the food industry, emerging from a growing trend to use natural antioxidants rather than their synthetic counterparts [69,70,71,72]. Antioxidant compounds can inhibit or slow the harm caused by oxidising agents through mechanisms such as metal complexation and the inhibition of free radicals [70,71,72,73]. Antioxidant compounds are particularly used in “antiaging” products, contributing to the restoration of the skin and the prevention of signs of ageing [74]. Antioxidants can also prevent hair loss and assist in the healthy growth of hair by increasing blood circulation [75].
The prevention of the oxidation of the DPPH radical by the biosurfactant and a standard (Trolox) was determined by the reduction in this compound to hydrazine, as demonstrated by the change in colour from purple to yellow and the corresponding reduction in absorbance [76].
The results in terms of the concentration (mg/mL) required to scavenge 50% (IC50) of the radical are shown in Table 3. Considering a concentration of 1 mg/mL of the standard, the biosurfactant exhibited considerable antioxidant activity using the DPPH radical reduction method, with a maximum of 58.25 ± 0.32%, compared to 87.54 ± 0.15% for Trolox. Fan et al. [77] studied the antioxidant activity of sophorolipids produced by S. bombicola cultivated in soybean oil and oleic acid using the DPPH method. The results showed antioxidant activity of 15 to 60% with sophorolipid concentrations of 3.125 mg/mL to 50 mg/mL. Hoa et al. [78] produced sophorolipids using fish oil and investigated antioxidant activity using the same method, reporting an inhibition rate of 10 to 80% with an IC50 of 4.45 mg/mL. The biosurfactant produced by the bacterial strain Marinobacter litoralis MB15 exhibited an antioxidant activity of 72.6% at 5.0 mg/mL; the biosurfactant also exhibited excellent antimicrobial properties against several pathogens, including S. aureus, Bacillus subtilis, Escherichia coli, Streptococcus pyogenes, Klebsiella pneumonia, Vibrio parahaemolyticus, and Candida albicans [79]. The biosurfactant produced by a strain of Bacillus subtilis RW-I isolated from refinery wastewater had DPPH scavenging activity of 85.2% at a concentration of 1.0 mg/mL [80].

Table 3.
Percentage of DPPH radical sequestration (% I) of different concentrations of biosurfactant produced by Starmerella bombicola ATCC 22214. Results expressed as means ± SD (n = 3); p < 0.05 indicative of significant difference.
The relatively low antioxidant activity of the biosurfactant produced by S. bombicola under the conditions of this work, compared to other biosurfactants, can be attributed to several structural and compositional factors. First, sophorolipids are glycolipids composed mainly of a sophorose sugar head and a long-chain hydroxylated fatty acid tail, lacking functional groups typically associated with strong antioxidant properties, such as phenolic hydroxyls, conjugated double bonds, or sulphur-containing moieties. In contrast, certain lipopeptides (e.g., surfactin from Bacillus subtilis) or rhamnolipids from Pseudomonas aeruginosa may contain amino acids or unsaturated lipid chains that can donate electrons or scavenge free radicals more effectively [81].
Secondly, the antioxidant potential of a biosurfactant is often influenced by the presence of associated minor metabolites or co-produced secondary compounds during fermentation. Biosurfactants from some bacterial strains may be co-extracted with peptides, or other redox-active molecules that enhance total antioxidant capacity, whereas sophorolipid preparations are typically more chemically homogeneous and may lack such bioactive impurities [82]. Furthermore, the degree of lactonization or acetylation in sophorolipids can affect their hydrophobicity and solubility, potentially limiting their interaction with aqueous-phase free radicals in standard antioxidant assays such as DPPH [83].
Lastly, the production medium and conditions used to cultivate S. bombicola can influence the final chemical structure and purity of sophorolipids. Media optimised for yield rather than bioactivity may favour the production of specific sophorolipid congeners with limited antioxidant function. As a result, compared to structurally diverse and functionally rich biosurfactants from other microbial sources, S. bombicola sophorolipids may inherently lack the chemical features necessary for high radical scavenging activity [83,84,85].
3.7. Toxicity of Biosurfactant
The results of the MTT assay conducted with mouse fibroblasts (L929) to determine the cytotoxicity of the biosurfactant revealed cell viability exceeding 95% at both concentrations tested—around the critical micelle concentration (CMC) and double the CMC. This indicates that the biomolecule exhibited no toxicity to this cell line, thus confirming its biocompatibility with the standards required across various industrial sectors.
Other biosurfactants derived from yeasts and bacteria have also been reported as non-toxic. For instance, the biosurfactant produced by C. sphaerica UCP 0995 had no cytotoxic effects on mouse fibroblast cells [86]. Additionally, biosurfactants obtained from S. cerevisiae and C. utilis exhibited no toxicity to the non-cancerous L929 and RAW 264.7 cells [48,49]. The same was reported for the biosurfactant produced by Rhodococcus sp. 51T7 [87]. The results of the cytotoxic test revealed that biosurfactant extracts produced from Lactobacillus pentosus and Bacillus sp. at a concentration of 1 g/L were also not cytotoxic to fibroblasts (fibroblast growth > 90%) [88].
4. Conclusions
The results of the present study demonstrate the considerable potential of the biosurfactant produced by the yeast S. bombicola ATCC 22214 as an active ingredient for cosmetic applications. The physicochemical properties, such as a significant reduction in surface tension as well as emulsifying capacity, foaming activity, and wetting profile, demonstrate its functional efficacy in oil-in-water formulations. Moreover, the biodegradability, absence of toxicity, as well as antioxidant and antimicrobial activities, demonstrate the viability of this biosurfactant as a sustainable alternative to synthetic surfactants traditionally used in the cosmetics industry. Thus, the biosurfactant studied here constitutes a promising option for the development of new products aligned with current ecological trends and the growing demand for safe natural ingredients. We are currently developing an innovative green formulation containing the biosurfactant produced under the conditions of this study.
Author Contributions
All authors contributed to this work. Conceptualisation, L.A.S.; methodology, A.P.B.C., G.P.d.A., K.G.d.O.B., F.C.G.d.A., M.d.G.C.d.S., C.J.G.d.S.J., A.S. and R.d.C.F.S.d.S.; validation, K.G.d.O.B., R.d.C.F.S.d.S. and L.A.S.; formal analysis, K.G.d.O.B., F.C.G.d.A. and L.A.S.; investigation, A.P.B.C., G.P.d.A., K.G.d.O.B., F.C.G.d.A., M.d.G.C.d.S. and C.J.G.d.S.J.; resources, L.A.S.; data curation, A.S., R.d.C.F.S.d.S. and L.A.S.; writing—original draft preparation, A.P.B.C., G.P.d.A., K.G.d.O.B. and C.J.G.d.S.J.; writing—review and editing, L.A.S. and A.S.; visualisation, L.A.S.; supervision, R.d.C.F.S.d.S. and L.A.S.; project administration, L.A.S.; funding acquisition, L.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the following Brazilian fostering agencies: Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES) (Finance Code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the laboratories of the Catholic University of Pernambuco (UNICAP) and the Advanced Institute of Technology and Innovation (IATI), Brazil.
Conflicts of Interest
Author Alessandra G. Sarubbo was employed by the company Caprichar srl. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lawrence, P.; Scacchi, B.; Dew, K.; Ceccoli, J. Reviving a more than century-old technology for modern skincare. J. Cosmet. Sci. 2024, 75, 536–552. [Google Scholar]
- Al Jbour, N.D. An overview of new trends in the cosmetics industry. Int. J. Appl. Pharm. 2025, 17, 136–147. [Google Scholar] [CrossRef]
- Wanjari, N.; Waghmar, J. A review on latest trend of cosmetics-cosmeceuticals. Int. J. Pharma Res. Rev. 2015, 4, 45–51. [Google Scholar]
- Naseri, R.N.N.; Esa, M.M.; Ibrahim, R.; Jais, Z. A review of makeup products, trends, and consumer behaviour. Int. J. Res. Innov. Soc. Sci. 2025, IX, 2077–2083. [Google Scholar] [CrossRef]
- Mondello, A.; Salomone, R.; Mondello, G. Exploring circular economy in the cosmetic industry: Insights from a literature review. Environ. Impact Assess. Rev. 2024, 105, 107443. [Google Scholar] [CrossRef]
- Couteau, C.; Diarra, H.; Schmitt, Z.; Coiffard, L. Study of the composition of 140 shampoos: Similarities and differences depending on the sales channel used. Eur. J. Dermatol. 2019, 29, 141–159. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour. Technol. 2017, 232, 389–397. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. In Application and Characterization of Surfactants; Najar, R., Ed.; InTech Open: London, UK, 2017; pp. 183–225. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061–102083. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Mahmoud, Z.H.; Hussein, U.A.-R.; Abduvalieva, D.; Alsultany, F.H.; Kianfar, E. Biosurfactants: Properties, applications and emerging trends. S. Afr. J. Chem. Eng. 2025, 53, 21–39. [Google Scholar] [CrossRef]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef]
- Vega, G.R.; Stampino, P.G. Bio-Based surfactants and biosurfactants: An overview and main characteristics. Molecules 2025, 30, 863. [Google Scholar] [CrossRef]
- Pires-Oliveira, R.; Joekes, I. UV–vis spectra as an alternative to the Lowry method for quantify hair damage induced by surfactants. Col. Surfaces B Biointerfaces 2014, 123, 326–330. [Google Scholar] [CrossRef]
- Farias, C.B.B.; De Almeida, F.C.G.; Da Silva, I.A.; Souza, T.C.; Meira, H.M.; Da Silva, R.C.F.S.; Luna, J.M.; Dos Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Paulino, B.N.; Pessôa, M.G.; Mano, M.C.R.; Molina, G.; Neri-Numa, I.A.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Aging skin: The role of diet: Facts and controversies. Clin. Dermatol. 2013, 31, 701–706. [Google Scholar] [CrossRef]
- Zahed, M.A.; Matinvafa, M.A.; Azari, A.; Mohajeri, L. Biosurfactant, a green and effective solution for bioremediation of petroleum hydrocarbons in the aquatic environment. Discov. Water 2022, 21, 5. [Google Scholar] [CrossRef]
- Kandasamy, R.; Rajasekaran, M.; Kv, S.; Uddin, M. New trends in the biomanufacturing of green surfactants: Biobased surfactants and biosurfactants. In Next-Generation Biomanufacturing Technologies; Rathinam, N.K., Sani, R.K., Eds.; ACS Publications: Washington, DC, USA, 2019; Volume 1329, pp. 243–260. [Google Scholar] [CrossRef]
- Gudina, E.J.; Pereira, J.F.B.; Costa, R.; Coutinho, J.A.P.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns. J. Hazard. Mater. 2013, 261, 106–113. [Google Scholar] [CrossRef]
- Bom, S.; Jorge, J.; Ribeiro, H.M.; Marto, J. A step forward on sustainability in the cosmetics industry: A review. J. Clean. Prod. 2019, 225, 270–290. [Google Scholar] [CrossRef]
- Vecino, X.; Rodríguez-Lopez, L.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Bioactivity of glycolipopeptide cell-bound biosurfactants against skin pathogens. Int. J. Biol. Macromol. 2017, 109, 971–979. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Rufino, R.D.; De Luna, J.M.; Sarubbo, L.A. Saponins and microbial biosurfactants: Potential raw materials for the formulation of cosmetics. Biotechnol. Prog. 2018, 34, 1482–1493. [Google Scholar] [CrossRef]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Moldes, A.D.; Rodrigues, L.R. Novel cosmetic formulations containing a biosurfactant from Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2017, 155, 522–529. [Google Scholar] [CrossRef]
- Farias, J.M.; Stamford, T.C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing a biosurfactant and chitosan: An eco-sustainable option for the control of cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef]
- Fontoura, I.C.C.; Saikawa, G.I.A.; Silveira, V.A.I.; Pan, N.C.; Amador, I.R.; Baldo, C.; Rocha, S.; Celligoi, M.A.P.C. Antibacterial activity of sophorolipids from Candida bombicola against human pathogens. Braz. Arch. Biol. Technol. 2020, 63, e20180568. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Soetaert, W. Sophorolipids. In Biosurfactants. Microbiology Monographsin; Soberón-Chávez, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 179–210. [Google Scholar] [CrossRef]
- Fukuoka, T.; Morita, T.; Konishi, M.; Imura, T.; Sakai, H.; Kitamoto, D. Structural characterization and surface-active properties of a new glycolipid biosurfactant, mono-acylated mannosylerythritol lipid, produced from glucose by Pseudozyma antarctica. Appl. Microbiol. Biotechnol. 2007, 76, 801–810. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Setapar, S.H.M.; Khatoon, A.; Ahmad, A. The potential use of biosurfactants in cosmetics and dermatological products: Current trends and future prospects. In Biosurfactants for a Sustainable Future: Production and Applications in the Environment and Biomedicine; Sarma, H., Prasad, M.N.V., Eds.; John Wiley & Sons: New York, NY, USA, 2021; pp. 397–421. [Google Scholar] [CrossRef]
- Hu, Y.; Ju, L.-K. Purification of lactonic sophorolipids by crystallization. J. Biotechnol. 2001, 87, 263–272. [Google Scholar] [CrossRef]
- Meylheuc, T.; van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, A. Determination of hydrophilic-lipophilic balance value. Int. J. Sci. Res. 2014, 3, 573–575. [Google Scholar]
- Al Badi, K.A.; Khan, S.A. Formulation, evaluation and comparison of the herbal shampoo with the commercial shampoos. J. Basic Appl. Sci. 2014, 3, 301–305. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: Production, characterization and surface active properties of biosurfactant. Bioresour. Technol. 2016, 221, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.D.; Steck, W.F. A modified HET-CAM assay approach to the assessment of anti-irritant properties of plant extract. Food Chem. Toxicol. 2000, 38, 867–872. [Google Scholar] [CrossRef]
- ICCVAM (Interagency Coordinating Committee on the Validation of Alternative Methods). Recommended Test Method Protocol: Hens Egg Test–Chorioallantoic Membrane (HET-CAM) Test Method; ICCVAM Test Method Eval Rep 13; ICCVAM: Research Triangle Park, NC, USA, 2010; pp. B30–B38. [Google Scholar]
- Steiling, W.; Bracher, M.; Courtellemont, P.; De Silva, O. The HET-CAM, a useful in vitro assay for assessing the eye irritation properties of cosmetic formulations and ingredients. Toxicol. Vitro 1999, 13, 375–384. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, P.A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.B.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1998, 48, 589–601. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—A new frontier for social and environmental safety: A mini review. Biotechnol. Res. Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Medeiros, A.O.; da Silva, M.G.C.; Converti, A.; de Almeida, F.C.G.; Sarubbo, L.A. Development of natural fungicidal agricultural defensives using microbial glycolipid and vegetable oil blends. Surfaces 2024, 7, 879–897. [Google Scholar] [CrossRef]
- Silva, I.A.; Fortunato, J.G.L.A.; Almeida, F.C.G.; Alves, R.N.; Cunha, M.C.C.; Rufino, R.D.; Fernandes, M.L.B.; Sarubbo, L.A. Production and application of a new biosurfactant for solubilisation and mobilisation of residual oil from sand and seawater. Processes 2024, 12, 1605. [Google Scholar] [CrossRef]
- Shah, M.U.H.; Sivapragasam, M.; Moniruzzaman, M.; Talukder, M.M.R.; Yusup, S.B.; Goto, M. Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem. Eng. J. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Konishi, M.; Yoshida, Y.; Horiuchi, J. Efficient production of sophorolipids by Starmerella bombicola using a corncob hydrolysate medium. J. Biosci. Bioeng. 2015, 119, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, J.V.; Pratap, A.P.; Kale, S.B. Evaluation of sunflower oil refinery waste as feedstock for production of sophorolipid. Process Biochem. 2019, 78, 15–24. [Google Scholar] [CrossRef]
- Lima, B.G.A.; Santos, J.C.V.; Silva, R.R.; Caldas, M.C.F.; Meira, H.M.; Rufino, R.D.; Sarubbo, L.A.; Luna, J.M. Sustainable production of biosurfactant grown in medium with industrial waste and use for removal of oil from soil and seawater. Surfaces 2024, 7, 537–549. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Veras, B.O.; Aguiar, J.S.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactant produced by Candida utilis UFPEDA1009 with potential application in cookie formulation. Electron. J. Biotechnol. 2021, 46, 14–21. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Potential food application of a biosurfactant produced by Saccharomyces cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020, 8, 434. [Google Scholar] [CrossRef]
- da Silva, P.F.F.; da Silva, R.R.; Sarubbo, L.A.; Guerra, J.M.C. Production and optimization of biosurfactant properties using Candida mogii and Licuri oil (Syagrus coronata). Foods 2024, 13, 4029. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Kamthan, M.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial biosurfactant as an alternate to chemical surfactants for application in cosmetics industries in personal and skin care products: A critical review. BioMed Res. Int. 2023, 2023, 375223. [Google Scholar] [CrossRef]
- Freeling, F.; Alygizakis, N.A.; von der Ohe, P.C.; Slobodnik, J.; Oswald, P.; Aalizadeh, R.; Cirka, L.; Thomaidis, N.S.; Scheurer, M. Occurrence and potential environmental risk of surfactants and their transformation products discharged by wastewater treatment plants. Sci. Total Environ. 2019, 681, 475–487. [Google Scholar] [CrossRef]
- Kaida, H.; Syed, M.E.; Shukor, Y.; Othman, A. Biodegradation of linear alkylbenzene sulfonates (LAS): A mini review. Bioremediat. Sci. Technol. Res. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Moldes, A.B.; Rodríguez-López, L.; Rincón-Fontán, M.; López-Prieto, A.; Vecino, X.; Cruz, J.M. Synthetic and bio-derived surfactants versus microbial biosurfactants in the cosmetic industry: An overview. Int. J. Mol. Sci. 2021, 22, 2371. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arch. Ind. Hyg. Toxicol. 2010, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Pilz, M.; Cavelius, P.; Qoura, F.; Awad, D.; Brück, T. Lipopeptides development in cosmetics and pharmaceutical applications: A comprehensive review. Biotechnol. Adv. 2023, 67, 108210. [Google Scholar] [CrossRef]
- AlQuadeib, B.T.; Eltahir, E.K.D.; Banafa, R.A.; Al-Hadhairi, L.A. Pharmaceutical evaluation of different shampoo brands in local Saudi market. Saudi Pharm. J. 2018, 26, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Selva Filho, A.A.P.; Faccioli, Y.E.; Converti, A.; da Silva, R.d.C.F.S.; Sarubbo, L.A. Maximization of the production of a low-cost biosurfactant for application in the treatment of soils contaminated with hydrocarbons. Sustainability 2024, 16, 7970. [Google Scholar] [CrossRef]
- Silveira, V.A.I.; Nishio, E.K.; Freitas, C.A.U.Q.; Amador, I.R.; Kobayashi, R.; Caretta, T.; Macedo, F.; Celligoi, M.A.P.C. Production and antimicrobial activity of sophorolipid against Clostridium perfringens and Campylobacter jejuni and their additive interaction with lactic acid. Biocatal. Agric. Biotechnol. 2019, 21, 101287. [Google Scholar] [CrossRef]
- Develter, D.W.G.; Lauryssen, L.M.L. Properties and industrial applications of sophorolipids. Eur. J. Lipid Sci. Technol. 2010, 112, 628–638. [Google Scholar] [CrossRef]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef]
- Moiset, G.; López, C.A.; Bartelds, R.; Syga, L.; Rijpkema, E.; Cukkemane, A.; Baldus, M.; Poolman, B.; Marrink, S.J. Disaccharides impact the lateral organization of lipid membranes. J. Am. Chem. Soc. 2014, 136, 16167–16175. [Google Scholar] [CrossRef]
- Valotteau, C.; Banat, I.M.; Mitchell, C.A.; Lydon, H.; Marchant, R.; Babonneau, F.; Pradier, C.-M.; Baccile, N.; Humblot, V. Antibacterial properties of sophorolipid-modified gold surfaces against Gram positive and Gram negative pathogens. Colloids Surf. B Biointerfaces 2017, 157, 325–334. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, L.; Rincon-Fontan, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Preservative and irritant capacity of biosurfactants from different sources: A comparative study. J. Pharm. Sci. 2019, 108, 2296–2304. [Google Scholar] [CrossRef]
- Freire, P.L.L.; Stamford, T.C.M.; Albuquerque, A.J.R.; Sampaio, F.C.; Cavalcante, H.M.M.; Macedo, R.O.; Galembeck, A.; Flores, M.A.P.; Rosenblatt, A. Action of silver nanoparticles towards biological systems: Cytotoxicity evaluation using hen’s egg test and inhibition of Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents 2015, 45, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Budai, P.; Lehel, J.; Tavaszi, J.; Kormos, É. HET-CAM test for determining the possible eye irritancy of pesticides. Acta Vet. Hung. 2010, 58, 369–377. [Google Scholar] [CrossRef]
- Krakowian, D.; Gądarowska, D.; Daniel-Wójcik, A.; Mrzyk, I. Cytotoxicity assay to assess eye irritation—A comparison with other methods and possible strategies for use. Toxicol. Vitro 2022, 81, 105343. [Google Scholar] [CrossRef]
- Dardouri, M.; Bettencourt, A.; Martin, V.; Carvalho, F.A.; Santos, C.; Monge, N.; Santos, N.C.; Fernandes, M.H.; Gomes, P.S.; Ribeiro, I.A.C. Using plasma mediated covalent functionalization of rhamnolipids on polydimethylsiloxane towards the antimicrobial improvement of catheter surfaces. Biomater. Adv. 2022, 34, 112563. [Google Scholar] [CrossRef]
- Resende, A.H.M.; Farias, J.M.; Silva, D.D.B.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Application of biosurfactants and chitosan in toothpaste formulation. Colloids Surfaces B Biointerfaces 2019, 181, 77–84. [Google Scholar] [CrossRef]
- Apak, R.; Mustafa, Ö.; Kubilay, G.; Çapanŏglu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Tancredi, M.; Carandente Coscia, C.; Russo Krauss, I.; D’Errico, G. Antioxidant properties of biosurfactants: Multifunctional biomolecules with added value in formulation chemistry. Biomolecules 2025, 15, 308. [Google Scholar] [CrossRef]
- Rashad, M.M.; Nooman, M.U.; Ali, M.M.; Al-kashef, A.S.; Mahmoud, A.E. Production, characterization and anticancer activity of Candida bombicola sophorolipids by means of solid state fermentation of sunflower oil cake and soybean oil. Grasas Aceites 2014, 65, e017. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Joshi, N.; Patidar, K.; Solanki, R.; Mahawar, V. Preparation and evaluation of herbal hair growth promoting shampoo formulation containing Piper betle and Psidium guajava leaves extract. Int. J. Green Pharm. 2018, 2018, S835–S839. [Google Scholar] [CrossRef]
- Turnes, J.M.; Bonetti, A.F.; Krause, M.S.; Canteli, V.C.D.; Paula, C.S.; Duarte, M.R.; Zanin, S.M.W.; Dias, J.F.G.; Miguel, M.D.; Miguel, O.G. Avaliação da atividade antioxidante e alelopática do extrato etanólico e frações das cascas do caule de Zanthoxylum rhoifolium Lam., Rutaceae. Rev. Ciênc. Farm. Bás. Apl. 2014, 35, 459–467. [Google Scholar]
- Fan, Y.; Xiaohui, Z.; Jing, H.; Ci, Z. Preliminary studies on surface properties and antioxidant activities of sophorolipids. Sci. Technol. Food Ind. 2012, 33, 166–168. [Google Scholar]
- Hoa, N.L.H.; Loan, L.Q.; Sang, V.T. Production and characterization of sophorolipids by Candida bombicola using catfish fat. Nat. Sci. Technol. 2017, 14, 152–159. [Google Scholar]
- Haque, E.; Kayalvizhi, K.; Hassan, S. Biocompatibility, antioxidant and anti-infective effect of biosurfactant produced by Marinobacter litoralis MB15. Int. J. Pharm. Investig. 2020, 10, 173–178. [Google Scholar] [CrossRef]
- Yalçın, E.; Çavuşoğlu, K. Structural analysis and antioxidant activity of a biosurfactant obtained from Bacillus subtilis RW-I. Turk. J. Biochem. 2010, 35, 243–247. [Google Scholar]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: Current trends and applications in biomedical, biotechnological and environmental fields. Curr. Opin. Colloid Interface Sci. 2019, 39, 117–129. [Google Scholar] [CrossRef]
- Shekhar, S.; Arumugam, S.; Tangavel, B. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Jezierska, S.; Claus, S.; Van Bogaert, I.N.A. Yeast glycolipid biosurfactants. FEBS Lett. 2017, 592, 1312–1329. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Silva, M.G.C.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Mendes da Silva Santos, E.; Alvares da Silva Lira, I.R.; Moraes Meira, H.; dos Santos Aguiar, J.; Diniz Rufino, R.; Germano de Almeida, D.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L.; Moura de Luna, J. Enhanced oil removal by a non-toxic biosurfactant formulation. Energies 2021, 14, 467. [Google Scholar] [CrossRef]
- Marqués, A.M.; Pinazo, A.; Farfan, M.; Aranda, F.J.; Teruel, J.A.; Ortiz, A.; Manresa, A.; Espuny, M.J. The physicochemical properties and chemical composition of trehalose lipids produced by Rhodococcus erythropolis 51T7. Chem. Phys. Lipids 2009, 158, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; López-Prieto, A.; Lopez-Álvarez, M.; Pérez-Davila, S.; Serra, J.; González, P.; Cruz, J.M.; Moldes, A.B. Characterization and cytotoxic effect of biosurfactants obtained from different sources. ACS Omega 2020, 5, 31381–31390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).