Exploring Exopolysaccharides Produced in Indigenous Mexican Fermented Beverages and Their Biotechnological Applications
Abstract
1. Introduction
2. Tibicos (Water Kefir Grains) and Their Microbial Communities
2.1. Microbial Communities Involved in the Production of Water Kefir
2.2. Techniques for Identifying the Microbial Contents in Water Kefir
3. Exopolysaccharides
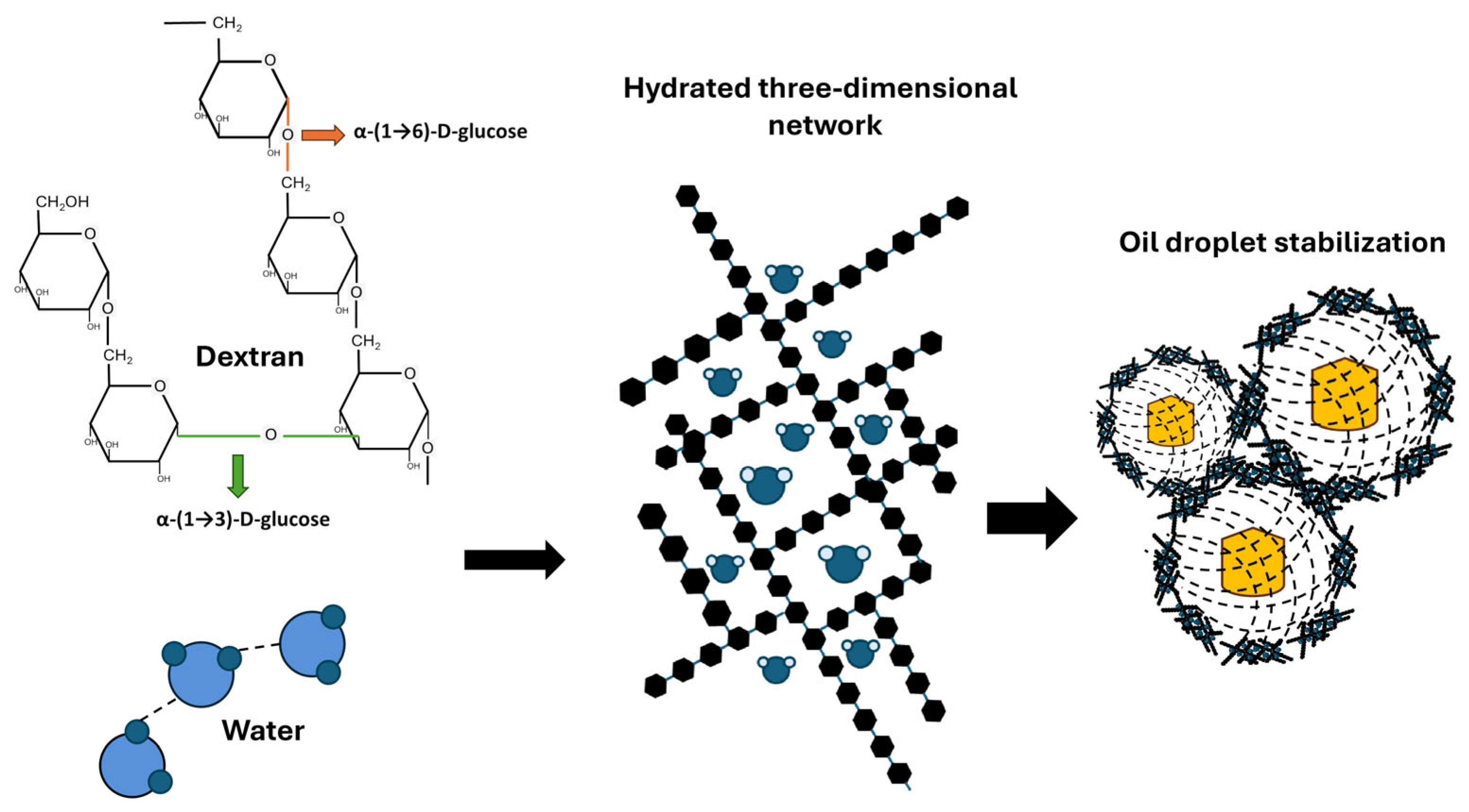
3.1. Classification, Chemical Composition, and Synthesis
3.2. Extraction, Purification, and Characterization of Exopolysaccharides
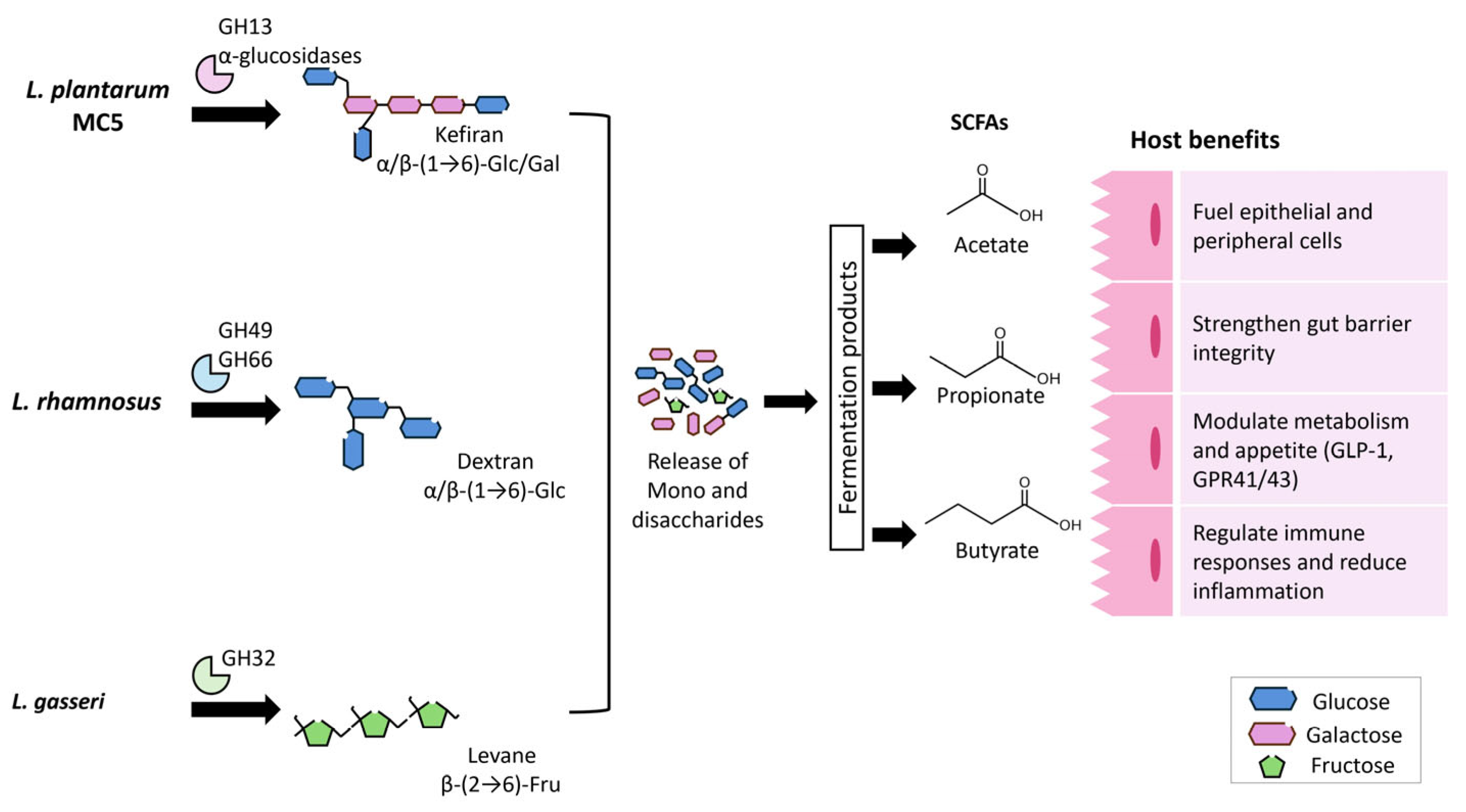
4. Functional Roles and Emerging Applications of Exopolysaccharides Derived from Microbial Community in Indigenous Mexican Fermented Beverages
4.1. Applications of Exopolysaccharides in the Food Industry
4.2. Recent Advances in the Health Benefits of Microbial Exopolysaccharides
4.2.1. Exopolysaccharides as Next-Generation Prebiotics
4.2.2. Antioxidant and Anti-Inflammatory Properties
4.2.3. Antibacterial Activity of Exopolysaccharides
4.2.4. Emerging Health Avenues and Research Gaps
4.3. Agro-Biotechnological Potential of Microbial Exopolysaccharides
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) |
| AAB | Acetic Acid Bacteria |
| CAZymes | Carbohydrate-active enzymes |
| CPS | Capsular Polysaccharides |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EPS | Exopolysaccharides |
| FTIR | Fourier Transform Infrared |
| HePs | Heteropolysaccharides |
| HoPs | Homopolysaccharides |
| LAB | Lactic Acid Bacteria |
| MRS | Man, Rogosa, and Sharpe |
| NGS | Next-Generation Sequencing |
| NMR | Nuclear Magnetic Resonance |
| PCR | Polymerase Chain Reaction |
| ROS | Reactive Oxygen Species |
| RSM | Response Surface Methodology |
| SCFAs | Short-Chain Fatty Acids |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| YGC | Yeast Glucose Chloramphenicol |
References
- Rojo Leyva, V.M.; Reyes Utrera, J.L. Bebidas Tradicionales de los Pueblos Indígenas de MÉXICO; Instituto nacional de los pueblos indígenas: Mexico City, Mexico, 2022. [Google Scholar]
- Quintero-Salazar, B.; Bernáldez Camiruaga, A.I.; Dublán-García, O.; Barrera García, V.D.; Favila Cisneros, H.J. Consumo y conocimiento actual de una bebida fermentada tradicional en Ixtapan del Oro, México: La sambumbia. Alteridades 2012, 22, 115–129. [Google Scholar]
- Koleff, P.; Urquiza-Haas, T.; Ruiz-GonzáLEZ, S.P.; Hernández-Robles, D.R.; Mastretta-Yanes, A.; Quintero, E.; Sarukhán, J. Biodiversity in Mexico: State of knowledge. In Global Biodiversity, 1st ed.; Apple Academic Press: Waretown, NJ, USA, 2018; pp. 285–337. [Google Scholar]
- Ramírez-Guzmán, K.N.; Torres-León, C.; Martinez-Medina, G.A.; de la Rosa, O.; Hernández-Almanza, A.; Alvarez-Perez, O.B.; Araujo, R.; González, L.R.; Londoño, L.; Ventura, J. Traditional fermented beverages in Mexico. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 605–635. [Google Scholar]
- Kaur, P.; Ghoshal, G.; Banerjee, U.C. Traditional bio-preservation in beverages: Fermented beverages. In Preservatives and Preservation Approaches in Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–113. [Google Scholar]
- Ojeda-Linares, C.; Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Torres-García, I.; Vallejo, M.; Casas, A. Traditional fermented beverages of Mexico: A biocultural unseen foodscape. Foods 2021, 10, 2390. [Google Scholar] [CrossRef]
- Hugenholtz, J. Traditional biotechnology for new foods and beverages. Curr. Opin. Biotechnol. 2013, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Romero-Luna, H.E.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Traditional fermented beverages from Mexico as a potential probiotic source. Ann. Microbiol. 2017, 67, 577–586. [Google Scholar] [CrossRef]
- Narzary, Y.; Brahma, J.; Brahma, C.; Das, S. A study on indigenous fermented foods and beverages of Kokrajhar, Assam, India. J. Ethn. Foods 2016, 3, 284–291. [Google Scholar] [CrossRef]
- Oviedo-León, J.F.; Yáñez Fernández, J.; Hernández-Sánchez, H.; Castro-Rodríguez, D. Probiotics as a Food Supplement: What are they and how do they work. JN Food Sci. Tech. 2023, 4, 1–7. [Google Scholar]
- Oviedo-León, J.F.; Cornejo-Mazón, M.; Ortiz-Hernández, R.; Torres-Ramírez, N.; Hernández-Sánchez, H.; Castro-Rodríguez, D.C. Exploration adhesion properties of Liquorilactobacillus and Lentilactobacillus isolated from two different sources of tepache kefir grains. PLoS ONE 2024, 19, e0297900. [Google Scholar] [CrossRef]
- Corona-González, R.I.; Ramos-Ibarra, J.R.; Gutiérrez-González, P.; Pelayo-Ortiz, C.; Guatemala-Morales, G.M.; Arriola-Guevara, E. The use of response surface methodology to evaluate the fermentation conditions in the production of tepache. Rev. Mex. Ing. Química 2013, 12, 19–28. [Google Scholar]
- Cota-Sánchez, J.H. Nutritional composition of the prickly pear (Opuntia ficus-indica) fruit. In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 691–712. [Google Scholar]
- Ulloa, M.; Herrera, T. Estudio de Pichia membranaefaciens y Saccharomyces cerevisiae, levaduras que constituyen parte de las zoogleas llamadas Tibicos en México. Sci. Fungorum 1981, 63–75. [Google Scholar]
- Martínez-Torres, A.; Gutiérrez-Ambrocio, S.; Heredia-del-Orbe, P.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Inferring the role of microorganisms in water kefir fermentations. Int. J. Food Sci. Technol. 2017, 52, 559–571. [Google Scholar] [CrossRef]
- Velázquez-Quiñones, S.E.; Moreno-Jiménez, M.R.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Álvarez, S.A.; Rosales-Villarreal, M.C.; Cervantes-Cardoza, V.; Rocha-Guzmán, N.E. Apple Tepache fermented with tibicos: Changes in chemical profiles, antioxidant activity and inhibition of digestive enzymes. J. Food Process. Preserv. 2021, 45, e15597. [Google Scholar] [CrossRef]
- Bozkir, E.; Yilmaz, B.; Sharma, H.; Esatbeyoglu, T.; Ozogul, F. Challenges in water kefir production and limitations in human consumption: A comprehensive review of current knowledge. Heliyon 2024, 10, e33501. [Google Scholar] [CrossRef] [PubMed]
- Gökırmaklı, Ç.; Şatır, G.; Guzel-Seydim, Z.B. Microbial viability and nutritional content of water kefir grains under different storage conditions. Food Sci. Nutr. 2024, 12, 4143–4150. [Google Scholar] [CrossRef]
- Limbad, M.; Gutierrez Maddox, N.; Hamid, N.; Kantono, K.; Higgins, C. Identification of the Microbiota in Coconut Water, Kefir, Coconut Water Kefir and Coconut Water Kefir-Fermented Sourdough Using Culture-Dependent Techniques and Illumina–MiSeq Sequencing. Microorganisms 2024, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.A.; Fernández, L.A.; Díaz, M.L.; Gallo, C.A.; Corona, M.; Evans, J.D.; Reynaldi, F.J. Bacterial diversity using metagenomics of 16s rDNA in water kefir, an innovative source of probiotics for bee nutrition. Rev. Argent. Microbiol. 2024, 55, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- de Almeida, K.V.; Sant’Ana, C.T.; Wichello, S.P.; Louzada, G.E.; Verruck, S.; Teixeira, L.J.Q. Water Kefir: Review of Microbial Diversity, Potential Health Benefits, and Fermentation Process. Processes 2025, 13, 885. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Fernández, L.A.; Díaz, M.L.; Pérez, M.; Corona, M.; Reynaldi, F.J. Microbiological and chemical characterization of water kefir: An innovative source of potential probiotics for bee nutrition. Rev. Argent. Microbiol. 2023, 55, 176–180. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.-P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Factories 2014, 13, S9. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Olivares-Illana, V.; Wacher-Rodarte, C.; Le Borgne, S.; López-Munguía, A. Characterization of a cell-associated inulosucrase from a novel source: A Leuconostoc citreum strain isolated from Pozol, a fermented corn beverage of Mayan origin. J. Ind. Microbiol. Biotechnol. 2002, 28, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, I.; Rodríguez-Alegría, M.E.; Miranda-Molina, A.; Giles-Gómez, M.; Conca Morales, R.; López-Munguía, A.; Bolívar, F.; Escalante, A. Screening and characterization of extracellular polysaccharides produced by Leuconostoc kimchii isolated from traditional fermented pulque beverage. SpringerPlus 2014, 3, 583. [Google Scholar] [CrossRef]
- Escalante, A.; Giles-Gómez, M.; Hernández, G.; Córdova-Aguilar, M.S.; López-Munguía, A.; Gosset, G.; Bolívar, F. Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food Microbiol. 2008, 124, 126–134. [Google Scholar] [CrossRef]
- Maribel, C.-M.; Humberto, H.-S.; Gustavo, G.L.; Lidia, D.-A.; de Jesús, C.S.A.; Antonio, J.-A.; Miguel, G.-S.; Abel, M.; María, J.-F. Production and partial characterization of an exopolysaccharide from Ustilago maydis in submerged culture. Afr. J. Biotechnol. 2012, 11, 7079–7087. [Google Scholar] [CrossRef][Green Version]
- Castro-Rodríguez, D.; Hernández-Sánchez, H.; Yáñez-Fernández, J. Structural characterization and rheological properties of dextran produced by native strains isolated of Agave salmiana. Food Hydrocoll. 2019, 90, 1–8. [Google Scholar] [CrossRef]
- Silbir, S.; Dagbagli, S.; Yegin, S.; Baysal, T.; Goksungur, Y. Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohydr. Polym. 2014, 99, 454–461. [Google Scholar] [CrossRef]
- Hernández-Oaxaca, D.; López-Sánchez, R.; Lozano, L.; Wacher-Rodarte, C.; Segovia, L.; Lopez Munguia, A. Diversity of Weissella confusa in pozol and its carbohydrate metabolism. Front. Microbiol. 2021, 12, 629449. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial exopolysaccharides: Structure, diversity, applications, and future frontiers in sustainable functional materials. Polysaccharides 2024, 5, 241–287. [Google Scholar] [CrossRef]
- Senko, H.; Kajić, S.; Huđ, A.; Palijan, G.; Petek, M.; Rajnović, I.; Šamec, D.; Udiković-Kolić, N.; Mešić, A.; Brkljačić, L. Will the beneficial properties of plant-growth promoting bacteria be affected by waterlogging predicted in the wake of climate change: A model study. Appl. Soil Ecol. 2024, 198, 105379. [Google Scholar] [CrossRef]
- Talbi, C.; Elmarrkechy, S.; Youssfi, M.; Bouzroud, S.; Belfquih, M.; Sifou, A.; Bouhaddou, N.; Badaoui, B.; Balahbib, A.; Bouyahya, A. Bacterial exopolysaccharides: From production to functional features. Prog. Microbes Mol. Biol. 2023, 6, 1–17. [Google Scholar] [CrossRef]
- Monsan, P.; Bozonnet, S.; Albenne, C.; Joucla, G.; Willemot, R.-M.; Remaud-Siméon, M. Homopolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef]
- Korakli, M.; Vogel, R.F. Structure/function relationship of homopolysaccharide producing glycansucrases and therapeutic potential of their synthesised glycans. Appl. Microbiol. Biotechnol. 2006, 71, 790–803. [Google Scholar] [CrossRef] [PubMed]
- van Hijum, S.A.F.T.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G.H. Structure-Function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Degeest, B.; Vaningelgem, F.; De Vuyst, L. Microbial physiology, fermentation kinetics, and process engineering of heteropolysaccharide production by lactic acid bacteria. Int. Dairy J. 2001, 11, 747–757. [Google Scholar] [CrossRef]
- De Vuyst, L.; De Vin, F.; Vaningelgem, F.; Degeest, B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- Jolly, L.; Stingele, F. Molecular organization and functionality of exopolysaccharide gene clusters in lactic acid bacteria. Int. Dairy J. 2001, 11, 733–745. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Salazar, N.; de los Reyes-Gavilán, C.G. Biosynthesis and chemical composition of exopolysaccharides produced by lactic acid bacteria. In Bacterial Polysaccharides: Current Innovations and Future Trends; Caister Academic Press: Norfolk, UK, 2009; pp. 279–310. [Google Scholar]
- Broadbent, J.R.; McMahon, D.J.; Welker, D.L.; Oberg, C.J.; Moineau, S. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: A review. J. Dairy Sci. 2003, 86, 407–423. [Google Scholar] [CrossRef]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of lactic acid bacteria: Production, purification and health benefits towards functional food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, S.; Liang, S.; Xiang, F.; Wang, X.; Lian, H.; Li, B.; Liu, F. Exopolysaccharides of lactic acid bacteria: Structure, biological activity, structure-activity relationship, and application in the food industry: A review. Int. J. Biol. Macromol. 2024, 257, 128733. [Google Scholar] [CrossRef]
- Hugenholtz, J.; Kleerebezem, M. Metabolic engineering of lactic acid bacteria: Overview of the approaches and results of pathway rerouting involved in food fermentations. Curr. Opin. Biotechnol. 1999, 10, 492–497. [Google Scholar] [CrossRef]
- Papagianni, M. Metabolic engineering of lactic acid bacteria for the production of industrially important compounds. Comput. Struct. Biotechnol. J. 2012, 3, e201210003. [Google Scholar] [CrossRef]
- Murugu, J.; Narayanan, R. Production, purification, and characterization of a novel exopolysaccharide from probiotic Lactobacillus amylovorus: MTCC 8129. Indian J. Microbiol. 2024, 64, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Gamar-Nourani, L.; Blondeau, K.; Simonet, J.M. Influence of culture conditions on exopolysaccharide production by Lactobacillus rhamnosus strain C83. J. Appl. Microbiol. 1998, 85, 664–672. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N.P. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Shimizu, H.; Shioya, S. Modelling and optimization of environmental conditions for kefiran production by Lactobacillus kefiranofaciens. Appl. Microbiol. Biotechnol. 2001, 57, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Petry, S.; Furlan, S.; Waghorne, E.; Saulnier, L.; Cerning, J.; Maguin, E. Comparison of the thickening properties of four Lactobacillus delbrueckii subsp. bulgaricus strains and physicochemical characterization of their exopolysaccharides. FEMS Microbiol. Lett. 2003, 221, 285–291. [Google Scholar] [CrossRef][Green Version]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef]
- Mozzi, F.; Oliver, G.; De Giori, G.S.; De Valdez, G.F. Influence of temperature on the production of exopolysaccharides by thermophilic lactic acid bacteria. Milchwissenschaft 1995, 50, 80–82. [Google Scholar]
- Shene, C.; Canquil, N.; Bravo, S.; Rubilar, M. Production of the exopolysacchzrides by Streptococcus thermophilus: Effect of growth conditions on fermentation kinetics and intrinsic viscosity. Int. J. Food Microbiol. 2008, 124, 279–284. [Google Scholar] [CrossRef]
- De Vuyst, L.; Zamfir, M.; Mozzi, F.; Adriany, T.; Marshall, V.M.; Degeest, B.; Vaningelgem, F. Exopolysaccharide-producing Streptococcus thermophilus strains as functional starter cultures in the production of fermented milks. Int. Dairy J. 2003, 13, 707–717. [Google Scholar] [CrossRef]
- Pham, P.L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. [Google Scholar] [CrossRef]
- Yadav, M.K.; Song, J.H.; Vasquez, R.; Lee, J.S.; Kim, I.H.; Kang, D.K. Methods for Detection, Extraction, Purification, and Characterization of Exopolysaccharides of Lactic Acid Bacteria—A Systematic Review. Foods 2024, 13, 3687. [Google Scholar] [CrossRef]
- Yáñez-Fernández, J.; Herrera Ovando, M.G.; Patlán Ramiírez, L.; Ramírez-Sotelo, G.; Guarin, C.A.; Castro-Rodríguez, D.C. Factorial design to optimize dextran production by the native strain Leuconostoc mesenteroides SF3. ACS Omega 2021, 6, 31203–31210. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and health-promoting properties of probiotics’ exopolysaccharides; isolation, characterization, and applications in the food industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 8194–8225. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mendoza, C.; Iván Roldán-Cruz, E.; Vázquez-Jahuey, M. Physicochemical, microbiological and organoleptic characterization of aguamiel and pulque from Alto Mezquital, Hidalgo. Agric. Soc. Desarro. 2022, 19, 63–75. [Google Scholar]
- Martínez-Ortiz, V.M.; Trujillo-López, M.A.; El-Kassis, E.G.; Bautista-Rodríguez, E.; Kirchmayr, M.R.; Hernández-Carranza, P.; Pérez-Armendáriz, B. Bacillus mojavensis isolated from aguamiel and its potential as a probiotic bacterium. Electron. J. Biotechnol. 2024, 67, 42–49. [Google Scholar] [CrossRef]
- Banerjee, A.; Mohammed Breig, S.J.; Gómez, A.; Sánchez-Arévalo, I.; González-Faune, P.; Sarkar, S.; Bandopadhyay, R.; Vuree, S.; Cornejo, J.; Tapia, J. Optimization and characterization of a novel exopolysaccharide from Bacillus haynesii CamB6 for food applications. Biomolecules 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a traditional Mexican alcoholic fermented beverage: Historical, microbiological, and technical aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, T.; Mu, Y.; Jing, H.; Obadi, M.; Xu, B. Enhancing the stabilization of β-carotene emulsion using ovalbumin-dextran conjugates as emulsifier. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 126806. [Google Scholar] [CrossRef]
- Cofelice, M.; Messia, M.C.; Marconi, E.; Cuomo, F.; Lopez, F. Effect of the xanthan gum on the rheological properties of alginate hydrogels. Food Hydrocoll. 2023, 142, 108768. [Google Scholar] [CrossRef]
- Bolaños-Núñez, S.; Santiago-Urbina, J.A.; Guyot, J.-P.; Díaz-Ruiz, G.; Wacher, C. Microbial interactions between amylolytic and non-amylolytic lactic acid bacteria strains isolated during the fermentation of Pozol. Foods 2021, 10, 2607. [Google Scholar] [CrossRef]
- Zavala-Díaz de la Serna, F.J.; Contreras-López, R.; Lerma-Torres, L.P.; Ruiz-Terán, F.; Rocha-Gutiérrez, B.A.; Pérez-Vega, S.B.; Elías-Ogaz, L.R.; Salmerón, I. Understanding the Biosynthetic Changes that Give Origin to the Distinctive Flavor of Sotol: Microbial Identification and Analysis of the Volatile Metabolites Profiles During Sotol (Dasylirion sp.) Must Fermentation. Biomolecules 2020, 10, 1063. [Google Scholar] [CrossRef]
- Song, J.; Qiu, Y.; Zhao, R.; Hou, J.; Tu, L.; Nie, Z.; Wang, J.; Zheng, Y.; Wang, M. Transcriptomics and metabolomics analysis of Sclerotium rolfsii fermented with differential carbon sources. Foods 2022, 11, 3706. [Google Scholar] [CrossRef]
- Afreen, A.; Ahmed, Z.; Khalid, N.; Ferheen, I.; Ahmed, I. Optimization and cholesterol-lowering activity of exopolysaccharide from Lactiplantibacillus paraplantarum NCCP 962. Appl. Microbiol. Biotechnol. 2023, 107, 1189–1204. [Google Scholar] [CrossRef]
- Yadav, M.; Sunita; Shukla, P. Probiotic potential of Weissella paramesenteroides MYPS5. 1 isolated from customary dairy products and its therapeutic application. 3 Biotech 2022, 12, 9. [Google Scholar] [CrossRef]
- Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of bacterial homopolysaccharide production and properties during food processing. Biology 2022, 11, 171. [Google Scholar] [CrossRef]
- Zhang, K.; Yue, L.; Cong, J.; Zhang, J.; Feng, Z.; Yang, Q.; Lu, X. Increased production of pullulan in Aureobasidium pullulans YQ65 through reduction of intracellular glycogen content. Carbohydr. Polym. 2025, 352, 123196. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, J.; Zhang, K.; Wang, Z.; Ma, J.; Yang, Q.; Lin, C. Transcriptome Analysis of Aureobasidium pullulans YQ65 Grown on Yeast Extract Peptone Glucose and Potato Dextrose Agar Media and Quantification of Their Effects on Pullulan Production. Foods 2024, 13, 3619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, M.; Chen, L.; Ma, L.; Huang, M.; Chen, L.; El-Seedi, H.R.A.; Teng, H. Modulation of gel characteristics in surimi-curdlan gel system by different valence metal cations: Mechanical properties, ionic distribution and microstructure visualization. Int. J. Biol. Macromol. 2025, 290, 138600. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, M. Genetic Engineering of Agrobacterium Increases Curdlan Production through Increased Expression of the crdASC Genes. Microorganisms 2023, 12, 55. [Google Scholar] [CrossRef]
- Khiabani, A.; Sarabi-Jamab, M.; Shakeri, M.-s.; Pahlevanlo, A.; Emadzadeh, B. Exploring the Acetobacteraceae family isolated from kombucha SCOBYs worldwide and comparing yield and characteristics of biocellulose under various fermentation conditions. Sci. Rep. 2024, 14, 26616. [Google Scholar] [CrossRef]
- Dailin, D.J.; Elsayed, E.A.; Malek, R.A.; Hanapi, S.Z.; Selvamani, S.; Ramli, S.; Sukmawati, D.; Sayyed, R.Z.; El Enshasy, H.A. Efficient kefiran production by Lactobacillus kefiranofaciens ATCC 43761 in submerged cultivation: Influence of osmotic stress and nonionic surfactants, and potential bioactivities. Arab. J. Chem. 2020, 13, 8513–8523. [Google Scholar] [CrossRef]
- Shakeri, M.-s.; Naji-Tabasi, S. Characterization and optimization of gellan gum production by natural Sphingomonas sp. SM2. LWT 2024, 200, 116164. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.L.; Hutkins, R.W. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Bouzaiene, T.; Mohamedhen Vall, M.; Ziadi, M.; Ben Rejeb, I.; Yangui, I.; Aydi, A.; Ouzari, I.; Moktar, H. Exopolysaccharides from Lactiplantibacillus plantarum C7 exhibited Antibacterial, Antioxidant, Anti-enzymatic, and prebiotic activities. Fermentation 2024, 10, 339. [Google Scholar] [CrossRef]
- Escalante, A.; Elena Rodríguez, M.; Martínez, A.; López-Munguía, A.; Bolívar, F.; Gosset, G. Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 2004, 235, 273–279. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, R.; Hernández-Oaxaca, D.; Escobar-Zepeda, A.; Ramos Cerrillo, B.; López-Munguía, A.; Segovia, L. Analysing the dynamics of the bacterial community in pozol, a Mexican fermented corn dough. Microbiology 2023, 169, 001355. [Google Scholar] [CrossRef] [PubMed]
- Cázares-Vásquez, M.L.; Rodríguez-Herrera, R.; Aguilar-González, C.N.; Sáenz-Galindo, A.; Solanilla-Duque, J.F.; Contreras-Esquivel, J.C.; Flores-Gallegos, A.C. Microbial exopolysaccharides in traditional Mexican fermented beverages. Fermentation 2021, 7, 249. [Google Scholar] [CrossRef]
- Akkerman, R.; Oerlemans, M.M.P.; Ferrari, M.; Fernández-Lainez, C.; de Haan, B.J.; Faas, M.M.; Walvoort, M.T.C.; de Vos, P. Exopolysaccharide β-(2, 6)-levan-type fructans have a molecular-weight-dependent modulatory effect on Toll-like receptor signalling. Food Funct. 2024, 15, 676–688. [Google Scholar] [CrossRef]
- Tintoré, M.; Cuñé, J.; Vu, L.D.; Poppe, J.; Van den Abbeele, P.; Baudot, A.; de Lecea, C. A Long-Chain Dextran Produced by Weissella Cibaria Boosts the Diversity of Health-Related Gut Microbes Ex Vivo. Biology 2024, 13, 51. [Google Scholar] [CrossRef]
- Kurakawa, T.; Kani, K.; Chudan, S.; Nishikawa, M.; Tabuchi, Y.; Sakamoto, K.; Nagai, Y.; Ikushiro, S.; Furusawa, Y. Rice Kefiran Ameliorates Obesity and Hepatic Steatosis Through the Change in Gut Microbiota. Microorganisms 2024, 12, 2495. [Google Scholar] [CrossRef]
- Savitskaya, I.; Zhantlessova, S.; Kistaubayeva, A.; Ignatova, L.; Shokatayeva, D.; Sinyavskiy, Y.; Kushugulova, A.; Digel, I. Prebiotic cellulose–pullulan matrix as a “vehicle” for probiotic biofilm delivery to the host large intestine. Polymers 2023, 16, 30. [Google Scholar] [CrossRef]
- Shimizu, H.; Miyamoto, J.; Hisa, K.; Ohue-Kitano, R.; Takada, H.; Yamano, M.; Nishida, A.; Sasahara, D.; Masujima, Y.; Watanabe, K. Sucrose-preferring gut microbes prevent host obesity by producing exopolysaccharides. Nat. Commun. 2025, 16, 1145. [Google Scholar] [CrossRef]
- Gościniak, A.; Lainé, E.; Cielecka-Piontek, J. How Do Cyclodextrins and Dextrans Affect the Gut Microbiome? Review of Prebiotic Activity. Molecules 2024, 29, 5316. [Google Scholar] [CrossRef]
- Nakamura, S.; Kurata, R.; Tonozuka, T.; Funane, K.; Park, E.Y.; Miyazaki, T. Bacteroidota polysaccharide utilization system for branched dextran exopolysaccharides from lactic acid bacteria. J. Biol. Chem. 2023, 299, 104885. [Google Scholar] [CrossRef]
- Zeng, M.; Oh, J.H.; van Pijkeren, J.P.; Pan, X. Selective utilization of gluco-oligosaccharides by lactobacilli: A mechanism study revealing the impact of glycosidic linkages and degree of polymerization on their utilization. J. Food Sci. 2024, 89, 523–539. [Google Scholar] [CrossRef]
- Wang, Y.; Svensson, B.; Henrissat, B.; Møller, M.S. Functional roles of N-terminal domains in pullulanase from human gut Lactobacillus acidophilus. J. Agric. Food Chem. 2023, 71, 18898–18908. [Google Scholar] [CrossRef]
- Deng, Z.; Li, D.; Wang, L.; Lan, J.; Wang, J.; Ma, Y. Activation of GABABR attenuates intestinal inflammation by reducing oxidative stress through modulating the TLR4/MyD88/NLRP3 pathway and gut microbiota abundance. Antioxidants 2024, 13, 1141. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X. Optimization of exopolysaccharide produced by Lactobacillus plantarum R301 and its antioxidant and anti-inflammatory activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef]
- Şirin, S. Lactic acid bacteria-derived exopolysaccharides mitigate the oxidative response via the NRF2-KEAP1 pathway in PC12 cells. Curr. Issues Mol. Biol. 2023, 45, 8071–8090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Bian, C.; Li, H.; Kang, Y.; Gao, Y.; Peng, Y.; Zhang, C. Structural Characterization and Antioxidant Activity of Exopolysaccharide Produced from Beet Waste Residue by Leuconostoc pseudomesenteroides. Antioxidants 2024, 13, 1289. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Boyajian, J.L.; Abosalha, A.; Nasir, A.; Ashfaq, I.; Islam, P.; Schaly, S.; Thareja, R.; Hayat, A.; Rehman, M.U. High-molecular-weight dextran-type exopolysaccharide produced by the novel Apilactobacillus waqarii improves metabolic syndrome: In vitro and in vivo analyses. Int. J. Mol. Sci. 2022, 23, 12692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, M.; Yao, M.; Naseeb, J.; Sarwar, A.; Yang, Z.; Aziz, T.; Alhomrani, M.; Alsanie, W.F.; Alamri, A.S. Lactiplantibacillus plantarum NMGL2 exopolysaccharide ameliorates DSS-induced IBD in mice mainly by regulation of intestinal tight junction and NF-κB p65 protein expression. Front. Microbiol. 2024, 15, 1491727. [Google Scholar] [CrossRef]
- Dentice Maidana, S.; Argañaraz Aybar, J.N.; Albarracin, L.; Imamura, Y.; Arellano-Arriagada, L.; Namai, F.; Suda, Y.; Nishiyama, K.; Villena, J.; Kitazawa, H. Modulation of the Gut–Lung Axis by Water Kefir and Kefiran and Their Impact on Toll-like Receptor 3-Mediated Respiratory Immunity. Biomolecules 2024, 14, 1457. [Google Scholar] [CrossRef]
- Tan, X.; Ma, B.; Wang, X.; Cui, F.; Li, X.; Li, J. Characterization of Exopolysaccharides from Lactiplantibacillus plantarum PC715 and Their Antibiofilm Activity Against Hafnia alvei. Microorganisms 2024, 12, 2229. [Google Scholar] [CrossRef]
- Ma, L.; Xu, X.; Peng, Q.; Yang, S.; Zhang, Y.; Tian, D.; Shi, L.; Qiao, Y.; Shi, B. Exopolysaccharide from Lactobacillus casei NA-2 attenuates Escherichia coli O157: H7 surface adhesion via modulation of membrane surface properties and adhesion-related gene expression. Microb. Pathog. 2022, 173, 105863. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-κB/MAPK pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wu, H.; Cao, X.; Gao, Z.; Tang, Z.; Fan, W.; Yan, L.; Liu, B.; Lin, H.; Song, S. Lactobacillus crispatus-derived exopolysaccharides with antibacterial activity limit Salmonella typhimurium invasion by inhibiting inflammasome-mediated pyroptosis. Food Funct. 2022, 13, 10501–10515. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, X.; Wei, T.; Wu, H.; Lu, J.; Lyu, M.; Wang, S. Anti-Helicobacter pylori activity of a Lactobacillus sp. PW-7 exopolysaccharide. Foods 2021, 10, 2453. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Pan, T.-M.; Wu, Y.-J.; Chang, S.-J.; Chang, M.-S.; Hu, C.-Y. Exopolysaccharide activities from probiotic bifidobacterium: Immunomodulatory effects (on J774A. 1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 2010, 144, 104–110. [Google Scholar] [CrossRef]
- Han, X.; Chen, Q.; Zhang, X.; Chen, X.; Luo, D.; Zhong, Q. Antibiofilm and antiquorum sensing potential of Lactiplantibacillus plantarum Z057 against Vibrio parahaemolyticus. Foods 2022, 11, 2230. [Google Scholar] [CrossRef]
- Malka, O.; Kalson, D.; Yaniv, K.; Shafir, R.; Rajendran, M.; Ben-David, O.; Kushmaro, A.; Meijler, M.M.; Jelinek, R. Cross-kingdom inhibition of bacterial virulence and communication by probiotic yeast metabolites. Microbiome 2021, 9, 70. [Google Scholar] [CrossRef]
- Kiran, N.S.; Yashaswini, C.; Singh, S.; Prajapati, B.G. Revisiting microbial exopolysaccharides: A biocompatible and sustainable polymeric material for multifaceted biomedical applications. 3 Biotech 2024, 14, 95. [Google Scholar] [CrossRef]
- Huang, C.-L.; Chu, H.-F.; Wu, C.-C.; Deng, F.-S.; Wen, P.-J.; Chien, S.-P.; Chao, C.-H.; Chen, Y.-T.; Lu, M.-K.; Tsai, Y.-C. Exopolysaccharide is the potential effector of Lactobacillus fermentum PS150, a hypnotic psychobiotic strain. Front. Microbiol. 2023, 14, 1209067. [Google Scholar] [CrossRef]
- Yue, F.; Han, H.; Xu, J.; Yao, X.; Qin, Y.; Zhang, L.; Sun, X.; Huang, J.; Zhang, F.; Lü, X. Effects of exopolysaccharides form Lactobacillus plantarum KX041 on high fat diet-induced gut microbiota and inflammatory obesity. Int. J. Biol. Macromol. 2025, 289, 138803. [Google Scholar] [CrossRef]
- Xie, Y.; Pei, F.; Liu, Y.; Liu, Z.; Chen, X.; Xue, D. Fecal fermentation and high-fat diet-induced obesity mouse model confirmed exopolysaccharide from Weissella cibaria PFY06 can ameliorate obesity by regulating the gut microbiota. Carbohydr. Polym. 2023, 318, 121122. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; An, S.; Kannan, P.R.; Wahab, A.; Ali, S.; Xiaoqing, L.; Suhail, M.; Iqbal, M.Z.; Kong, X. Development and characterization of biodegradable antibacterial hydrogels of xanthan gum for controlled ciprofloxacin release. Int. J. Biol. Macromol. 2025, 309, 142637. [Google Scholar] [CrossRef] [PubMed]
- López-Lozano, N.E.; Echeverría Molinar, A.; Ortiz Durán, E.A.; Hernández Rosales, M.; Souza, V. Bacterial diversity and interaction networks of Agave lechuguilla rhizosphere differ significantly from bulk soil in the oligotrophic basin of Cuatro Cienegas. Front. Plant Sci. 2020, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, K.; Liu, X.; Yao, L.; Chen, Z.; Han, H. Exopolysaccharide-Producing Bacteria Regulate Soil Aggregates and Bacterial Communities to Inhibit the Uptake of Cadmium and Lead by Lettuce. Microorganisms 2024, 12, 2112. [Google Scholar] [CrossRef]
- Prihatna, C.; Yan, Q. Exopolysaccharide is required by Paraburkholderia phytofirmans PsJN to confer drought-stress tolerance in pea. Front. Microbiol. 2024, 15, 1442001. [Google Scholar] [CrossRef]
- Bisson, G.; Comuzzi, C.; Giordani, E.; Poletti, D.; Boaro, M.; Marino, M. An exopolysaccharide from Leuconostoc mesenteroides showing interesting bioactivities versus foodborne microbial targets. Carbohydr. Polym. 2023, 301, 120363. [Google Scholar] [CrossRef]
- Azhar, M.; Chaudhary, A.; Gaba, S.; Bashir, L.; Kamra, A.; Kumar, S.N.; Singh, P.K.; Panwar, N. Evaluation of Antifungal and Biopreservative Potential of Orange Peel-derived Lactobacillus fermentum O1. 1 in Cherry Tomatoes. J. Food Prot. 2025, 88, 100541. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, K.-S. Enhancement of the phytoremediation performance in heavy metal-contaminated soil using a multifunctional EPS-producing bacterium Kosakonia sp. W18. Environ. Res. 2025, 274, 121355. [Google Scholar] [CrossRef]
- Vázquez-Vargas, C.C.; Cordero-Soto, I.N.; Flores-Maciel, H.A.; Lara-Ceniceros, T.E.; Gallegos-Infante, A.; González-Herrera, S.M.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Bioproduction of exopolysaccharides by lactic acid bacteria using agave by-products. Process Biochem. 2024, 146, 234–240. [Google Scholar] [CrossRef]
- Usmanova, A.; Brazhnikova, Y.; Omirbekova, A.; Kistaubayeva, A.; Savitskaya, I.; Ignatova, L. Biopolymers as Seed-Coating Agent to Enhance Microbially Induced Tolerance of Barley to Phytopathogens. Polymers 2024, 16, 376. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, G.; Guo, S.; Wang, G.; Ma, J.; Ma, C. Domain engineering of dextransucrase and soft nanoconfinement-enhanced biosynthesis of pharmaceutical-grade dextran. Int. J. Biol. Macromol. 2025, 319, 145257. [Google Scholar] [CrossRef]
- Wu, M.; Pakroo, S.; Nadai, C.; Molinelli, Z.; Speciale, I.; De Castro, C.; Tarrah, A.; Yang, J.; Giacomini, A.; Corich, V. Genomic and functional evaluation of exopolysaccharide produced by Liquorilactobacillus mali t6-52: Technological implications. Microb. Cell Factories 2024, 23, 158. [Google Scholar] [CrossRef]




| Group | Species Identified a |
|---|---|
| LAB | L. kefiranofaciens, Len. hilgardii, Ll. nagelii, Lp. plantarum, Lim. fermentum, Lac. casei, Lac. paracasei, Lim. reuteri, Len. diolivorans, Fr. farraginis, Ll. satsumensis, Lig. harbinensis, Lim. fermentum, Lp. plantarum, Api. kunkeei, Leuc. mesenteroides, Fr. hordei, Lev. brevis, Leuc. citreum |
| AAB | A. aceti, A. lovaniensis, A. tropicalis, A. indonesiensis, G. oxydans, A. pasteurianus, A. fabarum, A. orientalis, G. liquefaciens |
| Yeasts | S. cerevisiae, C. kefyr, C. guilliermondii, C. colliculosa, R. mucilaginosa, P. membranifaciens, P. occidentalis, H. valbyensis, Zt. florentina, D. bruxellensis, Z. lentus, T. delbrueckii, Lc. fermentati |
| Technique a | Purpose/Use | Advantages | Disadvantages |
| Culture-dependent isolation | Growth on selective media (e.g., MRS agar for LAB); colony morphology, Gram stain, catalase test | - Inexpensive and simple - Can isolate live strains for downstream use | - Misses non-culturable or slow-growing microbes - Biased toward dominant organisms |
| SDS-PAGE of whole-cell proteins | Phenotypic typing of LAB and related bacteria | - Provides strain-level fingerprints - Good for grouping isolates | - Labor-intensive - Low resolution for phylogeny |
| (GTG)5-PCR fingerprinting (Rep-PCR) | Genotyping of LAB strains | - High discriminatory power - Fast and reproducible for strain typing | - Requires reference patterns - Not ideal for unknown taxa |
| pheS gene sequencing | Precise identification of LAB to species level using phenylalanyl-tRNA synthase gene | - More specific than 16S rRNA - Good for closely related species | - Limited databases - Requires sequencing expertise |
| 16S rRNA gene sequencing (amplicon) | Culture-independent community profiling (mainly for bacteria) | - Detects both culturable and non-culturable species - Taxonomic assignment to genus/species level | - Low resolution for some genera - Bias from primers and PCR amplification |
| Shotgun metagenomics | Comprehensive analysis of total DNA from the microbial community | - Strain-level resolution - Can detect functional genes - Detects bacteria, archaea, yeasts, and viruses | - High cost and computational resources - DNA extraction must be efficient for all organisms |
| DGGE (Denaturing Gradient Gel Electrophoresis) | Community fingerprinting using 16S rDNA V3 region | - Detects dominant species - Allows visual comparison across samples | - Cannot resolve all species - Limited to most abundant populations |
| HPLC/GC (e.g., volatile/aromatic compounds) | Analyzes fermentation metabolites: organic acids, ethanol, and esters | - Indicates metabolic activity - Helps correlate microbes with functional traits | - Indirect method - Requires correlation with microbiota |
| Microscopy (light, SEM) | Visualizes kefir grain structure and microbial distribution | - Observes biofilms, morphology - Supports structural characterization | - Not taxonomically informative - Requires complementary methods |
| Co-culturing (e.g., Transwell system) | Assess microbial interaction (LAB/yeast mutualism) | - Reveals ecological relationships - Simulates natural consortia | - Not taxonomically specific - Complex to set up |
| Microbial Strain (Isolate) | Source Beverage | Monosaccharide Composition/EPS type | Potential Applications | Reference |
|---|---|---|---|---|
| Leuconostoc mesenteroides N06 | Pozol (maize) | Dextran–glucose (α-1→6 backbone, α-1→3 branches) | Food thickener; prebiotic carrier | [29] |
| Leuconostoc kimchii EPSA | Pulque | Dextran (α-1→6 main, α-1→2 and α-1→3 branches) | Bio-film matrix; functional food additive | [30] |
| Leuconostoc kimchii EPSB | Pulque | Cell-bound dextran + levan (β-2→6 fructan) | Encapsulation; viscosity modifier | [30] |
| Leuconostoc citreum/ L. kimchii | Pulque | Fructans (levan/inulin-type) | Prebiotics; viscosity enhancer | [31] |
| Ustilago maydis | Corn tejuino/native corn brew | β-(1→3/1→6) glucans (enzymatically confirmed) | Texture modifier; pharma precursor | [32] |
| Leuconostoc mesenteroides (SF-type) | Aguamiel (Agave salmiana) | Dextran (α-1→6 main, α-1→2 and α-1→3 branches) | Prebiotic fiber; food texture modifier | [33] |
| Zymomonas mobilis B-14023 | Aguamiel/Pulque | Levan–fructose (β-2→6) | Thickener; prebiotic; edible films | [34] |
| Weissella confusa WCP-3a | Pozol | Dextran (α-1→6 main, α-1→3 branches); high glucosyltransferase activity | Prebiotic soluble fiber; viscosity modifier | [35] |
| Leuconostoc citreum (pozol isolate) | Pozol | Inulin-type fructan (β-2→1) | Low-calorie sweetener; prebiotic | [29] |
| Type a | Main monomers | EPS | Microorganism | Application | Product | Ref. |
|---|---|---|---|---|---|---|
| HoPs | Glucose (α-1,6; α-1,3 branches) | Dextran | Leuconostoc mesenteroides, Lactobacillus | Thickener, texture enhancer, stabilizer | Bakery, beverages, additives, confectionery | [33,76] |
| HoPs | Fructose (β-2,6; β-2,1 branches) | Levan | Bacillus subtilis, Zymomonas mobilis | Sweetener, bulking agent, prebiotic | Pastries, beverages, dietary products | [76] |
| HoPs | Glucose (α-1,4; α-1,6 linkages) | Pullulan | Aureobasidium pullulans | Edible films, coatings, encapsulation | Fruits, candies, biodegradable films | [77,78] |
| HoPs | Glucose (β-1,3 linkages) | Curdlan | Agrobacterium spp. | Thermostable gels, stabilizer | Surimi, sausages, instant soups | [79,80] |
| HoPs | Glucose (β-1,4 linkages) | Bacterial cellulose | Komagataeibacter xylinus | Texture reinforcement, encapsulation matrices | Desserts, kombucha, low-calorie products | [81] |
| HoPs | Glucose (β-1,3 backbone, β-1,6 branches) | Scleroglucan | Sclerotium rolfsii | Viscosity agent, stabilizer | Sauces, dressings, soups | [73] |
| HePs | Glucose and galactose (1:1) | Kefiran | Lactobacillus kefiranofaciens | Viscosity, texture in fermented beverages | Kefir, yogurt | [82] |
| HePs | Glucose, rhamnose, glucuronic acid | Gellan | Sphingomonas elodea | Gelling agent, stable matrices | Desserts, confectionery, vegan gels | [83] |
| HePs | Glucose, mannose, glucuronic acid | Xanthan | Xanthomonas campestris | Thickener, stabilizer, viscosity | Sauces, dressings, gluten-free baking | [70] |
| HePs | β-D-mannuronic acid, α-L-guluronic acid | Bacterial alginate | Pseudomonas, Azotobacter | Encapsulation, gel formation | Minimally processed fruits, molecular caviar | [84] |
| Main Mechanism | Mode of Action | EPS (Strain) | Outcome Against GI Pathogens | Reference |
|---|---|---|---|---|
| Adhesion interference | Compete with bacterial lectins or modify surface charge/hydrophobicity, preventing initial attachment to epithelial cells or plastics | EPS-cn2 (Lactobacillus casei NA-2) | ↓ adhesion and ↓ early biofilm formation by E. coli O157:H7 | [107] |
| Direct structural disruption | Chelate Ca2+/Mg2+ and disorganize LPS or peptidoglycan via highly charged, branched conformations | EPS 7–4 (Lactobacillus crispatus); EPS from L. plantarum PC715 | Loss of membrane integrity and lysis of Salmonella Typhimurium | [109] |
| Gene-expression modulation/aggregation | Down-regulation of biofilm, motility and secretion-system genes; promotion of auto-aggregation that limits epithelial invasion | EPS-cn2 (L. casei NA-2); EPS from Lacticaseibacillus rhamnosus GG | ↓ LEE and other virulence genes in E. coli; aggregate formation and reduced invasion by S. Typhimurium | [107,108] |
| Quorum-sensing signal sequestration | Captures or blocks autoinducers (e.g., AI-2, AHL), reducing toxin production and biofilm maturation | Kefiran; EPS/extract from L. plantarum Z057 | ↓ reporter bioluminescence, ↓ toxins and thinner biofilm in Vibrio parahaemolyticus and other pathogens | [112,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oviedo-León, J.F.; Higuera, A.R.; Yáñez-Fernández, J.; Hernández-Sánchez, H.; Castro-Rodríguez, D.C. Exploring Exopolysaccharides Produced in Indigenous Mexican Fermented Beverages and Their Biotechnological Applications. Fermentation 2025, 11, 463. https://doi.org/10.3390/fermentation11080463
Oviedo-León JF, Higuera AR, Yáñez-Fernández J, Hernández-Sánchez H, Castro-Rodríguez DC. Exploring Exopolysaccharides Produced in Indigenous Mexican Fermented Beverages and Their Biotechnological Applications. Fermentation. 2025; 11(8):463. https://doi.org/10.3390/fermentation11080463
Chicago/Turabian StyleOviedo-León, Julián Fernando, Abril Ramírez Higuera, Jorge Yáñez-Fernández, Humberto Hernández-Sánchez, and Diana C. Castro-Rodríguez. 2025. "Exploring Exopolysaccharides Produced in Indigenous Mexican Fermented Beverages and Their Biotechnological Applications" Fermentation 11, no. 8: 463. https://doi.org/10.3390/fermentation11080463
APA StyleOviedo-León, J. F., Higuera, A. R., Yáñez-Fernández, J., Hernández-Sánchez, H., & Castro-Rodríguez, D. C. (2025). Exploring Exopolysaccharides Produced in Indigenous Mexican Fermented Beverages and Their Biotechnological Applications. Fermentation, 11(8), 463. https://doi.org/10.3390/fermentation11080463






