Production of Vegan Ice Cream: Enrichment with Fermented Hazelnut Cake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Fermented Hazelnut Oil Cake
2.3. Ice Cream Production
2.4. Characterization of Ice Cream Products
3. Results and Discussions
3.1. General Characterization of Ice Cream Products
3.2. Volatile Compounds in Ice Cream Products
3.3. Flow Behavior of Ice Cream Products
3.4. Overrun and Melting Properties of Ice Cream Products
3.5. Textural Properties of Ice Cream Products
3.6. Antioxidant Activity Profiles of Ice Cream Products Before and After In Vitro Digestion
3.7. Enzyme Inhibition Profiles of Ice Cream Products Before and After In Vitro Digestion
3.8. Sensorial Evaluation of Ice Cream Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goff, H.D.; Hartel, R.W.; Rankin, S.A. Ice Cream; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Clarke, C.; Cox, A. The Science of Ice Cream; Royal Society of Chemistry: London, UK, 2024. [Google Scholar] [CrossRef]
- Genovese, A.; Balivo, A.; Salvati, A.; Sacchi, R. Functional Ice Cream Health Benefits and Sensory Implications. Food Res. Int. 2022, 161, 111858. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-Based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Barker, S.; Falkeisen, A.; Gorman, M.; Knowles, S.; McSweeney, M.B. An Investigation into Consumer Perception and Attitudes towards Plant-Based Alternatives to Milk. Food Res. Int. 2022, 159, 111648. [Google Scholar] [CrossRef]
- Pontonio, E.; Montemurro, M.; Dingeo, C.; Rotolo, M.; Centrone, D.; Carofiglio, V.E.; Rizzello, C.G. Design and Characterization of a Plant-Based Ice Cream Obtained from a Cereal/Legume Yogurt-Like. LWT 2022, 161, 113327. [Google Scholar] [CrossRef]
- Boukid, F.; Rosell, C.M.; Rosene, S.; Bover-Cid, S.; Castellari, M. Non-Animal Proteins as Cutting-Edge Ingredients to Reformulate Animal-Free Foodstuffs: Present Status and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 6390–6420. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies, and Health Effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Ozdemir, M.B.; Kılıçarslan, E.; Demir, H.; Koca, E.; Salum, P.; Berktaş, S.; Çam, M.; Erbay, Z.; Aydemir, L.Y. Upgrading the Bioactive Potential of Hazelnut Oil Cake by Aspergillus Oryzae under Solid-State Fermentation. Molecules 2024, 29, 4237. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Available online: https://www.grandviewresearch.com/press-release/global-vegan-food-market# (accessed on 31 July 2025).
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Tabanelli, G.; Pasini, F.; Riciputi, Y.; Vannini, L.; Gozzi, G.; Balestra, F.; Caboni, M.F.; Gardini, F.; Montanari, C. Fermented Nut-Based Vegan Food: Characterization of a Home Made Product and Scale-Up to an Industrial Pilot-Scale Production. J. Food Sci. 2018, 83, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, F.D.; Adrar, N.; Bolling, B.W.; Capanoglu, E. Valorisation of Hazelnut By-Products: Current Applications and Future Potential. Biotechnol. Genet. Eng. Rev. 2022, 39, 586–621. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New Developments in Solid State Fermentation: I-Bioprocesses and Products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 409606. [Google Scholar] [CrossRef]
- Saharan, P.; Sadh, P.K.; Duhan, S.; Duhan, J.S. Bio-Enrichment of Phenolic, Flavonoids Content and Antioxidant Activity of Commonly Used Pulses by Solid-State Fermentation. J. Food Meas. Charact. 2020, 14, 1497–1510. [Google Scholar] [CrossRef]
- Atalar, I.; Kurt, A.; Gul, O.; Yazici, F. Improved Physicochemical, Rheological and Bioactive Properties of Ice Cream: Enrichment with High Pressure Homogenized Hazelnut Milk. Int. J. Gastron. Food Sci. 2021, 24, 100358. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. Some Physicochemical and Functional Properties of Alfalfa Soluble Leaf Proteins. LWT-Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Tamayo Tenorio, A.; Gieteling, J.; De Jong, G.A.H.; Boom, R.M.; Van Der Goot, A.J. Recovery of Protein from Green Leaves: Overview of Crucial Steps for Utilisation. Food Chem. 2016, 203, 402–408. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- AACC Method 30-25; Crude Fat in Wheat, Corn and Soy Flour, Feeds, and Mixed Feeds. AACC: Arnold, MD, USA, 2009.
- AACC Method 08-01; Ash–Basic Method. AACC: Arnold, MD, USA, 2009.
- Salum, P.; Erbay, Z.; Kelebek, H.; Selli, S. Optimization of Headspace Solid-Phase Microextraction with Different Fibers for the Analysis of Volatile Compounds of White-Brined Cheese by Using Response Surface Methodology. Food Anal. Methods 2017, 10, 1956–1964. [Google Scholar] [CrossRef]
- van Den Dool, H.; Dec Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Muse, M.R.; Hartel, R.W. Ice Cream Structural Elements That Affect Melting Rate and Hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef]
- Acu, M.; Kinik, O.; Yerlikaya, O. Probiotic Viability, Viscosity, Hardness Properties and Sensorial Quality of Synbiotic Ice Creams Produced from Goat’s Milk. Food Sci. Technol. 2021, 41, 167–173. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl Radical Scavenging Activity of Compatible Solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioglu, A. Antioxidant Activity of Protein Extracts from Heat-Treated or Thermally Processed Chickpeas and White Beans. Food Chem. 2007, 103, 301–312. [Google Scholar] [CrossRef]
- Aydemir, L.Y.; Gökbulut, A.A.; Baran, Y.; Yemenicioǧlu, A. Bioactive, Functional and Edible Film-Forming Properties of Isolated Hazelnut (Corylus avellana L.) Meal Proteins. Food Hydrocoll. 2014, 36, 130–142. [Google Scholar] [CrossRef]
- Vinutha, B.; Prashanth, D.; Salma, K.; Sreeja, S.L.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of Selected Indian Medicinal Plants for Acetylcholinesterase Inhibitory Activity. J. Ethnopharmacol. 2007, 109, 359–363. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Aydemir, L.Y.; Diblan, S.; Aktas, H.; Cakitli, G. Changes in Bioactive Properties of Dry Bean Extracts during Enzymatic Hydrolysis and in Vitro Digestion Steps. J. Food Meas. Charact. 2022, 16, 3682–3698. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different Polyphenolic Components of Soft Fruits Inhibit α-Amylase and α-Glycosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- ISO 11136; Sensory Analysis-Methodology-General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. ISO: Geneva, Switzerland, 2014.
- Salum, P.; Erbay, Z. Variation of Volatile Composition during the Production of Microencapsulated Cream Powder. Int. Dairy J. 2021, 118, 105047. [Google Scholar] [CrossRef]
- Ertekin, M.; Uğurlu, Ö.; Salum, P.; Erbay, Z. Effects of Milk Types Used in Antep Cheese Production on Some Cheese Organoleptic Quality Parameters and Brine Composition during 5-Month Ripening. J. Food Sci. 2023, 88, 1445–1465. [Google Scholar] [CrossRef]
- Santos, J.E.; Villarino, B.; Zosa, A.; Dayrit, F. Analysis of Volatile Organic Compounds in Virgin Coconut Oil and Their Sensory Attibutes. Philipp. J. Sci. 2011, 140, 161–171. [Google Scholar]
- Masson, F.; Hinrichsen, L.; Talon, R.; Montel, M.C. Factors Influencing Leucine Catabolism by a Strain of Staphylococcus Carnosus. Int. J. Food Microbiol. 1999, 49, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Tzia, C. Grape, Raisin and Sugarcane Molasses as Potential Partial Sucrose Substitutes in Chocolate Ice Cream: A Feasibility Study. Int. Dairy J. 2018, 76, 18–29. [Google Scholar] [CrossRef]
- Adapa, S.; Schmidt, K.A.; Jeon, I.J.; Herald, T.J.; Flores, R.A. Mechanisms of Ice Crystallization and Recrystallization in Ice Cream: A Review. Food Rev. Int. 2000, 16, 259–271. [Google Scholar] [CrossRef]
- Soukoulis, C.; Lebesi, D.; Tzia, C. Enrichment of Ice Cream with Dietary Fibre: Effects on Rheological Properties, Ice Crystallisation and Glass Transition Phenomena. Food Chem. 2009, 115, 665–671. [Google Scholar] [CrossRef]
- Aboulfazli, F.; Baba, A.S.; Misran, M. Effects of Fermentation by Bifidobacterium Bifidum on the Rheology and Physical Properties of Ice Cream Mixes Made with Cow and Vegetable Milks. Int. J. Food Sci. Technol. 2015, 50, 942–949. [Google Scholar] [CrossRef]
- Pon, S.Y.; Lee, W.J.; Chong, G.H. Textural and Rheological Properties of Stevia Ice Cream. Int. Food Res. J. 2015, 22, 1544–1549. [Google Scholar]
- Sofjan, R.P.; Hartel, R.W. Effects of Overrun on Structural and Physical Characteristics of Ice Cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Flores, A.A.; Goff, H.D. Ice Crystal Size Distributions in Dynamically Frozen Model Solutions and Ice Cream as Affected by Stabilizers. J. Dairy Sci. 1999, 82, 1399–1407. [Google Scholar] [CrossRef]
- Chang, Y.; Hartel, R.W. Development of Air Cells in a Batch Ice Cream Freezer. J. Food Eng. 2002, 55, 71–78. [Google Scholar] [CrossRef]
- Ervina; Surjawan, I.; Abdillah, E. The Potential of Avocado Paste (Persea americana) as Fat Substitute in Non-Dairy Ice Cream. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012006. [Google Scholar] [CrossRef]
- Güven, M.; Kalender, M.; Taşpinar, T. Effect of Using Different Kinds and Ratios of Vegetable Oils on Ice Cream Quality Characteristics. Foods 2018, 7, 104. [Google Scholar] [CrossRef]
- Syed, Q.; Anwar, S.; Shukat, R.; Zahoor, T. Effects of Different Ingredients on Texture of Ice Cream. J. Nutr. Health Food Eng. 2018, 8, 422–435. [Google Scholar] [CrossRef]
- Leahu, A.; Ropciuc, S.; Ghinea, C. Plant-Based Milks: Alternatives to the Manufacture and Characterization of Ice Cream. Appl. Sci. 2022, 12, 1754. [Google Scholar] [CrossRef]
- Ghaderi, S.; Mazaheri Tehrani, M.; Hesarinejad, M.A. Qualitative Analysis of the Structural, Thermal and Rheological Properties of a Plant Ice Cream Based on Soy and Sesame Milks. Food Sci. Nutr. 2021, 9, 1289–1298. [Google Scholar] [CrossRef]
- Varela, P.; Pintor, A.; Fiszman, S. How Hydrocolloids Affect the Temporal Oral Perception of Ice Cream. Food Hydrocoll. 2014, 36, 220–228. [Google Scholar] [CrossRef]
- Poursani, P.; Razavi, S.M.A.; Mazaheri Tehrani, M.; Javidi, F. Rheological, Physical, and Sensory Properties of Non-Fat Ice Creams as Affected by Selected Fat Replacers. J. Food Process Preserv. 2021, 45, e15010. [Google Scholar] [CrossRef]
- Barros, E.L.d.S.; Silva, C.C.; Canella, M.H.M.; Verruck, S.; Prestes, A.A.; Vargas, M.O.; Maran, B.M.; Esmerino, E.A.; Silva, R.; Balthazar, C.F.; et al. Effect of Replacement of Milk by Block Freeze Concentrated Whey in Physicochemical and Rheological Properties of Ice Cream. Food Sci. Technol. 2021, 42, e12521. [Google Scholar] [CrossRef]
- Karaca, O.B.; Güven, M.; Yasar, K.; Kaya, S.; Kahyaoglu, T. The Functional, Rheological and Sensory Characteristics of Ice Creams with Various Fat Replacers. Int. J. Dairy Technol. 2009, 62, 93–99. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Bohn, T. Dietary Factors Affecting Polyphenol Bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Soukoulis, C.; Fisk, I.D.; Bohn, T. Ice Cream as a Vehicle for Incorporating Health-Promoting Ingredients: Conceptualization and Overview of Quality and Storage Stability. Compr. Rev. Food Sci. Food Saf. 2014, 13, 627–655. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and Identification of Novel Antidiabetic and Anti-Obesity Peptides from Camel Milk Protein Hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Shamsia, S.; Conte, A. Release of Angiotensin Converting Enzyme-Inhibitory Peptides during in Vitro Gastro-Intestinal Digestion of Camel Milk. Int. Dairy J. 2016, 56, 119–128. [Google Scholar] [CrossRef]
- Simsek, S. Angiotensin I-Converting Enzyme, Dipeptidyl Peptidase-IV, and α-Glucosidase Inhibitory Potential of Hazelnut Meal Protein Hydrolysates. J. Food Meas. Charact. 2021, 15, 4490–4496. [Google Scholar] [CrossRef]
- Song, W.; Fu, J.; Zeng, Q.; Lu, H.; Wang, J.; Fang, L.; Liu, X.; Min, W.; Liu, C. Improving ACE Inhibitory Activity of Hazelnut Peptide Modified by Plastein: Physicochemical Properties and Action Mechanism. Food Chem. 2023, 402, 134498. [Google Scholar] [CrossRef]
- Keyf, P.; Uğurlu, Ö.; Erkin, Ö.C.; Aydemir, L.Y.; Erbay, Z. Bioactive Potential of Ripened White Cheeses Manufactured in Different Geographical Regions of Turkey. J. Food Sci. 2023, 88, 4731–4744. [Google Scholar] [CrossRef]
- Altınyüzük Akıllı, A.; Erkin, Ö.C.; Aydemir, L.Y.; Erbay, Z. Variation of Bioactive Potentials during the Production of Enzyme-Modified Cheese. Int. Dairy J. 2023, 147, 105788. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Pešić, M.M.; Kostić, A.; Stanojević, S.P.; Pešić, M.B. The Bioaccessibility of Grape-Derived Phenolic Compounds: An Overview. Foods 2025, 14, 607. [Google Scholar] [CrossRef]
- Pintać Šarac, D.; Tremmel, M.; Vujetić, J.; Torović, L.; Orčić, D.; Popović, L.; Mimica-Dukić, N.; Lesjak, M. How Do in Vitro Digestion and Cell Metabolism Affect the Biological Activity and Phenolic Profile of Grape Juice and Wine. Food Chem. 2024, 449, 139228. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.I.; Noh, S.K. Green Tea as Inhibitor of the Intestinal Absorption of Lipids: Potential Mechanism for Its Lipid-Lowering Effect. J. Nutr. Biochem. 2007, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.H.; Song, D.G.; Kim, S.M.; Pan, C.H. Inhibition of Gastrointestinal Lipolysis by Green Tea, Coffee, and Gomchui (Ligularia fischeri) Tea Polyphenols during Simulated Digestion. J. Agric. Food Chem. 2012, 60, 7152–7157. [Google Scholar] [CrossRef] [PubMed]
- Mathiassen, J.H.; Nejrup, R.G.; Frøkiær, H.; Nilsson, Å.; Ohlsson, L.; Hellgren, L.I. Emulsifying Triglycerides with Dairy Phospholipids Instead of Soy Lecithin Modulates Gut Lipase Activity. Eur. J. Lipid Sci. Technol. 2015, 117, 1522–1539. [Google Scholar] [CrossRef]


| Dairy Ice Cream (DIC) (100 g) | Hazelnut Cake Ice Cream (HIC) (100 g) | Fermented Hazelnut Cake Ice Cream (FHIC) (100 g) |
|---|---|---|
| 18 g powdered sugar | 13.45 g sweetener | 13.45 g sweetener |
| 0.75 g salep | 0.75 g salep | 0.75 g salep |
| 16.4 g dairy cream (35% oil content) | 8 g coconut oil | 8 g coconut oil |
| 73.25 g milk | 77.8 g hazelnut cake beverage (10% w/v) | 77.8 g fermented hazelnut cake beverage (5% w/v) |
| HOC | FHOC | |
|---|---|---|
| Dry matter (%) | 91.08 ± 0.02 a,† | 85.43 ± 0.23 b |
| Water activity (25 °C) | 0.733 ± 0.00 a | 0.704± 0.00 b |
| Protein (%) | 58.02 ± 0.77 b | 64.64 ± 0.28 a |
| Lipid (%) | 8.41 ± 0.12 b | 9.19± 0.35 a |
| Ash (%) | 6.05 ± 0.00 b | 7.11 ± 0.0 a |
| Carbohydrate (%) | 27.52 | 19.06 |
| Color values | ||
| L* | 77.34 ± 0.03 a | 54.83 ± 0.38 b |
| a* | 2.39 ± 0.010 b | 6.39 ± 0.07 a |
| b* | 13.18 ± 0.05 b | 16.47 ± 0.08 a |
| DIC | HIC | FHIC | |
|---|---|---|---|
| Dry matter (%) | 30.95 ± 0.21 c,† | 29.10 ± 0.14 b | 24.35 ± 0.35 a |
| Protein (%) | 2.33 ± 0.03 c | 3.67 ± 0.04 b | 2.08 ± 0.07 a |
| Lipid (%) | 7.88 ± 0.18 a | 8.00 ± 0.35 a | 8.25 ± 0.71 a |
| Ash (%) | 0.51 ± 0.01 c | 0.44 ± 0.00 b | 0.24 ± 0.03 a |
| Carbohydrate (%) | 20.07 | 16.99 | 14.02 |
| Dietary fiber (%) | 3.8 | 3.22 | 3.16 |
| Calorie (kcal)/100 g | 162 | 133 | 115 |
| pH | 6.80 ± 0.02 b | 6.60 ± 0.02 c | 8.17 ± 0.12 a |
| Color values | |||
| L* | 86.52 ± 0.40 c | 76.12 ± 0.06 b | 63.76 ± 0.87 a |
| a* | 0.21 ± 0.04 c | 1.63 ± 0.01 b | 3.33 ± 0.13 a |
| b* | 9.64 ± 0.13 b | 9.35 ± 0.08 b | 13.49 ± 0.42 a |
| LRI * | Compound | DIC | HIC | FHIC |
|---|---|---|---|---|
| 1210 | Limonene | - | 46.0 ± 2.2 | - |
| 1215 | 3-Methyl-1-butanol | - | - | 13.2 ± 0.2 |
| 1445 | Acetic acid | 15.3 ± 0.2 | 13.7 ± 0.6 | 14.5 ± 0.4 |
| 1506 | Pyrol | - | - | 39.2 ± 1.4 |
| 1525 | Benzaldehyde | - | - | 182.3 ± 2.7 |
| 1571 | 2-Methyl Propanoic acid | - | 32.2 ± 0.1 | |
| 1843 | Hexanoic acid | 15.5 ± 1.1 | 6.0 ± 0.1 | - |
| 1958 | δ-Octalactone | - | 108.3 ± 0.9 | 172.2 ± 2.6 |
| 2072 | Octanoic acid | 116.0 ± 4.9 | 43.0 ± 3.4 | 50.2 ± 3.0 |
| 2079 | p-Cresol | - | - | 9.1 ± 0.2 |
| 2180 | δ-Decalactone | - | 49.2 ± 1.6 | 94.8 ± 2.1 |
| 2413 | Benzoic acid | - | - | 7.5 ± 0.1 |
| Ice Cream | 25 °C | 35 °C | ||
|---|---|---|---|---|
| n (-) | k (Pa.sn) | n (-) | k (Pa.sn) | |
| DIC | 0.48 ± 0.01 a | 40.57 ± 1.49 c | 0.29 ± 0.04 a | 80.87 ± 14.08 b |
| HIC | 0.61 ± 0.02 c | 26.94 ± 2.14 b | 0.42 ± 0.11 a | 55.94 ± 21.41 b |
| FHIC | 0.51 ± 0.00 b | 2.63 ± 0.08 a | 0.63 ± 0.00 b | 1.85 ± 0.07 a |
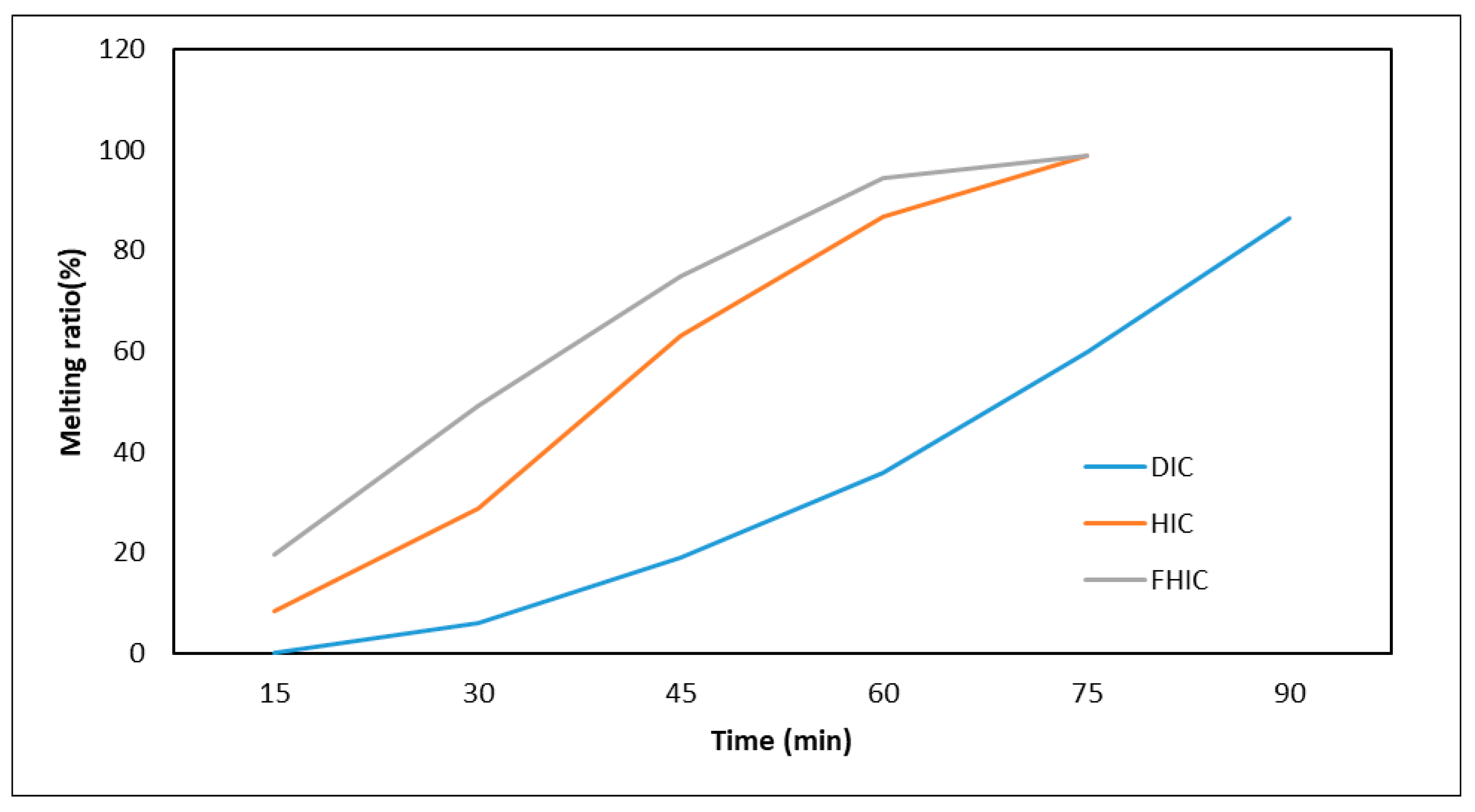
| Ice Cream | Overrun (%) | First Melting Time (min) | Melting Time (min) | Melting Ratio (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 15th min | 30th min | 45th min | 60th min | 75th min | 90th min | ||||
| DIC | 15.39 ± 0.41 c | 17.5 ± 0.7 c | 99.2 ± 0.0 b | - | 5.8 ± 0.6 | 19.0 ± 0.6 | 36.0 ± 7.8 | 59.8 ± 2.0 | 86.5 ± 4.7 |
| HIC | 4.01 ± 0.82 b | 6.0 ± 0.0 b | 68.0 ± 0.7 a | 8.40 ± 0.1 | 28.8 ± 0.9 | 63.0 ± 2.6 | 86.8 ± 2.1 | - | - |
| FHIC | 7.00 ± 0.50 a | 3.0 ± 0.3 a | 66.5 ± 1.1 a | 19.7 ± 0.3 | 49.1 ± 0.0 | 74.8 ± 0.3 | 94.3 ± 0.8 | - | - |
| Ice Cream | ABTS+ Radical Scavenging Activity (μmol Trolox/g) | DPPH Radical Scavenging Activity (μmol Trolox/g) | Hydroxyl Radical Scavenging Activity (mg Ascorbic Acid/g) | Metal (Fe+2) Chelating Activity (%) |
|---|---|---|---|---|
| DIC | 2.25 ± 0.04 a | N.D. | 183.7 ± 2.9 c | N.D. |
| HIC | 2.20 ± 0.05 a | 0.68 ± 0.28 a | 193.7 ± 3.4 a | 31.26 ± 1.10 |
| FHIC | 2.91 ± 0.00 a | 0.73 ± 0.23 a | 188.4 ± 1.8 b | 2.55 ± 0.09 |
| In vitro digestion | ||||
| DIC | 11.87 ± 0.39 c | 1.44 ± 0.04 b | N.D. | N.D. |
| HIC | 4.01 ± 0.09 b | 1.71 ± 0.06 c | N.D. | N.D. |
| FHIC | 10.03 ± 0.13 c | 4.73 ± 0.07 d | N.D. | N.D. |
| Ice Cream | α-Amylase/α-Glucosidase Inhibition (%) | ACE Inhibition (mg captopril/g) | AChE Inhibition (%) | Lipase Inhibition (%) |
|---|---|---|---|---|
| DIC | N.D. | N.D. | N.D. | 45.12 ± 0.34 a |
| HIC | N.D. | 0.76 ± 0.01 d | N.D. | 39.40 ± 2.98 b |
| FHIC | N.D. | N.D. | N.D. | 39.94 ± 2.23 b |
| In vitro digestion | ||||
| DIC | N.D. | 7.10 ± 0.47 b | 51.56 ± 0.23 c | N.D. |
| HIC | N.D. | 7.42 ± 0.08 a | 74.37 ± 1.56 a | N.D. |
| FHIC | N.D. | 4.85 ± 0.03 c | 57.69 ± 0.84 b | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydemir, L.Y.; Demir, H.; Erbay, Z.; Kılıçarslan, E.; Salum, P.; Ozdemir, M.B. Production of Vegan Ice Cream: Enrichment with Fermented Hazelnut Cake. Fermentation 2025, 11, 454. https://doi.org/10.3390/fermentation11080454
Aydemir LY, Demir H, Erbay Z, Kılıçarslan E, Salum P, Ozdemir MB. Production of Vegan Ice Cream: Enrichment with Fermented Hazelnut Cake. Fermentation. 2025; 11(8):454. https://doi.org/10.3390/fermentation11080454
Chicago/Turabian StyleAydemir, Levent Yurdaer, Hande Demir, Zafer Erbay, Elif Kılıçarslan, Pelin Salum, and Melike Beyza Ozdemir. 2025. "Production of Vegan Ice Cream: Enrichment with Fermented Hazelnut Cake" Fermentation 11, no. 8: 454. https://doi.org/10.3390/fermentation11080454
APA StyleAydemir, L. Y., Demir, H., Erbay, Z., Kılıçarslan, E., Salum, P., & Ozdemir, M. B. (2025). Production of Vegan Ice Cream: Enrichment with Fermented Hazelnut Cake. Fermentation, 11(8), 454. https://doi.org/10.3390/fermentation11080454








