1. Introduction
Changes in climate and climate variability will affect livestock production systems in all parts of the world, and will inevitably impact the 1.3 billion people in poverty whose livelihoods are wholly or partially dependent on livestock [
1]. Therefore, livestock keepers will have to mitigate emissions as well as adapt to the changing climate. The immediate need for livestock researchers aiming to counter climate change’s impact on livestock production is to understand the biology of climate change impact response components in depth and to study measures of animal well-being, giving a basis for predicting when an animal is under stress or distress and in need of attention [
2].
The future research needs for ameliorating heat stress in livestock are to identify strategies for developing and monitoring appropriate measures of heat stress; assess genetic components, including genomics and proteomics of heat stress in livestock; and develop alternative management practices to reduce heat stress and improve animal well-being and performance [
3]. Since climate change could result in an increase in heat stress, all methods to help animals cope with, or, at least, alleviate the impacts of heat stress could be useful to mitigate the impacts of global change on animal responses and performance [
4].
A recent literature survey clearly projects goats as being the ideal climate-resilient species [
5]. In the current climate change scenario, it is very essential to identify potential indigenous breeds which have the ability to survive in multiple environmental locations. Such an approach could help farming communities to capitalize on the most suited breeds to ensure their livelihood security [
6]. Further, assessing the adaptive capability based on rumen fermentation pattern and metagenomics analysis is a novel approach in elucidating the molecular mechanisms governing livestock adaptation [
7,
8].
Heat stress can either directly or indirectly affect the health and welfare of animals by impairing the rumen functions and intestinal mechanisms, resulting in reduced feed utilization [
9]. Thus, regulating rumen microbial fermentation is essential for the use of nutrients and additives to alleviate the negative effects of heat stress. Heat stress influences the rumen microbiome in ruminant animals [
9,
10]. The literature suggests that the energy supply deficiency during heat stress exposure can be reverted by manipulating the rumen bacteria [
10]. Similarly the rumen microbiota was also found to be altered at high altitude and this alteration was found to potentially aid adaptation to the harsh climate conditions on the plateau [
11]. Further, heat stress was found to have an effect on the microbiota and alter the concentration of some fatty acids in the rumen of cattle [
12]. Further, there are also reports reflecting a significant role of the rumen microbiome in inducing adaptive potential in heat-stressed animals [
13,
14].
Based on this background, a study was conducted to comparatively assess the adaptive capabilities of the indigenous Nandidurga and Bidri goat breeds. The primary objective of this study was to comparatively assess the resilience/adaptive capabilities of these two breeds based on changes associated with rumen fermentation pattern and the distribution pattern of the rumen microbiota. Such comparisons can aid in identifying the better-suited breed.
2. Materials and Methods
2.1. Study Location
This experiment was carried out at the experimental livestock farm of the National Institute of Animal Nutrition and Physiology, Bengaluru, India, on the southern Deccan plateau of the country, located at latitude 77°36′25.3″ E and longitude 12°57′04.3″ N, and at an altitude of 920 m above the mean sea level. The mean annual ambient temperature and relative humidity (RH) range from 15 to 36 °C and 20 to 85 percent, respectively. The experiment was conducted from May to July. The annual minimum and maximum temperature range between 15 and 22 °C and 27–34 °C, respectively. The annual RH variations during the study period ranged between 28 and 59 percent under the hot semi-arid environment.
2.2. Animals and Technical Design
Two different indigenous goat breeds of Karnataka in southern peninsular India, Nandidurga and Bidri, were used in this experiment. Both breeds belong to meat-type goats and are well known for their adaptive capabilities to the tropical climate. This study was conducted for a period of 45 days wherein 24 female goats of one year of age (12 Nandidurga breed and 12 Bidri breed) were used. This study was conducted after obtaining approval from the committee for the purpose of control and supervision of experiments on animals (CPCSEA), Ministry of Environment, Forest and Climate Change, Government of India, [Refernce: F.No.25/4/2019-CPCSEA dated 20 March 2019].
The animals were randomly allocated into four groups of six animals each, NC (n = 6; Nandidurga control), NHS (n = 6; Nandidurga heat stress), BC (n = 6; Bidri control) and BHS (n = 6; Bidri heat stress). The animals were stall-fed with a diet consisting of 60% roughage (Hybrid Napier) and 40% concentrate on the basis of a 3% body weight on a dry matter basis (
Supplementary Table S1). The NC and BC animals were maintained in the thermoneutral condition in the climatic chamber while the NHS and BHS animals were exposed to heat stress between 10:00 h to 16:00 h in the climate chamber during the experimental period. All group animals were fed and watered individually inside the climate chamber. All the cardinal weather parameters were recorded twice daily both inside and outside the climate chambers. After 16:00 h both the control and heating chambers were turned off and run again the next day before 10:00 h.
The weather variables, dry bulb temperature and wet bulb temperature, ambient temperature and relative humidity were recorded using dry bulb and wet bulb thermometers and a thermo-hygrometer, respectively. The weather variables were recorded twice daily (8:00 h and 14:00 h) during the entire duration of the study.
The McDowell [
15] equation was followed to calculate the temperature humidity index (
THI), as given below:
where
Tdb = dry bulb temperature and
Twb = wet bulb temperature
2.3. Climate Chamber Details
In order to conduct an environmental-stress-related study effectively, two climate chambers (thermal comfort chamber and heating climate chamber) are designed with a programmable thermostat, which can stabilize the required temperature and humidity within both chambers. Since it is well established that at the temperature range of 20–28 °C, small ruminant species remain thermally comfortable [
16], the thermal comfort chamber was maintained at 24 °C throughout the period. The simulation was carried out for six hours a day from 10:00 h to 16:00 h for the NC and BC groups of goats. Similarly, heat stress was induced to the NHS and BHS goats in the heating climate chamber. In the heating chamber, the temperature and relative humidity were gradually altered based on the simulated heat stress model. This depicted model was designed using baseline meteorological data of environmental temperature and relative humidity for every hour in the summer season. This model was exclusively designed to mimic the external heat stress conditions, especially at the time of grazing for a span of six hours (10.00 h to 16.00 h). Moreover, this model also eliminated the constant heat stress on animals, which is usually adopted on most of the heat stress associated studies. Thus, the weather variables are controlled for an experimental period of 10.00 h to 16.00 h in both the chambers to mimic the external environment’s comfort and the heat stress conditions in the climate chambers. In the heating chamber, the temperature and RH were 36 °C and 49% RH at 10:00 h; 37.5 °C and 44% RH at 11:00 h; 38.5 °C and 41% RH at 12:00 h; 38.8 °C and 40% RH at 13:00 h; 40.0 °C and 39% RH at 14:00 h; 38.3 °C and 42% RH at 15:00 h and 37.0°C and 45% RH at 16:00 h. Therefore, from an animal welfare perspective, the stimulated heat stress model was considered to be more practical and less stressful than traditional constant heat stress models.
2.4. Rumen Enzyme Assay
An aliquot (10 mL) of rumen fluid was centrifuged at 14,000×
g for 20 min at 4 °C to obtain the supernatant for extracellular (EC) enzyme activity and pellet for intracellular (IC) enzyme activity. The crude enzyme solutions from the supernatants and pellets were extracted following the procedure described previously [
7]. The activities of carboxy methyl cellulase (CMCase) and αamylase were assayed by using dinitrosalicylic acid (DNS) [
17]. The assay mixture contained 1 mL phosphate buffer, 0.5 mL of sample (both EC and IC enzyme fractions) and 0.5 mL of carboxy methyl cellulose solution (10 g/L) with an incubation time of 1 h at 39 °C for the estimation of carboxy methyl cellulose activity and 0.5 mL phosphate buffer, 0.25 mL starch solution (10 g/L) and 0.25 mL sample with an incubation time of 30 min at 39 °C for the estimation of αamylase. The amount of reducing sugar (glucose) released was measured colorimetrically [
7] using three replicates per sample. The enzyme activities were expressed as μmol of reducing sugars released per hour per mL under the assay conditions.
2.5. Volatile Fatty Acids
The rumen liquor collected was processed for VFA analysis as per the protocol by Chaidanya et al. [
7]. The total and individual VFA content in the rumen fluid samples were determined using gas chromatography (GC) (Agilent; Model 7890A GC System, Shanghai, China) equipped with a flame ionization detector (FID), programmable temperature vaporizer injector and capillary column (Agilent J&W DB-WAX GC Column; length 40 m; internal diameter 0.18 mm; film 0.18 µm). The analytical conditions for the fractionation of VFAs were an injection port temperature of 250 °C and a column temperature step up from 60 to 200 °C in 7 min with a hold time of 10 min and a detector temperature maintained at 300 °C with GC grade air; hydrogen and nitrogen were used as carrier gases with a flow rate of 1.0 mL/min. The samples were injected by an automatic injector at an injection volume of 1 μL using the split method and a 30:1 splitting ratio. Standard solutions of an appropriate concentration (mmol/L) were prepared from the individual substances, namely acetic acid, propionic acid, iso-butyric acid, butyric acid, iso valeric acid and valeric acid of analytical purity (Sigma-Aldrich, St. Louis, MO, USA) and the individual volatile fatty acids in the rumen liquor sample were identified based on the retention time of the standard. The concentrations of the individual VFAs in the rumen liquor samples were determined by recording the area of both the VFA mixed standards as well as the sample and expressed as mmol/L.
2.6. Rumen Metagenomics: 16S V3–V4 rRNA Sequencing
On the last day of the experimental study, rumen liquor was collected from a total of 16 goats (4 from each group) using a stomach tube connected to the handheld pump. The collected samples were processed for DNA extraction and the further V3–V4 16s rRNA sequencing pipeline at M/s Clevergene, Bangalore. From the collected rumen liquor, 250 μL was taken for DNA extraction, which was performed using the DNeasy PowerSoil Kit (Qiagen) following the manufacturer’s protocol. The extracted DNA were then checked for their quality and quantity using NanoDrop™ 2000 Spectrophotometer (ThermoScientific, Waltham, MA, USA). The samples were then subjected to library preparation wherein the V3&V4 hyper variable regions of the 16S gene were amplified using KAPA HiFi HotStart ReadyMix PCR Kit (KAPA BIOSYSTEMS, Wilmington, MA, USA). The amplified products were processed in the several stages of cleanup, elution, barcoding, purification and final library elution. The synthesized libraries were quantified in a Qubit.3 Fluorometer (Life technologies, Carlsbad, CA, USA) and QC checked using Agilent D5000 ScreenTape System. The QC-passed libraries were finally subjected to next-generation sequencing in the Illumina MiSeq. The sequence data generated using Illumina MiSeq were quality-checked using FastQC (Version 0.11.9) and MultiQC (Version 1.6) software. Trimgalore software (Version 0.6.5) was used to trim the reads to remove the degenerate primers, adapter sequences and low-quality bases. The QC-passed reads were imported into mothur and the pairs were aligned with each other to form contigs. The contigs were also screened for several criteria to eliminate all errors. Further, by using UCHIME software (v11), the gaps and the overhang at the ends from the contigs and chimera were removed. The filtered contigs were processed and classified into taxonomical outlines based on the GREENGENES v.13.8-99 database. The contigs were then clustered into OTUs (Operational Taxonomic Units). After the classification, OTU abundance was estimated. PICRUSt was used to predict gene family abundance. PICRUSt (version 1) program was designed to estimate the gene families contributing to a metagenome by bacteria or archaea identified using 16S rRNA sequencing. OTU contributions for the particular functions were estimated by metagenome_contributions.py script. LDA Effect Size (LefSe) was performed to compare the control and test groups. The number of discriminative features with an absolute LDA score > 2.0 was considered statistically significant.
2.7. Statistical Analysis
The data on the rumen enzyme assay and fermentation were analyzed by using SAS University Edition (SAS/STAT
®, 3.82 (Enterprise Edition) SAS Institute Inc., Cary, NC, USA), applying the MIXED procedure to infer fixed, interaction, random and repeated measurement effects by using mixed model equations. The below was the adopted statistical model:
where Y
ijklmn was the observation for the rumen enzyme assay and fermentation variables from the ijklmnop
th goat; µ denotes the overall mean effect; B
i denotes the fixed effect for the ith breed (i = Nandidurga, Bidri); T
j denotes the fixed effect for the jth treatment (j = thermoneutral, heat stress); D
k denotes the fixed effect for the kth day (k = day0, day15, day30 and day45); B × T × D
l was the lth interaction between breed, treatment and diet; A
m denotes the random animal effect for the mth animal and e
ijklmn denotes the random residual effect. The least square means were estimated for all the fixed and interaction effects and the means were compared based on the Tukey post hoc analysis.
4. Discussion
The rumen microbiome harbors highly abundant and diverse microbiota that play a significant role in metabolism, production and health [
18]. The ruminal composition is influenced by physical and chemical factors, diet, environmental, feeding regime, individual characteristics being the primary factors [
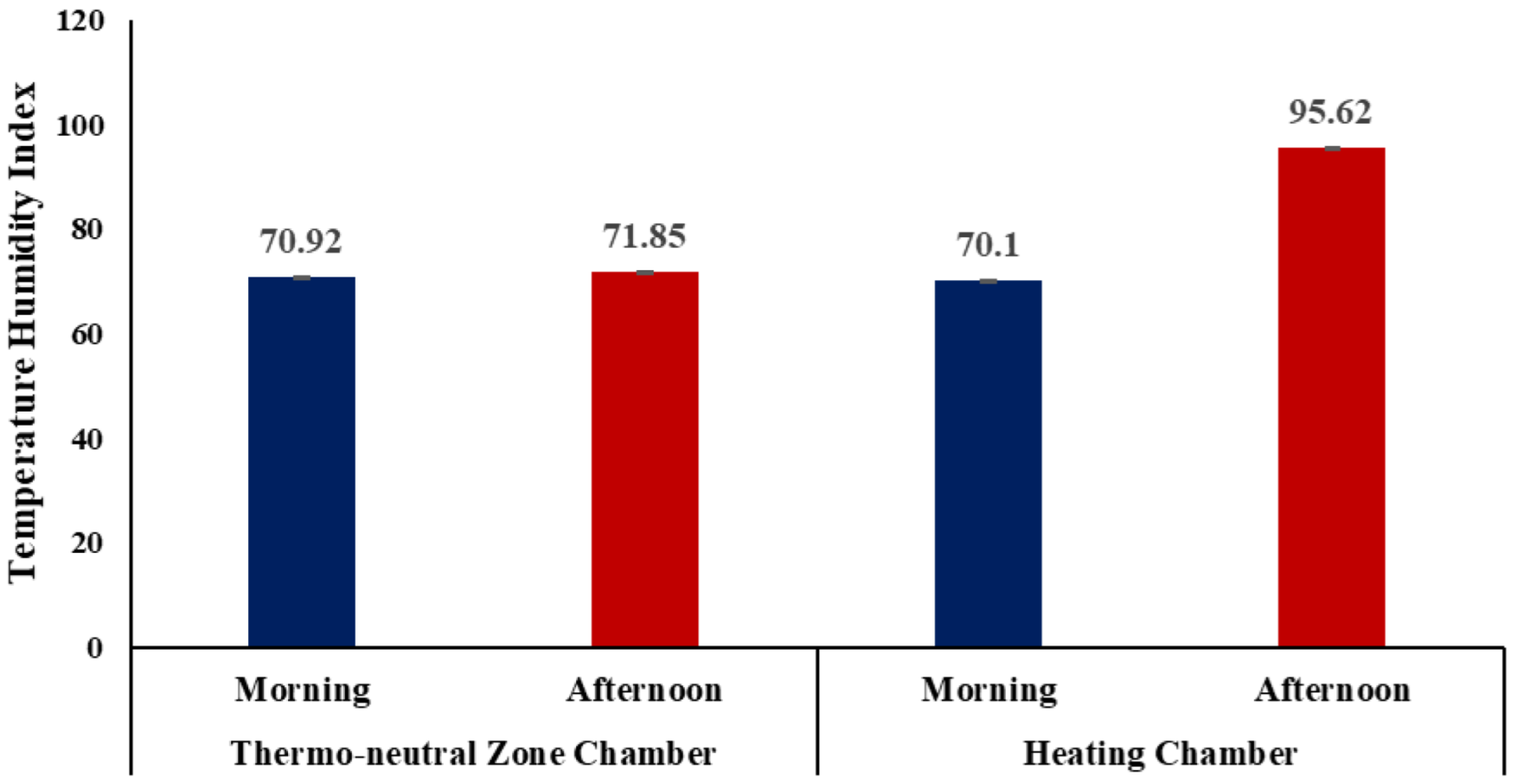
9]. There are relatively few documented reports assessing the rumen microbial dynamics in response to heat stress. Furthermore, scientific reports assessing the impact of heat stress on caprine rumen microbiota are even more scarcely reported. The present study was a first-of-its kind approach to assessing the impact of heat stress on caprine rumen microbiota and rumen variables in two indigenous goat breeds, Nandidurga and Bidri. The significantly higher mean afternoon THI of 95.62 ± 0.09 in the heating chamber when compared to the control (71.85 ± 0.08) indicates that the goats are under extreme heat stress [
15]. The THI above 78, as per McDowell [
15], demarcates the threshold for heat stress in goats, and previous studies from the same laboratory have also reported that when THI, as calculated as by McDowell [
15], is above 78, the animal is subject to severe stress conditions [
8,
19].
This study revealed heat stress to significantly alter the rumen ammoniacal nitrogen concentration in goats, wherein a significant reduction was observed in BHS in comparison to BS. Though most of the rumen fermentation variables, especially those associated with VFAs, did not reveal statistically significant differences between the breeds under stress, treatment as a fixed effect was significant for many of these factors. Heat stress was reported to reduce VFA and acetate levels in ruminants [
10,
14,
20]. Therefore, the findings from the present study point towards the impact of heat stress in reducing the VFA, acetate and other rumen variables in goats; however, they do not depict any differences between the breeds. Furthermore, this could be one of the many reasons why the rumen pH remained unaffected among the four groups. Though there were inconsistencies with alterations in rumen ammonia levels in heat-stressed ruminants, Cai et al. [
20] reported a significant reduction in ammonia–nitrogen in goats during the heat challenge period. Alterations in rumen fermentation variables have been reported to be attributed to a reduction in feed intake and a variation in the quantum and/or activity of rumen microbiota [
19,
21]. In addition to this theory, the water demand and water content in rumen are believed to increase, thereby resulting in a decrease in TVFA concentration in rumen [
22]
Heat stress was also observed to significantly reduce the extracellular CMCase, intracellular CMCase and total CMCase in both Nandidurga and Bidri goats. Chaidanya et al. [
7] reported a significant reduction of extracellular CMCase activity in heat-stressed Osmanabadi goats. The authors inferred the reduction in CMCase activity to probably be caused by the reduction in the ruminal protozoal concentration, especially cellulose-degrading microbes, due to heat stress, thereby influencing cellulose digestibility. Therefore, these results indicate that heat stress altered the ruminal fermentation and metabolism in goats.
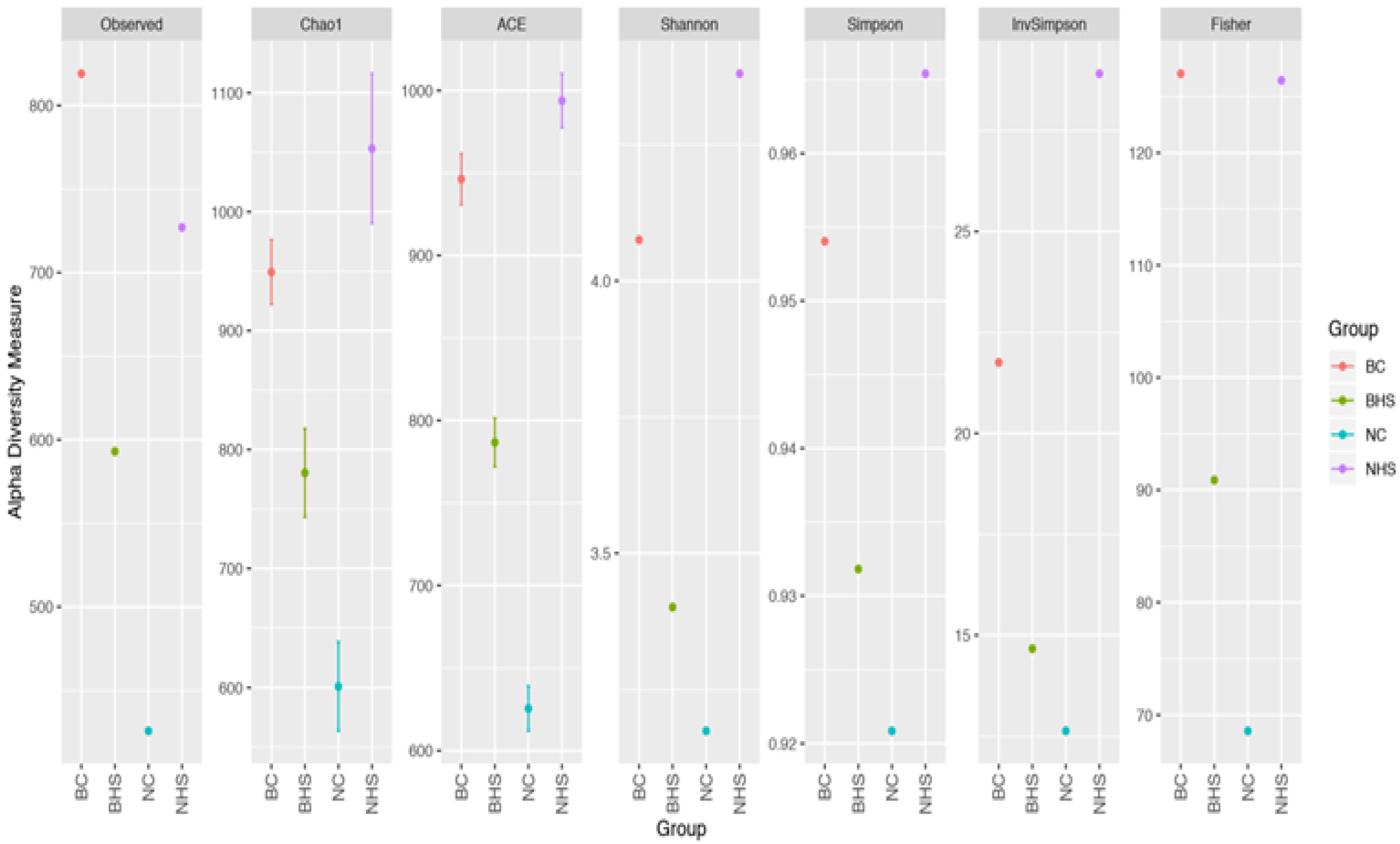
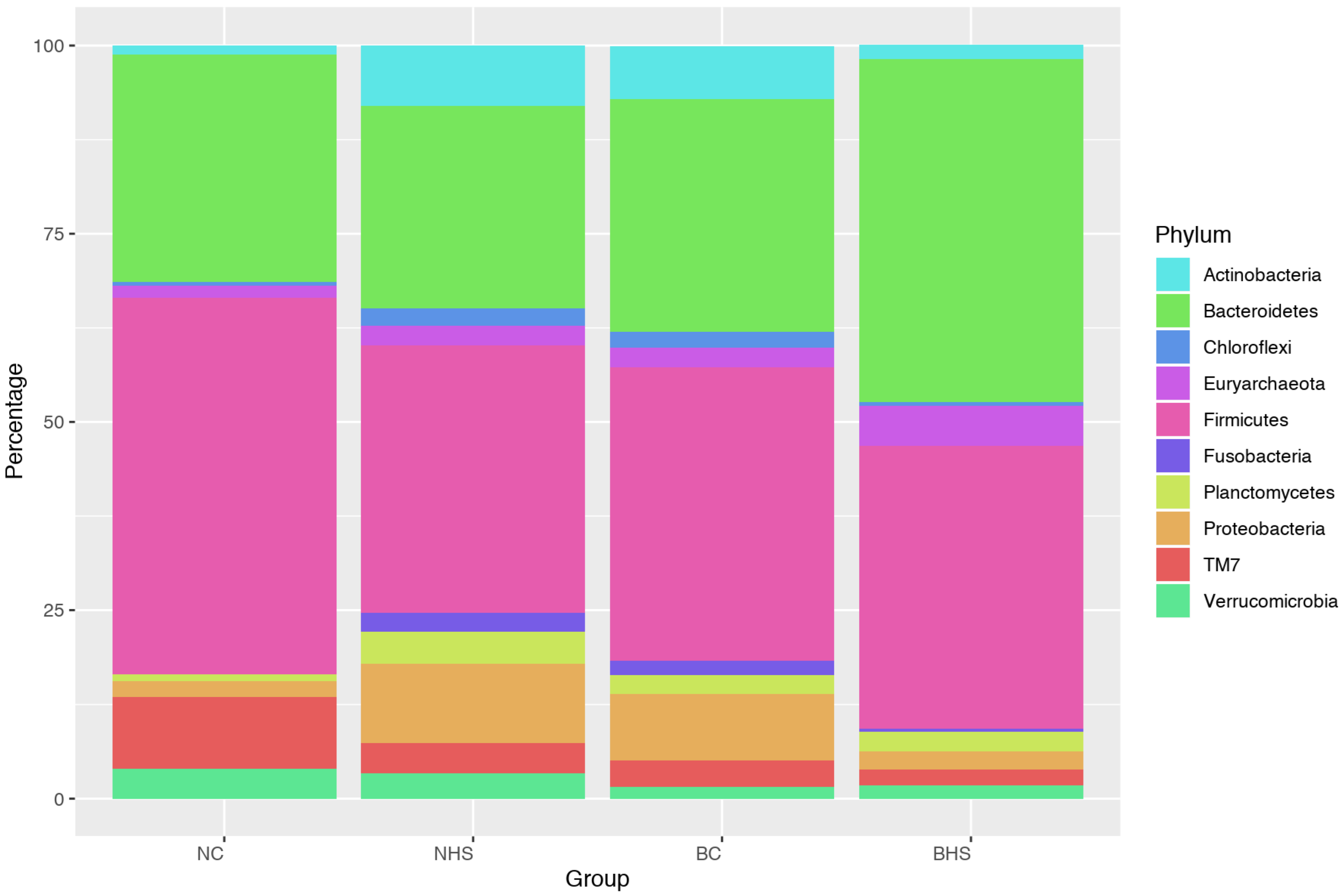
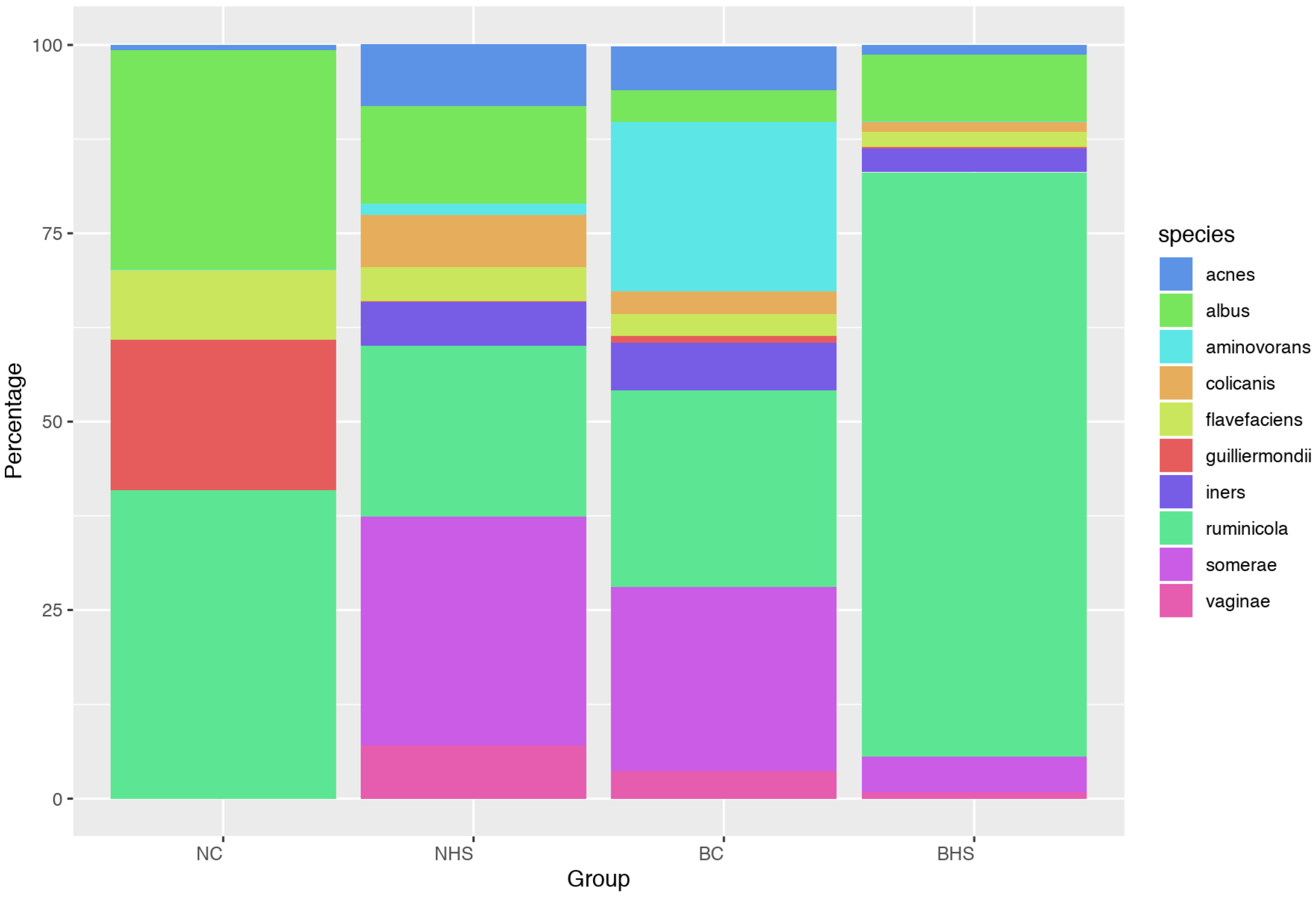
Rumen metabolites are produced by microbes; therefore, assessing the ruminal microbiome could provide better insight into the ruminal dynamics in heat-stressed goats. Studies assessing the impact of heat stress on rumen microbiota are scarcely documented; furthermore, this is a first-time report assessing the rumen microbial response to heat stress in two indigenous goat breeds. The Alpha diversity indices revealed heat stress altered the rumen microbial diversity in goats, with the two breeds depicting contrasting trends. However, the relative abundances revealed a nearly similar impact of heat stress on the rumen microbiota in Nandidurga and Bidri goats, with the reduction in acetate producing bacteria and an increased abundance of lactate-producing bacteria. Heat stress was observed to cause an evident reduction in the relative abundances of
Firmicutes,
TM7 and
Verrucomicrobia, and an increase in
Euryarchaeota, both in Nandidurga and Bidri goats subjected to heat stress when compared to their respective controls. However, contrasting relative abundances were observed for
Proteobacteria,
Actinobacteria,
Chloroflexi and
Fusobacteria, which were observed to have an increased abundance in NHS (in comparison to NC), while they also had reduced abundances in BHS (in comparison to BC). The reduction in the relative abundance of
Firmicutes in the present study is in accordance with previous reports [
14]. The bacteria under this phylum are dominated by fibrolytic and cellulolytic bacterial genera [
23]. It is vital to associate the findings from the rumen enzyme variables with the rumen metagenomic profile. A significant reduction in CMCase was observed due to heat stress in both breeds, indicating a probable reduction in rumen protozoa and especially in cellulose-degrading microbes, which is also the case with the altered ruminal microflora, including the cellulolytic bacteria. Furthermore, the phylum
Firmicutes is reported to have a high abundance of known H-producer species [
24]. Therefore, the lower abundance of
Firmicutes in the heat stress goats could be beneficial in terms of the low H contribution in the rumen environment.
It was interesting to observe the relative abundance of the predominant bacterial phylum
Bacteroidetes, associated with the breakdown of structural polysaccharides and fermentation of amino acids into acetate in the rumen [
23]. This phylum was reduced in NHS while it had an increased abundance in BHS. Zhong et al. [
14] reported an increased abundance of
Bacteroidetes with increasing THI in goats. The difference in the abundance levels between the two breeds could be explained by the variations at the genus and species levels.
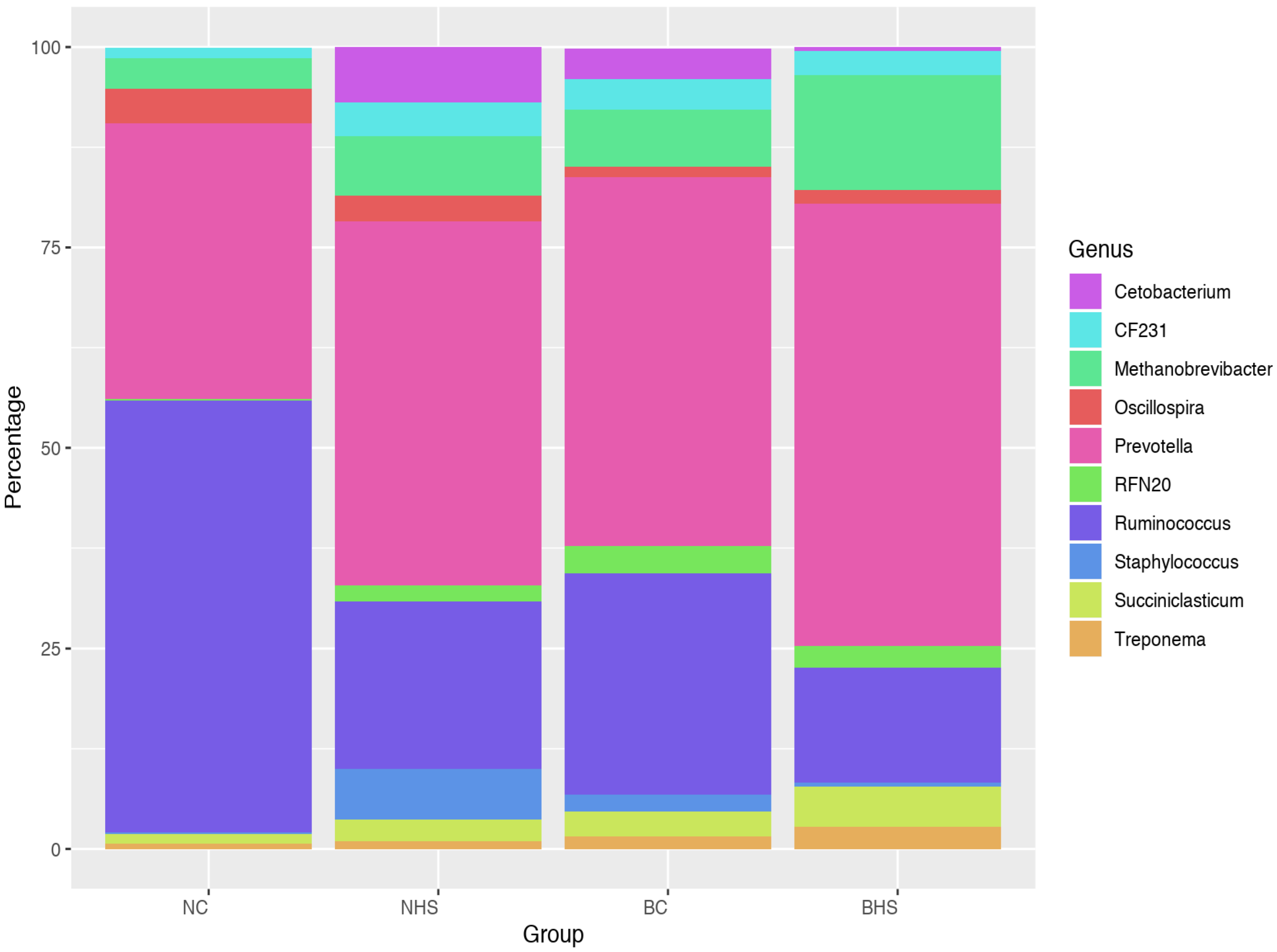
Prevotella and
Bacteroides are the two prime genera under the phylum
Bacteroidetes. The microbes under
Prevotella were reported to be involved in varied functions in the rumen, like digesting hemicelluloses, pectinolytic activity and proteolytic activity [
14]. Bacteria under the genus
Bacteroidetes were stated to utilize carbohydrates as an energy source and efficiently degrade structural carbohydrates. The relative abundances of both these genera were also reported to be altered during heat stress in ruminants [
10,
25]. A significantly increased abundance of
Prevotella was reported by Li et al. [
10] in heat-stressed goats. While the relative abundance of
Prevotella increased due to heat stress in both of the goat breeds, at the species level, there was a noteworthy difference. A drastic reduction in
P. ruminicola was observed in Nandidurga goats due to heat stress (34.74% in NC and 12.1% in NHS), while it increased in Bidri goats (15.53% in BC and 56.03% in BHS). Therefore, the overall increased abundance in the phylum
Bacteroidetes could be explained by the variation at the species level.
In addition to this, the bacteria under the phylum
Bacteroidetes are known to be better degraders of complex polysaccharides available in plant cell walls than Firmicutes [
26]. Therefore, the decreased abundance of
Firmucutes along with the increased abundance of
Bacteroidetes in heat-stressed Bidri goats could reflect a better adaptation of the breed to heat stress in comparison to Nandidurga goats.
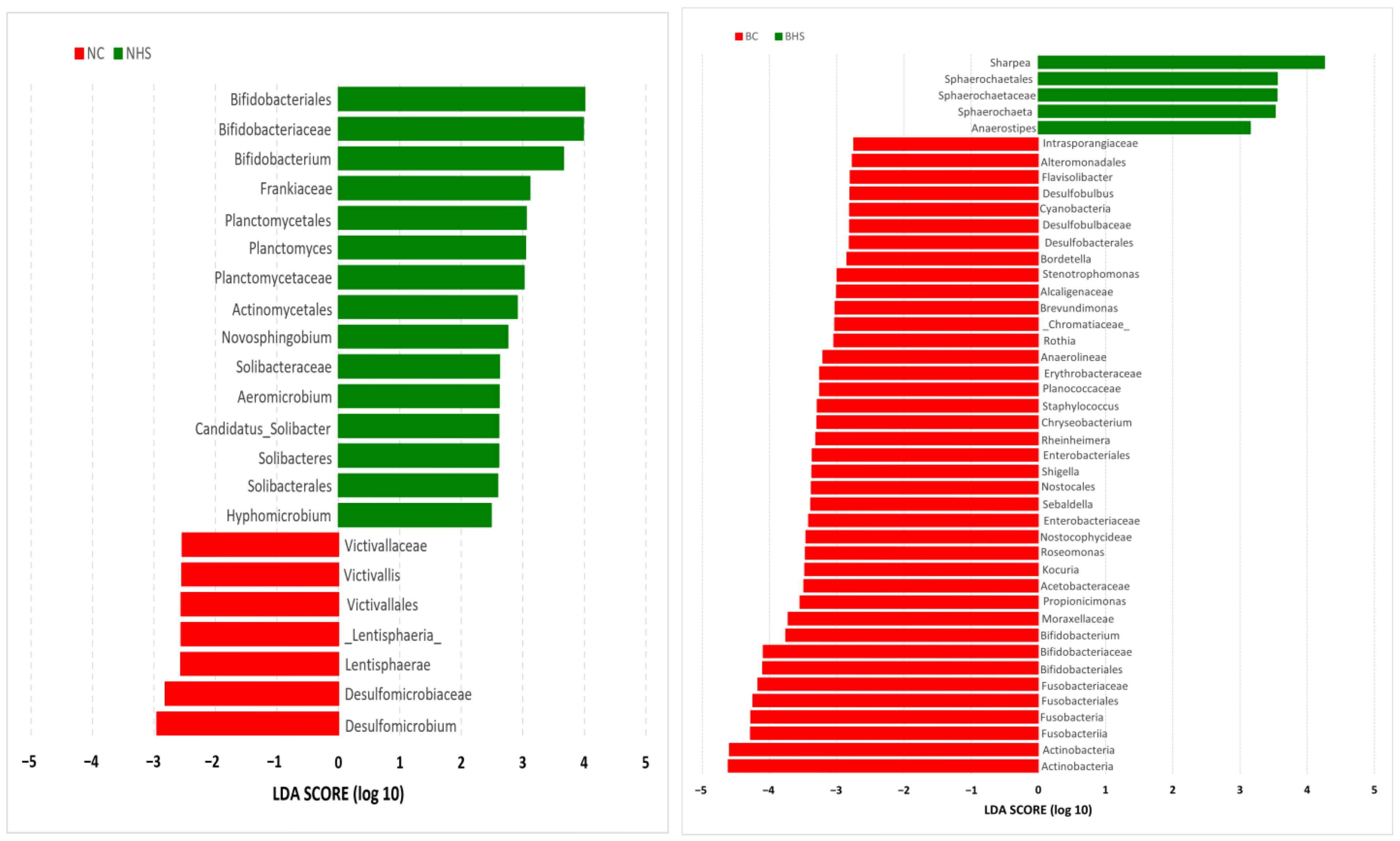
The LEfSe analysis revealed the abundances of three genera, Sharpea (phylum Firmicutes), Sphaerochaeta (phylum Spirochaetes) and Anaerostipes (phylum Firmicutes), to be significantly increased due to heat stress in Bidri goats. Furthermore, the relative abundances of 14 genera were found to be significantly reduced due to heat stress in Bidri goats. These genera included Kocuria (phylum Actinobacteria); Rothia (phylum Actinobacteria); Propionicimonas (phylum Actinobacteria); Bifidobacterium (phylum Actinobacteria); Flavisolibacter (phylum Bacteroidetes); Chryseobacterium (phylum Bacteroidetes); Staphylococcus (phylum Firmicutes); Sebaldella (phylum Fusobacteria); Brevundimonas (phylum Proteobacteria); Roseomonas (phylum Proteobacteria); Bordetella (phylum Proteobacteria); Desulfobulbus (phylum Proteobacteria); Rheinheimera (phylum Proteobacteria); Shigella (phylum Proteobacteria) and Stenotrophomonas (phylum Proteobacteria).
In Nandidurga goats, the proportion of microbial genera with increased abundances due to heat stress was relatively higher (six genera) than that of Bidri goats. Additionally, relatively higher proportions of genera were observed to have significantly lower abundances due to heat stress in Nandidurga goats. The genera Bifidobacterium (phylum Actinobacteria); Aeromicrobium (phylum Actinobacteria); Planctomyces (phylum Planctomycetes); Novosphingobium (phylum Proteobacteria); Hyphomicrobium (phylum Proteobacteria) and Candidatus_Solibacter (phylum Acidobacteria) were observed to have significantly higher abundances in NHS when compared to NC. The LEFSe analysis revealed only two phyla, Desulfomicrobium (phylum Proteobacteria) and Victivallis (phylum Lentisphaerae), to have significantly lower abundances in NHS when compared to NC.
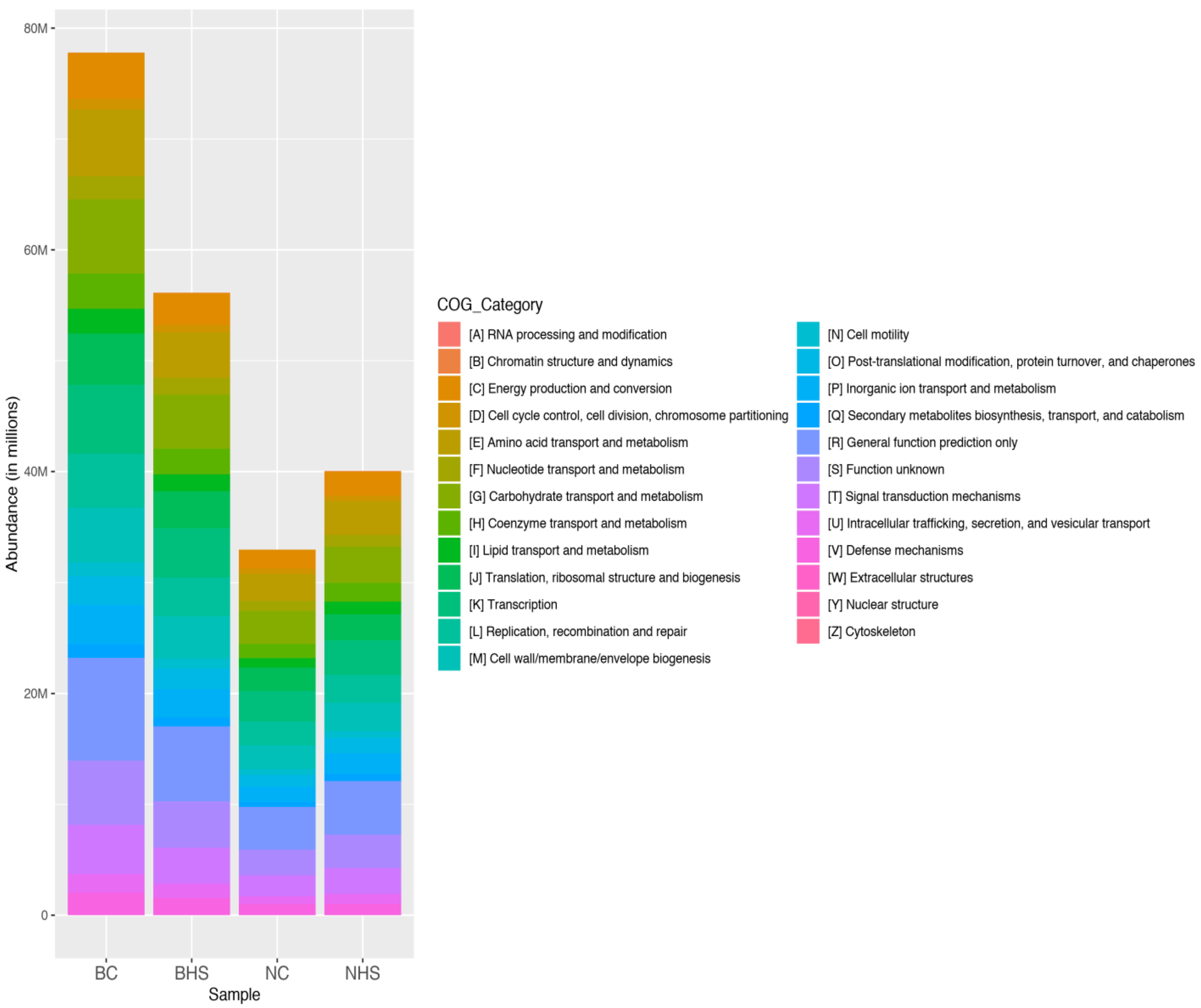
This difference in the proportion of the significantly altered ruminal microbiota could be attributed to the differences in the diversity indices wherein there were relatively more alterations in ruminal microbial diversity in Bidri goats than in Nandidurga goats. This difference in the ruminal composition can also be expected to alter the functional processes, which are reflected by the PICRUSt analysis. All the predicted functional pathways were observed to have lower abundances in Bidri goats due to heat stress, while the same pathways were increased in Nandidurga. The differences in the number of rumen microbial genera with significantly increased and reduced abundances between the two breeds could be linked to the lower abundance of functional processes in Bidri goats. Though this was the case, it was also observed that the abundances of the functional pathways in BHS were higher than those of NHS. This could indicate that heat-stress-associated ruminal alteration in Bidri goats could be relatively higher than in Nandidurga goats. The inference made from the rumen metagenome analysis corresponds with the phenotypic measurements.
5. Conclusions
The differences in heat stress responses between two indigenous goats, Nandidurga and Bidri, on rumen fermentation variables and rumen microbiota (assessed via 16s V3–V4 rRNA sequencing) were assessed. The THI obtained in the control chamber was 71.85 while in the heating chamber the THI was 95.62. Any value above 78 was considered extremely stressful. Therefore, the hypothesis of subjecting heat stress group animals to extremely severe heat stress was justified. This study revealed heat stress to have a significant impact on acetate, propionate, isobutyrate, total VFAs, extracellular CMCase, intracellular CMCase, total CMCase, extracellular amylase, intracellular amylase and total amylase. However the differences between the breeds were marginal, except for the extracellular CMCase, intracellular CMCase and total CMCase. Furthermore, a significant impact of heat stress on the rumen microbiota was observed. The rumen metagenome profile and rumen fermentation variables pointed towards a predominant alteration in the cellulose-degrading microbes in heat-stressed goats, as reflected by the decreased CMCase enzyme activity. While both the breeds exhibited nearly similar responses in the rumen microbial abundance levels due to heat stress, breed-specific differences were also observed. The microbial evenness and richness in Bidri goats were observed to be more altered due to heat stress when compared to Nandidurga goats. Thus, it could be understood that though Nandidurga and Bidri goats exhibited excellent resilience to the extremely high THIs, the ruminal microbial diversity and activity were relatively more impaired in Bidri goats. The findings from this study, being preliminary, need to be extended using better and more comprehensive aids such as rumen metabolomics, shotgun metagenomics and metatranscriptomics.