Impact of Selected Starter-Based Sourdough Types on Fermentation Performance and Bio-Preservation of Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Starter Culture
2.2. Preparation of Traditional Sourdough (Type 1)
2.3. Preparation of the Liquid Sourdough (Type 2)
2.4. Preparation of the Liquid Backslopped Sourdough (Type 3)
2.5. Preparation of the Lyophilized Inoculum for Sourdough (Type IV)
2.6. Doughs Preparation
2.7. Fermentation Parameters
- Sourdough fermentation: Each sourdough type (I–IV) was fermented for 24 h at 30 °C prior to dough preparation, with pH and TTA monitored hourly until stabilization (ΔpH < 0.1 over 2 h).
- Dough fermentation: All doughs (containing 20% sourdough) were fermented at 30 °C until peak dough rise (height plateau) and stable pH (<0.05 change over 30 min), as monitored by Panigraph. Fermentation time varied based on sourdough type (Table 2), and was terminated when the above endpoint criteria were met.
- Post-fermentation: Dough was then formed into round loaves and allowed to ferment for an additional 30 min at 40 °C before being baked in an electric oven (Itimat, Istanbul, Turkey) at 220 °C for about 15 min (Figure S1).
2.8. Total Titratable Acidity “TTA”
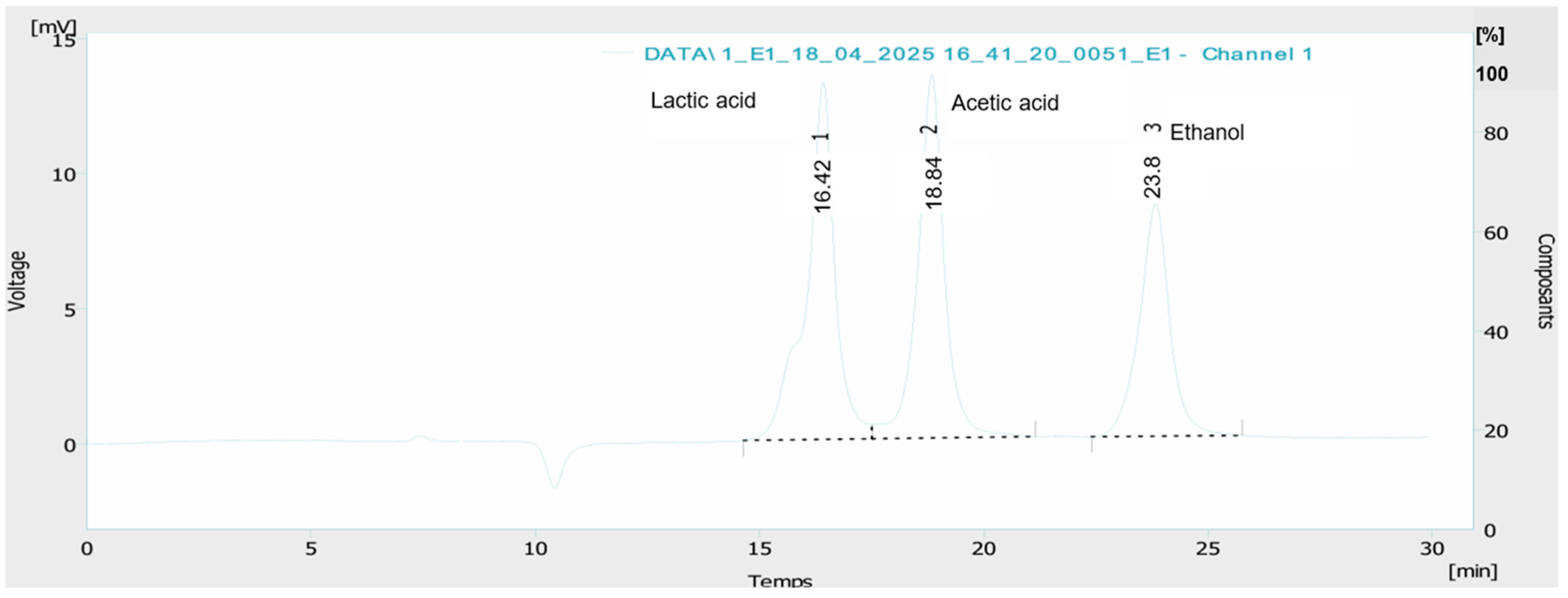
2.9. Analysis of Organic Acids and Ethanol
2.10. Bio-Preservation of Breads
2.11. Multivariate Analysis
2.12. Statistical Analysis
3. Results
3.1. Fermentation Parameters and TTA
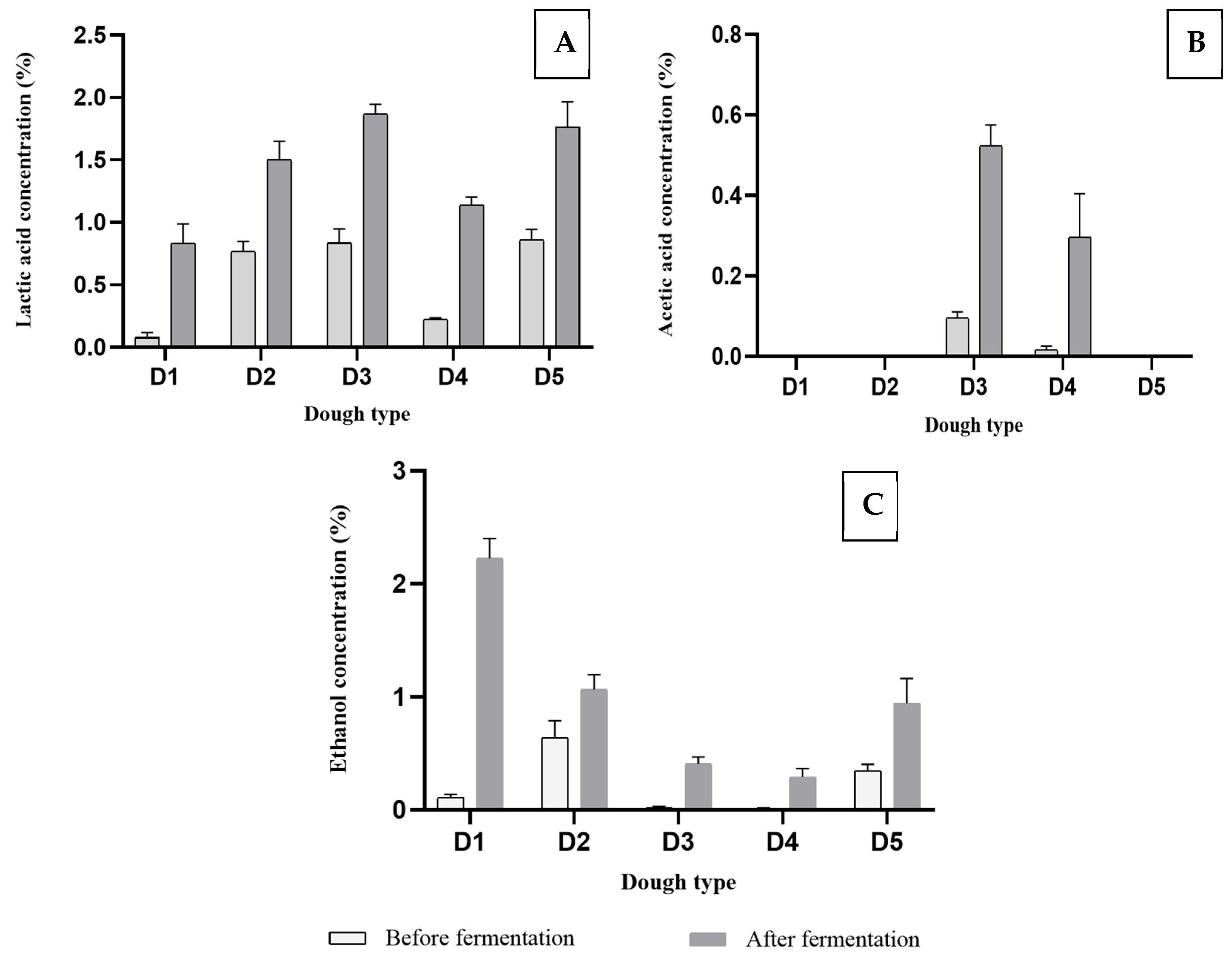
3.2. Analysis of Organic Acids and Ethanol
3.3. Multivariate Analysis and Correlation Study
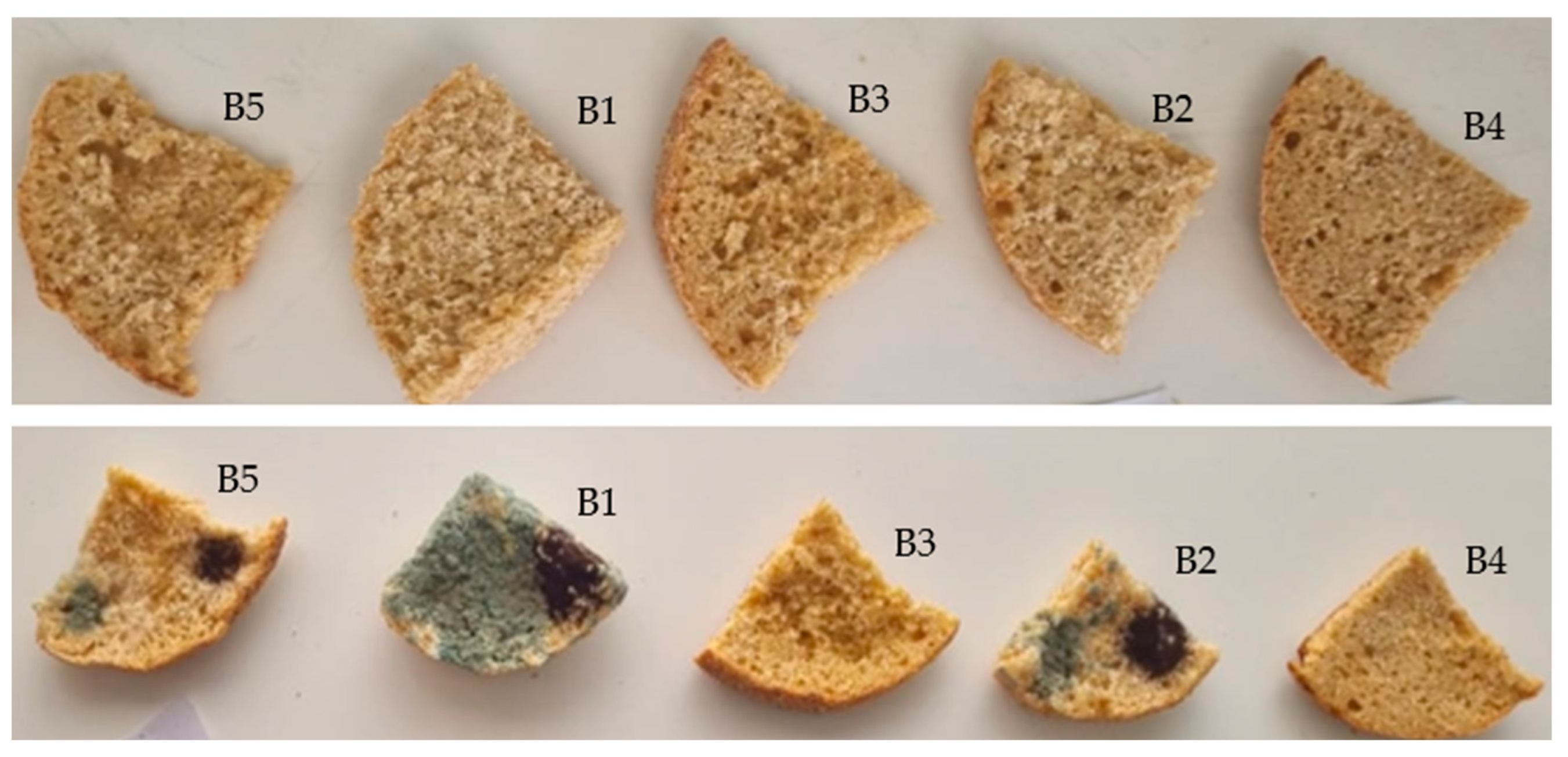
3.4. Shelf Life Improvement
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| AACC | American Association of Cereal Chemists |
| FQ | Fermentation quotient |
| LA | Lactic acid |
| LAB | Lactic acid bacteria |
| TTA | Total titratable acidity |
References
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Inter. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Jain, A.; Mathur, P. Evaluating Hazards Posed by Additives in Food-A Review of Studies Adopting a Risk Assessment Approach. Curr. Res. Nutr. Food Sci. J. 2015, 3, 243–255. [Google Scholar] [CrossRef]
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/natural-food-preservatives-market (accessed on 24 July 2025).
- Fraberger, V.; Ammer, C.; Domig, K.J. Functional properties and sustainability improvement of sourdough bread by lactic acid bacteria. Microorganisms 2020, 8, 1895. [Google Scholar] [CrossRef]
- Savkina, O.; Kuznetsova, L.; Parakhina, O.; Lokachuk, M.; Pavlovskaya, E. Impact of Using the Developed Starter Culture on the Quality of Sourdough, Dough and Wheat Bread. Agron. Res. 2019, 17, 1435–1451. [Google Scholar]
- De Bondt, Y.; Verdonck, C.; Brandt, M.J.; De Vuyst, L.; Gnzle, M.G.; Gobbetti, M.; Zannini, E.; Courtin, C.M. Wheat Sourdough Breadmaking: A Scoping Review. Annu. Rev. Food Sci. Technol. 2024, 15, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S. Innovations in Sourdough Bread Making. Fermentation 2021, 7, 29. Available online: https://pdfs.semanticscholar.org/1853/3bfc72d3e4f18bd2e360bc3736989a9886aa.pdf (accessed on 23 June 2025). [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Hammes, W.P.; Gänzle, M.G. Sourdough Breads and Related Products. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Springer: Boston, MA, USA, 1998; pp. 199–216. [Google Scholar] [CrossRef]
- Winters, M.; Panayotides, D.; Bayrak, M.; Rémont, G.; Viejo, C.G.; Liu, D.; Le, B.; Liu, Y.; Luo, J.; Zhang, P. Defined Co-Cultures of Yeast and Bacteria Modify the Aroma, Crumb and Sensory Properties of Bread. J. Appl. Microbiol. 2019, 127, 778–793. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and Cereal Fermentation in a Nutritional Perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough Production: Fermentation Strategies, Microbial Ecology, and Use of Non-Flour Ingredients. Crit. Rev. Food Sci. Nutr. 2023, 63, 2447–2479. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial Ecology and Process Technology of Sourdough Fermentation. Adv. Appl. Microbiol. 2017, 100, 49–160. [Google Scholar]
- Fekri, A.; Abedinzadeh, S.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Considering Sourdough from a Biochemical, Organoleptic, and Nutritional Perspective. J. Food Compos. Anal. 2024, 125, 105853. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold Spoilage of Bread and Its Biopreservation: A Review of Current Strategies for Bread Shelf Life Extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting Synergies of Sourdough and Antifungal Organic Acids to Delay Fungal Spoilage of Bread. Int. J. Food Microbiol. 2019, 302, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.S.; Ohstrom, A.; Rolon, M.L.; Smith, M.; Wolfe, B.E.; Wee, J.; Van Buiten, C.B. Sourdough Starter Culture Microbiomes Influence Physical and Chemical Properties of Wheat Bread. J. Food Sci. 2024, 89, 1414–1427. [Google Scholar] [CrossRef]
- Minervini, F.; De Angelis, M.; Di Cagno, R.; Gobbetti, M. Ecological Parameters Influencing Microbial Diversity and Stability of Traditional Sourdough. I. J. Food Microbiol. 2014, 171, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Figueroa, R.H.; Mani-López, E.; López-Malo, A. Antifungal Capacity of Poolish-Type Sourdough Supplemented with Lactiplantibacillus Plantarum and Its Aqueous Extracts in Vitro and Bread. Antibiotics 2022, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; D’Opazo, V.; Mañes, J.; Meca, G. Antifungal Activity and Shelf Life Extension of Loaf Bread Produced with Sourdough Fermented by Lactobacillus Strains. J. Food Process. Preserv. 2019, 43, 14126. [Google Scholar] [CrossRef]
- Teixeira, L.B.; Campos, J.Z.; Kothe, C.I.; Welke, J.E.; Rodrigues, E.; Frazzon, J.; Thys, R.C.S. Type III Sourdough: Evaluation of Biopreservative Potential in Bakery Products with Enhanced Antifungal Activity. Food Res. Int. 2024, 189, 114482. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef]
- Gerez, C.L.; Torino, M.I.; Rollán, G.; de Valdez, G.F. Prevention of Bread Mould Spoilage by Using Lactic Acid Bacteria with Antifungal Properties. Food Control 2009, 20, 144–148. [Google Scholar] [CrossRef]
- Atfaoui, K.; El Kabous, K.; Tarfaoui, K.; Erahioui, R.; Elwardani, H.; Chahboun, N.; Choukri, S.; ABDELKADER, Z.; Ouhssine, M. Determination of the optimal fertilization rate by a new bioorganic fertilizer to enhance the growth parameters and nutritional quality of red beetroot (Beta vulgaris L. ssp. Vulgaris). J. Microbiol. Biotechnol. Food Sci. 2024, 14, e10305. [Google Scholar] [CrossRef]
- Khadija, A.; Omar, B.; Abdessamad, E.; Rachid, I.; Imane, O.; Hicham, H.; Mohammed, O. Phenotypic and Genotypic Identification of the Most Acidifiers LAB Strains Isolated from Fermented Food. Biol. Bull. Russ. Acad. Sci. 2022, 49, 260–270. [Google Scholar] [CrossRef]
- Gobbetti, M.; Minervini, F.; Pontonio, E.; Di Cagno, R.; De Angelis, M. Drivers for the Establishment and Composition of the Sourdough Lactic Acid Bacteria Biota. I. J. Food Microbiol. 2016, 239, 3–18. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the Selected Antifungal LAB Isolate as a Protective Starter Culture in Pan Whole-Wheat Sourdough Bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Raffak, A.; Chafai, Y.; Hamouda, A.; Ouazzani Touhami, A.; Mounir, M. Assessment of the Fermentative Performance of Traditional Fresh Moroccan Sourdoughs and Their Freeze-Dried Forms Using Online Monitoring Device: Panigraph. Appl. Sci. 2023, 13, 12453. [Google Scholar] [CrossRef]
- Illueca, F.; Moreno, A.; Calpe, J.; Nazareth, T.D.M.; Dopazo, V.; Meca, G.; Quiles, J.M.; Luz, C. Bread Biopreservation through the Addition of Lactic Acid Bacteria in Sourdough. Foods 2023, 12, 864. [Google Scholar] [CrossRef]
- Martínez, R.M.; Cruz, M.; Loredo-Treviño, A.; Martínez, J.L.; Ruiz, H.A.; Rodríguez-Jasso, R.M.; Belmares, R. Evaluation of the Addition of Cassava Flour Fermented with Lactic Acid Bacteria on the Sensorial and Nutritional Properties of a Baked Product. Food Humanit. 2024, 3, 100329. [Google Scholar] [CrossRef]
- Raffak, A.; Chafai, Y.; Toure, I.; Hamouda, A.; Alaoui, M.I.; Touhami, A.O.; Mounir, M. Real-Time Monitoring Device “Panigraph” for Bread Fermentation with an Arduino-Based Data Acquisition and Management System. Moro. J. Agri. Sci. 2023, 4, 185–193. [Google Scholar]
- El Khaider, K.; Chafik, I.; Hamouda, A.; Afechtal, M.; Alaoui, M.I.; Mounir, M. Selection of Mixed Starters for the Preparation of Traditional Moroccan Bread. J. Biol. Res. 2023, 96. [Google Scholar] [CrossRef]
- Fadil, M.; Farah, A.; Ihssane, B.; Haloui, T.; Lebrazi, S.; Zghari, B. Chemometric investigation of light-shade effects on essential oil yield and morphology of Moroccan Myrtus communis L. SpringerPlus 2016, 5, 2749. [Google Scholar] [CrossRef]
- Gul, L.B.; Con, A.H.; Gul, O. Storage Stability and Sourdough Acidification Kinetic of Freeze-Dried Lactobacillus Curvatus N19 under Optimized Cryoprotectant Formulation. Cryobiology 2020, 96, 122–129. [Google Scholar] [CrossRef]
- Cakir, E.; Arici, M.; Durak, M.Z. Effect of Starter Culture Sourdough Prepared with Lactobacilli and Saccharomyces cerevisiae on the Quality of Hull-Less Barley-Wheat Bread. LWT 2021, 152, 112230. [Google Scholar] [CrossRef]
- Boscaino, F.; Ionata, E.; De Caro, S.; Sorrentino, A. Non-Conventional Yeasts from Mozzarella Cheese Whey and Artisanal Sourdoughs: Leavening Capacity and Impact on Bread Sensory Profile. Fermentation 2024, 10, 68. [Google Scholar] [CrossRef]
- Faria-Oliveira, F.; Diniz, R.H.; Godoy-Santos, F.; Piló, F.B.; Mezadri, H.; Castro, I.M.; Brandão, R.L. The Role of Yeast and Lactic Acid Bacteria in the Production of Fermented Beverages in South America. Food Prod. Ind. 2015, 4, 107–135. [Google Scholar]
- Gobbetti, M. The Sourdough Microflora: Interactions of Lactic Acid Bacteria and Yeasts. Trends Food Sci. Technol. 1998, 9, 267–274. [Google Scholar] [CrossRef]
- Geng, D.-H.; Liu, L.; Zhou, S.; Sun, X.; Wang, L.; Zhou, X.; Tong, L.-T. Effects of Lactobacillus Plantarum Inoculum on the Fermentation Rate and Rice Noodle Quality. J. Oleo Sci. 2020, 69, 1031–1041. [Google Scholar] [CrossRef]
- Pérez-Alvarado, O.; Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; Vinderola, G.; García-Cayuela, T. Role of Lactic Acid Bacteria and Yeasts in Sourdough Fermentation during Breadmaking: Evaluation of Postbiotic-like Components and Health Benefits. Front. Microbiol. 2022, 13, 969460. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.J.; Hammes, W.P.; Gänzle, M.G. Effects of Process Parameters on Growth and Metabolism of Lactobacillus sanfranciscensis and Candida humilis during Rye Sourdough Fermentation. Eur. Food Res. Technol. 2004, 218, 333–338. [Google Scholar] [CrossRef]
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; La Storia, A.; Gobbetti, M.; Di Cagno, R. Microbial Ecology Dynamics during Rye and Wheat Sourdough Preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [PubMed]
- Debonne, E.; Maene, P.; Vermeulen, A.; Van Bockstaele, F.; Depredomme, L.; Vermeir, P.; Eeckhout, M.; Devlieghere, F. Validation of In-Vitro Antifungal Activity of the Fermentation Quotient on Bread Spoilage Moulds through Growth/No-Growth Modelling and Bread Baking Trials. LWT 2020, 117, 108636. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Guerrini, S.; Granchi, L.; Vincenzini, M. Liquid and Firm Sourdough Fermentation: Microbial Robustness and Interactions during Consecutive Backsloppings. LWT 2019, 105, 9–15. [Google Scholar] [CrossRef]
- Torreggiani, A.; Beccaccioli, M.; Verni, M.; Cecchetti, V.; Minisci, A.; Reverberi, M.; Pontonio, E.; Rizzello, C.G. Combined Use of Trametes Versicolor Extract and Sourdough Fermentation to Extend the Microbiological Shelf-Life of Baked Goods. LWT 2023, 189, 115467. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Optimizing Lactic Acid Bacteria Proportions in Sourdough to Enhance Antifungal Activity and Quality of Partially and Fully Baked Bread. Foods 2024, 13, 2318. [Google Scholar] [CrossRef]
- Hansen, A.; Schieberle, P. Generation of Aroma Compounds during Sourdough Fermentation: Applied and Fundamental Aspects. Trends Food Sci. Technol. 2005, 16, 85–94. [Google Scholar] [CrossRef]
- Zhu, X.M.; Xia, X.L.; Yang, H.L.; Wang, W. Study on the Key Enzymes of Ethanol Oxidation and Acetic Acid Production in Acetobacter Pasteurianus HN 1.01. Sci. Tech. Food Ind. 2013, 34, 167–170. [Google Scholar]
- Hadaegh, H.; Seyyedain Ardabili, S.M.; Tajabadi Ebrahimi, M.; Chamani, M.; Azizi Nezhad, R. The Impact of Different Lactic Acid Bacteria Sourdoughs on the Quality Characteristics of Toast Bread. J. Food Qual. 2017, 2017, 7825203. [Google Scholar] [CrossRef]
- Alkay, Z.; Kilmanoğlu, H.; Durak, M.Z. Prevention of Sourdough Bread Mould Spoliage by Antifungal Lactic Acid Bacteria Fermentation. Eur. J. Sci. Technol. 2020, 18, 379–388. [Google Scholar] [CrossRef]
- Kam, P.V.; Bianchini, A.; Bullerman, L.B. Inhibition of Mold Growth by Sourdough Bread Cultures. Rural. Rev. Undergrad. Res. Agric. Life Sci. 2007, 2, 5. [Google Scholar][Green Version]
- Vollmar, A.; Meuser, F. Influence of Starter Cultures Consisting of Lactic Acid Bacteria and Yeasts on the Performance of a Continuous Sourdough Fermenter. Cereal Chem. 1992, 69, 20–27. [Google Scholar][Green Version]
- Gänzle, M.; Ripari, V. Composition and function of sourdough microbiota: From ecological theory to bread quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef]
- Brandt, M.J. Industrial production of sourdoughs for the baking branch–An overview. Int. J. Food Microbiol. 2019, 302, 3–7. [Google Scholar] [CrossRef]
- Lhomme, E.; Urien, C.; Legrand, J.; Dousset, X.; Onno, B.; Sicard, D. Sourdough microbial community dynamics: An analysis during French organic bread-making processes. Food Microbiol. 2016, 53, 41–50. [Google Scholar] [CrossRef]


| Dough Code | Sourdough Code | Sourdough Type | Durum Wheat Flour (g) | Soft Wheat Flour (g) | Water (g) | Salt (g) | Sugar (g) | Baking Yeast (g) | Sourdough (g) |
|---|---|---|---|---|---|---|---|---|---|
| D1 | PSC | Type 0 | 30.00 | 30.00 | 33.70 | 1.30 | 2.00 | 3.00 | 0.00 |
| D2 | PL4 | Type I | 30.00 | 30.00 | 26.70 | 1.30 | 0.00 | 0.00 | 20 |
| D3 | PS | Type II | 30.00 | 30.00 | 16.70 | 1.30 | 2.00 | 0.00 | 20 |
| D4 | PLS | Type III | 30.00 | 30.00 | 18.70 | 1.30 | 0.00 | 0.00 | 20 |
| D5 | PL4LYO | Type IV | 30.00 | 30.00 | 18.70 | 1.30 | 0.0 | 0.00 | 20 |
| Dough Code | Sourdough Code | Fermentation Time (min) | Leavening Capacity (cm) | pH | TTA (ml NaOH 0.1 N (10 g)−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial pH | Final pH | Variation | Initial TTA | Final TTA | Variation | ||||
| D1 | PSC | 664 ± 21.28 a | 3.74 ± 2.15 a | 4.92 | 4.74 | 0.18 ± 0.07 | 1.2 | 2 | 0.8 |
| D2 | PL4 | 1087 ± 20.42 d | 4.54 ± 0.08 c | 4.67 | 3.69 | 0.53 ± 0.09 | 3.5 | 12 | 8.5 |
| D3 | PS | 940 ± 38.30 c | 4.13 ± 0.13 b | 5.03 | 3.92 | 1.11 ± 0.09 | 3.5 | 13.5 | 10 |
| D4 | PLS | 740 ± 13.87 b | 5.52 ± 0.08 d | 4.72 | 3.69 | 0.50 ± 0.14 | 4.5 | 16 | 11.5 |
| D5 | PL4LYO | 1214 ± 16.52 e | 3.68 ± 0.11 a | 4.33 | 3.72 | 0.50 ± 0.05 | 3.5 | 11 | 7.5 |
| Dough Type | Δ Lactic Acid (%) | Δ Acetic Acid (%) | Δ Ethanol (%) | Fermentation Quotient FQ |
|---|---|---|---|---|
| D1 | 0.76 ± 0.08 a | n.d. | 2.12 ± 0.03 d | n.d. |
| D2 | 0.74 ± 0.01 a | n.d. | 0.43 ± 0.04 b | n.d. |
| D3 | 1.03 ± 0.09 c | 0.43 ± 0.03 a | 0.23 ± 0.19 a | 2.40 |
| D4 | 0.92 ± 0.02 b | 0.28 ± 0.04 b | 0.28 ± 0.02 a | 3.29 |
| D5 | 0.91 ± 0.05 b | n.d. | 0.60 ± 0.05 c | n.d. |
| Time of Fermentation (min) | Dough-Rise (cm) | pH Change (%) | ATT Change (%) | Lactic Acid Change (%) | Acetic Acid Change (%) | Ethanol Change (%) | |
|---|---|---|---|---|---|---|---|
| D1 | 664 | 3.74 | 3.66 | 62.16 | 978.05 | 0.00 | 1967.13 |
| D2 | 1087 | 4.54 | 20.99 | 241.51 | 95.91 | 0.00 | 66.17 |
| D3 | 940 | 4.12 | 22.07 | 282.08 | 123.23 | 448.85 | 2184.87 |
| D4 | 740.67 | 5.52 | 21.88 | 255.56 | 408.24 | 1723.47 | 2148.12 |
| D5 | 1214 | 3.68 | 14.22 | 213.21 | 104.67 | 0.00 | 170.25 |
| Breads | Fungi | Appearance of Fungal Growth (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| B1 | A. niger | - | - | - | + | + | + | + | + | + | + |
| B2 | - | - | - | - | - | + | + | + | + | + | |
| B3 | - | - | - | - | - | - | - | - | - | - | |
| B4 | - | - | - | - | - | - | - | - | - | - | |
| B5 | - | - | - | - | - | - | + | + | + | + | |
| B1 | P. commune | - | - | + | + | + | + | + | + | + | + |
| B2 | - | - | - | - | + | + | + | + | + | + | |
| B3 | - | - | - | - | - | - | - | - | - | - | |
| B4 | - | - | - | - | - | - | - | - | - | - | |
| B5 | - | - | - | - | - | + | + | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atfaoui, K.; Lebrazi, S.; Raffak, A.; Chafai, Y.; Kabous, K.E.; Fadil, M.; Ouhssine, M. Impact of Selected Starter-Based Sourdough Types on Fermentation Performance and Bio-Preservation of Bread. Fermentation 2025, 11, 449. https://doi.org/10.3390/fermentation11080449
Atfaoui K, Lebrazi S, Raffak A, Chafai Y, Kabous KE, Fadil M, Ouhssine M. Impact of Selected Starter-Based Sourdough Types on Fermentation Performance and Bio-Preservation of Bread. Fermentation. 2025; 11(8):449. https://doi.org/10.3390/fermentation11080449
Chicago/Turabian StyleAtfaoui, Khadija, Sara Lebrazi, Anas Raffak, Youssef Chafai, Karima El Kabous, Mouhcine Fadil, and Mohammed Ouhssine. 2025. "Impact of Selected Starter-Based Sourdough Types on Fermentation Performance and Bio-Preservation of Bread" Fermentation 11, no. 8: 449. https://doi.org/10.3390/fermentation11080449
APA StyleAtfaoui, K., Lebrazi, S., Raffak, A., Chafai, Y., Kabous, K. E., Fadil, M., & Ouhssine, M. (2025). Impact of Selected Starter-Based Sourdough Types on Fermentation Performance and Bio-Preservation of Bread. Fermentation, 11(8), 449. https://doi.org/10.3390/fermentation11080449






