Living Cultures in a Glass: The Health Promise of Probiotic Bacteria in Kombucha
Abstract
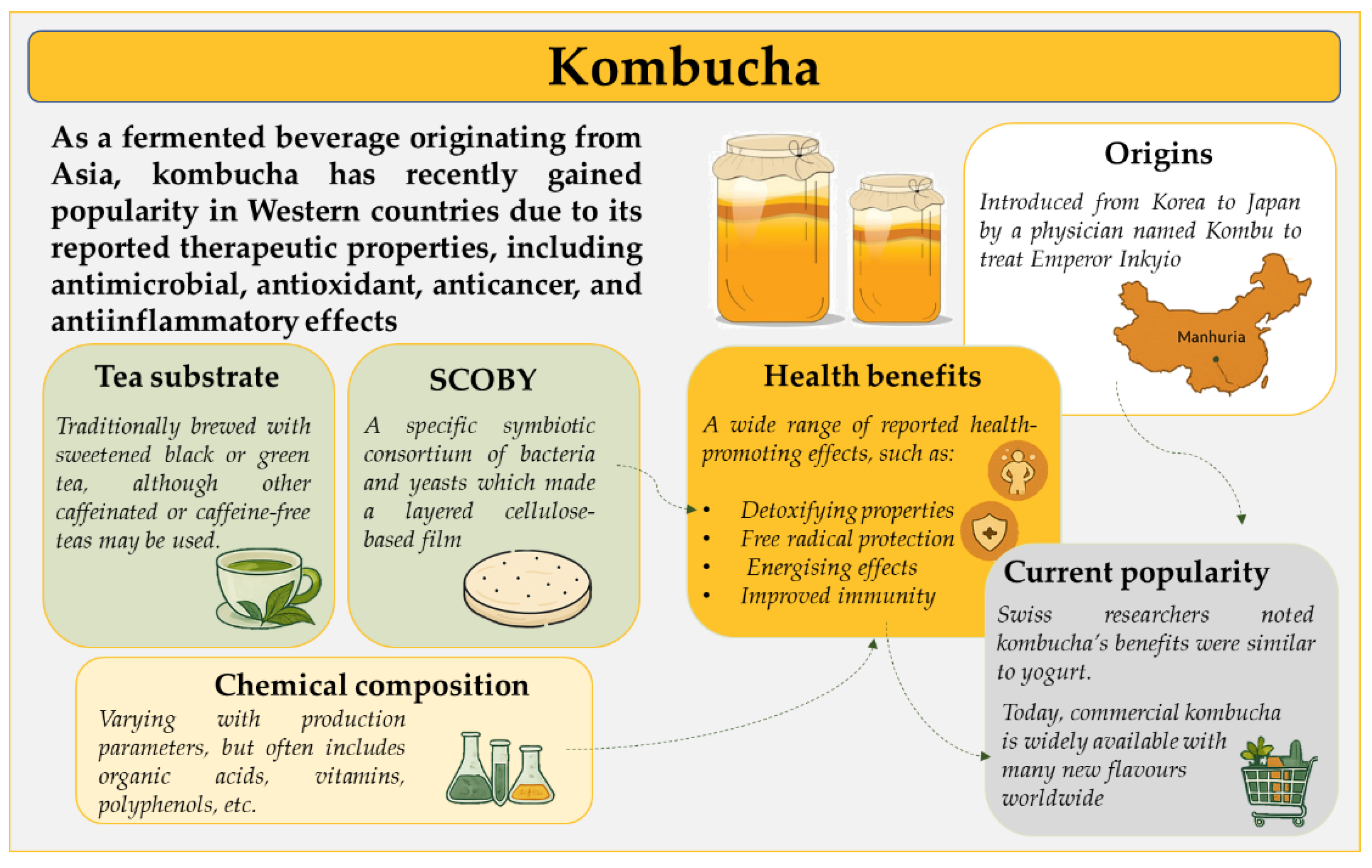
1. Kombucha
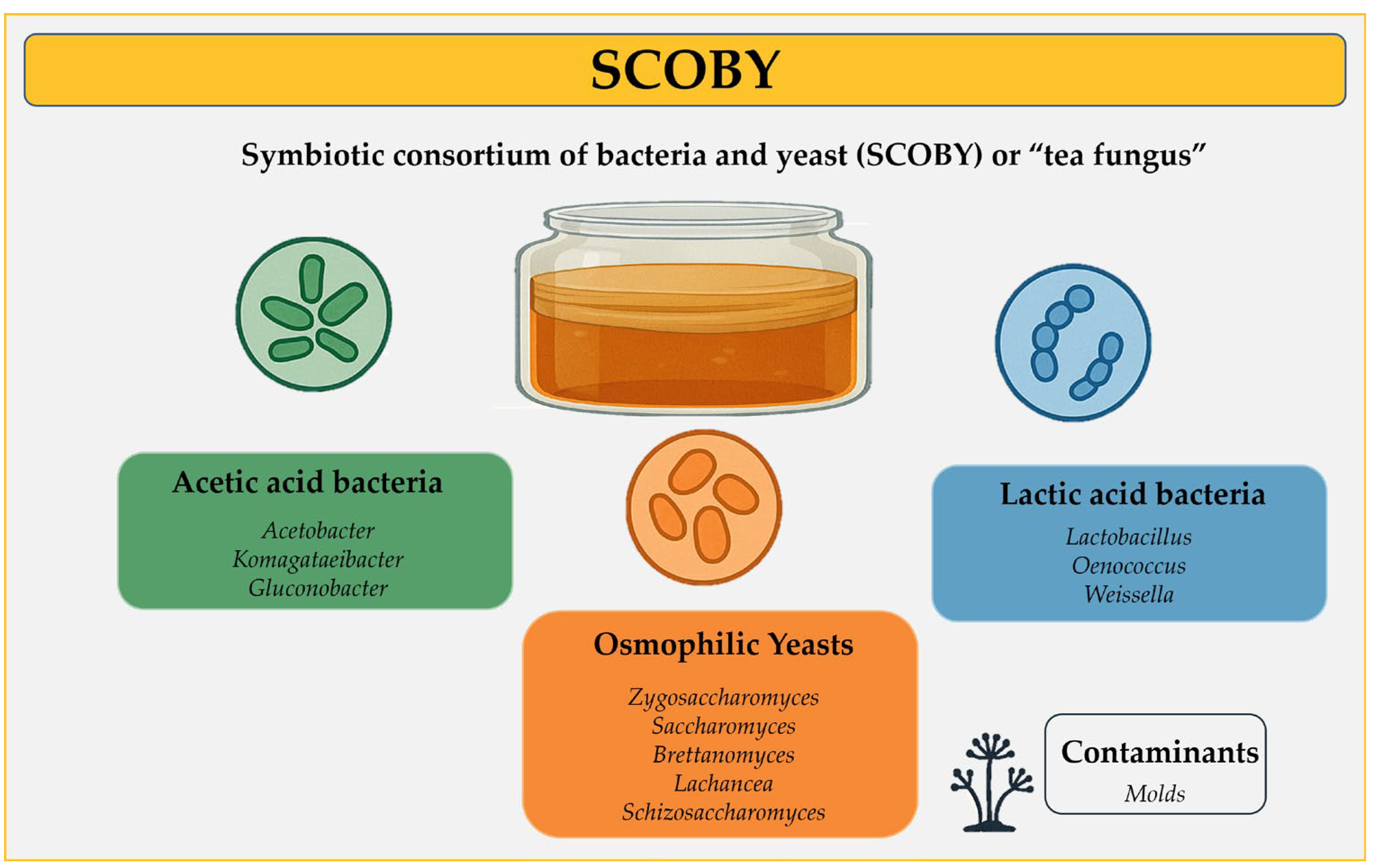
2. Lactic Acid Bacteria in Kombucha as Part of Natural Microbiota
| LAB | Method | Isolated from Kombucha/Pellicle | Origin | Sample Type (Commercial, Laboratory, Household) | Reference |
|---|---|---|---|---|---|
| Lactobacillus | DNA amplification and high-throughput sequencing | Kombucha | Canada, Ireland, UK, USA | Commercial | [31] |
| Lactococcus | DNA amplification and high-throughput sequencing | Kombucha | Canada, Ireland, UK, USA | Commercial | |
| Lactobacillus and Lactococcus | DNA amplification and high-throughput sequencing | Pellicle | Canada, Ireland, UK | Commercial | |
| Collinsella, Enterobacter, Weissella, Lactobacillus | Next-generation sequencing and data analysis | Kombucha | ns | Laboratory | [36] |
| nd | Morphological features followed by DNA amplification and high-throughput sequencing | Pellicle | Ukraine | Commercial | [39] |
| Oenococcus oeni, Lactobacillus nagelii, L. satsumensis | M13-PCR genetic profile clustering and sequence analysis | Kombucha | France | Commercial | [35] |
| Limosilactobacillus fermentum | DNA amplification and high-throughput sequencing | Kombucha | China | Household | [40] |
| Five isolates belonging to the Lactobacilli group | Molecular identification | Kombucha | Romania | Commercial | [32] |
| Lactobacillus | DNA amplification and high-throughput sequencing | Kombucha and pellicle | China | Commercial | [41] |
| Lactobacillaceae | Molecular identification | Kombucha and pellicle | UK | Commercial | [42] |
| nd | Diversity sequencing | Pellicle | USA | [27] | |
| Lactobacillus plantarum | Carbohydrate fermentation pattern followed by molecular identification | Pellicle | China | Commercial | [43] |
| nd | Metagenomic analyses | Kombucha and pellicle | France | Commercial and household | [44] |
| nd | Amplicon sequencing and shotgun metagenomics | Kombucha | Turkey | Household | [45] |
| Lactobacillus | Metabarcoding analyses | Kombucha and pellicle | USA | Commercial | [46] |
| nd | Metagenomics analysis | Kombucha | Australia | Commercial | [47] |
| Weissella spp., Lactobacillus rhamnosus, Lactobacillus acidophilus | DNA amplification and high-throughput sequencing | Kombucha | Iran | Commercial | [38] |
| nd | Phenotypic characterization followed by RNA sequence analyses | Kombucha | New Zealand | Commercial | [23] |
| Liquorilactobacillus and Ligilactobacillus | Metagenetic analysis | Kombucha | Brazil | Commercial | [33] |
| Lactobacillus nagelii and L. mali | Shotgun metagenomics analysis | Kombucha | USA | Commercial | [34] |
3. Kombucha with the Addition of Probiotic Cultures
| LAB Strain | Initial Number (log cfu/mL) | Survivability (Days) | Final Number(log cfu/mL) | pH Range (Initial–Final) | Reference |
|---|---|---|---|---|---|
| Lactobacillus plantarum | 8 | 8 | 3.43 | nr–3.15 | [51] |
| Lactobacillus plantarum | 6.81 | 3 | 4.39 | 4.16–2.78 | [57] |
| L. plantarum Lb-3 | 7.38 | 1 | 2.02 | 4.52–3.01 | [52] |
| L. plantarum Lb-4 | 7.54 | 3 | 2.95 | 4.30–3.03 | |
| L. plantarum Lb-5 | 7.13 | 3 | 1.90 | 4.29–3.06 | |
| Lactiplantibacillus plantarum subsp. plantarum | 6 | 28 | 6.26 | 3.32–2.68 | [56] |
| Lactiplantibacillus plantarum subsp. plantarum | 7 | 8 (not selected for storage analyses) | 8.32 | 3.34–2.72 | |
| Lactobacillus plantarum and Lactobacillus casei * | nr | nr | nr | nr | [54] |
| Lactobacillus casei | 8.72 | 15 | ~8.72 | 3.56–3.46 | [50] |
| Lactobacillus paracasei * | 3 | nr | nr | nr | [53] |
| Lactobacillus rhamnosus | 8.50 | 15 | 7.90 | 3.56–3.48 | [50] |
| Lactobacillus rhamnosus | 6.1 | 2 | 2.84 | 4.16–3.06 | [57] |
| Lactobacillus brevis and Lactobacillus fermentum | ~9 | 6 | <1 to 3 | nr–3 | [58] |
| Lactobacillus fermentum | 7.18 | 1 | <1 | 4.43–3.05 | |
| Lactobacillus hilgardii | 8.16 | 7 | 7.18 | 4.43–3.16 | [52] |
| Lactobacillus sp., Lactococcus lactis subsp. and Leuconostoc sp. * | 7.30 | nr | nr | 5.10–2.60 | [55] |
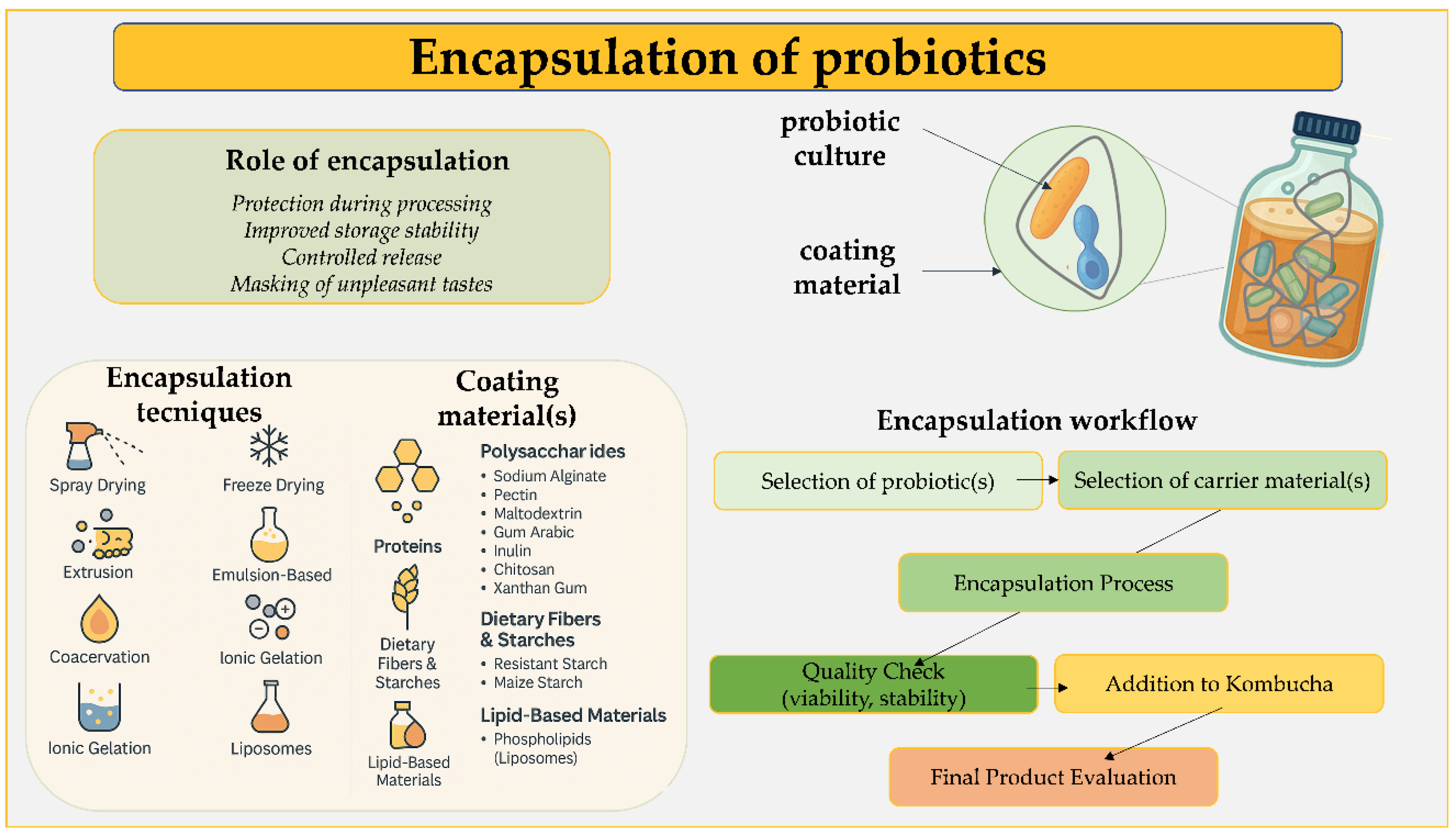
4. Encapsulation of Probiotic Bacteria as a Promising Method for Kombucha
| Probiotic Bacterial Strain | Carrier Material | Encapsulation Method | Food Product | Reference |
|---|---|---|---|---|
| Lactococcus lactis supsb. lactis 303 CFE | Liposomes | Microfluidization | Cheddar cheese ripening | [74] |
| Lactobacillus acidophilus and Bifidobacterium bifidum | Sodium alginate | Internal gelation | Grape juice | [72] |
| Enterococcus faecium | Sodium alginate | Extrusion | Sour cherry juice | [75] |
| Lactobacillus casei | Sodium alginate | Vibration technology | Pineapple, orange and raspberry juice | [76] |
| Lactobacillus casei and Lactobacillus acidophilus | Sodium alginate and cocoa powder | Lyophilization | Chocolate | [77] |
| Lacticaseibacillus rhamnosus | Sodium alginate | Ionic gelation following emulsification process as pretreatment | Apple juice and yogurt | [78] |
| Lactobacillus gasseri | Sodium alginate | Emulsification and extrusion | Apple juice | [79] |
| Lactobacillus rhamnosus and Lactobacillus plantarum | Whey protein isolate | Conventional and multilayer emulsion | Yogurt | [80] |
| Lactobacillus plantarum | Whey protein | Lyophilization | Apple juice | [81] |
| Probiotic Bacterial Strain | Carrier Material | Encapsulation Method | Food Product | Reference |
|---|---|---|---|---|
| Lactobacillus plantarum | Pectin, inulin and mixture of maltodextrin and glucose | Emulsion | Kombucha | [82] |
| Lactobacillus rhamnosus | Pectin, inulin, maltodextrin, pea protein, whey protein | Lyophilization | Kombucha | [13] |
5. Beneficial Effect of Probiotic Kombucha
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Šovljanski, O.; Budimac, T.; Tomić, A.; Cvetković, D.; Ranitović, A. Kombucha As a Functional Beverage Rich in Phenolic Compounds. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–42. [Google Scholar]
- Martins, H.F.; de Oliveira Santos, L.T.S.; de Carvalho, G.B.M.; Martinez, E.A. Kombucha: A literature review. Cuad. Educ. Y Desarro. 2023, 15, 11175–11197. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, wellness, and safety aspects of the consumption of kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef]
- Martínez Leal, J.; Valenzuela Suárez, L.; Jayabalan, R.; Huerta Oros, J.; Escalante-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA-J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Cetojević-Simin, D.D.; Bogdanovic, G.M.; Cvetkovic, D.D.; Velicanski, A.S. Antiproliferative and antimicrobial activity of traditional Kombucha and Satureja montana L. Kombucha. J. BUON 2008, 133, 395–401. [Google Scholar]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Chemical composition of kombucha. Beverages 2022, 8, 45. [Google Scholar] [CrossRef]
- Emiljanowicz, K.E.; Malinowska-Pańczyk, E. Kombucha from alternative raw materials–The review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef]
- Jayabalan, R.; Waisundara, V.Y. Kombucha as a functional beverage. In Functional and Medicinal Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 413–446. [Google Scholar]
- Bortolomedi, B.M.; Paglarini, C.S.; Brod, F.C.A. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef]
- Vargas, B.K.; Fabricio, M.F.; Ayub, M.A.Z. Health effects and probiotic and prebiotic potential of Kombucha: A bibliometric and systematic review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Maia, M.S.; Domingos, M.M.; de São José, J.F.B. Viability of Probiotic Microorganisms and the Effect of Their Addition to Fruit and Vegetable Juices. Microorganisms 2023, 11, 1335. [Google Scholar] [CrossRef]
- Budimac, T.; Pezo, L.; Šovljanski, O.; Cvetković, D.; Cvanić, T.; Vučetić, A.; Ranitović, A. An Optimal Probiotic Carrier: Multiple Steps Toward Selection and Application in Kombucha. Fermentation 2025, 11, 256. [Google Scholar] [CrossRef]
- Kim, J.; Adhikari, K. Current Trends in Kombucha: Marketing Perspectives and the Need for Improved Sensory Research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Market Growth Reports. Kombucha Beverage Market Report. 2024. Available online: https://www.marketgrowthreports.com/market-reports/kombucha-beverage-market-100707 (accessed on 17 June 2025).
- De Oliveira, P.V.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha benefits, risks and regulatory frameworks: A review. Food Chem. Adv. 2023, 2, 100288. [Google Scholar] [CrossRef]
- Batista, P.; Penas, M.R.; Pintado, M.; Oliveira-Silva, P. Kombucha: Perceptions and Future Prospects. Foods 2022, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.G.; de Lima, M.; Schmidt, V.C.R. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Cvetković, D.D.; Markov, S.L.; Velićanski, A. Antimicrobial activity of kombucha made from Rtanj tea. Hem. Ind. 2025, 59, 248–253. [Google Scholar] [CrossRef]
- De Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Bhattacharya, D.; Sarkar, S.; Gachhui, R. Kombucha: A promising functional beverage prepared from tea. In Non-Alcohol. Beverages; Woodhead Publishing: Cambridge, UK, 2019; pp. 285–327. [Google Scholar]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.-X.; Mutukumira, A.N. Kombucha: Production and Microbiological Research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation, J. Clean. Prod. 2021, 259, 126454. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea–Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- St-Pierre, D.L. Microbial Diversity of the Symbiotic Colony of Bacteria and Yeast (SCOBY) and Its Impact on the Organoleptic Properties of Kombucha. Master’s Thesis, The University of Maine, Orono, ME, USA, 2019. Available online: https://digitalcommons.library.umaine.edu/etd/3063/ (accessed on 12 June 2025).
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Enhancement of the antioxidant and starch hydrolase inhibitory activities of king coconut water (Cocos nucifera var. aurantiaca) by fermentation with Kombucha “tea fungus”. Int. J. Food Sci. Technol. 2016, 51, 490–498. [Google Scholar] [CrossRef]
- Laureys, D.; Britton, S.J.; de Clippeleer, J. Kombucha Tea Fermentation: A Review. J. Am. Soc. Brew. Chem. 2020, 78, 165–174. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea-A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Bogdan, M.; Justine, S.; Filofteia, D.C.; Petruta, C.C.; Gabriela, L.U.Ț.Ă.; Roxana, U.E.; Florentina, M.; Camelia Filofteia, D.; Călina Petruța, C.; Gabriela, L. Lactic acid bacteria strains isolated from Kombucha with potential probiotic effect. Rom. Biotechnol. Lett. 2018, 23, 13592–13598. [Google Scholar]
- Fabricio, M.F.; Mann, M.B.; Kothe, C.I.; Frazzon, J.; Tischer, B.; Flôres, S.H.; Ayub, M.A.Z. Effect of freeze-dried kombucha culture on microbial composition and assessment of metabolic dynamics during fermentation. Food Microbiol. 2022, 101, 103889. [Google Scholar] [CrossRef]
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and Chemical Profiles of Commercial Kombucha Products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Naghavi, N.S.; Khosravi-Darani, K.; Doudi, M.; Shahanipour, K. Isolation, molecular and phylogenetic identification of microorganisms from Kombucha solution and evaluation of their viability using flow cytometery. Food Sci. Technol. 2021, 42, e63220. [Google Scholar] [CrossRef]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional Efficacy of Probiotic Lactobacillus sanfranciscensis in Apple, Orange and Tomato Juices with Special Reference to Storage Stability and In Vitro Gastrointestinal Survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Podolich, O.; Zaets, I.; Kukharenko, O.; Orlovska, I.; Reva, O.; Khirunenko, L.; Sosnin, M.; Haidak, A.; Shpylova, S.; Rohutskyy, I.; et al. The first space-related study of a kombucha multimicrobial cellulose-forming community: Preparatory laboratory experiments. Orig. Life Evol. Biosph. 2017, 47, 169–185. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Sui, Y.C.; Wu, H.W.; Zhou, C.B.; Hu, X.C.; Zhang, J. Flavour chemical dynamics during fermentation of kombucha tea. Emir. J. Food Agric. 2018, 30, 732–741. [Google Scholar] [CrossRef]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef]
- Pei, J.; Jin, W.; Abd El-Aty, A.M.; Baranenko, D.A.; Gou, X.; Zhang, H.; Geng, J.; Jiang, L.; Chen, D.; Yue, T. Isolation, purification, and structural identification of a new bacteriocin made by Lactobacillus plantarum found in conventional kombucha. Food Control 2020, 110, 106923. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.P.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Arıkan, M.; Mitchell, A.L.; Finn, R.D.; Gürel, F. Microbial composition of Kombucha determined using amplicon sequencing and shotgun metagenomics. J. Food Sci. 2020, 85, 455–464. [Google Scholar] [CrossRef]
- Harrison, K.; Curtin, C. Microbial Composition of SCOBY Starter Cultures Used by Commercial Kombucha Brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Kaashyap, M.; Cohen, M.; Mantri, N. Microbial Diversity and Characteristics of Kombucha as Revealed by Metagenomic and Physicochemical Analysis. Nutrients 2021, 13, 4446. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Navarro, R.; Jensen, K.; Cayler, W.; Nielsen, T.; Curtin, C. Live, Probiotic, or Neither? Microbial Composition of Retail-Available Kombucha and “Hard” Kombucha in the Pacific Northwest of the United States. Beverages 2023, 9, 59. [Google Scholar] [CrossRef]
- Al-Dulaimi, F.K.; Abd-Alwahab, W.I.; Hasan, A.S. Bioactivity study of kombucha black tea and kombucha with skim milk on some of physiological and biochemical parameters in male albino rats. Int. J. Pharm. Res. 2018, 10, 301. [Google Scholar]
- Bueno, F.; Chouljenko, A.; Sathivel, S. Development of coffee kombucha containing Lactobacillus rhamnosus and Lactobacillus casei: Gastrointestinal simulations and DNA microbial analysis. LWT 2021, 142, 110980. [Google Scholar] [CrossRef]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef]
- Cvetković, D.; Ranitović, A.; Savić, D.; Joković, N.; Tomić, A.; Pezo, L.; Markov, S. Survival of wild strains of lactobacilli during Kombucha fermentation and their contribution to functional characteristics of beverage. Pol. J. Food Nutr. Sci. 2019, 69, 407–415. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.Y.; Yoo, D.G.; Jeon, Y.B.; Yoon, H.S.; Kim, C.H. Functional characteristics of kombucha fermented with lactic acid bacteria, yeast, and acetic acid bacteria derived from Korea traditional foods. J. Dairy Sci. Biotechnol. 2022, 40, 23–34. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Dong, N.T.N.; Nguyen, H.T.; Le, P.H. Lactic acid bacteria: Promising supplements for enhancing the biological activities of kombucha. Springerplus 2015, 4, 91. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, F.; Ji, B.; Li, B.; Luo, Y.; Yang, L.; Li, T. Symbiosis between microorganisms from kombucha and kefir: Potential significance to the enhancement of kombucha function. Appl. Biochem. Biotechnol. 2010, 160, 446–455. [Google Scholar] [CrossRef]
- Majid, A.A.; Suroto, D.A.; Utami, T.; Rahayu, E.S. Probiotic potential of kombucha drink from butterfly pea (Clitoria ternatea L.) flower with the addition of Lactiplantibacillus plantarum subsp. plantarum Dad-13. Biocatal. Agric. Biotechnol. 2023, 51, 102776. [Google Scholar] [CrossRef]
- Cvetković, D.; Ranitović, A.; Budimac, T.; Šovljanski, O. Examination of Lactobacillus plantarum and Lactobacillus rhamnosus during kombucha fermentation. In Proceedings of the 2nd International Symposium on Biotechnology, Čačak, Serbia, 14–15 March 2024; pp. 409–414. [Google Scholar]
- Bromley, A.; Perry, J. Survival of Probiotic Lactobacillus spp. During Kombucha Fermentation. Curr. Dev. Nutr. 2022, 6, 507. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Villarán, M.C. Encapsulation technology to protect probiotic bacteria. In Probiotics; IntechOpen: London, UK, 2012. [Google Scholar]
- Rama, G.R.; Führ, A.J.; da Silva, J.A.B.S.; Gennari, A.; Giroldi, M.; Goettert, M.I.; Volken de Souza, C.F. Encapsulation of Lactobacillus spp. using bovine and buffalo cheese whey and their application in orange juice. 3 Biotech 2020, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D.L.; Hoorfar, M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021, 3, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Subramanian, P. Encapsulation of probiotics: Past, present and future. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Kavitake, D.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods–A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Vallejo-Castillo, V. Probiotic encapsulation. Afr. J. Microbiol. Res. 2013, 7, 4743–4753. [Google Scholar] [CrossRef]
- Gul, O.; Atalar, I. Different stress tolerance of spray and freeze dried Lactobacillus casei Shirota microcapsules with different encapsulating agents. Food Sci. Biotechnol. 2019, 28, 807–816. [Google Scholar] [CrossRef]
- Tarifa, M.C.; Piqueras, C.M.; Genovese, D.B.; Brugnoni, L.I. Microencapsulation of Lactobacillus casei and Lactobacillus rhamnosus in pectin and pectin-inulin microgel particles: Effect on bacterial survival under storage conditions. Int. J. Biol. Macromol. 2021, 179, 457–465. [Google Scholar] [CrossRef]
- Barbosa, J.; Brandão, T.R.; Teixeira, P. Spray drying conditions for orange juice incorporated with lactic acid bacteria. Int. J. Food Sci. Technol. 2017, 52, 1951–1958. [Google Scholar] [CrossRef]
- Frakolaki, G.; Kekes, T.; Lympaki, F.; Giannou, V.; Tzia, C. Use of encapsulated Bifidobacterium animalis subsp. lactis through extrusion or emulsification for the production of probiotic yogurt. J. Food Process Eng. 2022, 45, e13792. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B. Legume proteins are smart carriers to encapsulate hydrophilic and hydrophobic bioactive compounds and probiotic bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1250–1279. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Saeed, M.; Azam, M.; Hussain, S.; Mohamed, A.A.; Alamri, M.S.; Anjum, F.M. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal and thermal conditions. Int. J. Food Prop. 2020, 23, 1899–1912. [Google Scholar] [CrossRef]
- Zeashan, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Tufail, T.; Ahmed, A.; Anjum, F.M. Survival and behavior of free and encapsulated probiotic bacteria under simulated human gastrointestinal and technological conditions. Food Sci. Nutr. 2020, 8, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci. Technol. Int. 2019, 25, 120–129. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Abrlova, M.; Kilcawley, K.N. Encapsulation of a Lactic Acid Bacteria Cell-Free Extract in Liposomes and Use in Cheddar Cheese Ripening. Foods 2013, 2, 100–119. [Google Scholar] [CrossRef]
- Azarkhavarani, P.R.; Ziaee, E.; Hosseini, S.M.H. Effect of encapsulation on the stability and survivability of Enterococcus faecium in a non-dairy probiotic beverage. Food Sci. Technol. Int. 2019, 25, 233–242. [Google Scholar] [CrossRef]
- Olivares, A.; Soto, C.; Caballero, E.; Altamirano, C. Survival of microencapsulated Lactobacillus casei (prepared by vibration technology) in fruit juice during cold storage. Electron. J. Biotechnol. 2019, 42, 42–48. [Google Scholar] [CrossRef]
- Hossain, M.N.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Impact of encapsulating probiotics with cocoa powder on the viability of probiotics during chocolate processing, storage, and in vitro gastrointestinal digestion. J. Food Sci. 2021, 86, 1629–1641. [Google Scholar] [CrossRef]
- Romero-Chapol, O.O.; Varela-Pérez, A.; Castillo-Olmos, A.G.; García, H.S.; Singh, J.; García-Ramírez, P.J.; Viveros-Contreras, R.; Figueroa-Hernández, C.Y.; Cano-Sarmiento, C. Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic Survival, In Vitro Digestion and Viability in Apple Juice and Yogurt. Appl. Sci. 2022, 12, 2141. [Google Scholar] [CrossRef]
- Varela-Pérez, A.; Romero-Chapol, O.O.; Castillo-Olmos, A.G.; García, H.S.; Suárez-Quiroz, M.L.; Singh, J.; Figueroa-Hernández, C.Y.; Viveros-Contreras, R.; Cano-Sarmiento, C. Encapsulation of Lactobacillus gasseri: Characterization, Probiotic Survival, In Vitro Evaluation and Viability in Apple Juice. Foods 2022, 11, 740. [Google Scholar] [CrossRef]
- Mahmoodi Pour, H.; Marhamatizadeh, M.H.; Fattahi, H. Encapsulation of different types of probiotic bacteria within conventional/multilayer emulsion and its effect on the properties of probiotic yogurt. J. Food Qual. 2022, 2022, 7923899. [Google Scholar] [CrossRef]
- Sun, W.; Nguyen, Q.D.; Sipiczki, G.; Ziane, S.R.; Hristovski, K.; Friedrich, L.; Visy, A.; Hitka, G.; Gere, A.; Bujna, E. Microencapsulation of Lactobacillus plantarum 299v Strain with Whey Proteins by Lyophilization and Its Application in Production of Probiotic Apple Juices. Appl. Sci. 2023, 13, 318. [Google Scholar] [CrossRef]
- Budimac, T.; Ranitović, A.; Cvetković, D.; Šovljanski, O.; Tomić, A.; Vučetić, A.; Cvanić, T. Survivability of microencapsulated Lactobacillus plantarum during kombucha fermentation. In Book of Abstracts: 5th International Congress “Food Technology, Quality and Safety–FoodTech 2024”, Proceedings of the 5th International Congress “Food Technology, Quality and Safety–FoodTech 2024”, Novi Sad, Serbia, 16–18 October 2024; University of Novi Sad, Institute of Food Technology: Novi Sad, Serbia, 2024. [Google Scholar]
- FAO/WHO. The Food and Agriculture Organization of the United Nations and the World Health Organization Joint FAO/WHO Expert Consultation on the Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria FAO/WHO Report No. 10-1-2001. 2001. Available online: https://openknowledge.fao.org/items/db384295-64d9-47e2-b65b-3c918efc5140 (accessed on 1 June 2025).
- Asar, R.; Erenler, S.; Devecioglu, D.; Ispirli, H.; Karbancioglu-Guler, F.; Ozturk, H.I.; Dertli, E. Understanding the Functionality of Probiotics on the Edge of Artificial Intelligence (AI) Era. Fermentation 2025, 11, 259. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Bellissimo, N.; Vandelanotte, C.; Williams, S.; Stanley, D.; Irwin, C. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019, 73, 24–37. [Google Scholar] [CrossRef]
- Kozyrovska, N.O.; Reva, O.M.; Goginyan, V.B.; de Vera, J.-P. Kombucha microbiome as a probiotic: A view from the perspective of post-genomics and synthetic ecology. Biopolymers Cell 2012, 28, 103–113. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budimac, T.; Ranitović, A.; Šovljanski, O.; Cvetković, D.; Tomić, A. Living Cultures in a Glass: The Health Promise of Probiotic Bacteria in Kombucha. Fermentation 2025, 11, 434. https://doi.org/10.3390/fermentation11080434
Budimac T, Ranitović A, Šovljanski O, Cvetković D, Tomić A. Living Cultures in a Glass: The Health Promise of Probiotic Bacteria in Kombucha. Fermentation. 2025; 11(8):434. https://doi.org/10.3390/fermentation11080434
Chicago/Turabian StyleBudimac, Tara, Aleksandra Ranitović, Olja Šovljanski, Dragoljub Cvetković, and Ana Tomić. 2025. "Living Cultures in a Glass: The Health Promise of Probiotic Bacteria in Kombucha" Fermentation 11, no. 8: 434. https://doi.org/10.3390/fermentation11080434
APA StyleBudimac, T., Ranitović, A., Šovljanski, O., Cvetković, D., & Tomić, A. (2025). Living Cultures in a Glass: The Health Promise of Probiotic Bacteria in Kombucha. Fermentation, 11(8), 434. https://doi.org/10.3390/fermentation11080434







