Abstract
Porphyra-derived polysaccharides (PPs) are promising prebiotic candidates due to their capacity to modulate gut microbiota and promote host health. However, their interactions with and utilization by probiotic microorganisms remain unclear. In this study, the fermentability of PPs by murine-derived lactic acid bacteria (LAB) strains was investigated, with particular attention to strain-specific metabolic activity, carbohydrate utilization, and potential exopolysaccharide (EPS) production. All tested strains were capable of utilizing PPs to varying extents, with strain A10 exhibiting the highest level of carbohydrate consumption. Notably, strain A5 showed increased mannose concentrations following fermentation, suggesting the biosynthesis of mannose-rich EPSs. HPLC analysis confirmed the presence of high-molecular-weight polysaccharides ranging from 2.6 to 8.1 × 105 Da, indicative of EPS production. FT-IR spectroscopy further revealed spectral features consistent with EPS structures. The antibacterial activity of postbiotic compounds produced by LAB strains fermenting PPs against Escherichia coli and Staphylococcus aureus was observed. These findings demonstrate distinct metabolic adaptations of LAB strains to PPs and emphasize their potential as prebiotic substrates.
1. Introduction
Porphyra, commonly known as nori, is a type of red algae that has been consumed as food for centuries, particularly in Asia [1]. It contains various bioactive compounds, including polysaccharides, proteins, and lipids, which exhibit a range of physiological activities [1,2]. Porphyra-derived polysaccharides (PPs) have been reported to consist of a linear backbone with 3-linked β-D-galactosyl units alternating with either 4-linked α-L-galactosyl 6-sulfate or 3,6-anhydro-α-L-galactosyl units [3]. These polysaccharides are being investigated for potential applications in food, medicine, and cosmetics due to their diverse biological properties, including immunomodulatory [4], anti-inflammatory [4,5], antioxidant [5,6], and anticancer effects [6].
Probiotics are live microorganisms that confer health benefits, including aiding digestion, supporting the host immune system, alleviating symptoms of certain intestinal diseases, and maintaining or restoring the balance of the gut microbiome [7]. Prebiotics are substrates selectively utilized by host microorganisms to confer health benefits [7]. Postbiotics are preparations of inactivated probiotics and/or their metabolic components that confer benefits to the host [8]. Among these components, substances such as lactic acid, bacteriocins, and exopolysaccharides (EPSs) have demonstrated antimicrobial activity against pathogenic microorganisms [8].
Recently, there has been growing interest in the potential applications of marine polysaccharides as dietary supplements, particularly due to their beneficial effects on the gastrointestinal tract [9]. Numerous studies have reported the potential and applications of red algae-derived polysaccharides as prebiotics. Sulfated polysaccharides from Gracilaria fisheri have demonstrated selective promotion of the growth of Lactobacillus paracasei TISTR 2389 and Lactobacillus acidophilus TISTR 875 [10]. Polysaccharides derived from Porphyra haitanensis have been shown to enhance the abundance of beneficial gut microbiota and stimulate the production of short-chain fatty acids (SCFAs) during in vivo fermentation in rats [11]. Furthermore, oral administration of these polysaccharides in mice significantly increased total SCFA levels in the gut and supported the integrity and function of the intestinal mucosal barrier [12]. Collectively, these findings suggest that red algae-derived polysaccharides have the potential to modulate gut microbiota composition and support intestinal health, demonstrating their promise as prebiotics. Nevertheless, most current research on algae-derived polysaccharides has primarily concentrated on their influence on gut microbial composition and SCFA production. Limited attention has been given to their roles as direct fermentation substrates and their specific effects on beneficial gut microorganisms, particularly probiotic strains such as Lactobacillus. Moreover, the mechanisms underlying the transformation and utilization of algae-derived polysaccharides during microbial fermentation remain inadequately characterized.
To build upon current understanding, this study investigated the prebiotic effects of PPs on lactic acid bacteria (LAB) strains isolated from the intestinal tract of mice subjected to long-term dietary supplementation with water extracts of Porphyra. These isolated strains were subsequently cultured with PPs to assess their growth responses. In parallel, compositional and structural analyses of the polysaccharides were conducted to evaluate their transformation and utilization during fermentation.
2. Materials and Methods
2.1. Chemicals and Materials
Chemicals, agar powder, and De Man, Rogosa, and Sharpe (MRS) medium were purchased from Sigma Aldrich Life Science (St. Louis, MO, USA). Nutrient broth (NB) was purchased from HiMedia Laboratories Private Limited (Mumbai, Maharashtra, India). Dried Porphyra dentata was purchased from Pin Jia Biotech (Pingtung, Taiwan). It was then ground into a fine powder using an electric grinder (RT-02, Mill Powder Tech Solutions, Tainan City, Taiwan) and passed through a 40-mesh sieve before use.
2.2. Preparation of Porphyra-Derived Polysaccharides
The preparation of PPs was conducted following previously established methods [13,14]. In summary, 40 g of Porphyra powder was suspended in 1000 mL of deionized water and autoclaved at 121 °C for 20 min. After hot-water extraction, the samples were centrifuged, and the supernatant was mixed with six times its volume of 95% ethanol to induce precipitation. The resulting precipitate was collected and freeze-dried to obtain a polysaccharide powder. To obtain polysaccharide fractions with different molecular weights, PP powder was dissolved in deionized water and subjected to ultrafiltration, yielding a high molecular weight fraction (>10 kDa) and a low molecular weight fraction (<10 kDa) for further experiments.
2.3. Protocol of Animal Experiments
In this study, a limited number of animals per group was used in accordance with the 3R principles to minimize animal use, as the primary aim was to isolate murine-derived LAB strains following dietary exposure to PPs and to profile gut microbial composition rather than to assess specific biological effects or intervention efficacy. The animal experiments were conducted in compliance with the ethical guidelines specified in the National Research Council’s Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee of National Taiwan Ocean University (NTOU IACUC-108040). Six-week-old female BALB/c mice were obtained from the National Laboratory Animal Center of Taiwan. The mice were housed in the laboratory animal facility at National Taiwan Ocean University (NTOU) and were given free access to food and water for one week before the experiment began. The mice were assigned to the following groups (three mice per group): the naïve group (NA) and the treatment groups. In the treatment groups, mice were orally administered PPs at a dose of 250 mg/kg body weight (b.w.) with either high- or low-molecular-weight fractions dissolved in 0.1 mL of PBS, once daily for 4 weeks. The dosage regimen was determined based on findings from a previous study [14]. On the final day of the experiment, the mice were humanely euthanized, and fecal materials were collected from the proximal colon. Fresh fecal materials were then used for next-generation sequencing (NGS) analysis of the full-length 16S rRNA gene, following previously established protocols [13,15].
2.4. Isolation and Identification of Murine LAB Strains
The isolation of bacterial strains from murine feces was conducted following previously established methods [16,17]. Briefly, fresh fecal material from each mouse was dissolved in sterile water and spread onto MRS agar plates for incubation (37 °C, 5% CO2). After 24 h of incubation, the isolated colonies were selected for identification and further experiments. The isolated bacteria were preserved in MRS broth with glycerol in a 1:1 ratio at −80 °C. Before each experiment, bacterial strains were cultivated in MRS broth under anaerobic conditions (37 °C, 5% CO2) for 24 h.
2.5. LAB Fermentation and the Preparation of Fermented Cell-Free Supernatants
The isolated LAB strains were cultured in standard MRS broth and modified MRS broth under anaerobic conditions (37 °C, 5% CO2) for 24 or 48 h. The modified MRS broth was prepared by adding 1% PPs. The optical density at 600 nm (OD600) and pH values were measured during the 48 h of fermentation. After 24 h of fermentation, the LAB cultures were centrifuged at 10,000× g, and the supernatants were collected and filtered through a 0.22 μm filter to remove any remaining cells.
2.6. Saccharides Detection and Chemical Analysis of Fermented Cell-Free Supernatants
Total sugar content was determined using the phenol–sulfuric acid method [18,19]. Briefly, fermented supernatants were diluted and mixed with phenol and sulfuric acid, followed by incubation at 100 °C for 30 min. After the reaction, absorbance was measured at 490 nm using a microplate reader (Synergy HT, BioTek Instrument, Winooski, VT, USA). The qualitative and quantitative analysis of monosaccharides in the fermented medium was conducted according to previously established methods [20]. Fermented supernatants were pre-treated with trifluoroacetic acid for 4 h, followed by removal of the acid using a rotary evaporator (N-1200A, EYELA, Tokyo, Japan). Monosaccharides in the processed samples were analyzed by high-performance liquid chromatography (HPLC) using a BP–100 Pb column (Benson Polymeric, Reno, NV, USA) under ligand-exchange mode, with an elution flow rate of 0.4 mL/min at 90 °C. The molecular weight distribution and chemical composition of the samples before and after fermentation were analyzed using HPLC and Fourier-transform infrared spectroscopy (FT-IR), respectively, following the procedures described by the previous study [14].
2.7. Antimicrobial Activity
The antibacterial activity of isolated LAB strains was tested using the agar–well diffusion method, as described in previous studies [16,17]. The indicator bacteria used were Staphylococcus aureus BCRC10451 and Escherichia coli BCRC11634, purchased from Food Industry Research and Development Institute (Hsinchu City, Taiwan). Briefly, the indicator bacterial strains were precultured for 18 h, and the bacterial suspensions were diluted with sterile water to an optical density equivalent to the 0.5 McFarland standard (OD600 = 0.37). The diluted bacterial suspensions were spread onto nutrient agar plates, which were then punctured to create wells (6 mm in diameter). The LAB fermented cell-free supernatants were introduced into the wells. All cultured plates were incubated at 37 °C with 5% CO2 for 18 h. After incubation, the plates were assessed, and the diameters of growth inhibition zones were measured.
2.8. Statistical Analysis
Comparisons between groups were performed using ANOVA, while differences between the test and control groups were analyzed using Tukey’s test (SigmaPlot V14, Systat Software Inc., San Jose, CA, USA). Data normality was assessed using the Shapiro–Wilk test before applying ANOVA. Statistical significance was defined as p < 0.05.
3. Results
3.1. Isolation and Identification of Fecal LAB Strains from Mice Treated with Water Extracts of Porphyra
To obtain murine-derived LAB strains potentially promoted by PP supplementation, fecal samples were collected from mice administered water extracts of Porphyra daily for four weeks. Colon-derived fecal material was processed for LAB isolation through serial dilution and plating on MRS agar, followed by incubation for 48 h. Distinct colonies were selected and identified by 16S rRNA gene sequencing using BLAST analysis (BLAST 2.14.1+). A total of nine LAB strains were isolated (Table 1). Strains showing ≥99% sequence similarity with type strains in the NCBI database were considered to belong to the same species. Notably, two species were of particular interest: Ligilactobacillus murinus and Limosilactobacillus reuteri, both known for their probiotic properties [21,22]. Among them, four LAB strains, including L. murinus A1, L. murinus A5, L. reuteri A9, and L. reuteri A10, exhibited stable growth over 48 h and were selected for further analysis.

Table 1.
The identification of the LAB strains isolated from the feces of mice treated with Porphyra-derived extracts.
3.2. Influence of Porphyra-Derived Polysaccharides on the Growth of LAB Strains
To assess the influence of PPs on the microbial activity of isolated LAB strains, the growth of L. murinus A1 and A5 and L. reuteri A9 and A10 co-cultured with PPs was determined. As shown in Figure 1, among the four strains, A1 exhibited significantly lower OD600 values, indicating weaker growth activity compared to the other strains. In contrast, the other LAB strains showed robust growth in standard MRS media, MRS supplemented with 1% PPs, and modified MRS media lacking glucose but containing 2% PPs as the sole carbon source. After 48 h of cultivation, OD600 values for A5, A9, and A10 all exceeded 1.0, with the lowest values observed in the 1% PPs-supplemented MRS group.
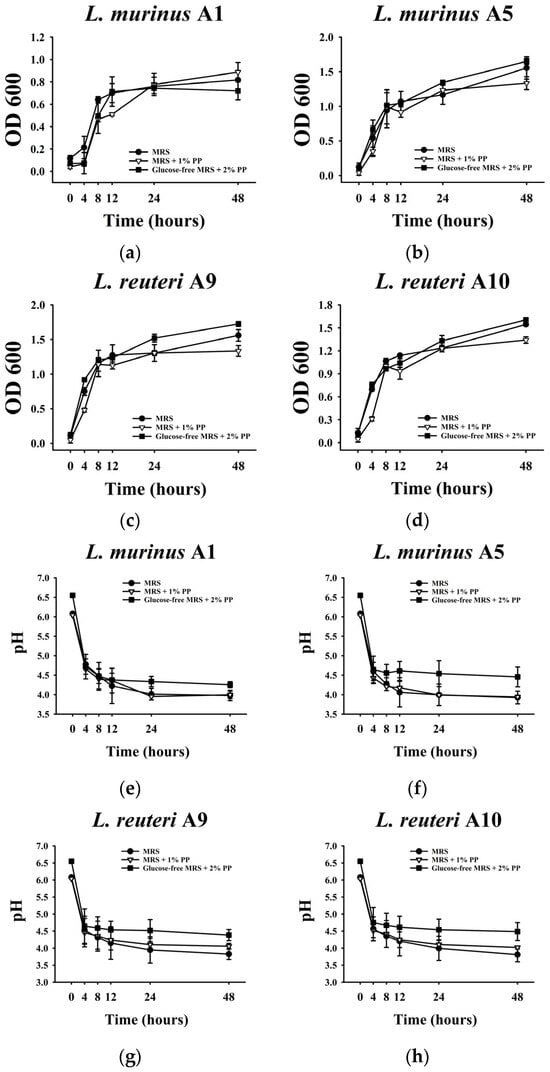
Figure 1.
Effect of Porphyra polysaccharides (PPs) on the growth and acid production of isolated LAB. (a–d) OD600 measurements of each isolated LAB at different fermentation times (0, 4, 8, 12, 24, and 48 h). (e–h) pH values of the LAB fermented supernatants at different fermentation times (0, 4, 8, 12, 24, and 48 h). All data are expressed as mean ± SD (n = 3).
To further assess the acid production capacity of these LAB strains, pH measurements were conducted. In the absence of glucose, when 2% PPs were provided as the sole carbon source, the pH values of the four strains were significantly higher than in the other conditions, suggesting diminished metabolic activity and acid production (Figure 1e–h). In standard MRS media and those supplemented with 1% PPs, the pH declined with bacterial growth (Figure 1a–h).
Additionally, total sugar content was measured before and after fermentation to evaluate carbohydrate utilization. As shown in Figure 2, LAB strains A5, A9, and A10 utilized 65–70% of the total sugars in both standard MRS media and those supplemented with 1% PPs after 24 h fermentation. LAB strain A10 exhibited the highest consumption of total sugar. Conversely, strain A1 consumed only 46–50% of the available sugars, indicating lower carbohydrate utilization efficiency. These findings are consistent with its growth and suggest strain A1 has lower overall microbial activity compared to the other three LAB strains. Given that probiotics typically grow in environments containing multiple carbohydrates, such as those found in the gastrointestinal tract, further experiments were conducted in the presence of glucose and 1% PPs to better simulate these conditions.
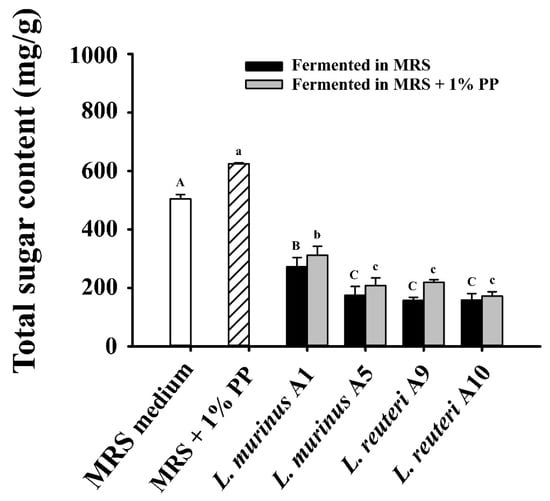
Figure 2.
Total sugar content in the supernatants of LAB strains after 24 h fermentation in MRS with or without 1% PPs. Data are expressed as mean ± SD (n = 3). Different letters (A–C, a–c) indicate significant differences (p < 0.05) among samples fermented in MRS medium and MRS medium supplemented with 1% PPs, respectively.
3.3. Utilization of PPs by LAB Fermentation
To assess the utilization of PPs by the four LAB strains during fermentation, changes in the molecular weight distribution of polysaccharide components in the fermentation medium were analyzed. As shown in Figure 3a, several molecular weight ranges were detected in both the non-fermented and fermented supernatant, including 7.8–8.1 × 105 Da, 2.6–3.9 × 105 Da, 0.8–2.3 × 104 Da, and 0.8–1.5 × 103 Da. Based on these results, four major molecular weight fractions were defined: F1 (2.6–8.1 × 105 Da), F2 and F3 (0.8–2.3 × 104 Da), and F4 (0.8–1.5 × 103 Da), which were consistently observed in both non-fermented and fermented supernatants. Notably, differences in peak areas, which represent the concentrations of polysaccharide fractions, were observed between non-fermented and fermented groups. In the fermented supernatants, the peak areas corresponding to the three lower molecular weight fractions (F2, F3, and F4) were significantly reduced compared to the non-fermented control (Figure 3b), indicating active degradation and utilization of PPs by the four LAB strains. Conversely, a significant increase in the peak area of the highest molecular weight fraction (F1) was detected in the fermented supernatants, suggesting the possible production of EPSs by the strains, with a molecular weight of approximately 7.8–8.1 × 105 Da.
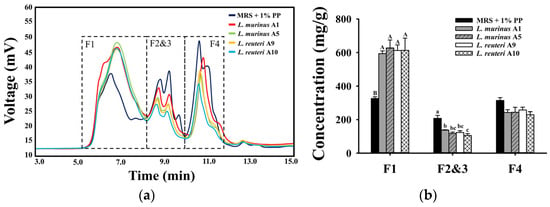
Figure 3.
Molecular weight analysis of polysaccharides using HPLC. F1–F4 represent four polysaccharide fractions with different molecular weights. (a) HPLC spectra of polysaccharide compounds in the supernatants before and after LAB fermentation in MRS with 1% PPs for 24 h. (b) Concentration of different polysaccharide fractions in the supernatants after 24 h fermentation. Data are expressed as mean ± SD (n = 3). Different letters (A and B for F1; a–c for F2 and F3) indicate significant differences (p < 0.05) among the respective sample groups.
To further investigate the metabolism of PPs during LAB fermentation, the changes in monosaccharide composition and concentrations of fermented supernatants were analyzed (Figure 4). Prior to fermentation, the standard MRS medium supplemented with 1% PPs contained glucose, galactose, and mannose as its primary monosaccharides. After 24 h of fermentation, four LAB strains demonstrated differential utilization of monosaccharides. As shown in Figure 4a, all four strains utilized a portion of the glucose. Specifically, LAB strain A1 exhibited the lowest glucose consumption, utilizing 20% of the available glucose, whereas strain A5 completely depleted it. LAB strains A9 and A10 showed comparable glucose consumption levels, each utilizing 50% (Figure 4a). With respect to mannose, LAB strains A1 and A9 each consumed 15% of mannose after 24 h of fermentation, whereas LAB strain A10 exhibited negligible utilization. Notably, the mannose concentration in the fermented supernatant of LAB strain A5 was increased (Figure 4b). Previous studies have identified galactose as the predominant monosaccharide in PPs [3]. In this study, LAB strains A1, A9, and A10 utilized a portion of the galactose during fermentation, as indicated by reduced galactose concentrations in the fermented supernatants. In contrast, the galactose level in the fermented supernatants of LAB strain A5 remained consistent after fermentation, indicating limited galactose utilization by this strain during fermentation with the supplementation of PPs (Figure 4c).
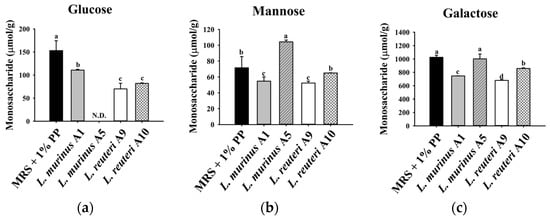
Figure 4.
The content of (a) glucose, (b) mannose, and (c) galactose in the supernatants of MRS with 1% PPs after 24 h fermentation of LAB strains. Data are expressed as mean ± SD (n = 3). Different letters (a–d) indicate a significant difference (p < 0.05). N.D.: not detected.
3.4. Structural Modification of Postbiotics After Fermentation by LAB Strains
FT-IR analysis was performed to compare the potential difference in chemical compositions of PPs, standard MRS medium, and the fermented supernatants of MRS with 1% PPs. As shown in Figure 5, the FT-IR spectra of the cell-free supernatant exhibited peaks that were also present in both PPs and the standard MRS medium groups, indicating components from both PPs and MRS were integrated into the LAB-fermented products. Specifically, the peaks at 1646, 1409, 1078, 926, and 817 cm−1, which are characteristic of porphyran, as well as those observed in the standard MRS medium (1601, 1457, 1121, 1044, 993, 853, 774 cm−1), were present in the supernatant of post-fermentation by the four LAB strains (Figure 5). These peaks correspond to the asymmetrical and symmetrical stretching vibrations of the carboxylate functional group (C=O at 1646 and 1408 cm−1), the stretching of the carboxyl group in the pyranose ring (C-OH at 1067 cm−1), and the sulfate group linked to a primary hydroxyl group (S=O at 817 cm−1). However, the additional peak (at 1250 cm−1) unique to LAB strains fermented supernatant was detected, which was absent from both PPs and the standard MRS medium (Figure 5).
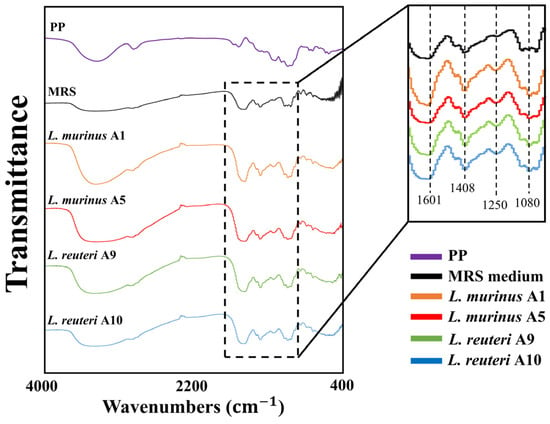
Figure 5.
Chemical characteristics and functional group analysis of fermented products using FT-IR. PP: Porphyra-derived polysaccharide.
3.5. Antimicrobial Ability of Postbiotics Against E. coli and S. aureus
To further evaluate the impact of PP supplementation on the antibacterial activity of the isolated LAB strains, each strain was cultured in two different media. One was standard MRS medium, and the other was MRS medium supplemented with 1% PPs. Antibacterial activity is considered an important indicator of probiotic potential. As shown in Figure 6, all four LAB strains exhibited promising antimicrobial activity against E. coli. However, the inhibition zones produced by strains A1 and A5 were reduced when cultured in MRS medium containing 1% PPs compared to the standard MRS medium. Compared to that in standard MRS medium, the inhibition zones of LAB strains against S. aureus were not statistically decreased when cultured in MRS medium containing 1% PPs (Figure 6).
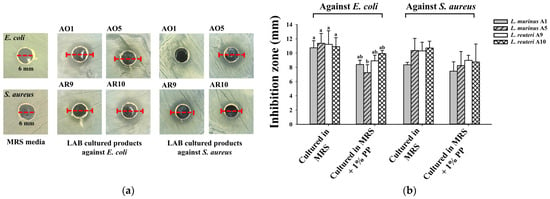
Figure 6.
Antibacterial activity of supernatants after LAB fermentation in MRS containing 1% PPs for 24 h. (a) Punctured wells (6 mm in diameter) and inhibition zones of test groups against E. coli and S. aureus from the isolated LAB. (b) The diameter of the inhibition zone of the supernatants fermented by isolated LAB strains. Data are expressed as mean ± SD (n = 3). Values with different letters (a and b) indicate statistically significant differences (p < 0.05) in the test against E. coli.
3.6. Gut Microbiota Modulation and Functional Microbial Shifts Induced by Porphyra-Derived Polysaccharides in Mice
β-diversity analysis revealed that mice treated with PPs, including both high (>10 kDa) and low (<10 kDa) molecular weight fractions, exhibited greater microbial diversity compared to the control group (Figure 7a). Specifically, PP treatment led to notable alterations in the relative abundance of several microbial taxa, indicating significant shifts in gut microbial composition. As shown in Figure 7b, the relative abundance of the class Clostridia increased in mice administered with high-molecular-weight PPs. Additionally, both PP-treated groups showed a marked increase in the class Bacteroidia, while a reduction in the class Bacilli. Finally, the Firmicutes/Bacteroidetes ratio was elevated in both PP groups compared to the control group (Figure 7c), suggesting broader ecological shifts in the gut microbiota.
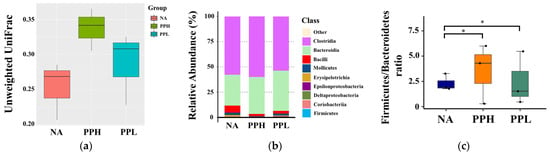
Figure 7.
Effects of PPs on gut microbiota composition in mice. NA: Non-treated mice. PPH and PPL: Mice administered daily with 250 mg/kg b.w. of high-molecular-weight PPs (PPH) and low-molecular weight PPs (PPL), respectively, for 4 weeks. (a) Microbiota diversity using unweighted UniFrac distance analysis. (b) Relative abundance of gut microbiota at the class level. (c) Firmicutes/Bacteroidetes ratio analysis. * indicates a significant difference compared to fresh MRS medium (p < 0.05).
4. Discussion
This study provides the first comprehensive evaluation of PPs as fermentable substrates for murine LAB strains, revealing strain-specific growth, metabolic activity, and carbohydrate utilization. These findings provide valuable insights into the potential of PPs to serve as novel marine-derived prebiotic compounds.
Algal polysaccharides such as ulvan, laminaran, and fucoidan have been reported to stimulate the growth of beneficial gut bacteria and modulate gut microbiota composition [23,24]. However, few studies have provided direct evidence that algal-derived dietary fibers specifically enhance the proliferation or metabolic activity of LAB. LAB can adapt metabolically to specific carbohydrate environments by inducing the production of enzymes such as glycoside hydrolases, glycosyltransferases, and carbohydrate esterases [25]. These enzymes allow the bacteria to break down complex polysaccharides and utilize the resulting sugars for growth [26]. This demonstrates the adaptive metabolic capacity of LAB shaped by long-term dietary exposure. In the present study, the LAB strains were isolated from mice that had undergone long-term dietary exposure to PPs. This treatment was intended to promote the enrichment of strains capable of fermenting PPs. Therefore, the murine-derived LAB strains were hypothesized to exhibit adaptive metabolic traits favoring the utilization of marine polysaccharides, thereby providing a more ecologically valid model for evaluating algal carbohydrate fermentation.
Previous studies have demonstrated that L. murinus supports gut barrier integrity by upregulating tight junction proteins and reducing intestinal permeability, thereby mitigating systemic inflammation [27,28]. It also displays antimicrobial activity against various pathogenic microorganisms [27]. L. reuteri, a well-characterized probiotic, produces antimicrobial compounds such as reuterin and has been shown to enhance intestinal barrier function and modulate gut microbiota composition, contributing to gastrointestinal health [29,30,31]. In our experiments, PPs supported the growth of the four LAB strains (L. murinus A1 and A5 and L. reuteri A9 and A10) but also appeared to influence their metabolic activity. Growth patterns and metabolic profiles of these strains exhibited strain-specific responses to PPs. L. murinus A1 showed significantly reduced growth, carbohydrate utilization, and acid production, suggesting a limited ability to metabolize PPs as a carbon source. In contrast, strains A5, A9, and A10 maintained robust growth under various conditions, including media where Porphyra polysaccharides served as the sole carbon source. Notably, pH values correlated with bacterial growth and sugar utilization. The observed decrease in pH during fermentation serves as a strong indirect indicator of organic acid production, a characteristic outcome of LAB metabolism. Among the metabolites typically produced, SCFAs such as acetate, propionate, and butyrate are known to play crucial roles in host physiology, including gut health and immune modulation [11,12]. In this study, although SCFAs were not directly quantified, the relatively higher pH levels observed in PP-supplemented media suggest lower acid production, potentially due to metabolic shifts or buffering effects associated with the polysaccharides. To further elucidate the fermentability of PPs and their metabolic impact, future investigations will employ metabolomic approaches to comprehensively profile fermentation products. These analyses will focus on SCFA production by probiotic strains and their potential relevance to the bioactivities observed in the host. Based on the metabolomic findings, key metabolites, including SCFAs and other organic acids, will be quantified and validated using targeted analytical techniques.
The measurement of total sugar concentration revealed that all LAB strains utilized carbohydrate sources during fermentation with PP supplementation. Among these strains, A10 exhibited the highest total sugar consumption, indicating enhanced carbohydrate metabolism and potentially more efficient polysaccharide degradation. Some LAB strains have been reported to metabolize a broad range of polysaccharides, including indigestible plant-derived polymers such as cellulose and hemicellulose [32]. This metabolic capacity also appears to extend to algal polysaccharides, as demonstrated by their ability to degrade macroalgal hydrolysates [3]. One study showed that L. acidophilus utilized hydrolysates from the macroalga G. corticata to produce lactic acid, indicating direct utilization of algal polysaccharides [33]. These compositional differences may have resulted in different selective pressures on the gut microbiota, which could explain the strain-specific differences observed in PP utilization and monosaccharide consumption. These findings suggest that PPs may be degraded and utilized by certain LAB strains, although the underlying mechanisms remain to be elucidated.
Low-molecular-weight polysaccharides, which are more readily metabolized by probiotics, have been shown to enhance probiotic growth and activity [34,35]. In the present study, HPLC analysis revealed a decrease in polysaccharide fractions F2, F3, and F4 following fermentation with the four LAB strains, indicating their utilization and supporting their potential as prebiotic substrates. Interestingly, the concentration of fraction F1 increased after fermentation, suggesting the synthesis of new polysaccharide molecules by LAB strains cultured with PPs. LAB are known to produce EPSs with immunomodulatory, anti-inflammatory, and antimicrobial properties [36,37,38]. Prior studies have reported varying EPS molecular weights for LAB, such as 8.0 × 105 Da for L. kefiranofaciens, 2.0 × 104 Da for L. helveticus, and 2.55–2.83 × 105 Da for L. plantarum [39,40,41]. In this study, the increase in F1 polysaccharides with molecular weights around 7.8–8.1 × 105 Da supports EPS production during PP fermentation by the four LAB strains. This indicates a synergistic interaction between PPs and LAB. Further studies are required to characterize the structure and functional roles of the EPSs produced, including their sulfate content and implications for microbial metabolism and host health.
In this study, strains A5, A9, and A10 effectively consumed galactose and glucose, consistent with efficient fermentation of PPs. Strain A5 exhibited complete glucose depletion, reflecting high metabolic activity, while strain A1 showed minimal glucose uptake, in line with its reduced growth and acid production. Conversely, strain A5 showed limited galactose uptake, indicating potential enzymatic limitations or substrate preferences. The effective utilization of specific monosaccharides by strains A5, A9, and A10 reinforces the selective prebiotic potential of PPs, indicating that such substrates may favor the enrichment of metabolically compatible probiotic strains in the gut. The distinct patterns of monosaccharide utilization and total sugar consumption observed after fermentation highlight strain-specific metabolic adaptations among the LAB strains. These differences suggest differential activation of carbohydrate catabolic pathways, likely mediated by unique sets of glycoside hydrolases and sugar transporters encoded by each strain. Such variability is well-documented among LAB and reflects their genetic and functional diversity, particularly in terms of carbohydrate-active enzymes involved in polysaccharide degradation. LAB are known to possess specialized enzymes and inducible transport systems that enable them to metabolize a wide range of carbohydrate sources across diverse ecological niches [42]. This metabolic flexibility is often achieved through the inducible expression of carbohydrate-active enzymes in response to specific substrates [26]. For instance, Lactiplantibacillus plantarum NCU116 has been shown to harbor strain-specific sugar transporters and glycoside hydrolases, supporting its metabolic versatility and efficient adaptation to plant-based fermentation environments [25]. In light of these findings, it is plausible that the LAB strains investigated in this study also possess distinct sets of carbohydrate-active enzyme genes and transporter systems that facilitate the efficient utilization of PPs. Further studies, such as comparative genomics, transcriptomic profiling, or enzyme activity assays, are warranted to elucidate the molecular mechanisms underlying these strain-specific metabolic responses. Interestingly, mannose concentrations increased in the culture supernatant of strain A5, suggesting metabolic transformation or the secretion of mannose-rich EPSs. EPSs containing mannose residues have been reported in Lacticaseibacillus casei, L. gasseri, and L. helveticus [38,43]. It has been reported that Lactobacillus-derived mannose-containing EPSs can increase macrophage viability and alleviate the symptoms of ulcerative colitis in mice [44]. The potential secretion of mannose-rich EPSs by strain A5 may have implications for host-microbe interactions, as mannose-containing EPSs have been associated with immunomodulatory activity and the inhibition of pathogen adhesion to intestinal epithelium.
Results of FT-IR analysis provided further evidence of metabolic activity during fermentation with PPs. The emergence of a distinctive absorption peak near 1250 cm−1 in fermented media suggests the synthesis of new biochemical compounds, possibly EPSs. Similar peaks have been documented in the FT-IR spectra of EPSs from L. plantarum and L. helveticus, corresponding to C–O stretching and O–H deformation vibrations [41,45]. While signals corresponding to both PPs and MRS medium were observed, the new peak suggests LAB-driven biochemical modifications. These changes may result from enzymatic degradation of algal substrates or the synthesis of postbiotic metabolites. The ability of PPs to stimulate EPS production and probiotic activity supports their potential role in promoting gut health. Further compositional analyses will help elucidate the structure of the newly synthesized molecules.
LAB exert antibacterial effects through various mechanisms, including the production of bacteriocins and organic acids [46,47]. LAB such as L. plantarum, L. rhamnosus, and L. reuteri have demonstrated strong antimicrobial activity [48,49,50]. In this study, the antibacterial activity of the four LAB strains was tested after fermentation with PPs. Surprisingly, the inhibitory effects of LAB-fermented supernatants against E. coli and S. aureus were slightly diminished compared to those cultured in standard MRS medium. This decrease may be due to shifts in metabolic pathways when PPs are used as carbon sources, potentially leading to reduced synthesis of antimicrobial compounds. Alternatively, algal polysaccharides may promote pathways favoring growth or EPS production over antibacterial metabolite synthesis. The composition of the fermentation medium can significantly influence bacteriocin production by LAB, particularly with respect to the types and concentrations of carbon and nitrogen sources [51]. In this study, the supplement of PPs provided an additional carbon source during LAB fermentation. This supplementation disrupted the original carbon-to-nitrogen ratio in the standard MRS medium. Such changes may have negatively affected the regulatory pathways involved in bacteriocin biosynthesis, leading to reduced production and consequently a diminished antibacterial effect against E. coli and S. aureus. Despite this reduction, PPs may still enhance other beneficial properties, such as EPS production, which contributes to probiotic functionality. Other products generated during probiotic fermentation, such as organic acids, bioactive peptides, and amino acid derivatives, are also known to exhibit various physiological activities, including antimicrobial, immunomodulatory, antioxidant, and gut barrier-supporting effects [52]. Future studies should include a more detailed characterization of these fermentation-derived compounds to better elucidate the mechanisms underlying the health benefits of postbiotics.
Algal polysaccharides are indigestible by the human gastrointestinal tract and instead serve as fermentable substrates for gut microbiota, producing bioactive metabolites such as SCFAs [30]. Numerous in vivo studies have reported beneficial effects of these polysaccharides through their interaction with gut microbiota [13,15]. One study on P. haitanensis polysaccharides demonstrated increased microbial diversity, enhanced growth of beneficial genera such as Akkermansia and Rikenellaceae, and a reduction in inflammation-associated bacteria [53]. In the present study, treatment with PPs significantly altered gut microbiota composition and diversity in mice. Notably, there was a specific increase in the class Bacteroidia in treated mice, which includes bacteria capable of degrading complex polysaccharides [54]. In contrast, the relative abundance of the class Bacilli was reduced. This class includes various species that can exert either beneficial or detrimental effects on the host, such as lactic acid bacteria and some opportunistic pathogens [55,56]. Further research is needed to elucidate the interactions between algal polysaccharides and specific probiotic strains in order to better demonstrate their potential as prebiotic candidates. Changes in the Firmicutes/Bacteroidetes ratio have been associated with metabolic and inflammatory states in both humans and animals. An elevated ratio is often linked to increased energy harvest and obesity, while a lower ratio may reflect anti-inflammatory conditions [57]. While an increased Firmicutes/Bacteroidetes ratio has been associated with metabolic disorders in humans, its implications in this context remain unclear [58]. It may reflect an adaptive microbial response or a shift in carbohydrate fermentation dynamics. However, the ratio alone is not a definitive marker of health, and further functional analysis of the microbial community is necessary to draw mechanistic conclusions. Overall, these findings suggest that PP treatments significantly influence the gut microbiome, potentially leading to favorable physiological outcomes.
5. Conclusions
This study highlights the prebiotic potential of PPs through their selective utilization by specific LAB strains, which demonstrated the ability to metabolize marine polysaccharides, produce EPSs, and modulate their metabolism in ways that may enhance probiotic functions. While a slight decline in antibacterial activity was observed, this may reflect a metabolic trade-off favoring EPS synthesis. Our findings support the sustainable use of algal polysaccharides in the food, health, and animal feed industries, with promising applications in the development of functional foods, dietary supplements, and microbiota-targeted interventions. Furthermore, the potential generation of bioactive postbiotic compounds opens new avenues for translational research. Although this study used murine-derived strains, which provided valuable insights into host–microbe–diet interactions, we acknowledge that extrapolation to other animals, including humans, must be made cautiously. Species-specific differences in microbiota composition and metabolism should be considered. Future research should aim to validate these findings in human-relevant models, elucidate the molecular mechanisms of EPS production, characterize the structures of fermentation by-products, and assess their health benefits in vivo.
Author Contributions
Y.-J.W.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing. H.-T.V.L. and C.-L.P.: Supervision. C.-H.H.: Conceptualization, Funding acquisition, Writing—review and editing, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the National Science and Technology Council, Taiwan (MOST 109-2320-B-019-007-MY3 and NSTC113-2320-B019-004-MY3).
Institutional Review Board Statement
Animal experiments were conducted in accordance with the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the NTOU Institutional Animal Care and Use Committee (NTOU IACUC-108040, approval date: 19 December 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request. Restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. The 16S rRNA gene sequences of the isolated LAB strains have been submitted to GenBank, and the accession numbers will be provided during peer review.
Conflicts of Interest
There are no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| PPs | Porphyra-derived polysaccharides |
| LAB | Lactic acid bacteria |
| EPSs | Exopolysaccharides |
| HPLC | High-performance liquid chromatography |
| FT-IR | Fourier-transform infrared spectroscopy |
| SCFAs | Short-chain fatty acids |
References
- Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical composition and potential practical application of 15 red algal species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health benefits and pharmacological effects of Porphyra species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Seong, H.; Bae, J.H.; Seo, J.S.; Kim, S.A.; Kim, T.J.; Han, N.S. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Wang, C.; Xie, M.; Huang, J.; Wang, Y. Two polysaccharides from Porphyra modulate immune homeostasis by NF-κB-dependent immunocyte differentiation. Food Funct. 2019, 10, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Huo, Y.F.; Wang, F.; Wang, C.; Zhu, Q.; Wang, Y.B.; Fu, L.L.; Zhou, T. Improved antioxidant and immunomodulatory activities of enzymatically degraded Porphyra haitanensis polysaccharides. J. Food Biochem. 2020, 44, e13189. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; Gao, W.; He, Y.; Wang, Y.; Sun, Y.; Kuang, H. Polysaccharides from Porphyra haitanensis: A review of their extraction, modification, structures, and bioactivities. Molecules 2024, 29, 3105. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in human health: A narrative review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-derived polysaccharides and their potential health benefits in nutraceutical applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef]
- Rormwong, T.; Sakpetch, P.; Choojit, S.; Kanjan, P. Extraction of sulfated polysaccharide from red seaweed (Gracilaria fisheri) and growth promotion of probiotic bacteria. Burapha Sci. J. 2023, 28, 752–771. [Google Scholar]
- Chen, P.; Tong, M.; Zeng, H.; Zheng, B.; Hu, X. Structural characterization and in vitro fermentation by rat intestinal microbiota of a polysaccharide from Porphyra haitanensis. Food Res. Int. 2021, 147, 110546. [Google Scholar] [CrossRef]
- Malairaj, S.; Veeraperumal, S.; Yao, W.; Subramanian, M.; Tan, K.; Zhong, S.; Cheong, K.L. Porphyran from Porphyra haitanensis enhances intestinal barrier function and regulates gut microbiota composition. Mar. Drugs 2023, 21, 265. [Google Scholar] [CrossRef]
- Fang, R.E.; Wei, Y.J.; Fang, S.Y.; Huang, C.H. Effects of Sargassum-derived oligosaccharides, polysaccharides and residues on ameliorating enteritis and dysbiosis in a murine model of food allergy. J. Funct. Foods 2023, 110, 105844. [Google Scholar] [CrossRef]
- Wei, Y.J.; Fang, R.E.; Ou, J.Y.; Pan, C.L.; Huang, C.H. Modulatory effects of Porphyra-derived polysaccharides, oligosaccharides and their mixture on antigen-specific immune responses in ovalbumin-sensitized mice. J. Funct. Foods 2022, 96, 105209. [Google Scholar] [CrossRef]
- Wei, Y.J.; Fang, R.E.; Liu, J.S.; Chen, Y.C.; Lin, H.T.V.; Pan, C.L.; Huang, C.H. Influence of Porphyra-derived polysaccharides and oligosaccharides on attenuating food allergy and modulating enteric microflora in mice. Food Agric. Immunol. 2023, 34, 2248419. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Pinto, R.A.; Rossi, E.A.; Vallance, B.A.; Cavallini, D.C. Isolation and characterization of potentially probiotic bacterial strains from mice: Proof of concept for personalized probiotics. Nutrients 2018, 10, 1684. [Google Scholar] [CrossRef] [PubMed]
- Marchwińska, K.; Gwiazdowska, D. Isolation and probiotic potential of lactic acid bacteria from swine feces for feed additive composition. Arch. Microbiol. 2022, 204, 61. [Google Scholar] [CrossRef]
- Ou, J.Y.; Wei, Y.J.; Liu, F.L.; Huang, C.H. Anti-allergic effects of Ulva-derived polysaccharides, oligosaccharides and residues in a murine model of food allergy. Heliyon 2023, 9, e22840. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Lee, M.C.; Huang, C.Y.; Lai, C.L.; Yeh, H.Y.; Huang, J.; Lung, W.Q.C.; Lee, P.T.; Nan, F.H. Colaconema formosanum, Sarcodia suae, and Nostoc commune as fermentation substrates for bioactive substance production. Fermentation 2022, 8, 343. [Google Scholar] [CrossRef]
- Chuandong, Z.; Hu, J.; Li, J.; Wu, Y.; Wu, C.; Lai, G.; Shen, H.; Wu, F.; Tao, C.; Liu, S. Distribution and roles of Ligilactobacillus murinus in hosts. Microbiol. Res. 2024, 282, 127648. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, Y.; Luo, Z.; Jiang, Y.; Xu, Z.; Yu, R. Lactobacillus reuteri in digestive system diseases: Focus on clinical trials and mechanisms. Front. Cell. Infect. Microbiol. 2023, 13, 1254198. [Google Scholar] [CrossRef]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The pros and cons of using algal polysaccharides as prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xiong, T.; Peng, Z.; Xiao, Y.S.; Liu, Z.G.; Hu, M.; Xie, M.Y. Genomic analysis revealed adaptive mechanism to plant-related fermentation of Lactobacillus plantarum NCU116 and Lactobacillus spp. Genomics 2020, 112, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Liu, C.; Li, Y.; Liu, Q.; Su, X.; Peng, Q.; Lei, X.; Li, W.; Menghe, B.; Bao, Q.; et al. Comparative genomics analysis of habitat adaptation by Lactobacillus kefiranofaciens. Foods 2023, 12, 1606. [Google Scholar] [CrossRef]
- Mukohda, M.; Yano, T.; Matsui, T.; Nakamura, S.; Miyamae, J.; Toyama, K.; Mitsui, R.; Mizuno, R.; Ozaki, H. Treatment with Ligilactobacillus murinus lowers blood pressure and intestinal permeability in spontaneously hypertensive rats. Sci. Rep. 2023, 13, 15197. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, L.; Li, M.; Hu, Y.; Zeng, B.; Yuan, H.; Zhao, L.; Zhang, C. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome 2018, 6, 54. [Google Scholar] [CrossRef]
- Elayaraja, S.; Annamalai, N.; Mayavu, P.; Balasubramanian, T. Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 2014, 4, S305–S311. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Gubelt, A.; Blaschke, L.; Hahn, T.; Rupp, S.; Hirth, T.; Zibek, S. Comparison of different Lactobacilli regarding substrate utilization and their tolerance towards lignocellulose degradation products. Curr. Microbiol. 2020, 77, 3136–3146. [Google Scholar] [CrossRef]
- Rajan, R.A.; Rizwana, H.; Elshikh, M.S.; Mahmoud, R.M.; Kalaiyarasi, M. Lactic acid production by fermentation of hydrolysate of the macroalga Gracilaria corticata by Lactobacillus acidophilus. BioResources 2024, 19, 8563–8576. [Google Scholar] [CrossRef]
- He, C.; Zhang, R.; Jia, X.; Dong, L.; Ma, Q.; Zhao, D.; Sun, Z.; Zhang, M.; Huang, F. Variation in characterization and probiotic activities of polysaccharides from litchi pulp fermented for different times. Front. Nutr. 2022, 9, 993828. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Cai, J.; Xiao, J.; Zhang, M.; Jia, X.; Dong, L.; Hu, K.; Yi, Y.; Zhang, R.; Huang, F. Purification, characterization and bioactivity of different molecular-weight fractions of polysaccharide extracted from litchi pulp. Foods 2023, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Dardis, C.; Gagliarini, N.; Garrote, G.L.; Abraham, A.G. Exopolysaccharides from Lactobacillus paracasei isolated from kefir as potential bioactive compounds for microbiota modulation. Front. Microbiol. 2020, 11, 583254. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, T.; Kurian, C.; Muthu, M.; Anand, A.; Anand, T.; Paari, K.A. Exopolysaccharides derived from probiotic bacteria and their health benefits. J. Pure Appl. Microbiol. 2023, 17, 35–50. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- De Paiva, I.M.; da Silva Steinberg, R.; Lula, I.S.; de Souza-Fagundes, E.M.; de Oliveira Mendes, T.; Bell, M.J.V.; Nicoli, J.R.; Nunes, Á.C.; Neumann, E. Lactobacillus kefiranofaciens and Lactobacillus satsumensis isolated from Brazilian kefir grains produce alpha-glucans that are potentially suitable for food applications. LWT-Food Sci. Technol. 2016, 72, 390–398. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Molecular characterization of an exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510 and its efficacy to improve the texture of starchy food. J. Food Sci. Technol. 2014, 51, 4012–4018. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Rui, X.; Yu, J.; Tang, W.; Chen, X.; Jiang, M.; Dong, M. Production of exopolysaccharides by Lactobacillus helveticus MB2-1 and its functional characteristics in vitro. LWT-Food Sci. Technol. 2014, 59, 732–739. [Google Scholar] [CrossRef]
- Barrangou, R.; Azcarate-Peril, M.A.; Duong, T.; Conners, S.B.; Kelly, R.M.; Klaenhammer, T.R. Global analysis of carbohydrate utilization by Lactobacillus acidophilus using cDNA microarrays. Proc. Natl. Acad. Sci. USA 2006, 103, 3816–3821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, R.; Xiao, Y.; Wang, H.; Chen, W.; Lu, W. Improvement effects of Lactobacillus-derived mannose-containing exopolysaccharides on ulcerative colitis. Food Biosci. 2024, 61, 104585. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qiu, L.; Zhang, F.; Xu, X.; Wei, H.; Tao, X. Characterization and bioactivities of the exopolysaccharide from a probiotic strain of Lactobacillus plantarum WLPL04. J. Dairy Sci. 2017, 100, 6895–6905. [Google Scholar] [CrossRef]
- Adeniyi, B.A.; Adetoye, A.; Ayeni, F.A. Antibacterial activities of lactic acid bacteria isolated from cow feces against potential enteric pathogens. Afr. Health Sci. 2015, 15, 888–895. [Google Scholar] [CrossRef]
- Islam, R.; Hossain, M.N.; Alam, M.K.; Uddin, M.E.; Rony, M.H.; Imran, M.A.S.; Alam, M.F. Antibacterial activity of lactic acid bacteria and extraction of bacteriocin protein. Adv. Biosci. Biotechnol. 2020, 11, 49–59. [Google Scholar] [CrossRef]
- Ma, E.; An, Y.; Zhang, G.; Zhao, M.; Iqbal, M.W.; Zabed, H.M.; Qi, X. Enhancing the antibacterial activity of Lactobacillus reuteri against Escherichia coli by random mutagenesis and delineating its mechanism. Food Biosci. 2023, 51, 102209. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.; Xu, Z. Identification of antibacterial substances of Lactobacillus plantarum DY-6 for bacteriostatic action. Food Sci. Nutr. 2020, 8, 2854–2863. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, L.; Sun, Z.; Ji, Y.; Wang, D.; Mei, L.; Shen, P.; Li, Z.; Tang, S.; Zhang, H. Fucoidan as a marine-origin prebiotic modulates the growth and antibacterial ability of Lactobacillus rhamnosus. Int. J. Biol. Macromol. 2021, 180, 599–607. [Google Scholar] [CrossRef]
- Veettil, V.N. Optimization of bacteriocin production by Lactobacillus plantarum using Response Surface Methodology. Cell. Mol. Biol. 2022, 68, 105–110. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating fermented: Health benefits of LAB-fermented foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Aweya, J.J.; Li, N.; Deng, R.Y.; Chen, W.Y.; Tang, J.; Cheong, K.L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Facimoto, C.T.; Clements, K.D.; White, W.L.; Handley, K.M. Bacteroidia and Clostridia are equipped to degrade a cascade of polysaccharides along the hindgut of the herbivorous fish Kyphosus sydneyanus. ISME Commun. 2024, 4, ycae102. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Ilinskaya, O.N.; Ulyanova, V.V.; Yarullina, D.R.; Gataullin, I.G. Secretome of intestinal Bacilli: A natural guard against pathologies. Front. Microbiol. 2017, 8, 1666. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).