Abstract
Biotechnological valorization of Flourensia cernua foliage was carried out using fungal solid-state fermentation; several outcomes of this bioprocess were identified which added value to the plant material. F. cernua leaves placed in aluminum trays were inoculated with Aspergillus niger; extracts of this plant were evaluated and the foliage was tested for in vitro digestibility. The solid bioprocess was carried out at 75% humidity for 120 h and after the fermentation, β-glucosidase activity; phenolics and in vitro digestibility were quantified and measured. Two high β-glucosidase production levels were detected at 42 and 84 h with 3192 and 4092 U/L, respectively. Several phenolics of industrial importance were detected with a HPLC-ESI-MS, such as glycosides of luteolin and apigenin. The other outcome was a substantial improvement in anaerobic digestibility. The unfermented sample registered a 30% in vitro degradability, whereas samples subjected to 84 h of fungal fermentation increased degradability by up to 51%. This bioprocess was designed to detect more than one product, which can contribute to an increase in the added value of F. cernua foliage.
1. Introduction
Valuable biomass resources are available in the Chihuahuan Desert of northern Mexico [1,2,3]. One example of these plant resources is F. cernua, whose foliage is used as feed for animals such as goats, but its chemical components often prevent it from being consumed [4,5]. This plant can be found primarily in the Mexican states of Coahuila and Chihuahua and in New Mexico, Arizona and Texas in the USA. This shrub is often browsed by cattle and goats but is less consumed even though it possesses similar nutritional traits to alfalfa. The reason for the low animal intake of F. cernua on rangeland could be attributed to its chemical composition [6,7,8]. This plant has been subject of phytochemical characterization where several compounds have been detected [9,10]. There have been attempts to biotransform the foliage of this shrub by biotechnological means [11]. In this regard, the valorization of plant material has been carried out to improve its characteristics or produce useful biomolecules and biocatalysts [12]; these products perform several functions, such as nutritional acquisition, defense and ecological associations. However, most organisms utilize its enzymes for the hydrolysis of oligosaccharides to glucose, which is the most utilizable form of carbon [13]. The foliage of this plant has also been the subject of microbial growth by filamentous fungi where it is indicative of the biodegradation capability and biomolecules release [14,15]. In these previous studies, it has been shown that it is possible to obtain useful biomolecules such as β-glucosidase from this plant by extraction and also by microbial processes [16,17]. This enzyme holds great importance for industrial purposes due to the ability of the hydrolytic activity to cleave the β-1, 4 glycosydic bond in cellobiose to release glucose from a cellulosic source. Furthermore, β-glucosidase can contribute to biomolecules release, releasing biomolecules such as phenolic compounds [18]. In this regard, β-glucosidase has recently been of increasing importance due to its ability to not only contribute to cellulose degradation but also phenolic glycoside release from plant tissues and bioactive phenolic deglycosylation [19,20]. Certain plants have been subject to microbial growth to increase protein content and also to improve digestibility in order to promote their use in animal feed [21]. In these types of bioprocesses, the microorganism develops biomass and produces enzymes that partially degrade plant components and support their digestion in the ruminal microbiome [22,23]. Since F. cernua is an available resource in arid areas, it is important to develop a process where high substrate loadings are used while remaining environmentally friendly. To achieve this, a solid bioprocess can be implemented where the ratio of substrate is much higher than moisture compared to conventional fermentation processes [24]. The advantage of using a solid-state bioprocess is that high loadings of substrate are used, lower amounts of moisture and less energy input are required and it emulates the natural conditions of fungi growth [25]. As it is known that the fungal strain of A. niger has an important background in enzyme production and bioactive compound release and can also be subjected to solid-state fermentation research, it was selected for this research. It was also chosen for this experiment since it has been shown to have the ability to improve the digestibility of forage proteins after fermentation processes [26]. The goal of this study was to develop three products from a single fungal solid-state fermentation process to increase the valorization of F. cernua.
2. Materials and Methods
2.1. Plant Material and Conditioning
Foliage along with petioles from F. cernua was collected in northeastern Mexico. Afterward, leaves were separated from petioles, dehydrated at 60 °C and stored in dark plastic bags at room temperature.
2.2. Fungal Strain and Inoculum Preparation
A. niger GH1 was kindly provided by the DIA-Autonomous University of Coahuila microbial collection. Spores were cryopreserved in glycerol–skim milk suspension at −20 °C. Spores were propagated in sterile potato dextrose agar medium at 30 °C for 72 h to be harvested later using 0.1% Tween 80 for spore counting in a hematocytometer.
2.3. Solid-State Culture and Crude Enzyme Extract
The substrate comprised 15 g of F. cernua leaves in an aluminum tray reactor (15 cm diameter × 3 cm depth). The mineral medium composed of (g/L): NaNO3 2.0, KH2PO4 0.5, KCl 0.25 and MgSO4 0.25, was adjusted to pH 5.5. Every reactor tray with the substrate was adjusted to 75% humidity and inoculated with 3 × 108 spores/g and was placed in an incubator at 30 °C for 120 h. The enzymatic extract was obtained with citrate buffer (0.1 M) at pH 4.6 using paper filters and a vacuum pump.
2.4. Analytical Methods
2.4.1. β-Glucosidase Activity Measurement
The activity quantification was carried out via modified procedure based on the method of Vattem & Shetty [27]. For this method, sodium citrate buffer (Na3C6H5O7) (0.1 M) at pH 4.6 was prepared along with p-nitrophenyl-β-glucopyranoside (pNPG) (0.009 M) as the enzymatic substrate and sodium carbonate (Na2CO3) (0.01 M) to stabilize and stop the reaction. The crude enzymatic extract was diluted (1:10). A standard reaction mixture contained 0.1 mL pNPG, 0.8 mL sodium citrate buffer and 0.1 mL of crude enzyme solution. After 10 min incubation at 50 °C, the reaction was stopped by addition of 1 mL of sodium carbonate and released p-nitrophenol was measured at 400 nm in a UV light spectrophotometer (Auxilab S.L., Beriáin, Spain). The standard curve was established using pure p-nitrophenol. One β-glucosidase unit was defined as the amount of enzyme required to release 1 micromol of p-nitrophenol per minute under the assay conditions.
2.4.2. Reducing Sugars and Total Hydrolyzable Phenolics Quantification
These compounds were measured using spectrophotometric methodologies. Reducing sugars was carried out as reported by Miller [28] using the DNS reagent and the results were obtained at a wavelength of 540 nm.
Phenolic content was measured as reported by Haile & Kang [29]. In this method, Folin–Ciocalteu reagent was used. The absorbances were taken at a wavelength of 750 nm.
2.4.3. HPLC-ESI-MS Methodology
For adequate use with the chromatographic equipment, the samples were filtered through a 0.45 µm nylon membrane and at a 1:10 dilution. Molecules were detected by sample injection during high-performance liquid chromatography (Varian ProStar, Grace, Columbia, MD, USA). The equipment uses a ternary pump, an autosampler, a photodiode array detector (320 nm) and a Denali C18 column (3.1 µm; 150 × 4.6 mm) at 30 °C. The mobile phases used were ethanol for the wash phase (solvent A), acetonitrile (solvent B) and 0.2% formic acid (solvent C). The solvents were used in gradients as follows: initial 3% B; 5–15 min, 16% B linear; 15–45 min, 50% B linear. After the analysis, the column was washed and reconditioned. The chromatographer was coupled with a mass spectrometer with ion trap (Varian 500 M/S), electrospray ionization (ESI), negative mode [M-H]−, 90 V of capillary voltage and 100–2000 m/z of mass range.
2.4.4. In Vitro Digestibility Assay
For buffer preparation, the reagents (g/L) were added as follows: buffer solution A: KH2PO4 (10), MgSO4 7 H2O (0.5), NaCl (0.5), CaCl2 2 H2O (0.1) and urea (0.5). Buffer solution B: Na2CO3 (15) and Na2S 9 H2O (1.0). For further steps of the digestibility assay, a mixture of solutions A + B was prepared at 1:5 with pH adjusted to 6.8. Ruminal liquid was collected from a ruminally cannulated steer and placed in airtight-seal cylinders and maintained at 39 °C for immediate use. The digestibility was carried out using a DAISY II Incubator (Ankom Technology Incorporation, Macedon, NY, USA) digestor under anaerobic conditions. The procedure was performed according to the instructions specified by the manufacturer. The determination of the digestibility was calculated using the following equation:
where W1 = weight of an empty F57 porous bag; W2 = sample weight; W3 = final weight after digestion and neutral detergent fiber measurement; C1 = correction factor (W3 − W1).
2.4.5. Statistical Analysis
The results (β-glucosidase activity, reducing sugars, phenolic content and in vitro digestibility) correspond to the mean of triplicate measurements with their respective standard deviation (±SD). Data were analyzed using an ANOVA procedure PROC GLM of SAS 8.2; (SAS Institute Inc., Cary, NC, USA) and the Tukey mean comparison test was used for mean comparison. The significance level was set at p < 0.05.
3. Results and Discussion
3.1. Enzyme Production
The performance of A. niger in the production of the enzyme under the conditions used in this experiment is shown in Figure 1. This fungus produces and concentrates the enzyme for various biological functions [29,30], from nutrient extraction, the hydrolysis of oligosaccharides, to defense mechanisms. In this study, an evolution was observed in the content of the enzyme produced in leaves of F. cernua, with a tendency to undergo a proportional growth from 12 h to 72 h of enzymatic fermentation, reaching maximum production with 4086.3 U/L at 84 h. Although it is not the most versatile of the microorganisms that have been reported to be the most prolific, such as those of the Bacillus genus [30], it showed high performance. Martins et al. [30], using Penicillium glabrum, obtained a β-glucosidase production of 22.45 ± 1.04 U mL−1 in the solid fermentation of canola flour in 6.5 days at a pH of 6.0. In this context, it can be stated that F. cernua has more potential in the synthesis of this enzyme [31]. β-glucosidases have been the subject of recent research due to the key role of these enzymes in biological processes and many biotechnological applications [31]. This enzyme catalyzes the hydrolysis of the glycosidic bond within carbohydrate fractions to produce non-reducing terminal glycosyl residues and oligosaccharides, as reported by Mol et al. [19], while also instigating the synthesis of bioactive aglycones from carbohydrate conjugates and the production of alkyl glucosides [32]. These mechanisms are mediated by the type of substrate and microorganisms employed during the fermentation process. This behavior is often related to the bioavailability of compounds susceptible to fungal degradation and so allows for formation of volatile compounds and release of bioactive aglycone compounds. In addition to endogenous β-glucosidase activity present in raw material, the function of β-glucosidases in fermenting microorganisms has been progressively clarified and increasingly appreciated [33]. In one case, F. cernua proved to be a source of glycosylated phenolic compounds, which could induce β-glucosidase production along with its content of celluloses and hemicelluloses [33]. In bacteria and fungi, β-glucosidase is a crucial element in the microbial cellulose multienzyme complex since it is responsible for the regulation of the entire cellulose hydrolysis process by easing cellobiose-mediated suppression and producing the final product glucose [33]. According to previous results, the cellulose and lignin contents in the bioprocess present a stable behavior, meaning that the enzymedoes not tend to decrease, particularly in cellulose, during at least the first hours of fermentation. Authors such as Das Neves et al. [34], using fodder palm and Penicillium roqueforti ATCC 10110, obtained 935.07 UI/g of enzyme, Dina and Thankamani [35] obtained 17.0 U/g using Aspergillus flavipes in wheat bran, El-Ghonemy [36] obtained 81.3 ± 4.2 U/g in Jojoba flour fermented with Aspergillus sp. DHE7 and Ezeilo et al. [37] obtained 131.76 U/g in palm oil leaves fermented with Trichoderma asperellum UC1. Hemicellulose is the polysaccharide that may exhibit change due to fungal biodegradation. Also, as previously stated, phenolics may play an important role in this regard. These types of compounds can be found in F. cernua [38,39,40] and were detected in this experiment, as seen in Figure 2. The importance of glycosylated phenolic compounds rests in the fact that they contain the required enzymes for fiber degradation, including β-glucosidase, which is one of the key enzymes for their degradation, along with endoglucanases, exoglucanases and xylanases, among others. The results of the chromatographic and mass spectrometry are shown in Figure 2 and Table 1. It has been reported that these types of compounds, along with xylose, as one of the main components of hemicellulose, can be used as inducers of many hydrolytic enzymes, along with β-glucosidase [41,42,43]. For this reason, β-glucosidase was considered as the main enzyme with its activity being the point of reference in this experiment. There is a high possibility that other hydrolytic enzymes were produced, such as endo and exoglucanase, but we were unable to detect them, apparently due to an excess of free sugars which interfere with the enzyme activity quantification. Concerning β-glucosidase, its hydrolytic activity can release phenolic compounds from plant tissue which is also evidence of ongoing degradation, which in turn makes fiber more susceptible to anaerobic digestion.
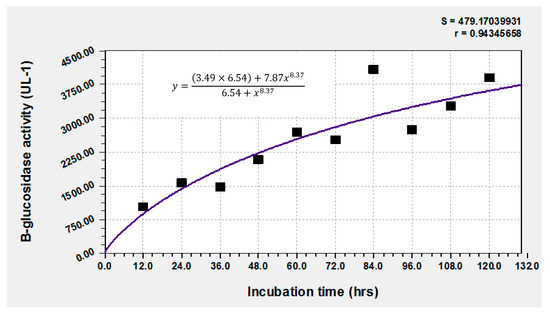
Figure 1.
Fungal production of β-glucosidase at 120 h of solid-state fermentation.
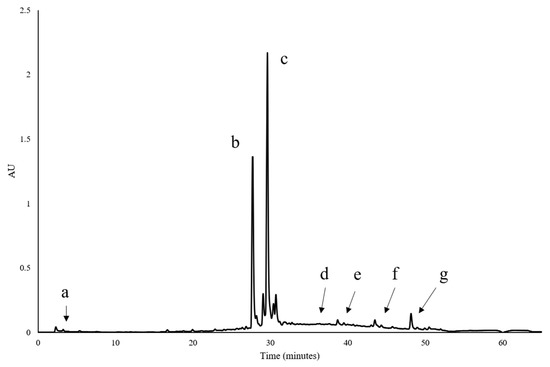
Figure 2.
Chromatographic profile of F. cernua extract at 48 h of fungal fermentation.

Table 1.
Compounds detected by HPLC-ESI-MS.
The Y-axis measures the absorbance units of the compounds as they elute from the column and this is expressed as AU (absorbance units). The X-axis measures the time it takes for a compound to elute from the column; thisis measured in minutes. The peaks indicated with letters correspond to the main compounds detected in the sample (see Table 1).
3.2. Bioactive Compounds Released
Most of the bioactive compounds detected can be considered among those that are able to be detected by HPLC-ESI-MS and quantified by the Folin–Ciocalteu reagent method [44]. These are shown in Figure 2 and Figure 3. The solid bioprocess showed that from the start, phenolic accumulation occurs up to 42 h. After that time, the phenolic content tend to decrease. This was expected, mainly because it has been reported that A. niger is able to release and degrade these molecules (Figure 4) [44]. The leaves of F. cernua are rich in these types of biocompounds. They represent a consortium of quite versatile compounds due to their diverse applications [45]. Reports on the antifungal capacity of bioactive extracts of F. cernua have been reported to be effective against pathogenic organisms such as Penicillium expansum, Fusarium oxysporum [46], Rhizoctonia solani, Rhizopus stolonifer, Botrytis cinerea, Colletotrichum gloeosporioides, Mucor sp., Penicillium sp., Alternaria alternate and Sclerotinia sclerotiorum [47,48], and in some cases can stimulate conidiospore germination on Alteraria solani [49] without antagonistic observations on strains such as A. niger. Recently, the efficacy of F. cernua extract components as bioactive agents in edible coatings to improve the shelf life of tomatoes and apples has been evaluated [50], hence their agroindustrial relevance. Considering that the point of the highest production of β-glucosidase is also at 42 h, the detection of compounds by HPLC/ESI/MS was carried out. Specifically, in this matter, the results are consistent with previous reports [50,51]. It was observed that apigenin and luteolin glycosides were present when sampling at 42 h. The assay was performed at that time in order to match the time of highest β-glucosidase production in the first half of the bioprocess. A number of compounds of industrial value were detected, along with the previously mentioned glycosylated phenolics (Table 1). One ion that was detected with a m/z value of 191 was scopoletin, which is a hydroxycoumarin with antioxidant, antidiabetic, anti-obesity and anti-hepatosteatosis effects [52,53]. Another ion detected with a m/z of 593.1 was luteolin 7-O-rutinoside, from which its aglycone has anti-inflammatory and neuroprotective activities [54,55]. An ion corresponding to apigenin–arabinoside–glucoside, with a m/z of 563.1, was also detected, which has chemotherapeutic effects and playsa role in the senescence of human colorectal cancer cells [56,57]. With a m/z of 261, the ion of homovanillic acid 4-sulfate was detected. This compound has demonstrated protective activities on β and skeletal muscle cells [58]. The mass that corresponds to 4-hydroxybenzoic acid 4-O-glucoside has a value of 299. This molecule promotes the inhibition of breast cancer cells with a driamycin [59]. The ion with a value of 313 corresponds to cirsimaritin. This compound has been linked to anti-inflammatory mechanisms [59]. Lastly, the ion with a m/z of 247.1 corresponded to vanillic acid 4-sulfate. This molecule, along with syringic acid, inhibits in vitro collagen fibril accumulation, which causes strokes and myocardial infarctions [60,61].
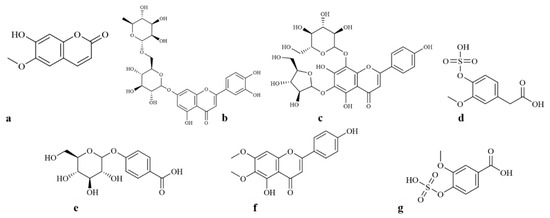
Figure 3.
Chemical structures of the bioactive compounds detected in the HPLC-ESI-MS analysis. (a = Scopoletin, b = Luteolin 7-O-rutinoside, c = Apigenin arabinoside–glucoside, d = Homovanillic acid 4-O-sulfate, e = Hydroxybenzoic acid 4-O-glucoside, f = Cirsimaritin, g = Vanillic acid 4-sulfate).
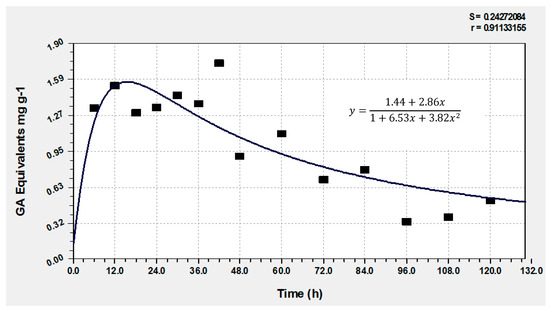
Figure 4.
Total hydrolyzable phenol presence and degradation in the fungal bioprocess.
3.3. Digestibility Assay
Figure 1 shows enzymatic production at 120 h, with three high production peaks observed: 42 h: 3192 UL-1, 84 h: 4092 UL-1 and 120 h: 3912 UL-1. In the first hours of the process, a correlation could be observed between the kinetics of enzymatic production and the percentage of in vitro dry matter digestibility (IVDMD). However, the maximum IVDMD was 51.3% at 48 h (Figure 5). The literature mentions that the maximum recommended time for the degradation of the dry matter of forages is 48 h; a longer period of times is not recommended because it increases costs. Toxicity of F. cernua depends on the part of the plant, with the fruits and flowers being toxic to livestock, while the leaves are relatively innocuous and can be used to obtain pharmacologically interesting compounds [62,63].
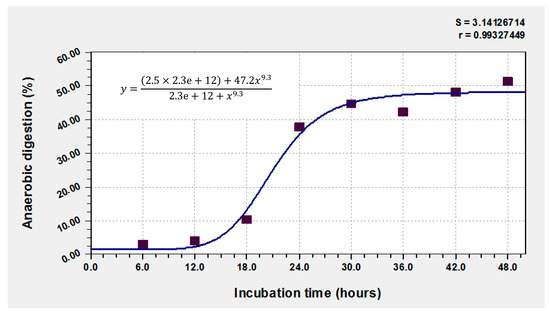
Figure 5.
In vitro anaerobic digestion of the fermented material at 48 h of incubation.
Filamentous fungi are naturally prone to lignocellulosic biomass degradation in different ecosystems due to their higher capacity for production and recovery of their extracellular enzyme pool [64]. There are several reports on filamentous fungi used in biomass biodegradation such as Aspergillus sp. YDJ14 [64], Coniophora putiana, Microdochium nivale [65] and Chaetomella species [66]. However, A. niger [66] and Trichoderma ressie [67] are the most commonly used fungi for commercial production of hydrolytic enzymes such as β-glucosidase due to their high production levels. This digestibility value could be influenced by several factors such as the physiological state and the animal species from which the ruminal fluid came, the time and type of feeding of the steer prior to harvesting the ruminal fluid, the handling of the liquid during the in vitro test and the degree of enzymatic interaction on the fiber–glycosides of the plant sample during biotechnological treatment and the physiological conditions of the microbial source. The in vitro test carried out by Sakita et al. [60] to evaluate the degradability of organic material in tropical forages reported that Trichoderma reesei was able to increase the actual degradability of organic material by up to 11.1% and neutral detergent fiber by up to 18% when the level of fibrolytic enzyme used was 5000 μL per kg of substrate, compared to that found in this work, which was higher [68]. Pech-Cervantes et al. [61] reported 55% in vitro degradability of Bermuda grass dry matter using commercial fibrolytic active enzymes, results that have a similar range obtained in the present work. Similarly, Putri et al. [62] reported a 58.9% to 75% in vitro digestibility of dry matter and crude protein of various forage samples fermented with ruminal liquid, and discovered that to achieve complete hydrolysis of the hemicellulosic chain, a consortium of enzymes must participate synergistically to degrade the xylan main chain, such as endo-β-(1,4)-xylanase and β-xylosidase [69].
Given the abundance of F. cernua in the semi-desert region of northern Mexico, it is important to manipulate this potential forage in order to achieve greater digestibility. This behavior could be related to the bioavailability of nutrients because the fungus needs compounds that have some glycosylated compounds as their main source of carbon and energy. However, the mechanism of absorption of these nutrients by the microorganism depends to a great extent on the disposition of the cell wall, the type of host–pathogen and its symbiotic association. This association must be successful because the microorganism hydrolyzes the toxic glycosides, making this plant less toxic and diminishing soluble-type aglycones [69,70]. Something similar was carried out by YangWang and Guo (2022) [69], who used an immobilized beta-glucosidase from Thermoascus aurantiacus to hydrolyze soy isoflavone glucosides into their aglycones such as daidzin and genistin, converting thisinto an amorphous cellulose which is easier to metabolize [70]. In relation to the results obtained in this study, the microorganism acted on the lignocellulosic fiber of the fermented sample through the enzyme β-glucosidase, thus helping to depolymerize the barriers that strongly protect the cellular content, as is the case of the glycosylated contents and proteins [70,71]. Therefore, it may have had some challenges in carrying out the hydrolysis of the structural compounds of the plant and subsequently increasing in the medium due to the enzymatic activity.
According to Figure 1, there may be indications of a relationship between the levels of β-glucosidase production excreted in the culture medium and the rupture of the lignocellulosic structures that reduce the possibility of achieving a better digestibility of the organic matter of the studied shrub.
4. Conclusions
Due to their abundance in the Chihuahuan Desert, F. cernua provides abundant biomass, in the form of foliage, which is very useful as a high-added value compound source. The single bioprocess described leads to the obtention of more than one product from this plant. This in mind, we were able to establish conditions using fungal solid-state fermentation to release several bioactive compounds. At the same time, the β-glucosidase enzyme was produced by A. niger, which plays a role in bioactive release and plant material degradation due to catalytic efficiency, versatility and ease of production. The in vitro digestibility of the fermented was substantial, which would benefit the small ruminant and cattle industry on rangeland. The overall advantage of the bioprocess is that in addition to the biomolecules released and their ease of production, the resulting fermented material could be used as ruminant feed or as part of a feed formulation for these animals; this would mitigate over-dependence on traditional feed ingredients for livestock. This result positions the use of fibrolytic enzymes as a reliable strategy to improve the degradability of low-quality forages, contributing to sustainability in livestock production.
Author Contributions
Conceptualization, M.Á.M.-M. and A.F.A.-C.; methodology, J.L.-T. and M.Á.M.-M.; software, M.M.-B.; validation, A.F.A.-C. and M.Á.M.-M.; formal analysis, C.N.A. and J.A.A.-V.; investigation, J.L.-T.; resources, A.F.A.-C.; data curation, M.M.-B.; writing—original draft preparation, J.L.-T.; writing—review and editing, M.Á.M.-M. and J.L.-T.; visualization, A.F.A.-C.; supervision, A.F.A.-C.; project administration, A.F.A.-C.; funding acquisition, A.F.A.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to mention that Antonio Francisco Aguilera Carbó passed away in 2024 and was responsible for funding the project that led to the experiments in this work. The first author expresses his gratitude to SECIHTI Mexico for scholarship awarded to pursue her master studies. To master Program in Agricultural Production Sciences of the Autonomous Agrarian University Antonio Narro.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| U/L | Unit per liter |
| g/L | gram per liter |
| U/g | Unit per gram |
| μL | Microliter |
| M | Molar |
| HPLC-ESI-MS | High Performance Liquid Chromatography–Electrospray Ionization–Mass Spectrometry |
| GH1 | Glycoside Hydrolases 1 |
| NaNO3 | Sodium nitrate |
| KH2PO4 | Potassium dihydrogen phosphate |
| KCl | Potassium chloride |
| MgSO4 | Magnesium sulfate |
| Na2CO3 | Sodium Carbonate |
| DNS | Dinitrosalicylicacid |
| NaCl | Sodium chloride |
| Na2S | Sodium sulfide |
| CaCl2 | Calcium chloride |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| ATCC | American Type Culture Collection |
| IVDMD | In Vitro Dry Matter Digestibility |
| pNPG | p-nitrophenyl-β-glucopyranoside |
References
- De León-Zapata, M.A.; Pastrana-Castro, L.; Rua-Rodríguez, M.L.; Alvarez-Pérez, O.B.; Rodríguez-Herrera, R.; Aguilar, C.N. Experimental protocol for the recovery and evaluation of bioactive compounds of tarbush against postharvest fruit fungi. Food Chem. 2016, 198, 62–67. [Google Scholar] [CrossRef]
- Estell, R.E.; Anderson, D.M.; James, D.K. Defoliation of Flourensia cernua (tarbush) with high-density mixed-species stocking. J. Arid Environ. 2016, 130, 62–67. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Pastrana-Castro, L.; Barbosa-Pereira, L.; Rua-Rodríguez, M.; Ventura, J.; Salinas, T.; Rodríguez, R.; Aguilar, C.N. Effect of Flourensia cernua Bioactive Compounds on Stability of an Oil-in-Water (O/W) Emulsion. Biointerface Res. Appl. Chem. 2021, 11, 13997–14006. [Google Scholar] [CrossRef]
- Castellanos-Perez, E.; Valencia-Castro, M.; Quiñones-Vera, J. Goats and the Need for Range Management in Mexico. Rangelands 2002, 24, 24–27. [Google Scholar] [CrossRef]
- García-Monjaras, S.; Santos-Díaz, R.E.; Flores-Najera, M.J.; Cuevas-Reyes, V.; Meza-Herrera, C.A.; Mellado, M.; Chay-Canul, A.J.; Rosales-Nieto, C.A. Diet selected by goats on xerophytic shrubland with different milk yield potential. J. Arid Environ. 2021, 186, 104429. [Google Scholar] [CrossRef]
- Salma, A.H.; Ayman, A.H.; Mona, M.M.Y.; Barbabosa-Pliego, A.; Mellado, M.; Salem, A.Z.M. Dietary Anti-nutritional Factors and Their Roles in Livestock Nutrition. Sustain. Agric. Rev. 2022, 57, 131–174. [Google Scholar]
- Morrone, J.J.A.; Fernández, R.; Jesús, A. Biogeographic unitsin the Chihuahuan Desert: Implications for regionalization and area nomenclature. Rev. Mex. Biodivers. 2022, 93, 933907. [Google Scholar]
- Estell, R.E.; Fredrickson, E.L.; James, D.K. Effect of light intensity and wavelength on concentration of plant secondary metabolites in the leaves of Flourensia cernua. Biochem. Syst. Ecol. 2016, 65, 108–114. [Google Scholar] [CrossRef]
- Linares-Braham, A.; Palomo-Ligas, L.; Nery-Flores, S.D. Bioactive Compounds and Pharmacological Potential of Hojasen (Flourensia cernua): A Mini review. Plant Sci. Today 2023, 10, 304–312. [Google Scholar] [CrossRef]
- Ventura, J.; Belmares, R.; Aguilera-Carbo, A.; Gutiérrez-Sanchez, G.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal biodegradation of tannins from creosote bush (Larrea tridentata) and tar bush (Flourensia cernua) for gallic and ellagic acid production. Food Technol. Biotechnol. 2008, 46, 213–217. [Google Scholar]
- Alvarez-Pérez, O.B.; Ventura-Sobrevilla, J.M.; Ascacio-Valdés, J.A.; Rojas, R.; Verma, D.K.; Aguilar, C.N. Valorization of Flourensia cernua DC as source of antioxidants and antifungal bioactives. Ind. Crops Prod. 2020, 152, 112422. [Google Scholar] [CrossRef]
- Godse, R.; Bawane, H.; Tripathi, J.; Kulkarni, R. Unconventional β-glucosidases: A promising biocatalyst for industrial biotechnology. Appl. Biochem. Biotechnol. 2021, 193, 2993–3016. [Google Scholar] [CrossRef]
- Lopez-Trujillo, J.; Medina-Morales, M.A.; Sanchez-Flores, A.; Arevalo, C.; Ascacio-Valdes, J.A.; Mellado, M.; Aguilar, C.N.; Aguilera-Carbo, A.F. Solid bioprocess of tarbush (Flourensia cernua) leaves for β-glucosidase production by Aspergillus niger: Initial approach to fiber–glycoside interaction for enzyme induction. 3 Biotech 2017, 7, 271. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Sun, Q.; Ma, J.; Xia, A.; Huang, Y.; Zhu, X.; Liao, Q. Elucidation of the interaction effects of cellulose, hemicellulose and lignin during degradative solvent extraction of lignocellulosic biomass. Fuel 2022, 327, 125–141. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Hernández-Castillo, D.; Angulo-Sánchez, J.L.; Rodríguez-García, R.; Villarreal Quintanilla, J.A.; Lira-Saldivar, R.H. Antifungal activity in vitro of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani, and Fusarium oxysporum. Ind. Crops Prod. 2007, 25, 111–116. [Google Scholar] [CrossRef]
- Nishida, V.S.; de Oliveira, R.F.; Brugnari, T.; Correa, R.C.G.; Peralta, R.A.; Castoldi, R.; de Souza, C.G.M.; Bracht, A.; Peralta, R.M. Immobilization of Aspergillus awamori β-glucosidase on commercial gelatin: An inexpensive and efficient process. Int. J. Biol. Macromol. 2018, 111, 1206–1213. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Pillai, S. Microbial β-glucosidases: Recent advances and applications. Biochimie 2024, 225, 49–67. [Google Scholar] [CrossRef]
- Ahmed, A.; Nasim, F.; Batool, K.; Bibi, A. Microbial β-Glucosidase: Sources, Production and Applications. J. Appl. Environ. Microbiol. 2017, 5, 31–46. [Google Scholar] [CrossRef]
- Mól, P.C.G.; Júnior, J.C.Q.; Veríssimo, L.A.A.; Boscolo, M.; Gomes, E.; Minim, L.A.; Da Silva, R. β-glucosidase: An overview on immobilization and some aspects of structure, function, applications and cost. Process Biochem. 2023, 130, 26–39. [Google Scholar] [CrossRef]
- Shuai, S.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Huang, Z.; Yu, J.; Mao, X.; Yan, H.; He, J. Effect of fermented rapeseed meal on growth performance, nutrient digestibility, and intestinal health in growing pigs. Anim. Nutr. 2023, 15, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Villas-Bôas, S.G.; Esposito, E.; Mitchell, D.A. Microbial conversion of lignocellulosic residues for production of animal feeds. Anim. Feed Sci. Technol. 2002, 98, 1–12. [Google Scholar] [CrossRef]
- Graminha, E.B.N.; Goncalves, A.Z.L.; Pirota, R.D.P.B.; Balsalobre, M.A.A.; Da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef]
- Izábal-Carvajal, A.L.; Sepúlveda, L.; Chávez-González, M.L.; Torres-León, C.; Aguilar, C.N.; Ascacio-Valdés, J.A. Extraction of Bioactive Compounds via Solid-State Fermentation Using Aspergillus niger GH1 and Saccharomyces cerevisiae from Pomegranate Peel. Waste 2023, 1, 806–814. [Google Scholar] [CrossRef]
- Stodolak, B.; Starzynska-Janiszewska, A.; Baczkowicz, M. Aspergillus oryzae (Koji Mold) and Neurospora Intermedia (Oncom Mold) Application for Flaxseed Oil Cake Processing. LWT 2020, 131, 109651. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus edodes using a solid-state system. Process Biochem. 2003, 39, 367–379. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Martins, E.H.; Ratuchne, A.; De Oliveira Machado, G.; Knob, A. Canola meal as a promising source of fermentable sugars: Potential of the Penicillium glabrum crude extract for biomass hydrolysis. Biocatal. Agric. Biotechnol. 2020, 27, 101713. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 3. [Google Scholar] [CrossRef]
- Paventi, G.; Di Martino, C.; Coppola, F.; Iorizzo, M. β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health. Foods 2025, 14, 1451. [Google Scholar] [CrossRef]
- Bhatia, Y.; Mishra, S.; Bisaria, V.S. Microbial β-Glucosidases: Cloning, Properties, and Applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef]
- Das Neves, C.A.; De Menezes, L.H.S.; Soares, G.A.; Dos Santos Reis, N.; Tavares, I.M.C.; Franco, M.; De Oliveira, J.R. Production and biochemical characterization of halotolerant β-glucosidase by Penicillium roqueforti ATCC 10110 grown in forage palm under solid-state fermentation. Biomass Convers. Biorefin. 2020, 12, 3133–3144. [Google Scholar] [CrossRef]
- Dina, S.; Thankamani, V. Optimization of cellulase production from Aspergillus flavipes by submerged and solid state fermentation. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e4754. [Google Scholar] [CrossRef]
- El-Ghonemy, D.H. Optimization of extracellular ethanol-tolerant b-glucosidase production from a newly isolated Aspergillus sp. DHE7 via solid state fermentation using jojoba meal as substrate: Purification and biochemical characterization for biofuel preparation. J. Genet. Eng. Biotechnol. 2021, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Ezeilo, U.R.; Wahab, R.A.; Huyop, F.; David, E.E.; Tin, L.C. Solid-state valorization of raw oil palm leaves by novel fungi Trichoderma asperellum UC1 and Rhizopus oryzae UC2 for sustainable production of cellulase and xylanase. J. Chem. Technol. Biotechnol. 2022, 97, 520–533. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Nimtz, M.; Wissing, J.; Zeng, A.; Rinas, U. The intra- and extracellular proteome of Aspergillus niger growing on defined medium with xylose or maltose as carbon substrate. Microb. Cell Fact. 2010, 20, 9–23. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Buenrostro, J.J.; De la Cruz, R.; Sepúlveda, L.; Aguilera-Carbó, A.F.; Prado, A.; Contreras, J.C.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal biodegradation of pomegranate ellagitannins. J. Basic Microbiol. 2014, 54, 28–34. [Google Scholar] [CrossRef]
- Aranda-Ledesma, N.E.; González-Hernández, M.D.; Rojas, R.; Paz-González, A.D.; Rivera, G.; Luna-Sosa, B.; Martínez-Ávila, G.C.G. Essential Oil and Polyphenolic Compounds of Flourensia cernua Leaves: Chemical Profiling and Functional Properties. Agronomy 2022, 12, 2274. [Google Scholar] [CrossRef]
- Castillo-Reyes, F.; León-Juárez, E.D.; Nery-Flores, S.D.; Flores-Gallegos, A.C.; Campos-Muzquiz, L.G.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R. Polyphenols extraction from creosote bush, tarbush, and soursop using ultrasound-microwave and their effect against Alternaria alternata and Fusarium solani. Rev. Mex. Fitopatol. 2022, 40, 349. [Google Scholar] [CrossRef]
- Ganchev, D. Antisporulation Action of Tarbush Plant (Flourensia cernua) Towards Conidiospores of Plant Pathogens. J. Sustain. Agric. 2022, 6, 81–84. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Aguirre-Joya, J.A.; Rojas, R.; Vicente, A.; Aguilar-González, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Recubrimiento comestible de cera de candelilla con bioactivos de Flourensia cernua para prolongar la calidad de los frutos de tomate. Alimentos 2020, 9, 1303. [Google Scholar]
- Ri, J.; Lee, H.; Choi, R.; Sim, M.; Choi, M.; Kwon, E.; Won, K.; Kim, M.; Lee, M. Anti-obesity and anti-hepatosteatosis effects of dietary scopoletin in high-fat diet fed mice. J. Funct. Foods 2016, 25, 433–446. [Google Scholar]
- Jang, J.H.; Park, J.E.; Han, J.S. Scopoletin inhibits α-glucosidase in vitro and alleviates postprandial hyperglycemia in mice with diabetes. Eur. J. Pharmacol. 2018, 834, 152–156. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Banerjee, K.; Mandal, M. Oxidative stress triggered by naturally occurring flavone apigenin results in senescence and chemotherapeutic effect in human colorectal cancer cells. Redox Biol. 2015, 5, 153–162. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Torres-Moreno, H.; López-Romero, J.C.; Vidal-Gutiérrez, M.; Villarreal-Quintanilla, J.Á.; Carrillo-Lomelí, D.A. Antioxidant, anti-inflammatory, and antiproliferative activities of Flourensia spp. Biocatal. Agric. Biotechnol. 2023, 47, 102552. [Google Scholar] [CrossRef]
- Bitner, B.F.; Ray, J.D.; Kener, K.B.; Herring, J.A.; Tueller, J.A.; Johnson, D.K.; Tellez, C.M.; Fausnacht, D.W.; Allen, M.E.; Thomson, A.H.; et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in β-cells and skeletal muscle cells. J. Nutr. Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, K.; Zhang, X.; Yang, C.; Li, X. 4-Hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/P53 pathway. Biochem. Biophys. Res. Commun. 2018, 504, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Yeon, J.; Lee, J.; Hyun, H.; Hahm, D.; Cheon, S.; Lee, S.; Seo, G.; Jung, K.; Sung, K. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. maackii Maxim. Bioorg. Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef] [PubMed]
- Rasheeda, K.; Bharathy, H.; Fathima, N.N. Vanillic acid and syringic acid: Exceptionally robust aromatic moieties for inhibiting in vitro self-assembly of type I collagen. Int. J. Biol. Macromol. 2018, 113, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Szucs, C.; Farkas, A.; Szuhaj, M.; Maroti, G.; Bagi, Z.; Rakhely, G.; Kovacs, K.L. Pretreatment of lignocellulosic biogas substrates by filamentous fungi. J. Biotechnol. 2022, 360, 160–170. [Google Scholar] [CrossRef]
- Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, R.S.; Gupta, V.K.; Mazumder, P.B.; Khan, A.F. Microbial beta glucosidase enzymes: Recent advances in biomass conversation for biofuels application. Biomolecules 2019, 9, 220. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, Q.; Zhang, Y.F.; Chen, D.D.; Yi, X.N.; Chen, D.S.; Cheng, X.P.; Li, M.; Wang, H.Y.; Chen, K.Q.; et al. Improving the catalytic activity of b-glucosidase from Coniophora puteana via semi-rational design for efficient biomass cellulose degradation. Enzym. Microb. Technol. 2023, 164, 110188. [Google Scholar] [CrossRef]
- Singh, N.; Sithole, B.; Govinden, R. Screening for cellulases and preliminary optimisation of glucose tolerant b-glucosidase production and characterization. Mycology 2023, 14, 91–107. [Google Scholar] [CrossRef]
- Chauve, H.; Mathis, H.; Huc, D.; Casanave, D.; Monot, F.; Lopes, N. Comparative kinetic analysis of two fungal b-glucosidases. Biotechnol. Biofuels 2010, 3, 3. [Google Scholar] [CrossRef]
- Da Costa, S.G.; Pereira, O.L.; Teixeira-Ferreira, A.; Valente, R.H.; De Rezende, S.T.; Guimaraes, V.M.; Genta, F.A. Penicillium citrinum UFV1 β-glucosidases: Purification, characterization, and application for biomass saccharification. Biotechnol. Biofuels 2018, 11, 226. [Google Scholar] [CrossRef]
- Sakita, G.Z.; Bompadre, T.F.V.; Dineshkumar, D.; Lima, P.d.M.T.; Filho, A.L.A.; Campioni, T.S.; Neto, P.d.O.; Neto, H.B.; Louvandini, H.; Abdalla, A.L. Fibrolytic enzymes improving in vitro rumen degradability of tropical forages. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1267–1276. [Google Scholar] [CrossRef]
- Pech-Cervantes, A.A.; Muhammad, I.; Ogunade, I.M.; Jiang, Y.; Kim, D.H.; Gonzalez, C.F.; Adesogan, A.T. Exogenous fibrolytic enzymes and recombinant bacterial expansis synergistically improve hydrolysis and in vitro digestibility of Bermuda grasshaylage. J. Dairy Sci. 2019, 102, 8059–8073. [Google Scholar] [CrossRef]
- Putri, E.M.; Zain, M.; Warly, L.; Hermon, H. Effects of rumen-degradable-to-undegradable protein ratio in ruminant diet on in vitro digestibility, rumen fermentation, and microbial protein synthesis. Vet. World 2021, 14, 640–648. [Google Scholar] [CrossRef]
- Hati, S.; Ningtyas, D.W.; Khanuja, J.K.; Prakash, S. β-Glucosidase from almonds and yoghurt cultures in the biotransformation of isoflavones in soy milk. Food Biosci. 2020, 34, 100542. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, X.; Li, T.; Guo, H.; Chang, R.; Liu, J.; Liu, Q.; Hao, H.; Huang, T.; Chen, W.; et al. Quantification of isoflavone glycosides and aglycones in rat plasma by LC-MS/MS: Troubleshooting of interference from food and its application to pharmacokinetic study of Semen sojae Praeparatum extract. J. Pharm. Biomed. Anal. 2018, 161, 444–454. [Google Scholar] [CrossRef]
- Hu, S.; Wang, D.; Hong, D. A simple method for beta-glucosidase immobilization and its application in soybean isoflavone glycosides hydrolysis. Biotechnol. Bioprocess Eng. 2018, 23, 39–48. [Google Scholar] [CrossRef]
- Rana, V.; Rana, D. Role of microorganisms in lignocellulosic biodegradation. In Renewable Biofuels; Springer Briefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2017; pp. 19–67. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current perspective on production and applications of microbial cellulases: A review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Guo, Q.; Deng, W.; Du, G.; Li, R. Isolation of the termostable β-glucosidase-secreting strain Bacillus altitudinis JYY-02 and its application in the production of gardenia blue. Microbiol. Spectr. 2022, 10, e0153522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, X.; Wang, Z.; Wang, Y.; Dai, Y.; Yin, L.; Xu, Z.; Zhou, J. Cloning and expression of Lactobacillus brevis b-glucosidase and its effect on the aroma of strawberry wine. J. Food Process. Preserv. 2023, 46, e16368. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, T.; Li, Z.; Liu, P.; Wang, Y.; He, N.; Liang, D. Characterization of a beta-glucosidase from Bacillus licheniformis and its effect on bioflocculant degradation. AMB Express 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).