Abstract
The study aimed to evaluate how different production methods and fermentation processes using two different lactic acid bacteria (LAB) affect the chemical composition and bioactive properties of pistachio beverages. The beverages were prepared with two varieties of pistachios, one from Argentina and the other from Italy. The pistachios were processed with two technologies: a domestic processor and a colloidal mill. For the fermentation, pistachio beverages were inoculated with two different LAB strains and incubated at 28 °C for 24 h. The beverages were analyzed for proximal composition (including protein, fat, fiber, and minerals) and bioactive properties such as antioxidant activity, angiotensin-converting enzyme inhibition (ACE-I), and dipeptidyl peptidase-4 inhibition (DPP-4). The colloidal milling allowed the inclusion of the whole pistachio nut, resulting in beverages with higher solid content and no waste. Beverages treated with colloidal milling exhibited higher acidity, improved microbial fermentation performance, and generally showed higher bioactivity compared to those obtained by the domestic processor. Bioactivity varied according to the pistachio variety, the processing method and LAB strains used. Lactic acid bacteria fermentation decreased antioxidant properties of the beverages by ~40% but improved anti-hypertensive and hypoglycaemic activities. Fermented pistachio-based beverages showed promising health-promoting properties, indicating their potential as functional foods.
1. Introduction
Pistachios (Pistacia vera L.) are nuts widely consumed worldwide, particularly in the European Mediterranean region, as well as in Iran, Turkey, Syria, and the United States [1]. They are renowned for their nutritional aspects, making them a valuable addition to a healthy diet. Pistachios are a good source of proteins, dietary fiber, fat (particularly oleic and linoleic fatty acids), minerals like potassium (K), phosphorous (P), magnesium (Mg), and calcium (Ca), and vitamins including B6, E, and K, as well as other bioactive compounds such as carotenoids, phenolic acids, flavonoids, and anthocyanins [2].
Pistachio nuts are widely appreciated for their sensory properties and are included as an ingredient in numerous food formulations, such as ice-creams, pesto, sauces, salads, and desserts, or as salted snacks [3]. However, with the growing number of vegetarian, vegan, and flexitarian consumers, there has been a surge in the demand for dairy alternatives to meet different dietary preferences and health concerns [4,5]. Rich in protein, healthy fats, vitamins, and minerals, pistachio-based beverages offer a unique combination of taste, nutrition, and health benefits, making them an attractive alternative to traditional dairy products. Moreover, since they do not contain lactose, they are suitable for people with lactose intolerance. Pistachio nuts contain bioactive phenolic compounds that have antioxidant [6] and anti-inflammatory properties [7], which can help to protect against oxidative stress and inflammatory processes. Other beneficial effects on blood pressure, endothelial function, and markers of glucose and insulin metabolism have also been described [3]. As reported by different authors, the fermentation of plant-based matrices with selected microbial strains has proven to be a powerful tool for improving the flavor, texture, and nutritional value, offering a sustainable and nutritious beverage option [8,9]. Recently, some authors [10] have reported that pistachio resulted in an optimal matrix for the growth of lactic acid bacteria, opening up the possibility of the production of new plant-based fermented beverages. However, as pointed out by Di Renzo & Reale [11], in order to improve food quality, the optimization of production and fermentation processes plays a crucial role, which usually requires a holistic approach involving several key factors, such as the selection of raw materials and microbial strains, the control of fermentation conditions, and technological innovations. Each of these factors plays an important role in achieving the consistency, flavor, texture, and health benefits in fermented food products.
Despite the growing demand for plant-based beverages as dairy alternatives, pistachio-based drinks remain underexplored both in the scientific literature and in commercial markets. Most studies and products to date have focused on soy, almond, and oat beverages, while pistachio beverages are still niche, with limited data available on their nutritional, functional, and technological properties. Additionally, although pistachios are known for their high antioxidant activity, unsaturated fatty acids, and potential cardioprotective effects, little is known about how these health benefits are retained or enhanced in fermented beverage form. This creates a notable research gap: the lack of systematic studies on how processing and fermentation impact the market potential (e.g., stability, acceptability, and scalability) and health-promoting properties of pistachio-based beverages. The present study aims to address this gap by evaluating how different processing approaches such as the origin of raw materials, production methods (domestic processor or colloidal milling), and fermentation by microbial strains affect the physicochemical, microbiological, and functional features of beverages made from Italian and Argentinian pistachios.
2. Materials and Methods
2.1. Raw Materials
Pistachio (Pistacia vera L.) used for the experiments consisted of shelled, unsalted, and unroasted nuts from Argentina and Italy. In detail, Argentinean pistachio nuts (A) (Iranian varieties) were cultivated in San Juan, Argentina, harvested in 2019, and kindly donated by Pistacho de los Andes (San Juan, Argentina). Italian pistachios (I) (Pistacia vera L.) were harvested in 2019 and purchased from the Aroma Sicilia farm (Bronte, Sicily, Southern Italy).
2.2. Microbial Strains
Two lactic acid bacteria strains, Leuconostoc pseudomesenteroides PD4 and Companilactobacillus alimentarius PG3, belonging to the microbial culture collection of the Institute of Food Sciences—National Research Council (ISA-CNR, Avellino, Italy) were used in this study. The strains were maintained as frozen stocks in 25% (v/v) glycerol and routinely propagated in De Man, Rogosa, and Sharpe (MRS) medium (Oxoid, Milan, Italy), pH 6.8, for 24 h at 30 °C.
2.3. Pistachio Beverage Preparation
The pistachio-based beverages were obtained in two different ways. In the first procedure, a domestic food mixer (Thermomix TM31, Wuppertal, Germany) was used and the beverages were obtained as described by Di Renzo et al. [10]. Briefly, 500 g of pistachios were washed and then soaked for 5 h at 25 °C. The nuts were then drained and mixed with hot water (80 °C) at a ratio of 1:5 (pistachios–water) and then ground using the domestic mixer. The samples were then filtered through a double fine mesh strainer to remove solid particles (waste), heat-treated at 70 °C for 30 min and cooled at 4 °C prior to inoculation with lactic acid bacteria. Samples obtained using the domestic food mixer were named as AD or ID for the Argentinean or Italian pistachio beverages, respectively.
For the second procedure, a colloidal mill (Homomaster 120, S.A.R. Galliate Lombardo, Varese, Italy) was used to obtain the beverage. Briefly, 1 kg of pistachios at a ratio of 1:5 (pistachios–water) was ground in the mill with recirculation for about 5 min. The beverage was then heat-treated at 70 °C for 30 min and cooled to 4 °C until the lactic acid bacteria were inoculated. Samples obtained using colloidal mill were named AC or IC for the Argentinean and Italian pistachio beverages, respectively.
2.4. Inoculum of Pistachio-Based Beverages
The beverages obtained were inoculated with two different strains of lactic acid bacteria, L. pseudomesenteroides PD4 (S1) and C. alimentarius PG3 (S2). The inoculum was prepared by subculturing each LAB strain in MRS broth and incubating at 28 °C for 16 h. This was followed by centrifugation at 4000 rpm for 10 min, washing the pellet with sterile 0.9 g/100 mL NaCl saline, and finally resuspending in the beverage at the desired concentration. Each strain of LAB was used at a final concentration of approximately 6 log cfu/mL beverage. The beverages were fermented at 28 °C for 24 h and then stored at 4 °C. An uninoculated beverage was used as control.
Eight different pistachio beverages were obtained and labeled as follows: AD-S1, AD-S2, AC-S1, and AC-S2 for Argentinean pistachio beverages obtained by domestic (D) or colloidal mill (C) and fermented with L. pseudonesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains; and ID-S1, ID-S2, IC-S1, and IC-S2 for Italian pistachio beverages obtained by domestic or colloidal mill and fermented with L. pseudonesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains. The fermented beverages were then lyophilized and stored at −20 °C for further analysis.
2.4.1. Microbiological Analysis of the Beverage
Lactic acid bacteria counts in the beverage were verified by viable counts on De Man, Rogosa, and Sharpe (MRS) agar (Oxoid, Milan, Italy) supplemented with 0.1 g/L cycloheximide (SIGMA Aldrich, Taufkirchen, Germany) at 28 °C for 72 h under anaerobic conditions (Gas Pack AnaeroGenTM, OXOID Ltd., Basingstoke, UK). Counts were taken at the time of inoculation and after 24 h of fermentation. In detail, 10 g of each beverage was aseptically transferred to a sterile stomacher bag and diluted with 90 mL of physiological solution (9 g/L NaCl). After shaking for 1 min in a stomacher apparatus (BAG MIXER 400, Interscience, France), the samples were serially diluted and plated in the appropriate medium. The results of the viable counts were expressed as log colony-forming units per gram of beverage (log cfu/g).
2.4.2. Beverage Acidity
The pH of the fermented beverages was measured after 24 h of fermentation at 30 °C using a Medidor PH Basic 20 pHmeter (CRISON, Alella, Spain).
2.4.3. Proximate Composition
Protein, lipid, and ash contents were determined according to the Association of Official Analytical Chemists methods [12]. Total dietary fiber was assessed using the Megazyme® commercial kit. The results were expressed as g/100 g dry matter (d.b.).
2.4.4. Enzymatic Assays
Acetic acid, lactic acid, sucrose, fructose, glucose, and ethanol concentrations in the pistachio beverages were determined using the RIDA® CUBE enzymatic assay kits (R-Biopharm, Darmstadt, Germany) according to the manufacturer’s instructions (R-Biopharm, Darmstadt, Germany). Specifically, kits RCS4226 for acetic acid, RCS4240 for D/L-Lactic acid, RCS4140 for D-Glucose, RCS4160 for D-Glucose/D-Fructose, RCS4180 Sucrose/D-Glucose, and RCS4340 for ethanol were utilized. The samples were analyzed in triplicate at time zero and after 24 h fermentation.
2.4.5. Phenolic Acid Analysis
Phenolic compounds of the aqueous extracts of the pistachio beverages were determined by RP-HPLC. Briefly, a Shimadzu series SCL-40 equipment, with a Shimadzu SPD-M40 diode array detector, Shimadzu LC-40B XR pump, Shimadzu SIL-40C XR autosampler, and Shimadzu CTO-40S column oven, was used on a Poroshell 120 EC-C18 3 × 100 mm, 2.7 µm column (Agilent Technologies, Santa Clara, CA, USA). The mobile phase was delivered at 0.4 mL/min, with a gradient mixture of water containing 0.1 mL/100 mL formic acid (eluent A) and acetonitrile (eluent B) as follows: 0–2 min, 5–6% B; 2–4 min, 6–7% B; 4–7 min, 7% B; 7–9 min, 7–9% B; 9–12 min, 9% B; 12–16 min, 9–12% B; 16–17 min, 12–14% B; 17–18 min, 14–16% B, 18–22 min, 16–18% B; 22–28 min, 18–25% B; 28–30 min, 25–28% B; 30–38 min, 28–30% B; 38–40 min, 30–100% B; 40–45 min, 100% B; 45–47 min, 100–5% B; 47–52 min, 5% B. The column oven was set at 35 °C. The analyzed phenolic compounds were detected at their maximum absorbance. Peak identification was performed by comparing the retention times and spectral characteristics with those of the external standards. The compounds analyzed were gallic acid, 4-hydroxybenzoyl alcohol, 3,4 dihydroxybenzoic acid (protocatechuic acid), 4-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid, catechin, chlorogenic and vanillic acid, 3-hydroxybenzoic acid, caffeic acid, syringic acid, epicatechin, p-coumaric acid, 3-(3-hydroxyphenyl) propanoic acid, ferulic acid, sinapic acid, ellagic acid, rutin hydrated, myricetin, 3-phenyl propionic acid, daidzein, quercetin, genistein, apigenin, and kaempferol. Data were processed using Shimadzu LC solution software.
2.4.6. Color Determination
The color of the freeze-dried beverages was assessed using a Minolta CM-508d spectrophotometer (Osaka, Japan), based on the color space defined by the CIE (Commission International de l’Eclairage) standard, as follows: chromatic plane of coordinate a* (positive values correspond to red and negative to green tones) and coordinate b* (positive values correspond to yellow and negative to blue tones), and the L* axis (luminosity) being perpendicular to them. The measurement conditions were Illuminant D65, observation angle 10º, and occluded specular component. Values were expressed as the mean of three measurements. The color was measured on the surface of the standard plate (color parameters L* = 93.4, a* = −1.8, b* = 4.4), and the total color difference (ΔE*) was calculated as follows:
The browning index (BI) was also calculated according to the next equation:
where x = (a* + 1.75L*)/(5.645L* + a* − 0.3012b*).
BI = [100 (x − 0.31)]/0.172,
2.4.7. Determination of Bioactive Properties
Lyophilized samples were dispersed in water at a concentration of 10 g of sample per 100 mL. The dispersions were mixed in a vortex for 15 min, sonicated for 30 min, shaken again for 15 min, and centrifuged at 10,000× g for 15 min. Proper dilutions were performed for each assay.
The antioxidant activity (AOA) was assessed by measuring the ferric reducing antioxidant power (FRAP), according to Benzie and Strain [13], and expressed as µmol Trolox/g sample d.b. Additionally, inhibition of 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) was measured according to Cian et al. [14]. The absorbance was measured at 734 nm after 6 min of initial mixing, using a spectrophotometric plate reader (Asys UVM340). Results were expressed as Trolox equivalent scavenging (µmol Trolox/g sample d.b.).
Anti-diabetogenic activity (ADA) was evaluated by measuring the inhibition of dipeptidyl peptidase IV (DPP-IV) activity, according to Wang et al. [15]. Results were expressed as inhibition percentage of DPP-IV (%).
Angiotensin-converting enzyme-I (ACE-I) inhibition was measured according to Hayakari et al. [16], and was used to estimate the antihypertensive activity (AHA). Results were expressed as ACE-I inhibition (%).
2.5. Statistical Analysis
Each experiment was performed in triplicate. All results were expressed as the mean ± standard deviation (SD). Statgraphics Centurion XV 15.2.06 software was used to analyze the data by analysis of variance (one-way ANOVA). Differences between samples were analyzed by Duncan’s multiple range tests, and Pearson’s correlation was utilized to determine significant correlations between the compounds analyzed (p < 0.05).
3. Results and Discussion
3.1. Nutritional Composition of Pistachio Nuts
The fermented pistachio-based beverages were produced using Italian and Argentinean pistachios with the nutritional composition showed in Table 1. Italian pistachios had a moisture content of 4.1 ± 0.2 g/100 g, while Argentinean pistachios had a moisture content of 5.2 ± 0.0 g/100 g. The Italian pistachios had higher lipid and total dietary fiber contents and lower protein content compared to the Argentinean cultivar. The Italian and Argentinean pistachios had the same ash content. These differences in nutritional composition are likely influenced by environmental factors such as climate, soil type, and cultivation practices in the respective countries. Our results align with those of previous studies [17] showing significant variations in the composition of pistachios from different countries, including Iran, the United States, Turkey, Syria, Kyrgyzstan and Italy, highlighting that geographical origin substantially influences pistachio composition. Multiple and complex environmental factors influence the composition of pistachios such as the genetic variability (cultivar differences), climate conditions (temperature, rainfall, and sunlight), soil characteristics (soil pH, mineral composition, and salinity), and agronomic practices (irrigation, fertilization, harvest timing, and post-harvest handling), as also stated by different authors [18,19,20,21].

Table 1.
Composition of Italian and Argentinean pistachio nuts.
3.2. Microbiological Characterization of Fermented Pistachio Beverages
Table 2 shows the LAB counts (log cfu/g) and the pH values of Italian (I) and Argentinean (A) pistachio beverages made with domestic processor (D) or colloidal mill (C) and fermented for 24 h with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains. As expected, the uninoculated samples (ID, IC, AD, and AC) had lactic acid bacteria counts below 1 log cfu/g. All the beverages were inoculated with the same LAB concentration comprised between 6.16 ± 0.42 and 6.3 ± 0.01 log cfu/g. After 24 h fermentation at 28 °C, the lactic acid bacteria counts increased in all the beverages, reaching values between 8.36 ± 0.06 and 9.21 ± 0.23 log cfu/g, confirming that pistachios, like other plant matrices, are an effective substrate for the growth of this microbial group [9,22].

Table 2.
Lactic acid bacteria load (log cfu/g) and pH values of Italian (I) and Argentinean (A) pistachio beverages made with domestic processor (D) or colloidal mill (C) and fermented with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains.
The pH values at time zero ranged from 6.45 ± 0.07 (sample AC) to 6.74 ± 0.04 (sample AD), which is consistent with the findings of Erem & Kilic-Akyilmaz [23], who observed that the pH of plant materials used in the preparation of plant-based dairy analogs is generally within the range of 6–7. As reported by other authors, the fermentation of plant-based beverages, which usually lasts 12 to 24 h, generally ends when the pH level reaches approximately 4.2–4.5 [9]. In our study, a high level of acidification was achieved after 24 h of fermentation with both strains of lactic acid bacteria (S1 and S2), with pH values between 4.19 ± 0.10 (A-S1) and 4.59 ± 0.05 (A-S2), ensuring acidification necessary for preservation and safety. No significant differences were found between the different samples.
3.3. Nutritional Composition of Fermented Pistachio Beverages
Table 3 shows the chemical composition of the beverages. The solid content of beverages produced by domestic processing was around 9 g/100 mL, while that of beverages produced by colloidal milling was around 18 g/100 mL, regardless of whether the pistachios were Argentinean or Italian.

Table 3.
Chemical composition of Italian (I) and Argentinean (A) pistachios beverages made with domestic processor (D) or colloidal mill (C) and fermented for 24 h with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains.
The domestic processing of pistachios produced waste representing around 12.1% of solids for both cultivars. The composition of the waste (d.b.) for Argentinean and Italian pistachios, respectively, was as follows: 35.0 ± 0.0 and 35.7 ± 0.0 g/100 g of lipids, 24.7 ± 0.1 and 22.3 ± 0.2 g/100 g of proteins, and 21.7 ± 0.3 and 24.3 ± 0.2 g/100 g of total dietary fiber. However, the colloidal milling did not produce any waste since the finely milled particles remained dispersed in the beverage.
The main components of the beverages were lipids, followed by proteins. Since some lipids, proteins, and fiber were lost in the waste, the beverages made using domestic processor had about 2 times less lipids and proteins and 3 times less fiber than the beverages made with colloidal mill.
On the other hand, comparing the control samples obtained by colloidal mill, the Argentinean beverages had approximately the same lipid and ash content, a lower content of fiber, and a higher content of proteins than the Italian ones, in agreement with the content of nuts. However, in the case of domestic processing, the content of proteins, ash, and lipids was the same for both cultivars, but the AD samples showed lower TDF content than the ID samples.
After fermentation, a distinct behavior between cultivars and treatments was observed. A decrease in fiber and protein content was detected for the Italian domestic beverage, which was more pronounced for S2, while the lipid content did not change. In the case of the Argentinean cultivar, only the lipid content decreased with fermentation. On the other hand, for beverages produced with colloidal mill and for both cultivars, the fermentation with S2 decreased protein and lipid content, while TDF and ash were not affected. The higher lipid content of fermented beverages could be related to the fat release from the matrix, which led to a better solvent extraction.
As expected, lactic acid bacteria fermentation produced organic acids and ethanol, while the sugars were consumed (Table 4). The main acid produced by fermentation was lactic acid, whose values ranged from 2.7–3.3 g/L to 5.5–6.1 g/L for domestic and colloidal mill, respectively. Acetic acid changed from 0.07–0.14 to 0.2–1.9 g/L by fermentation and ethanol from 0.03–0.19 g/L to 0.54–1.9 g/L, and 0.08–0.62 for S1 and S2, respectively.

Table 4.
Organic acids, ethanol, disaccharide, and monosaccharide contents (g/L) from Italian (I) and Argentinean (A) pistachio beverages made with domestic processor (D) or colloidal mill (C) and fermented for 24 h with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains.
A higher content of acids and ethanol was found in the beverages obtained with colloidal processing for both pistachio cultivars. However, the ethanol levels remain below the threshold typically considered significant in non-alcoholic beverages and do not pose concerns from either a technological or regulatory perspective. According to the Food and Drug Administration (FDA), beverages containing less than 0.5% ethanol by volume are classified as “non-alcoholic,” while those with 0.5% or higher require appropriate labeling [24,25]. Glucose and fructose were completely consumed during fermentation, but the final sucrose content varied depending on the LAB strain and the method used for the preparation of beverages.
3.4. Polyphenolic Profile of Fermented Pistachio Beverages
The analysis of phenolic profile is another important issue that complements the nutritional properties. Table 5 shows water-soluble polyphenols present in the different beverages, with gallic acid being the predominant phenolic acid identified. Syringic and ferulic acids were also detected, but at lower concentrations. Regarding flavonoids, only catechin, epicatechin, rutin and myricetin were detected, with catechin and epicatechin present in higher amounts. Other studies identified flavanols, flavonols, flavanones, isoflavones, and anthocyanins as the main flavonoids in pistachio nuts, with concentrations ranging from 16 to 70 mg/100 g, depending on the variety. The amount of polyphenols can vary significantly depending on the genotype, pre- and post-harvest, and storage conditions. Ballistreri et al. [26] have reported that the anthocyanin content increased with ripening, but sun-drying led to a substantial loss, and flavonoids and tocopherols decreased during both ripening and drying. Fabani et al. [27] studied the chemical profile and antioxidant activities of three cultivars of Pistacia vera cv Kerman from Argentina. The main polyphenols, separated by HPLC and identified by electrospray ionization (ESI) coupled to quadrupole time-of-flight mass spectrometry (LC–ESI–QTOF–MS), were gallic acid and (+)-catechin. The presence of myricetin, isoquercitin, and a dimer of procyanidin, was also found. Moreover, these authors found no significant differences in the total polyphenol content between cultivars of different ages.

Table 5.
Phenolic compounds (µg/g b.s.) detected in aqueous extracts for Italian (I) and Argentinean (A) pistachio beverages made with domestic processor (D) or colloidal mill (C) and fermented for 24 h with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2).
When control beverages obtained by colloidal mill were compared, the Italian cultivar was found to contain higher levels of gallic and ferulic acids, catechin, and epicatechin than the Argentinean beverages. However, it contained lower levels of syringic acid and rutin, while myricetin was not detected. On the other hand, the fermentation of colloidal milled beverages reduced the polyphenolic content for both strains evaluated, while in the case of domestically processed beverages, the effect depended on the phenolic compound, the cultivar, and the strain used.
A clear point was that colloidal milling produced a beverage with a higher amount of polyphenols than domestic processing, due to the loss of the non-dispersed fraction (waste). The flavonoid distribution and predominance differ between the skin and the kernel. Pistachio kernels are characterized by the presence of catechin, eriodictyol-7-O-glucoside, genistein-7-O-glucoside, naringenin-7-O-neohesperidoside, rutin (quercetin-3-O-rutinoside), isoquercetin, genistein, eriodictyol, daidzein, and apigenin [28]. Pistachio skins contain these flavonoids, except for isoflavones and apigenin. They also contain epicatechin, quercetin, naringenin, luteolin, kaempferol, and anthocyanidins (e.g., cyanidin-3-O-galactoside and cyanidin-3-O-glucoside) [2,29,30]. Thus, the polyphenol profile was affected by both the milling process used to prepare the beverages and the fermentation with lactic acid bacteria, at least in terms of the ratio of the different compounds.
3.5. Color
Color parameters of the Argentinean and Italian fermented pistachio beverages are presented in Table 6. When all the data were analyzed by ANOVA Multifactor, significant effects of variety (Argentinean or Italian) were observed. Thus, the statistical analysis was performed separately for each variety. Lower L* values were detected in all Italian beverages compared to Argentinean ones, suggesting that Italian pistachios presented lower luminosity due to differences in chemical composition. Bronte pistachios presented a violet-colored skin. Differences in the a* and b* parameters were found, but not to a greater proportion than in L*.

Table 6.
Color values for Italian (I) and Argentinean (A) pistachio beverages made with domestic (D) or colloidal mill (C) and fermented for 24 h with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains.
Regarding the Italian cultivar, ANOVA Multifactor (Table 7) showed that the method of processing (colloidal or domestic), and the fermentation (without fermentation—W— or fermented with S1 or S2 strain) had a significant effect, as did the interaction between the two processes, except for the b* parameter. In the case of Argentinean beverage, neither the method nor fermentation had a significant effect on the b* parameter. On the other hand, for both types of pistachios, in the absence of fermentation, colloidal milling produced lighter beverages with lower L* values; fermentation increased the L* values of C compared to D beverages. ΔE and BI increased with fermentation in domestic beverages, whereas the opposite behavior was observed for colloidal samples. Moreover, fermentation with S1 produced a more colored beverage (lower values of L* and higher a*, ΔE, and BI values) than S2.

Table 7.
Results of ANOVA Multifactor. Effects of the method of processing (colloidal, C or domestic, D) and fermentation (without starter, W or fermentation with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains).
Thus, the variety, the method of processing and the strain used for fermentation are involved in determining the final color of the beverages, with Italian pistachio beverages being more colored than those made with the Argentinian variety. Furthermore, it is reasonable to hypothesize that microbial metabolism could influence color through the production or degradation of pigments, changes in pH, or oxidation-reduction reactions affecting the color. In this context, the lactic acid bacteria strains used produced organic acids that may have altered the chemical environment and, consequently, the color parameters of the matrix.
3.6. Bioactive Properties
Results on the antioxidant properties are shown in Figure 1. Unfermented Argentinean and Italian beverages made with domestic processor showed similar ABTS+ scavenging and FRAP activities. In general, colloidal milling allowed for the obtaining of beverages with higher bioactivity, due to all components being included in the beverage. Moreover, the Italian pistachio beverage obtained by colloidal milling showed significantly higher antioxidant activity levels than the Argentinean pistachio beverage. Fermentation decreased this activity in all the beverages by around 40% for ABTS+ scavenging (except for AC-S1), and by 30–60% for FRAP. This decrease in AOA could be related to the concentration of phenolics after processing. In this sense, colloidal milling enabled greater extraction of phenolic compounds from the nuts, while fermentation reduced the content of the most active phenolic structures through metabolism. ABTS+ inhibition correlated with the content of epicatechin (p: 0.0323; r: 0.9094) and gallic acid (p: 0.0241; r: 0.9255).

Figure 1.
Antioxidant properties of pistachio beverages from Italian (I) and Argentinian (A) varieties made with domestic processor (ν) or colloidal mill (ν) and fermented with L. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains. (A) ABTS+ scavenging; (B) FRAP assay. Different letters mean significant differences among samples (p < 0.05). * means significant difference between domestic or colloidal milling.
Tomaino et al. [6] showed that pistachio shells had higher levels of phenolic compounds and exhibit greater antioxidant activity than the kernels. It is likely that fine grinding improved the extraction of phenolics from both parts of the nut, avoiding the loss of phenolics bound to the waste produced by domestic processor. Additionally, Grace et al. [7] reported that the polar skin extract had the highest phenolic content, and that antioxidant activity was associated with the polyphenolics instead of lipophilic constituents of the nuts. The antioxidant potential of phenolic compounds is related to the scavenging of free radicals, which may reduce the oxidative stress markers observed in healthy subjects under clinical studies after pistachio consumption [2].
Additionally, other phenolic compounds (e.g., proanthocyanidins and flavonoids) present in in vitro digested and fermented pistachios, like γ-tocopherol [31], and natural occurring phylloquinone, carotenoids, and chlorophyll [29] could be involved in the pistachio antioxidant properties.
Figure 2 shows the anti-diabetogenic (ADA-inhibition of DPP-IV) and antihypertensive (AHA-inhibition of ACE-I) activities of pistachio beverages. No DPP IV inhibition was detected in the Argentinean pistachio beverages. However, colloidal milling of Italian pistachio favored this activity (Figure 2A). The fermentation increased the inhibition of DPP-IV, particularly for S2 strain fermentation. Regarding ACE-I inhibition (Figure 2B), all samples had a high AHA, except for the ID-S2 sample. The AHA was higher for beverages obtained using the colloidal mill. Fermentation increased ACE-I inhibition for Argentinian pistachio beverages, mainly when S1 strain was used. While LAB fermentation did not change the activity of IC beverages, it decreased ID beverages bioactivity. Since fermentation decreased polyphenols and there was no correlation among AHA or ADA and the content of polyphenols, the results of AHA and ADA suggest that bioactive peptides could be involved in these bioactivities. However, there are few reports about bioactive peptides generated by fermentation or digestion of pistachio proteins. Recently, Marulo et al. [32] analyzed the bioactive peptides released by lactic acid bacteria fermenting pistachio beverages and found that a variety of peptides with different sequences and lengths are generated in beverage samples as a result of LAB-induced proteolysis. Some of the released peptides possess potential bioactivities, such as ACE and DPP-IV inhibition and antioxidant properties.
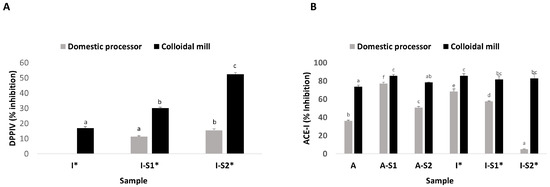
Figure 2.
Bioactive properties from pistachio-beverages from Italian (I) and Argentinian (A) varieties made with domestic processor (ν) or colloidal mill (ν) and fermented with Leuc. pseudomesenteroides PD4 (S1) or C. alimentarius PG3 (S2) strains. (A) DPP IV inhibition; (B) ACE-I inhibition. Different letters mean significant differences among samples (p < 0.05). * means significant difference between domestic or colloidal milling.
Systematic reviews and meta-analyses have suggested that consuming pistachios can have beneficial effects on blood pressure, endothelial function, and on markers of glucose and insulin metabolism [2]. Asbaghi et al. [33] reported that pistachios could reduce systolic blood pressure, especially in trials with a follow-up period of at least 12 weeks. Kendall et al. [34] reported that the addition of pistachios (56 g) to foods with a high glycaemic index (such as pasta, parboiled rice, and instant mashed potatoes) reduced the total postprandial glycaemic response by 20–30%.
4. Conclusions
Pistachio beverages have the potential to serve as functional milk-alternatives with multiple bioactivities, including antioxidant, anti-diabetogenic, and antihypertensive properties. Our findings highlight the importance of considering the geographical origin of pistachios when evaluating their nutritional properties, as these can vary significantly between regions. Industrial processing to make pistachio drinks would be desirable, since it allows the whole nut to be incorporated into the product without generating waste.
The different processing treatments had distinct impacts on the functional properties of the pistachio-based beverages. Beverages treated with colloidal milling exhibited higher acidity, increased production of organic acids and ethanol, and improved microbial fermentation performance, especially when combined with L. pseudomesenteroides PD4. In contrast, beverages produced with a domestic food processor showed lower fermentation intensity and occasional sedimentation during storage. These differences are likely attributable to particle size, matrix stability, and protein dispersion, all of which influence microbial activity and physicochemical behavior.
Fermentation could enhance some bio-functional properties such as anti-diabetogenic and antihypertensive activities, which could be mainly attributed to bioactive peptides produced by LAB. To this regard, the strain L. pseudomesenteroides PD4 (S1) probed to be an interesting candidate for the development of fermented pistachio beverages.
Finally, the industrial processing of pistachios for the production of milk analogs and fermented beverages is encouraged, since it allows for obtaining a better extraction of nutrients and health-promoting compounds.
Furthermore, we highlighted that future research should focus on optimizing processing parameters (e.g., homogenization intensity and thermal treatment), performing sensory and volatile compound analyses, investigating the interaction between specific strains and different plant matrices, and studying the effect of gastrointestinal digestion on the bioaccessibility of bioactive compounds, and their properties related to health benefits.
Author Contributions
Conceptualization, T.D.R. and A.R.; data curation, A.G.G., S.R.D. and A.R.; formal analysis, T.D.R., A.G.G., L.P.S. and A.R.; funding acquisition, M.C.P. and A.R.; investigation, T.D.R., A.G.G., L.P.S. and A.R.; methodology, T.D.R., M.C.P., S.R.D. and A.R.; project administration, M.C.P. and A.R.; resources, M.C.P., S.R.D. and A.R.; supervision, A.R.; writing—original draft, T.D.R., S.R.D. and A.R.; writing—review and editing, T.D.R., M.C.P., S.R.D. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bilateral project between ISA-CNR and Centro de Investigación y Desarrollo en Criotecnología de Alimentos (CONICET-UNLP-CIC) entitled “Development of functional pistachio-based fermented beverage” (SAC.AD002.001.024). The project was also supported by the European Commission—Next—GenerationEU, Project SUS- MIRRI.IT “Strengthening the MIRRI Italian Research Infrastructure for Sustainable Bioscience and Bioeconomy”, code n. IR0000005 (within Italy’s National Recovery and Resilience Plan) and Project PICT-2020-Serie A-03116.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LAB | Lactic acid bacteria |
| A | Argentinean pistachio nuts |
| I | Italian pistachio nuts |
| D | Domestic processor |
| C | Colloidal mill |
| S1 | Leuconostoc pseudomesenteroides PD4 |
| S2 | Companilactobacillus alimentarius PG3 |
| BI | Browing index |
| AOA | Antioxidant activity |
| ADA | Antidiabetogenic activity |
| AHA | Antihypertensive activity |
| ACE-I | Angiotensin converting enzyme -I |
| DPP-IV | Dipeptidyl peptidase -IV |
| TDF | Total dietary fiber |
References
- Noguera-Artiaga, L.; Salvador, M.D.; Fregapane, G.; Collado-González, J.; Wojdyło, A.; López-Lluch, D.; Carbonell-Barrachina, Á.A. Functional and sensory properties of pistachio nuts as affected by cultivar. J. Sci. Food Agric. 2019, 99, 6696–6705. [Google Scholar] [CrossRef]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, nutrients, bioactives and novel health effects. Plants 2022, 11, 18. [Google Scholar] [CrossRef]
- Costa, J.; Silva, I.; Villa, C.; Mafra, I. A novel single-tube nested real-time PCR method to quantify pistachio nut as an allergenic food: Influence of food matrix. J. Food Comp. Anal. 2023, 115, 105042. [Google Scholar] [CrossRef]
- Pakzadeh, R.; Goli, S.A.H.; Abdollahi, M.; Varshosaz, J. Formulation optimization and impact of environmental and storage conditions on physicochemical stability of pistachio milk. Food Meas. 2021, 15, 4037–4050. [Google Scholar] [CrossRef]
- Mertdinç, Z.; Aydar, E.F.; Kadı, I.H.; Demircan, E.; Çetinkaya, S.K.; Özçelik, B. A new plant-based milk alternative of Pistacia vera geographically indicated in Türkiye: Antioxidant activity, in vitro bio-accessibility, and sensory characteristics. Food Biosci. 2023, 53, 102731. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. In vitro lipolytic, antioxidant and anti-inflammatory activities of roasted pistachio kernel and skin constituents. Food Funct. 2016, 7, 4285–4298. [Google Scholar] [CrossRef]
- Fernández-Varela, R.; Hansen, A.H.; Svendsen, B.A.; Moghadam, E.G.; Bas, A.; Kračun, S.K.; Harlé, O.; Poulsen, V.K. Harnessing fermentation by Bacillus and lactic acid bacteria for enhanced texture, flavor, and nutritional value in plant-based matrices. Fermentation 2024, 10, 411. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziębicka, A.; Ziarno, M. Properties of rice-based beverages fermented with lactic acid bacteria and Propionibacterium. Molecules 2022, 27, 2558. [Google Scholar] [CrossRef]
- Di Renzo, T.; Osimani, A.; Marulo, S.; Cardinali, F.; Mamone, G.; Puppo, M.C.; Garzón, A.G.; Drago, S.R.; Laurino, C.; Reale, A. Insight into the role of lactic acid bacteria in the development of a novel fermented pistachio (Pistacia vera L.) beverage. Food Biosci. 2023, 53, 102802. [Google Scholar] [CrossRef]
- Di Renzo, T.; Reale, A. Process optimization and quality improvement of fermented foods and beverages. Foods 2025, 14, 1238. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis, 18th ed.; Horwitz, W., Ed.; AOAC INTERNATIONAL: Washington, DC, USA, 2005. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzón, A.G.; Betancur Ancona, D.; Chel Guerrero, L.; Drago, S.R. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Wang, T.Y.; Hsieh, C.H.; Hung, C.C.; Jao, C.L.; Lin, P.Y.; Hsieh, Y.L.; Hsu, K.C. A study to evaluate the potential of an in silico approach for predicting dipeptidyl peptidase-IV inhibitory activity in vitro of protein hydrolysates. Food Chem. 2017, 234, 431–438. [Google Scholar] [CrossRef]
- Hayakari, M.; Kondo, Y.; Izumi, H. A rapid and simple spectrophotometric assay of angiotensin-converting enzyme. Anal. Biochem. 1978, 84, 361–369. [Google Scholar] [CrossRef]
- Boukid, F.; Abbattangelo, S.; Carini, E.; Marseglia, A.; Caligiani, A.; Vittadini, E. Geographical origin discrimination of Pistachio (Pistacia vera L.) through combined analysis of physical and chemical features. Eur. Food Res. Technol. 2019, 245, 143–150. [Google Scholar] [CrossRef]
- Tsantili, E.; Takidelli, C.; Christopoulos, M.V.; Lambrinea, E.; Rouskas, D.; Roussos, P.A. Physical, compositional and sensory difference in nut among pistachio (Pistachia vera L.) varieties. Sci. Hortic. 2010, 125, 562–568. [Google Scholar] [CrossRef]
- Ak, B.E.; Kaska, N. Effects of climatic conditions on pistachio nut development and quality. Acta Hortic. 1999, 488, 229–234. [Google Scholar]
- Ozdemir, M.; Topuz, A. Influence of different drying techniques on the retention of nutrients and oils in pistachios. J. Food Eng. 2004, 62, 89–93. [Google Scholar]
- Tavakolipour, H. Postharvest operations of pistachio nuts. J. Food Sci. Technol. 2015, 52, 1124–1130. [Google Scholar] [CrossRef]
- Mesquita, M.C.; dos Santos Leandro, E.; de Alencar, F.R.; Assunção Botelho, R.B. Fermentation of chickpea (Cicer arietinum L.) and coconut (Coccus nucifera L.) beverages by Lactobacillus paracasei subsp paracasei LBC 81: The influence of sugar content on growth and stability during storage. LWT-Food Sci. Technol. 2020, 132, 109834. [Google Scholar] [CrossRef]
- Erem, E.; Kilic-Akyilmaz, M. The role of fermentation with lactic acid bacteria in quality and health effects of plant-based dairy analogues. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13402. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. GRAS Notice No. GRN 000679—Dealcoholized Fermented Orange Juice; 2010; GRAS Notice (GRN) No. 679. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm (accessed on 25 June 2025).
- Okaru, A.O.; Lachenmeier, D.W. Defining No and Low (NoLo) Alcohol Products. Nutrients 2022, 14, 3873. [Google Scholar] [CrossRef]
- Ballistreri, G.; Arena, E.; Fallico, B. Influence of ripeness and drying process on the polyphenols and tocopherols of Pistacia vera L. Molecules 2009, 14, 4358–4369. [Google Scholar] [CrossRef]
- Fabani, M.P.; Luna, L.; Baroni, M.V.; Monferran, M.V.; Ighani, M.; Tapia, A.; Wunderlin, D.A.; Feresin, G.E. Pistachio (Pistacia vera var Kerman) from Argentinean cultivars. A natural product with potential to improve human health. J. Funct. Foods 2013, 5, 1347–1356. [Google Scholar] [CrossRef]
- Liu, Y.; Blumberg, J.B.; Chen, C.-Y.O. Quantification and bioaccessibility of California pistachio bioactives. J. Agric. Food Chem. 2014, 62, 1550–1556. [Google Scholar] [CrossRef]
- Bulló, M.; Juanola-Falgarona, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Nutrition attributes and health effects of pistachio nuts. Br. J. Nutr. 2015, 113 (Suppl. S2), S79–S93. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Pistachio oil (Pistacia vera L. cv. Uzun): Characterization of key odorants in a representative aromatic extract by GC-MS-olfactometry and phenolic profile by LC-ESI-MS/MS. Food Chem. 2018, 240, 24–31. [Google Scholar] [CrossRef]
- Glei, M.; Ludwig, D.; Lamberty, J.; Fischer, S.; Lorkowski, S.; Schlörmann, W. Chemopreventive potential of raw and roasted pistachios regarding colon carcinogenesis. Nutrients 2017, 9, 1368. [Google Scholar] [CrossRef]
- Marulo, S.; De Caro, S.; Nitride, C.; Di Renzo, T.; Di Stasio, L.; Ferranti, P.; Reale, A.; Mamone, G. Bioactive peptides released by lactic acid bacteria fermented pistachio beverages. Food Biosci. 2024, 59, 103988. [Google Scholar] [CrossRef]
- Asbaghi, O.; Hadi, A.; Campbell, M.S.; Venkatakrishnan, K.; Ghaedi, E. Effects of pistachios on anthropometric indices, inflammatory markers, endothelial function and blood pressure in adults: A systematic review and meta-analysis of randomized controlled trials. Br. J. Nutr. 2021, 126, 718–729. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Josse, A.R.; Esfahani, A.; Jenkins, D.J.A. The impact of pistachio intake alone or in combination with high carbohydrate foods on post-prandial glycemia. Eur. J. Clin. Nutr. 2011, 65, 696–702. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).