Abstract
The objective of this study was the evaluation of fungal solid-state fermentation (SSF) for the production of alginate lyase and extraction of uronic acids from Sargassum sp. For this purpose, the fungi Trichoderma asperellum, Aspergillus oryzae, and Rhizopus oryzae were applied (alone or combined) to Sargassum sp. biomass through SSF (107 spores gbiomass−1, 30 °C, and 7 days of treatment). In general, individual SSF with all three fungi degraded the biomass, achieving a marked synergy in the production of cellulase, laminarinase, and alginate lyase activities (especially for the last one). Trichoderma was the most efficient species in producing laminarinase, whereas Rhizophus was the best option for producing alginate lyase. However, when dual combinations were tested, the maximal values of alginate lyase activities were reached (13.4 ± 0.2 IU gbiomass−1 for Aspergillus oryzae and Rhizopus oryzae). Remarkably, uronic acids were the main monomeric units from algal biomass solubilization, achieving a maximum yield of 14.4 mguronic gbiomass−1, with the A + R condition being a feasible, eco-friendly alternative to chemical extraction of this monomer. Additionally, the application of all the fungal pretreatments drastically decreased the total phenolic content (TPC) in the biomass from 369 mg L−1 to values around 44–84 mg L−1, minimizing the inhibition for possible subsequent biological processes in which the residual solid can be used.
1. Introduction
The so-called blue bioeconomy incorporates economic activities that generate non-food products from renewable aquatic biological resources [1]. In this sense, macroalgae have been used to obtain different high-value products. Among them, Sargassum sp. is a brown macroalgae that is globally abundant [2]. These marine algae have their origin in the Sargasso Sea and are the most common subtidal marine brown macroalga from the bottom of the ocean in tropical latitudes, where they form aquatic forests. They also act as an invasive seaweed on the Mexican coasts of the Caribbean Sea and Latin America [3], causing negative impacts on ecosystems, with societal and economic triggers related to fishing and tourism [4] due to their high growth rate. For example, the removal of the increasing volume of Sargassum sp. in Mexico in 2019 (85,000 tons) incurred a cost of USD 2.6 million [5]. In fact, the sea temperature elevation due to climate change has a relevant role in the spread of invasive seaweed from tropical latitudes [6].
Brown macroalgae are rich in a variety of polysaccharides, such as laminarin, cellulose, fucoidans [7], and alginates [8], and other organic compounds such as biopeptides [9], natural brown dye, and bio-oil [7]. Due to this composition and because of the above-mentioned problems caused by their accumulation on the coasts, different alternatives for the potential valorization of this algal biomass have been evaluated. Thus, macroalgae have been used as raw material for third-generation biorefinery, providing different high-content products such as pigments, lipids, proteins [5], and specific sugars [10] with several applications such as food additives [11], biostimulants [12], and biofuel production [13].
Among different sugars, alginates are structural carbohydrates composed of uronic acids (β-D-mannuronic and α-L-guluronic acid) that comprise 40% of their dry cell weight. This linear polymer shows properties such as stabilizing, thickening, and emulsifying and allows for the formation of gels and films, making it valuable for applications in different sectors such as food, biomedicine, and bioremediation [14,15]. For these reasons, the global alginate market is in constant growth, reaching USD 728.4 million in 2020, and an expected annual growth rate of 5% up to 2028 [16].
Currently, different methods are being optimized to break down cell walls in order to release uronic acids. So, the extraction of uronic acids from brown seaweed in general, and Sargassum in particular, is based mainly on thermo-chemical methods involving a sequential application of basic (NaOH, Na2CO3) and acid agents (HCl, H2SO4) [15,17,18,19]. Also, physicochemical methods are being tested with the assistance of ultrasound [20] and microwave irradiation [21]. More recently, the use of a combination of enzymatic and acid methods has been investigated by using lyases as pretreatments due to the specificity of these enzymes toward the various glycosidic linkages. In a combined treatment, the mild chemical conditions, which need to be compatible with the enzymatic environment (low temperature, pH 6–8), permit high yields in comparison with only chemical hydrolysis [22].
However, the enzymatic step includes commercial enzymes, which are very expensive and unsuitable for industrial production and can be inactivated due to high temperatures and acid/alkali conditions [23]. In this sense, other alternatives have arisen, such as molecular-modifying processes, to resist strong operational conditions, increasing the catalytic properties of the original enzyme [23].
To overcome this problem, solid-state fermentation (SSF) is an effective process to achieve the secretion of specific enzymes [24] that could be used in an eco-friendly, simple, and economical process. In this sense, it has been reported that some hydrolytic fungus strains express specific enzymes during SSF, favoring the solubilization of the brown macroalgae biomass. Trichoderma, as an example, is an anaerobic facultative fungus with different ecological habitats, including natural soils and agricultural residues [25]. The secretion of lytic enzymes such as chitinases, glucanases, and proteases is well known in Trichoderma species [26]. Thus, Wang et al. [27] measured cellulase, xylanase, and beta-glucosidase activities after SSF with T. asperellum. Trichoderma asperellum has been determined to be useful for the production of polysaccharide lyases (such as alginate lyases), glycoside hydrolases (such as chitinases), glycosyl transferases (such as laminarinase), and carbohydrate esterases [26,28,29]. The production of alginate lyases and other enzymes capable of degrading specific polymers from brown algae by some species of the Aspergillus genus has also been detailed. For example, Aspergillus oryzae, which is an aerobic facultative fungus commonly used in the food and chemical industries, produces valuable enzymes such as cellulase, alginate lyase, and laminarinase [30,31,32]. Similarly, the genus Rhizopus has been widely used, at an industrial scale, for enzyme production (glucoamylases, cellulases, and tannases) and in traditional food production such as tempeh, peka, ragi, and loog-pang [33]. Some authors have determined the capacity of R. oryaze to produce cellulases and laminarinases or β-1,3-glucanases [34]. Recently, it has been determined that the potential to secrete endo-β-1,4 glucuronan lyase and different alginate lyases is also higher for Rhizopus spp. than for Aspergillus spp. [35].
In previous studies applied to the Caribbean biomass of Sargassum spp., Gordillo-Sierra [15] employed SSF in combination with physico-chemical extraction, utilizing three distinct strains (Aspergillus niger ATCC 6275; Rhizopus oryzae BIOTEC 018; and Trichoderma asperellum BT01). In addition, a series of experiments was conducted to ascertain the most effective conditions for the SSF process, with the objective of achieving an average total reducing sugars (TRS) release of 0.54 g L−1. These experiments were conducted within the following ranges of conditions: 4–8 days (duration); 25–35 °C (temperature); and 40–70% (humidity). The maximum sugar concentration attained was 2.05 g L−1 at an incubation time of 8 days, a temperature of 35 °C, and a humidity of 70%, using R. oryzae BIOTEC018. However, to the best of our knowledge, a combination of these strains has not been reported previously in the literature. Furthermore, they were never applied directly to biomass without prior extraction.
The main objective of this study was to produce sugars (predominantly uronic acids) from the brown seaweed Indonesian Sargassum spp. through the utilization of fungal SSF, employing a combination of distinct fungal strains (Trichoderma asperellum (T), Aspergillus oryzae (A), and Rhizopus oryzae (R)). Furthermore, the secretion of several enzymes of significant commercial value in algal biomass processing, including cellulase, laminarinase, and alginate lyase, has been the focus of the study, as well as the production of uronic acid and other monomeric sugars.
2. Materials and Methods
2.1. Substrate Sampling and Conditioning
The biomass used was Sargassum spp., collected from Makassar Island (Indonesia) in December 2022. The samples were washed with tap water to remove salt and impurities. The washed biomass was then prepared for the SSF procedure by drying it using two methods:
(i) Freeze-drying using a Labcoco® FreeZone series benchtop freeze dryer (Kansas City, MO, USA) to avoid the effect of high temperatures on the phenolic content during phenolic compound extraction;
(ii) Hot drying in a 50 °C KRIS® Food dehydrator (Foshan, Guangdong, China) for four days. The samples were then milled using a Philips® HR2116 domestic blender (Bogor, Indonesia) and stored in a hermetically sealed 50 L can to avoid humidity before their utilization.
2.2. Fungal Inoculum Obtention
To obtain the inoculum of Aspergillus oryzae KKB4, Rhizopus oryzae GAP1, and Trichoderma asperellum MLT1J1, the strains were grown on potato dextrose agar (PDA) slants at 30 °C for seven days [36]. Spores were harvested by adding 5 mL of a 0.05% Tween 80 suspension with vigorous manual agitation for 2 minutes. The number of spores in the suspension was counted using an improved Neubauer chamber and an Olympus® BX51 optical microscope (Tokyo, Japan), yielding concentrations of 3.95 × 107, 0.53 × 107, and 1.68 × 107 spores mL−1 for each strain, respectively.
2.3. Solid-State Fermentation (SSF)
In the enzyme production procedure, 1 g of dry algal biomass was inoculated with 0.6 mL of the aforementioned fungal inoculum. When using a single strain (individual tests), a total of 1.8 × 107 spores gbiomass−1 was used. Conversely, for the fungal combinations (dual and triple tests), the same final spore concentration was achieved by adding each fungus individually. For instance, 0.3 mL of T and 0.3 mL of A were added for the dual T + A combination and 0.2 mL of T, 0.2 mL of A, and 0.2 mL of R were added for the T + A + R combination (where A is Aspergillus oryzae KKB4, R is Rhizopus oryzae GAP1, and T is Trichoderma asperellum MLT1J1). The SSF tests were conducted at a solid-to-liquid ratio of 1:3 using Mandels’ salt solution according to Agabo [24], achieving a final humidity level of 77%. All assays were performed in flasks that were incubated under static conditions in a Memmert® IN55 incubator (Schwabach, Germany) at 30 °C for seven days. Moreover, all SSF tests were performed in triplicate.
2.4. Enzyme Extraction
After the SSF procedure, the sugars and enzymes were extracted. The extraction was carried out in a Memmert® WNB45 water bath (Schwabach, Germany) at 4 °C for 30 min with agitation (150 rpm), using ice and adding 20 mL of Tween 80 (0.1%) per gram of fermented biomass. The samples were centrifuged for 10 minutes at 10,000 rpm in a Thermo Fisher Scientific® Sorvall ST-8R centrifuge (Langenselbold, Germany). The liquid extracts were preserved at 4 °C. The TRS, cellulase (FPase), alginate lyase, and laminarinase activities, as well as the monomeric sugars produced during the SSF assays, were measured in the liquid fraction. The total phenolic content (TPC) was also measured in the solid and liquid fractions to evaluate their removal.
2.5. Sugar Characterization
TRS was analyzed using the dinitrosalicylic (DNS) method [37], measuring the absorbance at 540 nm with a Thermo Scientific® Genesys 10s UV-Vis spectrophotometer (Waltham, MA, USA). Monomeric sugars were also determined using a Shimadzhu® LC 10ADvp HPLC system (Kyoto, Japan) with a Bio-Rad® HPX-87H 300 × 7.8 mm aminex® column (Hercules, CA, USA), and a Shimadzu® RID-10A. refractometry detector (Kyoto, Japan) with 0.5 mM H2SO4 as the mobile phase at a flow rate of 0.6 mL·min−1.
2.6. Enzymatic Activity Determination
The activities of cellulase (EC 3.2.1.91) and alginate lyase (EC 4.2.99.4) were determined by measuring the TRS formed using the DNS method after incubating the enzymatic extract under standard assay conditions [24] in a Memmert® WNB45 water bath (Schwabach, Germany). Laminarinase (EC 3.2.1.39) activity was estimated using the modified protocol of [38] with a reference of 0.3% laminarin in a 50 mM potassium phosphate-buffer solution (pH 6.5). The activity was analyzed after a 20-min incubation at 30 °C of a reaction mixture containing 0.25 mL of substrate and 0.25 mL of enzyme solution. TRS formation was then quantified using the DNS method. The incubated substrate with buffer served as the blank. Each SSF replicate was also incubated in triplicate. One unit of FPase, alginate lyase, and laminarinase activity is defined as the amount of enzyme that catalyzes the liberation of reducing sugar equivalent to 1 µM d-glucose per minute under standard assay conditions of each specific enzyme [24,38].
Assuming that the combined activity would be obtained as the sum of the experimental individual activities in each respective proportion, the theoretical combined activity could be estimated. For example, the theoretical cellulase activity for the T + A combination can be calculated as it is expressed in Equation (1):
where Tcellulase is the theoretical cellulase activity and expcellulase(T) and (A) are the experimental cellulase activities determined after individual pretreatment with Trichoderma and Aspergillus, respectively. Similarly, the triple theoretical cellulase activity for T + A + R can be calculated using Equation (2):
2.7. Sample Characterization
The following determinations were performed for the characterization of the samples: total solids (TS) in a Memmert® UN110 oven (Schwabach, Germany), volatile solids (TVS) in an Advantec® FUW222PB furnace (Tokyo, Japan), chemical oxygen demand (COD) using an Advantec® TB-620 hot block bath (Tokyo, Japan), and total Kjeldahl nitrogen (N) using a FOSS® Kjeltec system (Suzhou, China), developed according to the APHA-AWWA-WPCF standard methods [39].
2.8. Phenolic Extraction and Determination
Phenolic determination was carried out for both solid and liquid samples. For the solid samples (raw macroalgae after conditioning and the solid obtained after enzymatic production from pre-treated macroalgae), the phenolic compounds were previously extracted. For this purpose, the samples were mixed with 70% ethanol at a ratio of 1:30 w/v (g mL−1) [40]. The mixtures were sonicated in a Hielscher Ultrasonics GmbH® UP200St ultrasound system (Teltow, Germany) under the optimal conditions described by Putra et al. [41]. The samples were then centrifuged at 4500 rpm for 10 min, and the supernatant was concentrated using an IKA® RV 10-Basic rotary evaporator (KG, Staufen, Germany) at 72 °C and 175 mbar of absolute pressure until the final volume was 5 mL. In liquid samples (enzymatic extract), the TPC was determined after filtering through a 0.47 µm filter. In all cases, TPC was determined using the Folin–Ciocalteu method [42]. The results were calculated by comparison with the calibration curves of gallic acid and expressed as mg of gallic acid per g of dry weight (DW) of seaweed, or per L in the case of the liquid extracts.
2.9. Statistical Analysis
The mean values obtained from the analysis of enzymatic activities, sample characterization, and phenolic determinations have a 95% confidence interval (α = 0.05; n = 3). All analyses were performed using Origin Pro8© software.
3. Results
3.1. Characterization of Sargassum spp.
The characterization of the algal biomass showed a TS content of 92.8%, with an organic matter content of 75.1% on a dry basis, of which 12 ± 1.6% of the total organic matter was alginate according to references [15,43,44]. This biomass also exhibited a COD-to-N ratio of 90.8. Conversely, a high TPC (369 mg L−1 or 1.79 mg gbiomass−1) was extracted from the algal biomass.
3.2. Enzymatic Production
Figure 1 shows the enzymatic activities determined after 7 days of fungal pretreatment. The three fungi showed cellulase, alginate lyase, and laminarinase activities, and in most cases, the fungal combinations were better than expected.
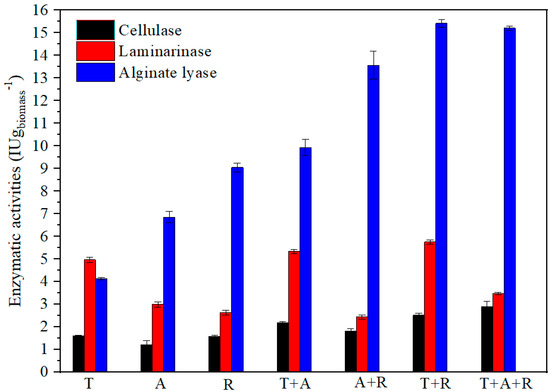
Figure 1.
Cellulase, laminarase, and alginate lyase activities (International Units per gram of biomass: IU gbiomass−1) in enzymatic extracts obtained from Sargassum spp. biomass after 7 days of various biological pretreatments with T: Trichoderma asperellum, A: Aspergillus oryzae, and R: Rhizopus oryzae, either individually or in combination.
Regarding cellulase activity, individual treatments generated similar cellulase activities of 1.5 ± 0.18 FPU gbiomass−1, on average. Cellulase activity in dual combinations was 1.5 times over-registered in individual fungus tests. In fact, the best results were obtained in the triple (T + A + R) test, reaching 2.9 FPU gbiomass−1, which was double the level obtained with the individual fungi (Figure 1). In general, cellulase activities in combined tests increased by 30–100%, reaching maximum efficiency when all three fungi were applied simultaneously.
Focusing on laminarinase activity, the Trichoderma individual test showed greater activity (4 IU gbiomass−1) than the other individual tests for the other fungi (2 IU/gbiomass for Aspergillus, and 1.7 IU gbiomass−1 for Rhizopus). This activity was maintained when combined with Aspergillus or Rhizopus (4.3 and 4.7 IU gbiomass−1, respectively). Nevertheless, laminarinase activity decayed when the combinations were A + R and T + A + R (1.4 and 2.5 IU gbiomass−1, respectively).
The alginate lyase activity values of Rhizopus are particularly notable. It appears to be a successful producer of alginate lyase with an activity value of 7 IU gbiomass−1 compared to Aspergillus (4.8 IU gbiomass−1) or Trichoderma (2.1 IU gbiomass−1). On average, however, all the dual and triple combinations increased the alginate lyase activity compared to the individual tests (2.3 and 2.8, respectively), with maximal activity obtained when the A + R or T + A + R combinations were applied (13.4 ± 0.2 and 13.2 ± 0.0 IU gbiomass−1, respectively).
Regarding the data on experimental enzymatic production, it is possible to calculate all the theoretical enzyme activities and TRS release for double and triple combinations (“t” in Table 1). The ratio of the experimental to theoretical values of the enzymatic activities and TRS can also be calculated for each fungi combination (“e/t” in Table 1). According to the TRS “e/t” ratios, the combination of strains increased the TRS release beyond the expected theoretical value. An improvement range of 6–29% has been observed, with no evidence of antagonism. The maximum e/t ratio of TRS levels was found when Aspergillus was absent, coinciding with maximum alginate lyase values.

Table 1.
Theoretical enzyme activities and TRS calculated (t) and their respective experimental/theoretical ratios and TRS calculated (e/t), for double and triple combinations. T: Trichoderma asperellum; A: Aspergillus oryzae; R: Rhizopus oryzae.
3.3. Total Phenolic Compounds
Table 2 shows the TPC released after SSF in the liquid extract, as well as the TPC remaining in the solid fraction, in terms of concentration (mg L−1) and percentage. Table 2 also shows the TPC in the exhausted solids and the TPCresidual after different pretreatments. The release of TPC in the enzymatic extract reached concentrations of 37.8–52.4 mg L−1, with a maximum release occurring with the co-culture of Trichoderma and Rhizopus. In this sense, the Trichoderma species optimally released TPC in all the cases when applied, obtaining around 50 mg L−1. Conversely, the applied fungal pretreatment was found to drastically decrease the TPC from 366 ± 4 mg L−1 to values of around 44–84 mg L−1 in the exhausted solids in all cases, with a % TPCresidual ranging from 12.1 to 22.9%, which was maximal when the co-culture of three strains was applied. Finally, it is important to note that most phenolic liquid–solid ratios were around 1, with an equal distribution in both the solid and liquid extracts after SSF application.

Table 2.
TPC in the liquid enzymatic extract and in solid algae, % TPCresidual after different pretreatments. T: Trichoderma asperellum; A: Aspergillus oryzae; R: Rhizopus oryzae.
Table 3 shows the TPC ratios in relation to the enzymatic activities. As can be seen, the TPC/FPU values are similar and increase with separate fungi (0.62 ± 0.01), compared to the combined fungi. The TPC/IUlaminarinase ratio of the Trichoderma fermentation extract was 0.25, which is lower than that obtained from the other combinations. Their presence reduces the value of the combinations. In contrast, the TPC/IUAlginate lyase ratio showed the opposite trend. Trichoderma increased this ratio, as did the combined fungal fermentation extracts.

Table 3.
TPC in the liquid enzymatic extract and in solid algae, % TPCresidual after different pretreatments, and TPC/enzymatic activity ratio. T: Trichoderma asperellum; A: Aspergillus oryzae; R: Rhizopus oryzae.
3.4. Sugar Release
Figure 2 shows the final TRS concentrations after 7 days of fungal pretreatment as well as the monomeric composition of the enzymatic extracts.
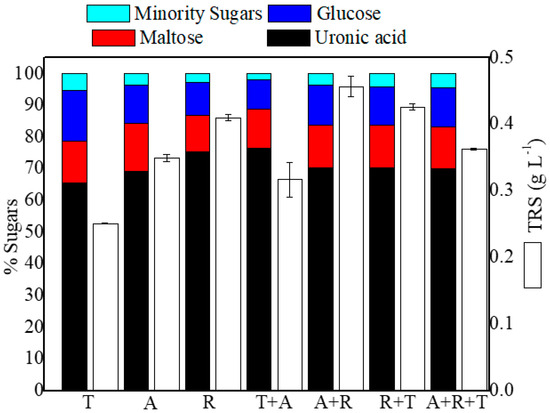
Figure 2.
Final total reducing sugars (TRS) and their monomeric sugar composition (as a percentage) as measured in the enzymatic extracts obtained from the Sargassum spp. biomass after 7 days of different biological pretreatments with T: Trichoderma asperellum, A: Aspergillus oryzae, and R: Rhizopus oryzae, either individually or in combination.
As can be seen, Rhizopus oryzae (R) was the most efficient fungal agent in terms of TRS release, reaching values of 0.41 g L−1, with even higher values observed in dual combinations with Aspergillus oryzae (A + R) and Trichoderma asperellum (T + R) at 0.46 and 0.43 g L−1, respectively. It is also important to note that the TRS value for the crude (non-biologically pretreated) Sargassum samples was 0.2 ± 0.01 g L−1, which is slightly lower than the values obtained when Aspergillus and Trichoderma were applied alone (0.25 ± 0.01 and 0.35 ± 0.01, respectively).
Conversely, due to the high level of alginate lyase activity, the main monomers obtained were uronic acids, with concentrations ranging from 67.9 to 74.7% and similar values. As expected, the concentrations were slightly higher for R and A + R, given their increased alginate lyase enzymatic profile. The maximum uronic yield produced in this case was 14.4 mguronic gbiomass−1, for A + R (Figure 3). The others obtained a similar yield of 10.5 ± 0.84 mguronic gbiomass−1, except for the Trichoderma pretreatment, which generated only 7.79 ± 0.2 mg g−1.
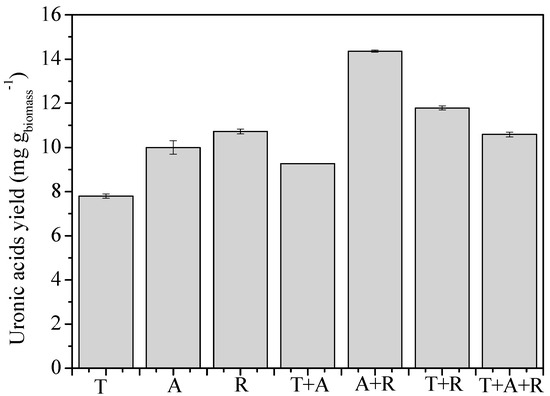
Figure 3.
Uronic acid yields (mg·gbiomass−1) measured in the enzymatic extract obtained from Sargassum spp. biomass after 7 days of different biological pretreatments with T: Trichoderma asperellum, A: Aspergillus oryzae, and R: Rhizopus oryzae, either individually or in combination.
On the other hand, the other more representative monomers were maltose (13.2 ± 1%) and glucose (12 ± 2%).
4. Discussion
4.1. Sargassum spp. As Sugary Biomass for Bio-Processes
The obtained COD/N ratio is a relevant parameter for characterizing biomass as a substrate for bio-processes [45]. However, the COD/N ratio values are strongly influenced by the specific brown algae, cultivation conditions, and harvesting time [46]. The composition is also crucial for designing the bio-process to be applied. In this sense, the high content of alginate and other cell wall sugars necessitates enzymatic treatment to produce their fermentable oligomers and monomers known as uronic acid. These can be used to produce various products, such as volatile fatty acids [47] or polyhydroxybutyrate [48], using a biorefinery approach. Co-culture in SSF, which is widely used as a biocontrol technique for identifying plant pathogens, can also be applied to macroalgae biomass as an eco-friendly and economical treatment for subsequent bio-processes, or as the main bioprocess to obtain sugars.
However, the presence of polyphenols could be counterproductive. This is particularly important when considering the release of phenolic compounds after SSF. Therefore, if the main objective is to use the Sargassum spp. biomass for enzyme production, the algal polyphenols could contribute to maintaining a chemical balance and play a fundamental role in protecting against UV radiation and microbial contamination [49].
However, if the Sargassum spp. biomass is destined for subsequent fermentative processes involving uronic acids, the TPC should be reduced to avoid inhibiting microbial growth [50,51]. Therefore, it is critical to determine the TPC when extracting valuable products, such as enzymes or sugars, from Sargassum spp. The TPC values in this study are consistent with those reported in the literature for Sargassum species by Fagundo-Mollineda et al. [49].
4.2. Effect of Co-Culture in Enzymatic Production
The comparison between the experimental and expected theoretical activities (Table 2) is an important aspect to be studied because some fungal species have been shown to have an antagonistic effect on other plant fungal parasites [52].
In all combinations, the experimental activity exceeded the theoretical activity by a factor of at least two. This is noteworthy, considering the mycoparasitic nature of Trichoderma against fungi such as Fusarium solani, Rhizoctonia solani, and Sclerotinia sclerotiorum. Previous studies have shown that the cell walls of other fungi often induce alginate lyase expression in Trichoderma species [52]. As expected, this study found that the individual cultures of different fungi (including Trichoderma) induced alginate expression when grown with algae biomass. Alginate lyase degrades alginate, a complex polysaccharide composed of α-L-guluronate and β-D-mannuronate, found in both macroalgae and fungal cell walls. Synergistic effects in alginate lyase production were observed in nearly all combinations in this study.
4.3. Effect of Total Phenolic Compounds in Enzymatic Production
The main polyphenols found in Sargassum species are bound to proteins and alginates within the cell walls [53]. Therefore, the cell wall breakdown provokes the release of these phenolic compounds into the liquid extract. It is known that there is a negative correlation between certain enzymatic activities and the presence of certain phenolic compounds [54].
In this sense, Ximenes et al. [55] studied the inhibitory and/or deactivating effects of polymeric and monomeric phenols on cellulases and β-glucosidases in this context. Some of these phenolic compounds are present in Sargassum extracts, including gallic acid, ferulic acid, ρ-coumaric acid, 4-hydroxybenzoic acids, and vanillin [56]. The results showed that, after 24 h, gallic acid, hydroxy-cinnamic acid, 4-hydroxybenzoic acid, and vanillin caused 20–80% deactivation of cellulases and/or β-glucosidases using different fungi, such as Trichoderma reesi or Aspergillus niger, with a TPC/FPU ratio of 0.5. They also concluded that the strength of the inhibition or deactivation depended on the enzyme type and the microorganism from which the enzyme was derived.
In this study, the TPC/FPU values (0.32–0.63) were found to be within the range of the inhibitory ratios previously tested by Ximenes et al. [55]. Conversely, this could explain the lower values of cellulase and laminarinase (which is a β-glucanase) enzymatic activities in all the samples from this study in the liquid extract (Figure 1) compared to alginate lyases. This effect can be counteracted by the effect of other enzymes. In this sense, Walle et al. and Abdallah et al. [57,58] determined that glucosidase enzymes could hydrolyze the glycosidic bonds in certain glycosylated phenolic compounds such as quercetin, releasing the aglycone (the phenolic core) without losing their activity. This occurs with glycosylated quercetin, which is also present in Sargassum [40]. In this context, Borisova et al. [54] studied the inhibitory effect of soluble phenolic compounds on the enzymatic hydrolysis of wheat straw using some Trichoderma enzymes and commercial enzyme mixtures. They found that the yields of cellobiohydrolase hydrolysis dropped by 70–75%, compared to the reference hydrolysis without phenolic compounds when genz/gTPC = 0.02. However, Cellic Ctec2 exhibited reduced inhibition (60%) due to the impact of the presence of β-glucosidase. This is because these endoglucanases have an open-cleft architecture in their active sites, which allows similar phenolic compounds to bind.
For this reason, controlling the balance between TPC and the enzyme activity I is important for determining possible inhibition in the final enzymatic extracts and, consequently, diminishing inhibition problems in the possible subsequent enzymatic bioprocesses applied to the residual solid.
4.4. Fungal Co-Cultures Improve the Release of Sugars
The sum of all the enzymatic activities demonstrates that Rhizopus is the main enzyme producer, either on its own or in co-culture. As can be seen in the results, Rhizopus produced the highest TRS release when grown alone or in co-culture with Aspergillus and Trichoderma, doubling the TRS release compared to crude biomass (2.0, 2.3, and 2.1 times, respectively).
However, the T + A and T + A + R combinations were less successful in terms of TRS production. The TRS increase was only 1.6 and 1.8 times higher than the crude biomass. This effect may be related to a competition between Trichoderma and Aspergillus, given that certain species of Trichoderma and Aspergillus are antagonists by different mechanisms such as mycoparasitism (the secretion of enzymes that degrade fungal cell walls), competition for space and nutrients, and/or the secretion of metabolites that produce antibiosis [59]. Certain Trichoderma species, for example, are widely used in agriculture as biocontrol agents against various plant diseases. This is attributed to their ability to inhibit the growth of other fungi through the production of secondary metabolites [25] or the high expression of enzymes such as chitinases, glucanases, proteases, and alginate lyases, which degrade fungal cell walls [28].
Among the different sugar monomers present in the extract, maltose has been identified. This monosaccharide is not commonly obtained from the Sargassum species. It was previously determined in low quantities by Vaghela et al. [60]. Furthermore, this study obtained glucose at values very similar to those reported by Tonon et al. [44] and, above all, uronic acids (between 67.9 and 74.7%).
4.5. SSF as a Feasible Alternative for Uronic Acid Production
The results obtained for uronic acid production are in the same range as those obtained with commercial enzymes by Davis et al. [43] (65–57%) in Jamaica, where the monosaccharides released by individual enzymatic treatments of three different Sargassum morphotypes were compared: S. natans I (SnI), S. fluitans III (Sf), and S. natans VIII (SnVIII) (Table 4).

Table 4.
Comparison of uronic acids produced by enzyme applications on Sargassum algae. Note: “S-L” means “solid to liquid”.
Among the different monosaccharides obtained, the release of uronic acids was higher in the SnI samples that underwent enzymatic treatment with pronase, yielding 11.7 ± 1.1 mguronics gbiomass−1 (Table 4), which is very similar to the results obtained in this study (Figure 3). This work also obtained higher uronic acid values than Tonon et al. [44] in the Dominican Republic (40%), despite applying a chemical carbohydrate extraction process. Hence, considering that the total uronic acid content in the Sargassum spp. species can vary from 15 to 140 mg gbiomass−1, depending on the season, year, and location of collection [44], this process could be a feasible option for uronic acid production.
Optimizing this biological method for enzyme production could be a crucial step in replacing the more contaminating thermochemical processes that use chemicals such as trifluoroacetic acid or ethanol to completely extract these uronic acids [43,44,61].
Also, this method allows the uronic acids to be easily fractionated, as other monomers, such as glucose from laminarin and cellulose, can be consumed by the fungal strains over long pre-treatment periods (7 days). This fact has previously been reported in studies focusing on the effect of pre-treatment time on the process when operating with individual fungi and other brown seaweeds [24]. Consequently, long pre-treatment times could result in only the most difficult-to-consume substances, such as uronic acids, remaining in the medium.
It is important to note that Sargassum spp. is a key species among the different brown seaweeds used for industrial alginate production despite their low yield of alginate for the different morphotypes, they can be harvested when the main commercial sources are unavailable [62]. On the other hand, the proposed uronic acid-producing methods could be transferred to other types of brown macroalgae with higher alginate contents. This procedure can be used as both a uronic production method and a biological pre-treatment in a biorefinery approach.
5. Conclusions
This study examined the ability of Trichoderma asperellum (T), Aspergillus oryzae (A), and Rhizophus sp (R), as well as their combinations, to extract uronic acids from Sargassum biomass using biological methods. In general, SSF involving individual and combinations of the fungi degraded the biomass, and cellulase, alginate lyase, and laminarinase activities were determined in all the SSF assays. Trichoderma produced the most laminarinase, whereas Rhizophus produced the most alginate lyase. Alginate lyase activity was higher than the other enzymatic activities, particularly cellulase, due to the inhibition of this specific enzyme by solubilized phenolic compounds. Significant synergy was observed in cellulase, laminarinase, and alginate lyase production when dual and triple combinations were tested, particularly with regard to the latter. The maximum alginate lyase activity value was thus reached (13.4 ± 0.2 IU gbiomass−1), leading to a higher proportion of uronic acids (84 ± 3% of total monomers). Final values of 7.8–14.4 mgUronics gbiomass−1 were obtained, which are in the same range as those reported in the literature for commercial enzymes.
It is also noteworthy that the application of all fungal pretreatments drastically decreased the total phenolic content (TPC) in the biomass from 369 mg L−1 to values around 44–84 mg L−1, minimizing inhibition of subsequent biological processes for which the residual solid could be used.
Author Contributions
Conceptualization, C.A.-G., W.S. and M.N.C.; methodology, C.A.-G. and W.S.; software, C.A.-G.; validation, L.I.R.-G., C.J.Á.-G. and A.B.; formal analysis, L.I.R.-G., C.J.Á-G. and A.B.; investigation, C.A.-G.; resources, W.S. and M.N.C.; data curation, C.A.-G.; writing—original draft preparation, C.A.-G.; writing—review and editing, L.I.R.-G., C.J.Á.-G. and A.B.; visualization, C.A.-G.; supervision, L.I.R.-G., C.J.Á.-G. and A.B.; project administration, W.S. and M.N.C.; funding acquisition, L.I.R.-G., C.J.Á.-G. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Grant TED2021-130891B-I00 funded by MICIU/AEI/10.13039/501100011033 and by European Union NextGenerationEU/PRTR and the R + D + I project PID2019-104525RB-I00 funded by MCIN/AEI/10.13039/501100011033. Ph.D. Cristina Agabo García was granted a Postdoctoral Contract funded by the Department of Economic Transformation, Industry, Knowledge and Universities from the Andalusian Government in the call for hiring of PhD research staff by the agents of the Andalusian Knowledge System, allowing the short research stay co-funded by “Research Plan from University of Cádiz 2022–2023”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work has been supported by the Technical Staff of the Faculty of Agricultural Technology of the Universitas Gadjah Mada, Yogyakarta.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| A | Aspergillus oryzae |
| COD | Chemical Oxygen Demand |
| FPU | Filter paper unit |
| IU | International unit |
| R | Rhizopus oryzae |
| SSF | Solid-state fermentation |
| T | Trichoderma asperellum |
| TKN | Total Kjeldahl nitrogen |
| TPC | Total phenolic compounds |
| TRS | Total reducing sugars |
| TS | Total Solids |
| TVS | Volatile Solids |
References
- EUMOFA. Blue Bioeconomy Report; Luxembourg: European Commission. 2023. Available online: https://eumofa.eu/documents/20178/84590/blue+bioeconomy+report+2022+final.pdf/eb889d94-74a6-2c15-e136-4d2204118c6a?t=1673441855108 (accessed on 31 July 2024).
- Farobie, O.; Amrullah, A.; Bayu, A.; Syaftika, N.; Anis, L.A.; Hartulistiyoso, E. In-depth study of bio-oil and biochar production from macroalgae Sargassum sp. via slow pyrolysis. RSC Adv. 2022, 12, 9567–9578. [Google Scholar] [CrossRef] [PubMed]
- Marx, U.C.; Roles, J.; Hankamer, B. Sargassum blooms in the Atlantic Ocean—From a burden to an asset. Algal Res. 2021, 54, 102188. [Google Scholar] [CrossRef]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspective. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- González-Gloria, K.D.; Rodríguez-Jasso, R.M.; Aparicio, S.E.; Chávez González, M.L.; Kostas, E.T.; Ruiz, H.A. Macroalgal biomass in terms of third-generation biorefinery concept: Current status and techno-economic analysis. A review. Bioresour. Technol. Rep. 2016, 16, 100863. [Google Scholar] [CrossRef]
- Pereira, L. Non-indigenous seaweeds in the Iberian Peninsula, Macaronesia Islands (Madeira, Azores, Canary Islands) and Balearic Islands: Biodiversity, ecological impact, invasion dynamics, and potential industrial applications. Algal Res. 2024, 78, 103407. [Google Scholar] [CrossRef]
- González-Fernández, L.A.; Castillo Ramos, V.; Sánchez Polo, M.; Medellín Castillo, N.A. Fundamentals in applications of algae biomass: A review. J. Environ. Manag. 2023, 338, 117830. [Google Scholar] [CrossRef]
- Morales-Contreras, B.E.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H.; Rodríguez-Jasso, R.M.; Ruiz, H.A. Hydrothermal systems to obtain high value-added compounds from macroalgae for bioeconomy and biorefineries. Bioresour. Technol. 2022, 343, 126017. [Google Scholar] [CrossRef]
- Baghel, R.S. Developments in seaweed biorefinery research: A comprehensive review. Chem. Eng. J. 2023, 454, 140177. [Google Scholar] [CrossRef]
- Kwon, O.M.; Kim, D.H.; Kim, S.K.; Jeong, G.T. Production of sugars from macro-algae Gracilaria verrucosa using combined process of citric acid-catalyzed pretreatment and enzymatic hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Jumadi, O.; Annisi, A.D.; Djawad, Y.A.; Bourgougnon, N.; Amaliah, N.A.; Asmawati, A.; Baso Manguntungi, A.; Inubushi, K. Brown algae (Sargassum sp.) extract prepared by indigenous microbe fermentation enhanced tomato germination parameters. Biocatal. Agric. Biotechnol. 2023, 47, 102601. [Google Scholar] [CrossRef]
- Yuhendra, A.P.; Farghali, I.; Mohamed, M.A.; Iwasaki, M.; Tangtaweewipat, S.; Ihara, I.; Sakai, R.; Umetsu, K. Potential of biogas production from the anaerobic digestion of Sargassum fulvellum macroalgae: Influences of mechanical, chemical, and biological pretreatments. Biochem. Eng. J. 2021, 175, 108140. [Google Scholar] [CrossRef]
- Ramírez-Partida, A.E.; García-Cayuela, T.; Amador-Castro, L.F.; Alper, H.S.; Carrillo-Nieves, D. Towards a biorefinery processing Sargassum seaweed: Techno-economic assessment of alginate and fucoidan production through SuperPro Designer® process simulation. Environ. Technol. Innov. 2024, 34, 103587. [Google Scholar] [CrossRef]
- Gordillo-Sierra, A.R.G.; Amador-Castro, L.F.; Ramírez-Partida, A.E.; García-Cayuela, T.; Carrillo-Nieves, D.; Alper, H.S. Valorization of Caribbean Sargassum biomass as a source of alginate and sugars for de novo biodiesel production. J. Environ. Manag. 2022, 324, 116364. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Inc. Alginate Market Size, Share & Trends Analysis Report by Type (High M, High G), by Product (Sodium, Propylene Glycol), By Application (Pharmaceutical, Industrial), by Region, and Segment Forecasts, 2021–2028 (No. GVR-2-68038-244-0). 2021. Available online: https://www.grandviewresearch.com/industry-analysis/alginate-market. (accessed on 30 September 2024).
- Sari-Chmayssem, N.; Taha, S.; Mawlawi, H.; Guégan, J.P.; Jeftić, J.; Benvegnu, T. Extracted and depolymerized alginates from brown algae Sargassum vulgare of Lebanese origin: Chemical, rheological, and antioxidant properties. J. Appl. Phycol. 2016, 28, 1915–1929. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef]
- Nogueira, M.T.; Chica, L.R.; Yamashita, C.; Nunes, N.S.S.; Moraes, I.C.F.; Branco, C.C.Z.; Branco, I.G. Optimal conditions for alkaline treatment of alginate extraction from the brown seaweed Sargassum cymosum C. Agardh by response surface methodology. Appl. Food Res. 2022, 2, 100141. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave assisted step-by-step process for the production of fucoidan, alginate sodium, sugars and biochar from Ascophyllum nodosum through a biorefinery concept. Bioresour. Technol. 2015, 198, 819–827. [Google Scholar] [CrossRef]
- Gravdahl, M.; Aarstad, O.; Petersen, A.; Karlsen, S.; Donati, I.; Czjzek, M.; Alexander, O.; Åstrand, H.; Rye, P.D.; Tøndervik, A.; et al. A Chemo-Enzymatic Method for Preparation of Saturated Oligosaccharides from Alginate and Other Uronic Acid-Containing Polysaccharides. Carbohydr. Polym. 2024, 343, 122487. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Lu, C.; Zhou, X.; Chen, L.; Qiu, C.; Long, J.; Qiu, C.; Jin, Z.; Long, J. Advances in alginate lyases and the potential application of enzymatic prepared alginate oligosaccharides: A mini review. Int. J. Biol. Macromol. 2024, 260 Pt 1, 129506. [Google Scholar] [CrossRef] [PubMed]
- Agabo-García, C.; Romero-García, L.I.; Álvarez-Gallego, C.J.; Blandino, A. Valorisation of the invasive alga Rugulopteryx okamurae through the production of monomeric sugars. Appl. Microbiol. Biotechnol. 2023, 107, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Liu, C.; Zhang, C.; He, X.; Wang, H.; Peng, W.; Zheng, C. Trichoderma species from plant and soil: An excellent resource for biosynthesis of terpenoids with versatile bioactivities. J. Adv. Res. 2023, 49, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Salwan, R.; Sharma, V. Extracellular proteins of Trichoderma and their role in plant health. S. Afr. J. Bot. 2022, 147, 359–369. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Yu, D.; Lin, H.; Shen, Q.; Zhao, Y. Excellent waste biomass-degrading performance of Trichoderma asperellum T-1 during submerged fermentation. Sci. Total Environ. 2017, 31, 1329–1339. [Google Scholar] [CrossRef]
- Zheng, F.; Han, T.; Basit, A.; Liu, J.; Miao, T.; Jiang, W. Whole-genome sequence and comparative analysis of Trichoderma asperellum ND-1 reveal its unique enzymatic system for efficient biomass degradation. Catalysts 2022, 12, 437. [Google Scholar] [CrossRef]
- Vargas-Hoyos, H.A.; Gilchrist-Ramelli, E. Producción de enzimas hidrolíticas y actividad antagónica de Trichoderma asperellum sobre dos cepas de Fusarium aisladas de cultivos de tomate (Solanum lycopersicum). Sci. Fungorum 2015, 42, 9–16. [Google Scholar]
- Agabo-García, C.; Sarafidou, M.; Psaki, O.; Argeiti, C.; Blandino, A.; Álvarez-Gallego, C.; Romero-García, L.I.; Koutinas, A. Bioprocess development for the production of poly (3-hydroxybutyrate) using the invasive macroalgae Rugulopteryx okamurae. Environ. Technol. Innov. 2025, 40, 104342. [Google Scholar] [CrossRef]
- Romero-Vargas, A.; Cala, K.; Blandino, A.; Díaz, A.B. Bioconversion of the invasive seaweed Rugulopteryx okamurae into enzymes and polyhydroxyalkanoates. Algal Res. 2024, 81, 103587. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, Y.; Long, S.; Feng, S.; Jia, X.; Hu, Y.; Maomao, M.; Jingxin, L.; Zeng, B. Aspergillus oryzae as a cell factory: Research and applications in industrial production. J. Fungi 2024, 10, 248. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae–Ancient microbial resource with importance in modern food industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, E.; Benoit, I.; van den Brink, J.; Wiebenga, A.; Coutinho, P.M.; Henrissat, B.; de Vries, R.P. Carbohydrate-active enzymes from the zygomycete fungus Rhizopus oryzae: A highly specialized approach to carbohydrate degradation depicted at genome level. BMC Genom. 2011, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Vellozo-Echevarría, T.; Barrett, K.; Vuillemin, M.; Meyer, A.S. Mini-Review: The distinct carbohydrate active enzyme secretome of Rhizopus spp. represents fitness for mycelium remodeling and solid-state plant food fermentation. ACS Omega 2024, 9, 34185–34195. [Google Scholar] [CrossRef] [PubMed]
- Villasante, J.; Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Heredia-Olea, E.; Almajano, M. Extrusion and solid-state fermentation with Aspergillus oryzae on the phenolic compounds and radical scavenging activity of pecan nut (Carya illinoinensis) shell. Br. Food J. 2021, 123, 4367–4382. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Koteshwara, A.; Philip, N.V.; Aranjani, J.M.; Hariharapura, R.C.; Mallikarjuna, S.V. A set of simple methods for detection and extraction of laminarinase. Sci. Rep. 2021, 11, 2489. [Google Scholar] [CrossRef]
- APHA-AWWA-WPCF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J.; et al. Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef]
- Putra, V.G.P.; Mutiarahma, S.; Chaniago, W.; Rahmadi, P.; Kurnianto, D.; Hidayat, C.; Carrera, C.; Palma, M.; Setyaningsih, W. An ultrasound-based technique for the analytical extraction of phenolic compounds in red algae. Arab. J. Chem. 2022, 15, 103597. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, G.G.; Barbero, C.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 15, 23–32. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef]
- Tonon, T.; Machado, C.B.; Webber, M.; Webber, D.; Smith, J.; Pilsbury, A.; Allen, M.J. Biochemical and elemental composition of pelagic Sargassum biomass harvested across the Caribbean. Phycology 2022, 2, 204–215. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Zeng, W.; Chen, R.; Lin, D.; Li, G.; Liang, H. Bacterial-algae biofilm enhance MABR adapting a wider COD/N ratios wastewater: Performance and mechanism. Sci. Total Environ. 2021, 781, 146663. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. The effect of seasonal variation on biomethane production from seaweed and on application as a gaseous transport biofuel. Bioresour. Technol. 2016, 209, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, Q.; Han, X.; Du, Y.; Jiang, Y.; Yan, X.; Cui, Y.; Kang, W.; Meng, L.; Cao, Z. Unleashing the potential of short-chain fatty acids: Alginate-degrading consortium boosts anaerobic sludge fermentation. J. Environ. Chem. Eng. 2024, 12, 112178. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Narsico, J.; Kobayashi, T.; Inoue, A.; Ojima, T. Production of poly (3-hydroyxybutylate) by a novel alginolytic bacterium Hydrogenophaga sp. strain UMI-18, using alginate as a sole carbon source. J. Biosci. Bioeng. 2019, 128, 203–208. [Google Scholar] [CrossRef]
- Fagundo-Mollineda, A.; Robledo, D.; Vásquez-Elizondo, R.M.; Freile-Pelegrín, Y. Antioxidant activities in holopelagic Sargassum species from the Mexican Caribbean: Temporal changes and intra-thallus variation. Algal Res. 2023, 76, 103289. [Google Scholar] [CrossRef]
- Fernández-Medina, P.; Álvarez-Gallego, C.J.; Caro, I. Yield evaluation of enzyme hydrolysis and dark fermentation of the brown seaweed Rugulopteryx okamurae hydrothermally pretreated by microwave irradiation. J. Environ. Chem. Eng. 2022, 10, 108817. [Google Scholar] [CrossRef]
- Sharma, P.; Melkania, U. Impact of furan derivatives and phenolic compounds on hydrogen production from the organic fraction of municipal solid waste using co-culture of Enterobacter aerogenes and E. coli. Bioresour. Technol. 2017, 239, 49–56. [Google Scholar] [CrossRef]
- Fernandes-Qualhato, T.; Alvares Cardoso Lopes, F.; Stecca Steindorff, A.; Silva Brandão, R.; Santos, R.; Jesuino, A.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: Evaluation of antagonism and hydrolytic enzyme production. Biotechnol. Lett. 2013, 35, 1461–1468. [Google Scholar] [CrossRef]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From isolation and structural characterization, to the evaluation of their antidiabetic and anticancer potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef]
- Borisova, A.S.; Pihlajaniemi, V.; Kont, R.; Niemelä, K.; Koitto, T.; Mikkelson, A.; Väljamäe, P.; Kristiina Kruus, K.; Marjamaa, K. The effect of soluble phenolic compounds from hydrothermally pretreated wheat straw on Trichoderma reesei cellulases and commercial enzyme cocktails. Biomass Convers. Biorefinery 2024, 14, 971–984. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzyme Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, A.V.; Khilare, C.J. RP-HPLC profile of major phenolics from brown marine macro algae. J. Appl. Pharm. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Kashegari, A.T.; Shalabi, A.A.; Darwish, K.M.; El-Halawany, A.M.; Algandaby, M.M.; Ibrahim, S.R.M.; Abdel-Naim, A.B.; Koshak, A.E.; Mohamed, G.; et al. Phenolics from Chrozophora oblongifolia aerial parts as inhibitors of α-glucosidases and advanced glycation end products: In-vitro assessment, molecular docking and dynamics studies. Biology 2022, 11, 762. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol 2020, 10, 604923. [Google Scholar] [CrossRef]
- Vaghela, P.; Trivedi, K.; Anand, K.V.; Brahmbhatt, H.; Nayak, J.; Khandhediya, K.; Ghosh, A. Scientific basis for the use of minimally processed homogenates of Kappaphycus alvarezii (red) and Sargassum wightii (brown) seaweeds as crop biostimulants. Algal Res. 2023, 70, 102969. [Google Scholar] [CrossRef]
- Darko, C.N.S.; Premarathna, A.D.; Humayun, S.; Agyei-Tuffour, B.; Goosen, N.J.; Tuvikene, R. Physico- and biochemical properties of alginates extracted from Ecklonia maxima and Sargassum fluitans using a simple cascade process. J. Appl. Phycol. 2024, 36, 661–674. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate Production from Marine Macroalgae, with Emphasis on Kelp Farming. In Alginates and Their Biomedical Applications; Series in Biomaterials Science and Engineering; Rehm, B., Moradali, M., Eds.; Springer: Singapore, 2018; Chapter 2; pp. 27–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).