Characterization of SCOBY and Lactiplantibacillus plantarum ELB90 Fermented Coffee Kombucha from Different Coffee Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Fermented Coffee Beverages
2.3. pH, Titratable Acidity (TA%), Organic Acid Determination
2.4. Enumeration of Lactic Acid Bacteria (LAB), Acetic Acid Bacteria (AAB), and Yeast
2.5. Bioactive Compound Analysis
2.5.1. Extraction of Free and Bound Phenolics
2.5.2. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC), and Antioxidant Activity Assays
2.5.3. HPLC Analysis of Phenolics
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Fermented Coffee Drinks
3.2. Lactic Acid Bacteria (LAB), Acetic Acid Bacteria (AAB), and Yeast Counts
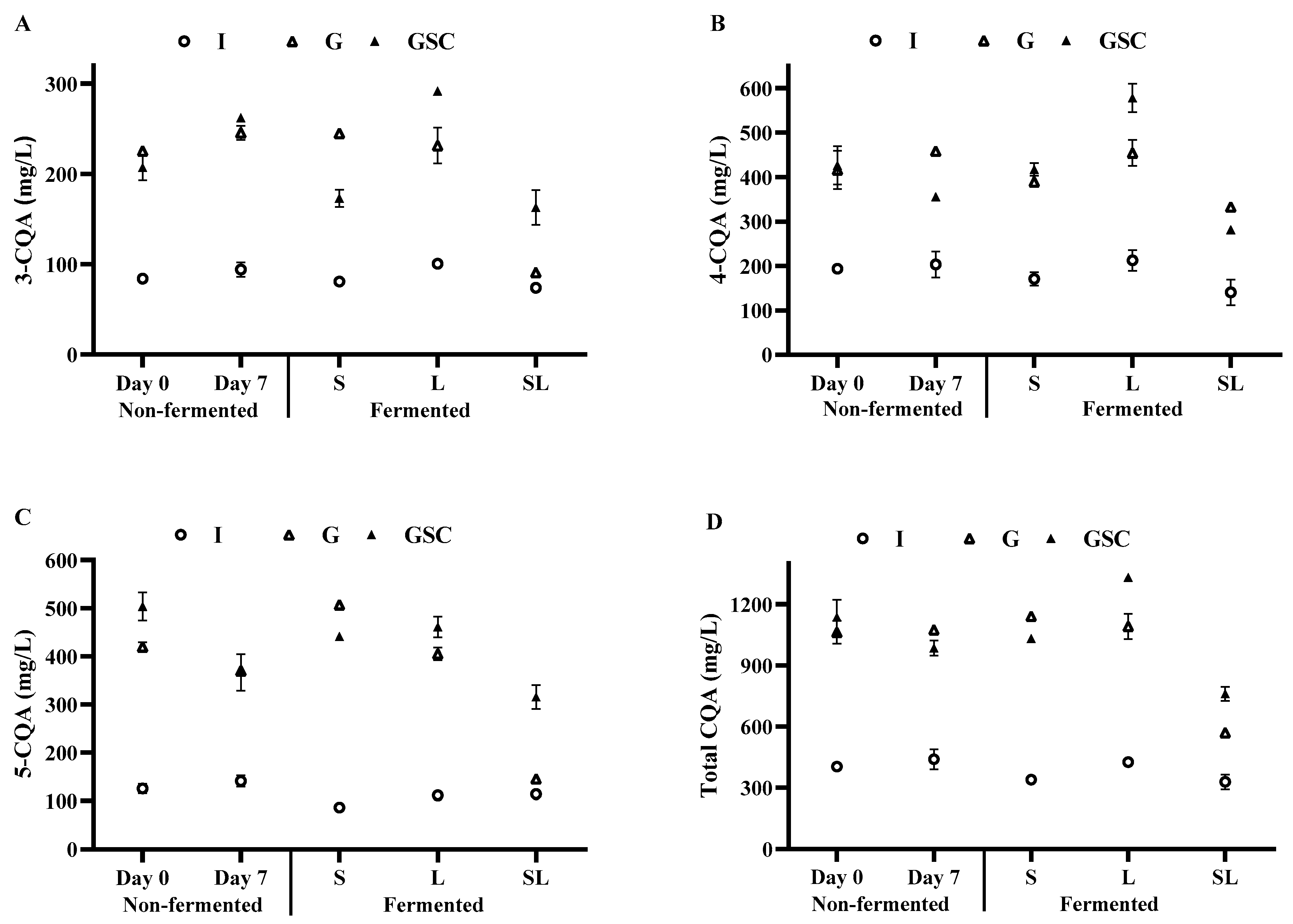
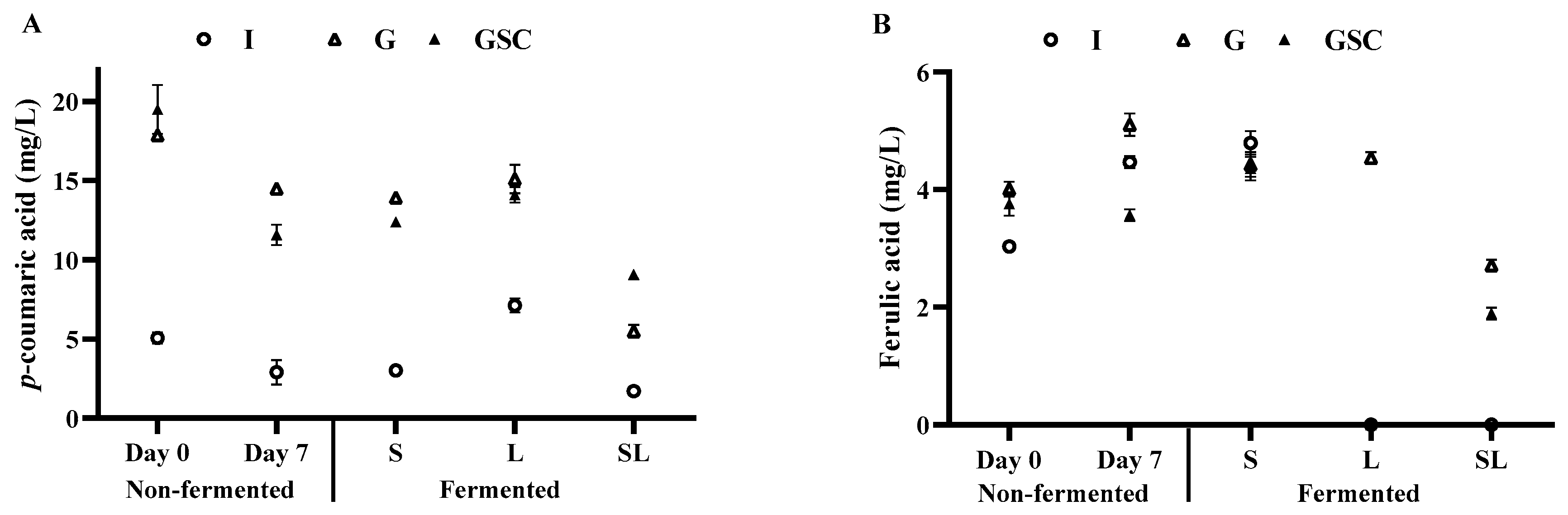
3.3. TPC, TFC, Antioxidant Activities, Caffeine, and Phenolic Constituents of Fermented Coffee Drinks
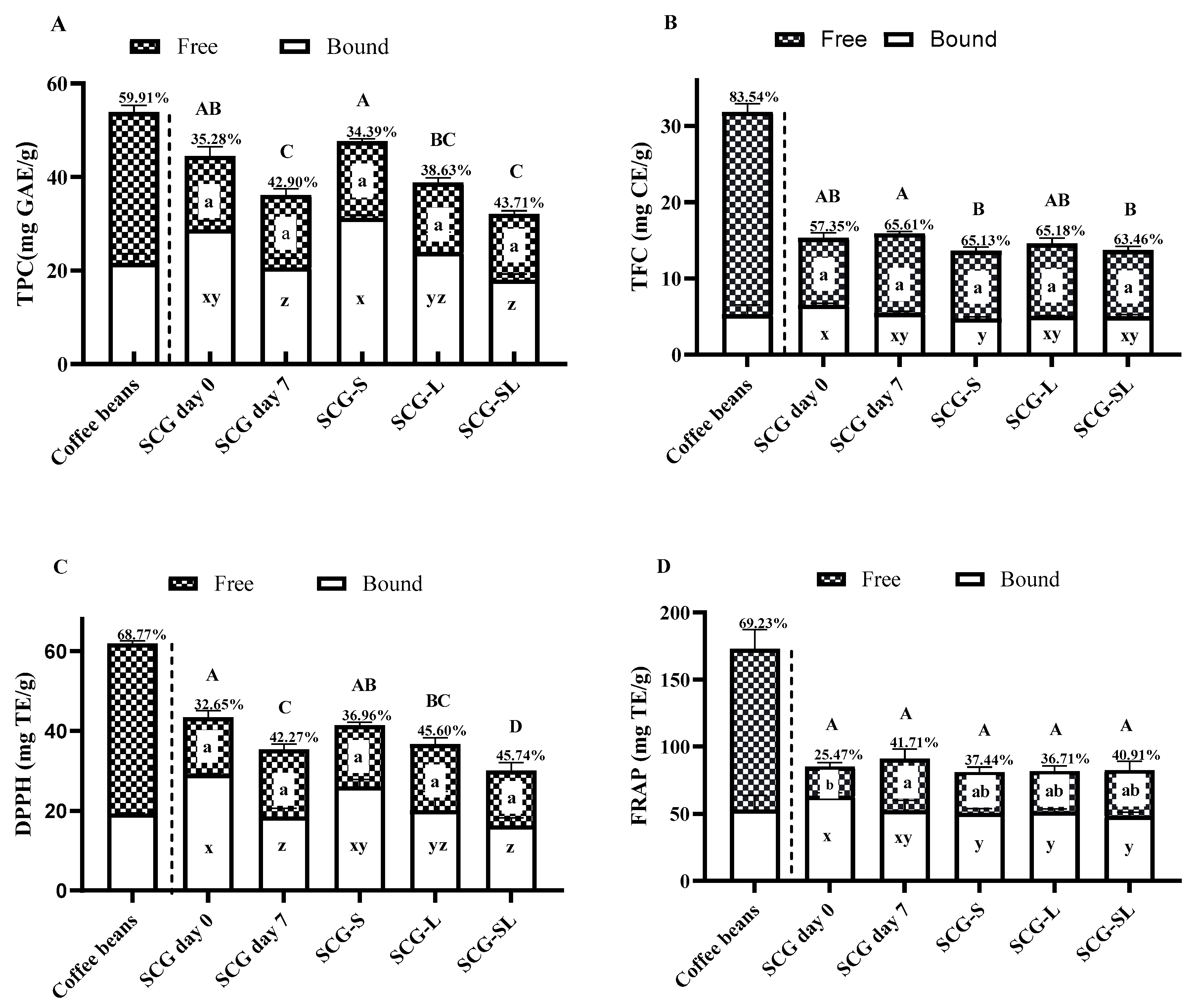
3.4. TPC, TFC, Antioxidant Activities, Caffeine, and Phenolic Constituents of SCG
3.5. Sensory Properties of Fermented Coffee Drinks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, K.; Curtin, C. Microbial Composition of SCOBY Starter Cultures Used by Commercial Kombucha Brewers in North America. Microorganisms 2021, 9, 1060. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbial Dynamics between Yeasts and Acetic Acid Bacteria in Kombucha: Impacts on the Chemical Composition of the Beverage. Foods 2020, 9, 963. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Yang, T.; Mac Regenstein, J.; Zhou, P. Functional Properties and Sensory Characteristics of Kombucha Analogs Prepared with Alternative Materials. Trends Food Sci. Technol. 2022, 129, 608–616. [Google Scholar] [CrossRef]
- Sanwal, N.; Gupta, A.; Bareen, M.A.; Sharma, N.; Sahu, J.K. Kombucha Fermentation: Recent Trends in Process Dynamics, Functional Bioactivities, Toxicity Management, and Potential Applications. Food Chem. Adv. 2023, 3, 100421. [Google Scholar] [CrossRef]
- Freitas, A.; Sousa, P.; Wurlitzer, N. Alternative Raw Materials in Kombucha Production. Int. J. Gastron. Food Sci. 2022, 30, 100594. [Google Scholar] [CrossRef]
- Wu, H.; Gu, J.; BK, A.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of Processing on Bioaccessibility and Bioavailability of Bioactive Compounds in Coffee Beans. Food Biosci. 2022, 46, 101373. [Google Scholar] [CrossRef]
- Min, J.; Cao, Z.; Cui, L.; Li, F.; Lu, Z.; Hou, Y.; Yang, H.; Wang, X.; Xu, C. The Association between Coffee Consumption and Risk of Incident Depression and Anxiety: Exploring the Benefits of Moderate Intake. Psychiatry Res. 2023, 326, 115307. [Google Scholar] [CrossRef] [PubMed]
- Monente, C.; Ludwig, I.A.; Irigoyen, A.; De Peña, M.-P.; Cid, C. Assessment of Total (Free and Bound) Phenolic Compounds in Spent Coffee Extracts. J. Agric. Food Chem. 2015, 63, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.S.; Mertz, C.; Morel, G.; Lacour, S.; Belleville, M.-P.; Durand, N.; Dornier, M. Alcoholic Fermentation as a Potential Tool for Coffee Pulp Detoxification and Reuse: Analysis of Phenolic Composition and Caffeine Content by HPLC-DAD-MS/MS. Food Chem. 2020, 319, 126600. [Google Scholar] [CrossRef]
- Santos da Silveira, J.; Durand, N.; Lacour, S.; Belleville, M.-P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-State Fermentation as a Sustainable Method for Coffee Pulp Treatment and Production of an Extract Rich in Chlorogenic Acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- Milić, M.D.; Buntić, A.V.; Mihajlovski, K.R.; Ilić, N.V.; Davidović, S.Z.; Dimitrijević-Branković, S.I. The Development of a Combined Enzymatic and Microbial Fermentation as a Viable Technology for the Spent Coffee Ground Full Utilization. Biomass Convers. Biorefinery 2023, 13, 6747–6759. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Liu, S.-Q. Coffee Brews as Food Matrices for Delivering Probiotics: Opportunities, Challenges, and Potential Health Benefits. Trends Food Sci. Technol. 2022, 119, 227–242. [Google Scholar] [CrossRef]
- Bueno, F.; Chouljenko, A.; Sathivel, S. Development of Coffee Kombucha Containing Lactobacillus rhamnosus and Lactobacillus casei: Gastrointestinal Simulations and DNA Microbial Analysis. LWT 2021, 142, 110980. [Google Scholar] [CrossRef]
- Ferreira de Miranda, J.; Martins Pereira Belo, G.; Silva de Lima, L.; Alencar Silva, K.; Matsue Uekane, T.; Gonçalves Martins Gonzalez, A.; Naciuk Castelo Branco, V.; Souza Pitangui, N.; Freitas Fernandes, F.; Ribeiro Lima, A. Arabic Coffee Infusion Based Kombucha: Characterization and Biological Activity during Fermentation, and in Vivo Toxicity. Food Chem. 2023, 412, 135556. [Google Scholar] [CrossRef]
- Pavlović, M.O.; Stajić, M.; Gašić, U.; Duletić-Laušević, S.; Ćilerdžić, J. The Chemical Profiling and Assessment of Antioxidative, Antidiabetic and Antineurodegenerative Potential of Kombucha Fermented Camellia sinensis, Coffea arabica and Ganoderma lucidum Extracts. Food Funct. 2023, 14, 262–276. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the Functional Properties of Coffee Through Fermentation by “Tea Fungus” (Kombucha). J. Food Process. Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Zofia, N.-Ł.; Aleksandra, Z.; Tomasz, B.; Martyna, Z.-D.; Magdalena, Z.; Zofia, H.-B.; Tomasz, W. Effect of Fermentation Time on Antioxidant and Anti-Ageing Properties of Green Coffee Kombucha Ferments. Molecules 2020, 25, 5394. [Google Scholar] [CrossRef]
- Fibrianto, K.; Zubaidah, E.; Muliandari, N.A.; Wahibah, L.Y.; Putri, S.D.; Legowo, A.M.; Al-Baarri, A.N. Antioxidant Activity Optimisation of Young Robusta Coffee Leaf Kombucha by Modifying Fermentation Time and Withering Pre-Treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 12029. [Google Scholar] [CrossRef]
- Zubaidah, E.; Iastika, A.R.; Widyaningsih, T.D.; Febrianto, K. Immunomodulatory Activity of Black Tea Kombucha (Camellia sinensis) and Arabica Coffee Leaves Tea Kombucha (Coffee arabica) for Salmonella Typhi-Infected Mice. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 12128. [Google Scholar] [CrossRef]
- Muzaifa, M.; Andini, R.; Sulaiman, M.I.; Abubakar, Y.; Rahmi, F. Nurzainura Novel Utilization of Coffee Processing By-Products: Kombucha Cascara Originated from ‘Gayo-Arabica’. IOP Conf. Ser. Earth Environ. Sci. 2021, 644, 12048. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Arici, M. Effect of the Fermentation Temperature on the Degradation of Phytic Acid in Whole-Wheat Sourdough Bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea—Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Bozkurt, F.; Bekiroglu, H.; Sagdic, O. Use of Principal Component Analysis and Cluster Analysis for Differentiation of Traditionally-Manufactured Vinegars Based on Phenolic and Volatile Profiles, and Antioxidant Activity. Pol. J. Food Nutr. Sci. 2020, 70, 347–360. [Google Scholar] [CrossRef]
- Ozkan, K.; Karadag, A.; Sagdic, O.; Ozcan, F.S.; Ozer, H. The Effects of Different Drying Methods on the Sugar, Organic Acid, Volatile Composition, and Textural Properties of Black ‘Isabel’ Grape. J. Food Meas. Charact. 2023, 17, 1852–1861. [Google Scholar] [CrossRef]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A.R. Impact of Roasting on the Phenolic and Volatile Compounds in Coffee Beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef]
- de Almeida, S.S.; da Costa, G.B.M.; Barreto, M.S.; Freire, D.M.G.; Lobo, L.A.; Domingues, R.M.C.P.; Moura-Nunes, N.; Monteiro, M.; Perrone, D. Bioaccessibility and Gut Metabolism of Phenolic Compounds of Breads Added with Green Coffee Infusion and Enzymatically Bioprocessed. Food Chem. 2020, 333, 127473. [Google Scholar] [CrossRef]
- Yener, E.; Saroglu, O.; Sagdic, O.; Karadag, A. The Effects of Different Drying Methods on the In Vitro Bioaccessibility of Phenolics, Antioxidant Capacity, and Morphology of European Plums (Prunes domestica L.). ACS Omega 2024, 9, 12711–12724. [Google Scholar] [CrossRef]
- Ozcan, B.E.; Saroglu, O.; Karakas, C.Y.; Karadag, A. Encapsulation of Purple Basil Leaf Extract by Electrospraying in Double Emulsion (W/O/W) Filled Alginate-Carrageenan Beads to Improve the Bioaccessibility of Anthocyanins. Int. J. Biol. Macromol. 2023, 250, 126207. [Google Scholar] [CrossRef]
- Zhi, R.; Zhao, L.; Shi, J. Improving the Sensory Quality of Flavored Liquid Milk by Engaging Sensory Analysis and Consumer Preference. J. Dairy Sci. 2016, 99, 5305–5317. [Google Scholar] [CrossRef]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in Kombucha Tea Fermentation: A Review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Bellumori, M.; Angeloni, G.; Guerrini, L.; Masella, P.; Calamai, L.; Mulinacci, N.; Parenti, A.; Innocenti, M. Effects of Different Stabilization Techniques on the Shelf Life of Cold Brew Coffee: Chemical Composition, Flavor Profile and Microbiological Analysis. LWT 2021, 142, 111043. [Google Scholar] [CrossRef]
- Andreson, M.; Kazantseva, J.; Kuldjärv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.-L.; Vilu, R. Characterisation of Chemical, Microbial and Sensory Profiles of Commercial Kombuchas. Int. J. Food Microbiol. 2022, 373, 109715. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and Microbiological Impacts on the Chemical and Flavor Profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Barbosa, C.D.; Trovatti Uetanabaro, A.P.; Rodrigues Santos, W.C.; Caetano, R.G.; Albano, H.; Kato, R.; Cosenza, G.P.; Azeredo, A.; Góes-Neto, A.; Rosa, C.A.; et al. Microbial–Physicochemical Integrated Analysis of Kombucha Fermentation. LWT 2021, 148, 111788. [Google Scholar] [CrossRef]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in Content of Organic Acids and Tea Polyphenols during Kombucha Tea Fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling Microbial Ecology of Industrial-Scale Kombucha Fermentations by Metabarcoding and Culture-Based Methods. FEMS Microbiol. Ecol. 2017, 93, 48. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-Based Analysis of the Bacterial and Fungal Compositions of Multiple Kombucha (Tea Fungus) Samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, X.; Xiao, Y.; Sheng, Q.; Tu, L.; Chen, F.; Yan, Y.; Zheng, Y.; Wang, M. Interaction of Acetic Acid Bacteria and Lactic Acid Bacteria in Multispecies Solid-State Fermentation of Traditional Chinese Cereal Vinegar. Front. Microbiol. 2022, 13, 964855. [Google Scholar] [CrossRef]
- Gobbetti, M. The Sourdough Microflora: Interactions of Lactic Acid Bacteria and Yeasts. Trends Food Sci. Technol. 1998, 9, 267–274. [Google Scholar] [CrossRef]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic Activity and Symbiotic Interactions of Lactic Acid Bacteria and Yeasts Isolated from Water Kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the Mechanisms of Positive Microbial Interactions That Benefit Lactic Acid Bacteria Co-Cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C. Antioxidant Properties of Ready-to-Drink Coffee Brews. J. Agric. Food Chem. 2003, 51, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant Activity, Polyphenols, Caffeine and Melanoidins in Soluble Coffee: The Influence of Processing Conditions and Raw Material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of Coffee Roasting on the Incorporation of Phenolic Compounds into Melanoidins and Their Relationship with Antioxidant Activity of the Brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Ashihara, H.; Crozier, A. Caffeine: A Well Known but Little Mentioned Compound in Plant Science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [CrossRef]
- Purwoko, T.; Suranto; Setyaningsih, R.; Marliyana, S.D. Chlorogenic Acid and Caffeine Content of Fermented Robusta Bean. Biodiversitas 2022, 23, 902–906. [Google Scholar] [CrossRef]
- Wang, X.; Wan, X.; Hu, S.; Pan, C. Study on the Increase Mechanism of the Caffeine Content during the Fermentation of Tea with Microorganisms. Food Chem. 2008, 107, 1086–1091. [Google Scholar] [CrossRef]
- Kučera, L.; Papoušek, R.; Kurka, O.; Barták, P.; Bednář, P. Study of Composition of Espresso Coffee Prepared from Various Roast Degrees of Coffea arabica L. Coffee Beans. Food Chem. 2016, 199, 727–735. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic Compounds in Coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Macheiner, L.; Schmidt, A.; Mayer, H.K. A Novel Basis for Monitoring the Coffee Roasting Process: Isomerization Reactions of 3-Caffeoylquinic and 4-Caffeoylquinic Acids. LWT 2021, 152, 112343. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and Functional Paths of Lactic Acid Bacteria in Plant Foods: Get out of the Labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Maksimowski, D.; Oziembłowski, M.; Kolniak-Ostek, J.; Stach, M.; Zubaidi, M.A.; Nawirska-Olszańska, A. Effect of Cold Brew Coffee Storage in Industrial Production on the Physical-Chemical Characteristics of Final Product. Foods 2023, 12, 3840. [Google Scholar] [CrossRef] [PubMed]

| I | G | GSC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Fermented | Fermented | Non-Fermented | Fermented | Non-Fermented | Fermented | |||||||||||
| Unit | Day 0 | Day 7 | S | L | SL | Day 0 | Day 7 | S | L | SL | Day 0 | Day 7 | S | L | SL | |
| Acetic Acid | mg/L | - | - | 1425.26 ± 15.93 a,x | 193.22 ± 4.82 c,y | 1314.43 ± 39.61 b,y | - | - | 1493.02 ± 13.67 b,x | 336.98 ± 25.72 c,x | 1683.53 ± 64.18 a,x | - | - | 1488.34 ± 117.66 a,x | 241.85 ± 0.74 c,y | 1399.13 ± 23.25 b,y |
| Lactic Acid | - | - | nd | 583.31 ± 5.84 a,x | 378.69 ± 70.45 b,y | - | - | nd | 502.44 ± 12.47 b,xy | 584.62 ± 92.48 a,x | - | - | nd | 448.74 ± 16.45 a,y | 320.67 ± 31.66 b,y | |
| Caffeine | 961.1 ± 40.18 b,y | 1058.55 ± 70.09 a,x | 941.70 ± 4.27 b,z | 841.41 ± 0.72 c,z | 883.08 ± 0.11 bc,x | 1137.72 ± 13.76 a,x | 1022.83 ± 6.8 b,xy | 1176.50 ± 9.39 a,x | 1137.14 ± 44.59 a,y | 754.66 ± 1.33 c,y | 1087.72 ± 51.62 b,x | 967.0 ± 39.58 c,y | 1102.2 ± 25.67 b,y | 1226.82 ± 41.06 a,x | 733.39 ± 6.92 d,y | |
| TPC | mg GAE/mL | 2.54 ± 0.47 a,y | 1.18 ± 0.03 b,x | 1.31 ± 0.11 b,y | 1.06 ± 0.23 b,z | 2.30 ± 0.14 a,x | 2.48 ± 0.26 ab,y | 1.14 ± 0.13 c,x | 1.58 ± 0.15 c,y | 2.10 ± 0.20 b,y | 2.70 ± 0.13 a,x | 3.27 ± 0.11 a,x | 1.15 ± 0.04 c,x | 2.30 ± 0.14 b,x | 2.92 ± 0.21 a,x | 2.39 ± 0.15 b,x |
| TFC | mg CE/mL | 2.16 ± 0.04 b,y | 2.05 ± 0.06 b,y | 2.17 ± 0.07 b,x | 2.58 ± 0.17 a,y | 2.21 ± 0.08 b,x | 2.56 ± 0.02 a,x | 2.30 ± 0.17 ab,xy | 2.03 ± 0.05 b,x | 2.32 ± 0.07 ab,y | 2.20 ± 0.04 b,x | 2.69 ± 0.05 b,x | 2.31 ± 0.0 c,x | 2.15 ± 0.11 c,x | 3.22 ± 0.36 a,x | 1.71 ± 0.08 d,y |
| DPPH | mg TE/mL | 3.43 ± 0.05 a,y | 3.43 ± 0.11 a,y | 3.06 ± 0.21 a,z | 2.30 ± 0.17 b,y | 3.20 ± 0.21 a,y | 2.57 ± 0.14 cd,z | 3.26 ± 0.03 b,y | 4.06 ± 0.36 a,y | 2.36 ± 0.39 d,y | 3.12 ± 0.06 bc,y | 11.50 ± 0.14 a,x | 8.78 ± 0.52 b,x | 7.24 ± 0.10 c,x | 7.74 ± 0.40 c,x | 8.93 ± 0.31 b,x |
| FRAP | 3.52 ± 0.07 bc,y | 2.89 ± 0.07 c,y | 4.54 ± 0.22 a,y | 4.47 ± 0.35 a,x | 4.24 ± 0.40 ab,y | 4.71 ± 0.51 ab,x | 3.75 ± 0.10 c,x | 5.31 ± 0.68 a,x | 3.58 ± 0.07 c,y | 4.17 ± 0.10 bc,y | 4.20 ± 0.16 b,x | 3.31 ± 0.35 c,xy | 4.73 ± 0.57 ab,xy | 4.85 ± 0.07 ab,x | 5.01 ± 0.18 a,x | |
| Samples | 5-CQA | 4-CQA | 3-CQA | Total CQA | Caffeic Acid | Ferulic Acid | p-Coumaric Acid | Caffeine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | B | F | B | F | B | F | B | F | B | F | B | F | B | |||
| Coffee beans | 549.45 ± 9.78 | nd | 978.30 ± 5.14 | nd | 417.68 ± 4.81 | nd | 1945.43 ± 9.45 | nd | nd | 213.15 ± 35.74 | 11.74 ± 1.19 | 25.74 ± 2.68 | 11.98 ± 1.36 | 2.71 ± 0.68 | 1105.81 ± 32.95 | |
| Non-fermented SCG | day 0 | 195.81 ± 8.58 a | nd | 233.14 ± 8.14 a | nd | 105.90 ± 4.30 a | nd | 534.85 ± 21.02 a | nd | nd | 242.23 ± 1.33 a | 1.52 ± 0.07 bc | 33.50 ± 0.78 a | 11.89 ± 0.51 bc | 3.84 ± 0.04 a | 271.26 ± 34.26 b |
| day 7 | 130.78 ± 10.49 c | 147.76 ± 9.50 d | 72.88 ± 5.58 b | 351.42 ± 14.76 c | 198.75 ± 1.07 b | 0.82 ± 0.14 c | 25.65 ± 0.78 b | 10.65 ± 0.86 c | 2.41 ± 0.02 c | 366.41 ± 25.96 a | ||||||
| Fermented SCG | S | 154.72 ± 5.93 b | 200.93 ± 9.35 b | 73.85 ± 3.24 b | 429.49 ± 18.52 b | 184.96 ± 3.54 b | 4.88 ± 0.17 a | 23.63 ± 1.69 b | 14.35 ± 0.41 a | 2.26 ± 0.20 c | 330.21 ± 12.22 a | |||||
| L | 151.48 ± 2.53 b | 176.00 ± 12.49 c | 73.66 ± 1.55 b | 401.15 ± 16.57 bc | 196.97 ± 3.89 b | 1.96 ± 0.43 b | 26.45 ± 0.58 b | 12.51 ± 0.34 b | 3.27 ± 0.37 b | 359.00 ± 6.44 a | ||||||
| SL | 126.43 ± 7.25 c | 241.99 ± 2.69 a | 77.10 ± 2.63 b | 445.52 ± 12.56 b | 201.70 ± 19.02 b | 4.86 ± 0.63 a | 28.17 ± 3.18 b | 11.82 ± 0.35 bc | 2.63 ± 0.19 c | 255.67 ± 5.76 b | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saroglu, O.; Irmak, Y.G.; Yildirim, R.M.; Karadag, A. Characterization of SCOBY and Lactiplantibacillus plantarum ELB90 Fermented Coffee Kombucha from Different Coffee Sources. Fermentation 2025, 11, 428. https://doi.org/10.3390/fermentation11080428
Saroglu O, Irmak YG, Yildirim RM, Karadag A. Characterization of SCOBY and Lactiplantibacillus plantarum ELB90 Fermented Coffee Kombucha from Different Coffee Sources. Fermentation. 2025; 11(8):428. https://doi.org/10.3390/fermentation11080428
Chicago/Turabian StyleSaroglu, Oznur, Yagmur Gulce Irmak, Rusen Metin Yildirim, and Ayse Karadag. 2025. "Characterization of SCOBY and Lactiplantibacillus plantarum ELB90 Fermented Coffee Kombucha from Different Coffee Sources" Fermentation 11, no. 8: 428. https://doi.org/10.3390/fermentation11080428
APA StyleSaroglu, O., Irmak, Y. G., Yildirim, R. M., & Karadag, A. (2025). Characterization of SCOBY and Lactiplantibacillus plantarum ELB90 Fermented Coffee Kombucha from Different Coffee Sources. Fermentation, 11(8), 428. https://doi.org/10.3390/fermentation11080428






