Response of Akkermansia muciniphila to Bioactive Compounds: Effects on Its Abundance and Activity
Abstract
1. Introduction
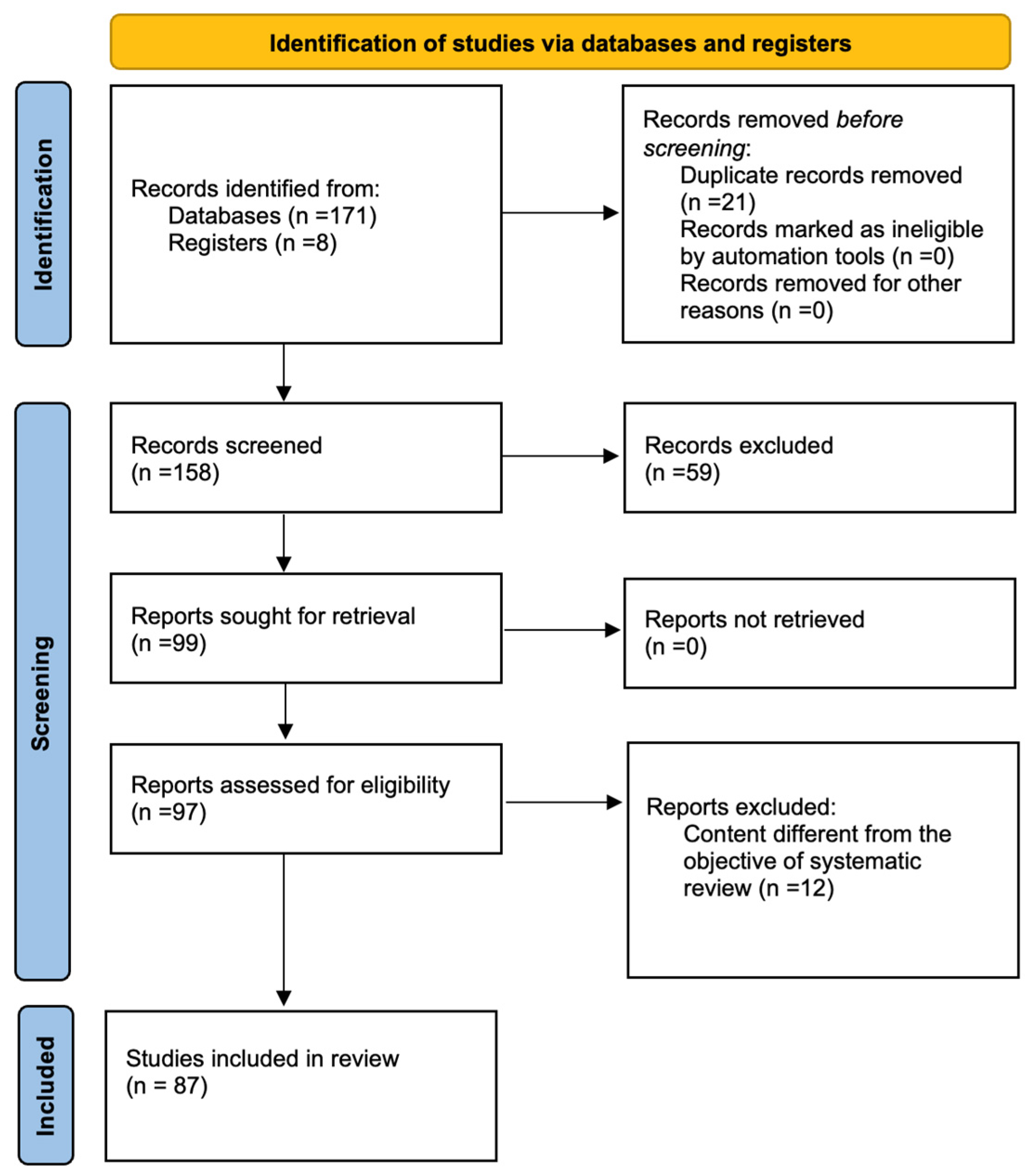
2. Materials and Methods
3. Results
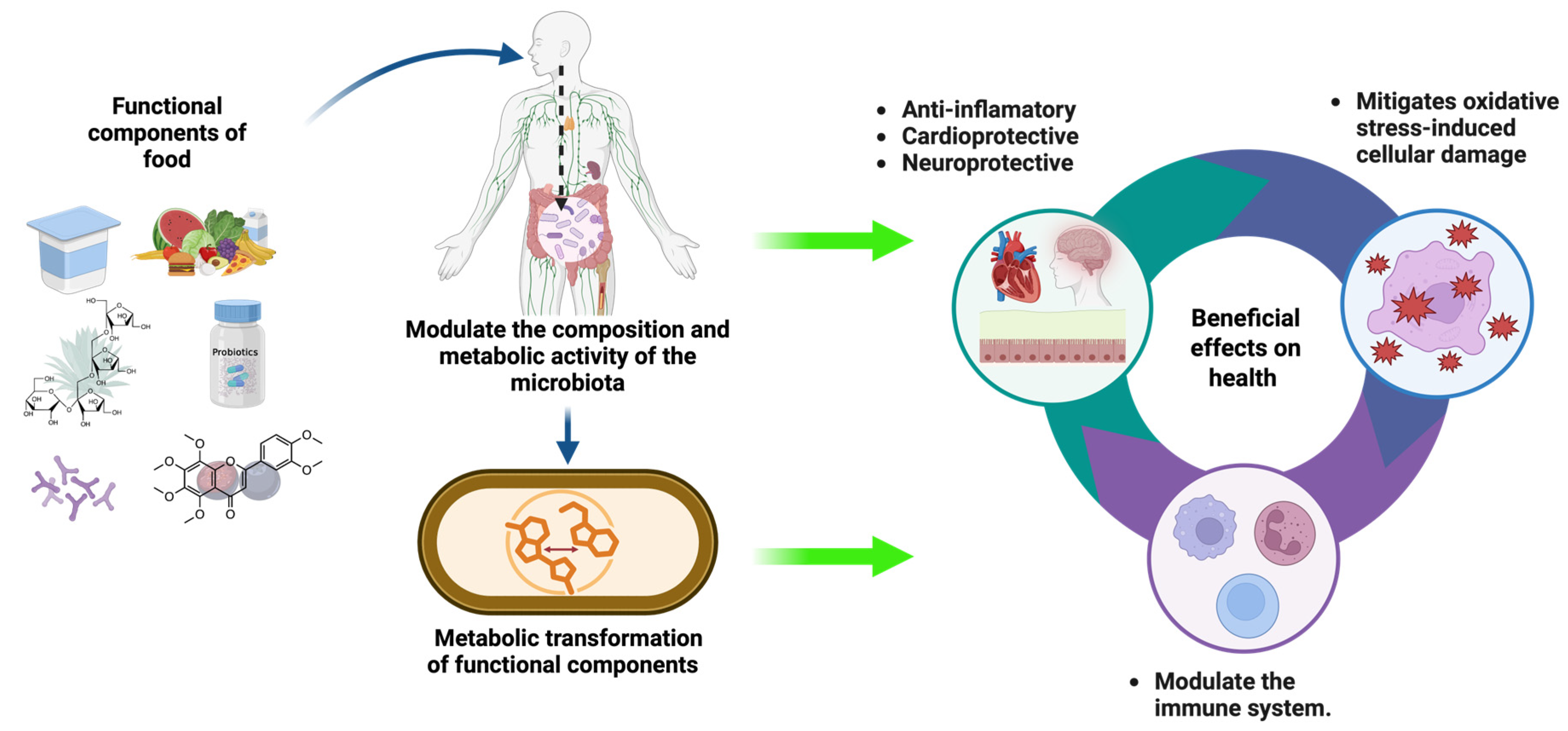
3.1. Gut Microbiota and Human Health
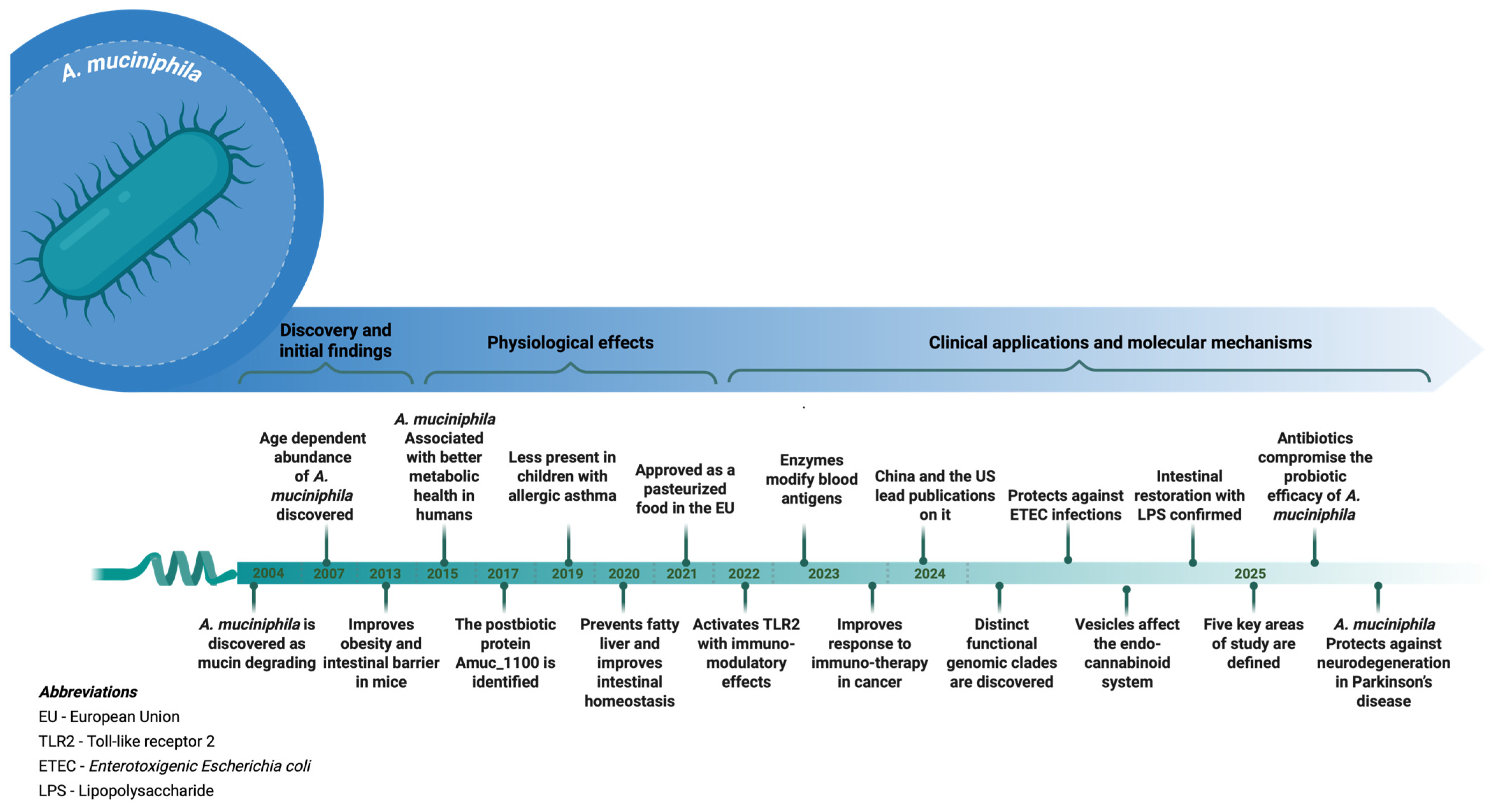
3.2. A. muciniphila: Biological and Functional Characteristics
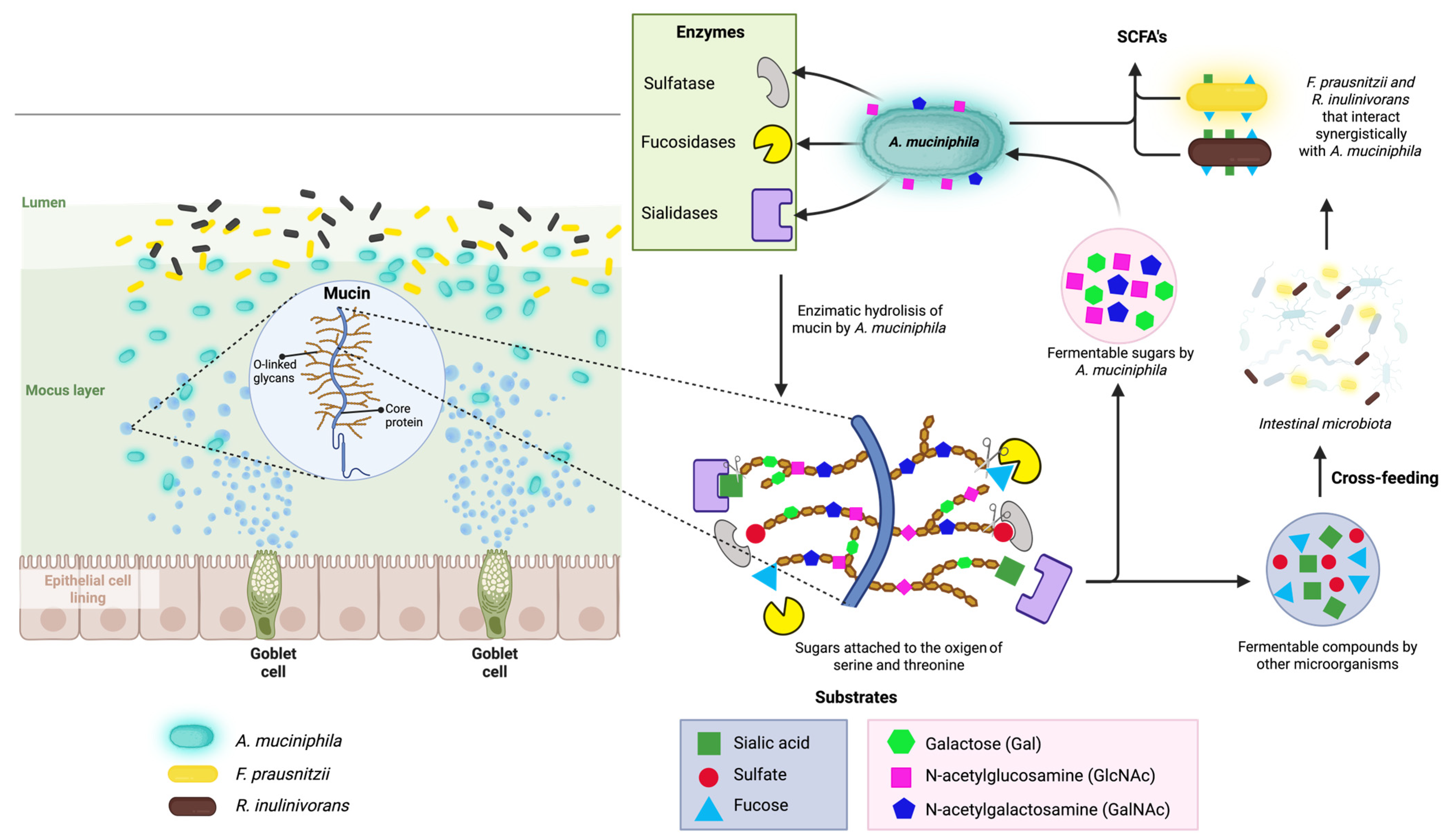
3.3. Mucin Degradation and Metabolic Role of A. muciniphila
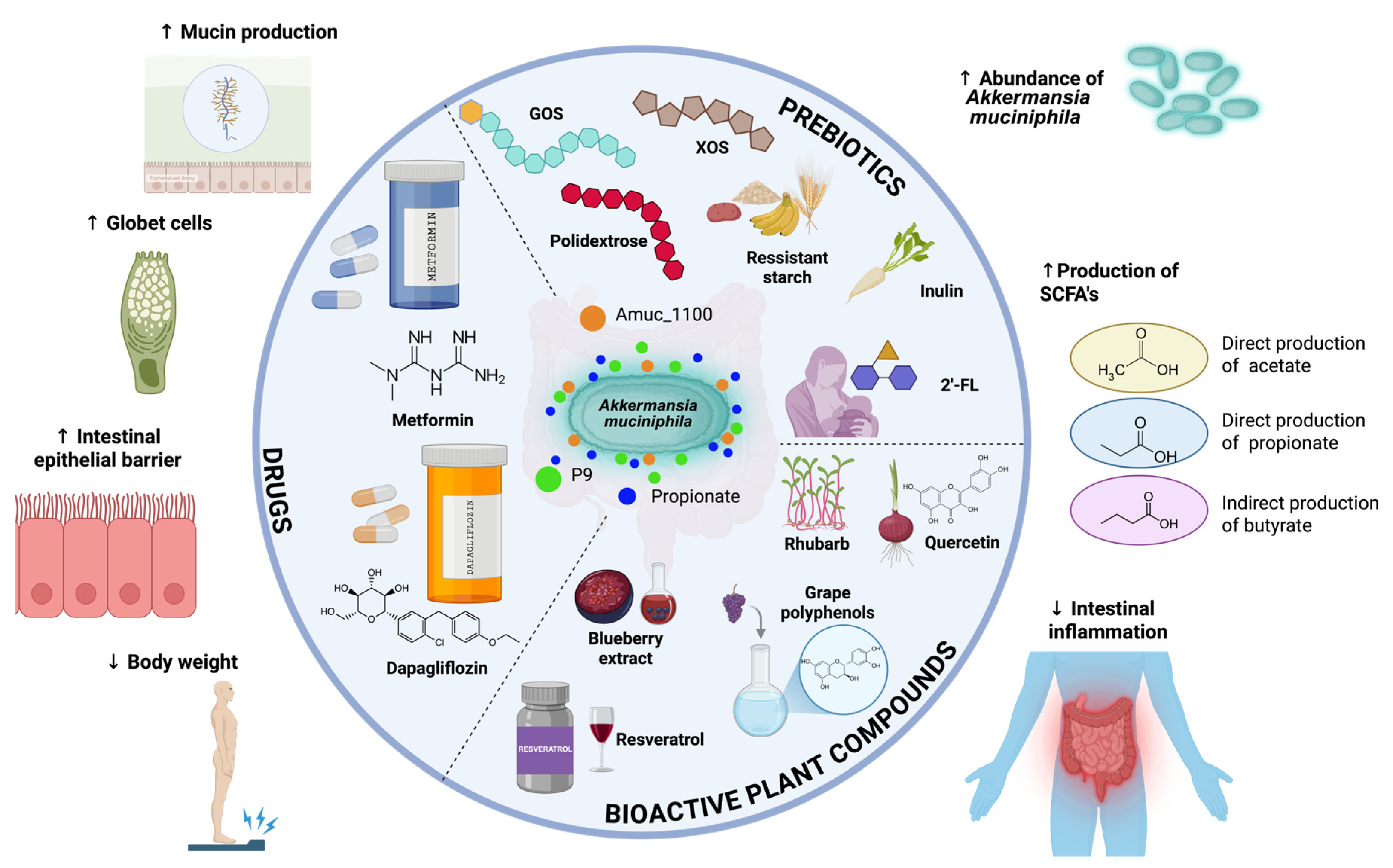
3.4. Bioactive Compounds That Modulate A. muciniphila
3.4.1. Non-Digestible Fibers
3.4.2. Plant-Derived Bioactive Compounds
3.4.3. Human Milk Oligosaccharides
3.4.4. Drugs
4. Discussion
- Acetate: A. muciniphila produces acetate through the fermentation of mucin-derived sugars. Acetate acts as a substrate for peripheral tissues and is also involved in cholesterol metabolism and lipogenesis. Furthermore, acetate can serve as a precursor for butyrate synthesis by other colonic microbes.
- Propionate: Propionate is generated via the succinate pathway in A. muciniphila. This SCFA contributes to gluconeogenesis in the liver and has been associated with satiety regulation and improved insulin sensitivity.
- Butyrate: Although not directly synthesized by A. muciniphila, its activity supports butyrate producers by supplying fermentation intermediates. Butyrate is essential for colonic epithelial health, anti-inflammatory responses, and maintaining the integrity of the gut barrier—effects indirectly reinforced by the presence of A. muciniphila.
5. Conclusions
6. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-Martínez, A.B.; Lamban-Per, B.M.; Lezaun, M.; Rezusta, A.; Arbones-Mainar, J.M. Exploring Functional Products and Early-Life Dynamics of Gut Microbiota. Nutrients 2024, 16, 1823. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; do Socorro Silva Costa, P.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia municiphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Bazzaz, S.; Da Cruz, A.G.; Khorshidian, N.; Saadat, Y.R.; Sabahi, S.; Ozma, M.A.; Lahouty, M.; Aslani, R.; Mortazavian, A.M. A Critical Review on Akkermansia muciniphila: Functional Mechanisms, Technological Challenges, and Safety Issues. Probiotics Antimicrob. Proteins 2024, 16, 1376–1398. [Google Scholar] [CrossRef] [PubMed]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Chen, M.; Liu, Y.; Zhou, Y.; Liu, H.; Wang, S.; Ji, Y. Role of Akkermansia muciniphila in insulin resistance. J. Gastroenterol. Hepatol. 2024, 40, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhou, M.; Zogona, D.; Xing, Z.; Wu, T.; Chen, R.; Cui, D.; Liang, F.; Xu, X. Akkermansia muciniphila: A potential candidate for ameliorating metabolic diseases. Front. Immunol. 2024, 15, 1370658. [Google Scholar] [CrossRef] [PubMed]
- Van Buiten, C.B.; Seitz, V.A.; Metcalf, J.L.; Raskin, I. Dietary Polyphenols Support Akkermansia muciniphila Growth via Mediation of the Gastrointestinal Redox Environment. Antioxidants 2024, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Somasundaram, I.; Das, D.; Manoj, S.J.; Banu, H.; Mitta Suresh, P.; Paul, S.; Bisgin, A.; Zhang, H.; Sun, X.-F.; et al. Functional Foods: A Promising Strategy for Restoring Gut Microbiota Diversity Impacted by SARS-CoV-2 Variants. Nutrients 2023, 15, 2631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, J.; Lin, X. Akkermansia muciniphila helps in the recovery of lipopolysaccharide-fed mice with mild intestinal dysfunction. Front. Microbiol. 2025, 16, 1523742. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- WGO (World Gastroenterology Organisation). Directrices Mundiales de la Organización Mundial de Gastroenterología; World Gastroenterology Organisation: Milwaukee, WI, USA, 2023. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chen, C.; Patil, S.; Dong, S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front. Nutr. 2024, 11, 1355542. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Chen, Y.; Yu, Z.; Cheng, P.; Yu, Z.; Cheng, W.; Zhang, W.; Wang, Z.; Gao, X.; et al. Function and therapeutic prospects of next-generation probiotic Akkermansia muciniphila in infectious diseases. Front. Microbiol. 2024, 15, 1354447. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Derrien, M.; Isolauri, E.; De Vos, W.M.; Salminen, S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Tokman, H.B.; Uysal, H.K.; Demiryas, S.; Karakullukcu, A.; Saribas, S.; Cokugras, H.; Kocazeybek, B. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol. Immunopathol. 2019, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06780. [Google Scholar] [CrossRef] [PubMed]
- Anso, I.; Naegeli, A.; Cifuente, J.O.; Orrantia, A.; Andersson, E.; Zenarruzabeitia, O.; Moraleda-Montoya, A.; García-Alija, M.; Corzana, F.; Del Orbe, R.A.; et al. Turning universal O into rare Bombay type blood. Nat. Commun. 2023, 14, 1765. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.-M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y.; et al. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Ghali, E.N.H.K.; Pranav; Chauhan, S.C.; Yallapu, M.M. Inulin-based formulations as an emerging therapeutic strategy for cancer: A comprehensive review. Int. J. Biol. Macromol. 2024, 259, 129216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Dang, S.; Liu, X.; Zhang, Y.; Zhang, H. The worldview of Akkermansia muciniphila, a bibliometric analysis. Front. Microbiol. 2025, 16, 1500893. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, M.; Zhang, Y.; Xu, T.; Zhou, X.; Qian, M.; Yang, Z.; Han, X. Akkermansia muciniphila identified as key strain to alleviate gut barrier injury through Wnt signaling pathway. Elife 2024, 12, RP92906. [Google Scholar] [CrossRef]
- Noori, P.; Sotoodehnejadnematalahi, F.; Rahimi, P.; Siadat, S.D. Akkermansia muciniphila and Its Extracellular Vesicles Affect Endocannabinoid System in in vitro Model. Digestion 2025, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cheng, C.; Li, R.; Chen, Z.; Tang, K.; Du, G. The role of Akkermansia muciniphila in maintaining health: A bibliometric study. Front. Med. 2025, 12, 1484656. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Teng, T.M.; Han, J.; Kim, H.S. Antibiotic-associated changes in Akkermansia muciniphila alter its effects on host metabolic health. Microbiome 2025, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, G.; Gong, J.; Yang, X.; Cheng, Y.; Li, D.; Sheng, S.; Zhang, F. Akkermansia muciniphila protects against dopamine neurotoxicity by modulating butyrate to inhibit microglia-mediated neuroinflammation. Int. Immunopharmacol. 2025, 152, 114374. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.G.; Song, E.J.; Yoon, H.J.; Chung, W.H.; Seo, H.Y.; Kim, D.; Lee, D.; Seo, J.-G.; Lee, H.; Kim, S.I.; et al. Clade-specific extracellular vesicles from Akkermansia muciniphila mediate competitive colonization via direct inhibition and immune stimulation. Nat. Commun. 2025, 16, 2708. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Menjivar, C.; Pagella, E.; Biswas, I. Akkermansia muciniphila. Trends Microbiol. 2024, 32, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Shuoker, B.; Pichler, M.J.; Jin, C.; Sakanaka, H.; Wu, H.; Gascueña, A.M.; Liu, J.; Nielsen, T.S.; Holgersson, J.; Karlsson, E.N.; et al. Sialidases and fucosidases of Akkermansia muciniphila are crucial for growth on mucin and nutrient sharing with mucus-associated gut bacteria. Nat. Commun. 2023, 14, 1833. [Google Scholar] [CrossRef] [PubMed]
- Ndongo, S.; Armstrong, N.; Raoult, D.; Fournier, P.E. Reclassification of eight Akkermansia muciniphila strains and description of Akkermansia massiliensis sp. nov. and Candidatus Akkermansia timonensis, isolated from human feces. Sci. Rep. 2022, 12, 21747. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Kang, H.; You, H.J.; Ko, G.P. Revisiting the role of Akkermansia muciniphila as a therapeutic bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, R.; Chen, S.; Feng, R.; Chang, S.K.; Luo, Y.; Hong, H.; Tan, Y. Silver carp by-product hydrolysate: A promising mucin substitute for stimulating Akkermansia muciniphila growth and metabolic activity. Food Biosci. 2025, 67, 106310. [Google Scholar] [CrossRef]
- Khalili, L.; Park, G.; Nagpal, R.; Salazar, G. The Role of Akkermansia muciniphila on Improving Gut and Metabolic Health Modulation: A Meta-Analysis of Preclinical Mouse Model Studies. Microorganisms 2024, 12, 1627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, H.; Wu, P.; Yang, S.; Xue, W.; Xu, B.; Zhang, S.; Tang, B.; Xu, D. Akkermansia muciniphila: A promising probiotic against inflammation and metabolic disorders. Virulence 2024, 15, 2375555. [Google Scholar] [CrossRef] [PubMed]
- Segers, A.; de Vos, W.M. Mode of action of Akkermansia muciniphila in the intestinal dialogue: Role of extracellular proteins, metabolites and cell envelope components. Microbiome Res. Rep. 2023, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Zhang, S.; Duan, Z.; Ding, Y.; Chen, T.; Wang, R.; Feng, Z.; Shi, G.; Zhou, J.; Chen, Y. A critical review on the relationship of herbal medicine, Akkermansia muciniphila, and human health. Biomed. Pharmacother. 2020, 128, 110352. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Yuan, J.; Li, D. Biological activity of galacto-oligosaccharides: A review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. Effect of inulin, galacto-oligosaccharides, and polyphenols on the gut microbiota, with a focus on Akkermansia muciniphila. Food Funct. 2024, 15, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottiè, H.M.; De Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Nicaud, J.M. Metabolic Engineering for Expanding the Substrate Range of Yarrowia lipolytica. Trends Biotechnol. 2016, 34, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Monter, C.; Álvarez-Arce, A.; Nuño-Lambarri, N.; Escalona-Nández, I.; Juárez-Hernández, E.; Chávez-Tapia, N.C.; Uribe, M.; Barbero-Becerra, V.J. Inulin Improves Diet-Induced Hepatic Steatosis and Increases Intestinal Akkermansia Genus Level. Int. J. Mol. Sci. 2022, 23, 991. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Fructooligosaccharides (FOSs): A Condensed Overview. Compounds 2025, 5, 8. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.T.; Eller, L.K.; Nettleton, J.E.; Reimer, R.A. Postnatal prebiotic fibre intake mitigates some detrimental metabolic outcomes of early overnutrition in rats. Eur. J. Nutr. 2016, 55, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Nveena, B.S.N.; Arora, S. Polydextrose as a Functional Ingredient and its Food Applications: A Review. Indian J. Dairy Sci. 2015, 69, 239–251. [Google Scholar]
- Hooda, S.; Vester Boler, B.M.; Rossoni Serao, M.C.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C.; Swanson, K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Verhoog, S.; Taneri, P.E.; Díaz, Z.M.R.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary factors and modulation of bacteria strains of Akkermansia muciniphila and faecalibacterium prausnitzii: A systematic review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Leszczyñski, W. Resistant Starch-Classification, Structure, Production. Pol. J. Food Nutr. Sci. 2004, 54, 37–50. [Google Scholar]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of dietary resistant starch on the human gut Microbiome, Metaproteome, and Metabolome. MBio 2017, 8, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, J.; Tan, H.; Zhong, Y.; Nie, S. Akkermansia muciniphila, an important link between dietary fiber and host health. Curr. Opin. Food Sci. 2022, 47, 100905. [Google Scholar] [CrossRef]
- Fareed, S.Z.; Tangjaidee, P.; Khumsap, T.; Klangpetch, W.; Phongthai, S.; Kanpiengjai, A.; Khanongnuch, C.; Unban, K. Xylooligosaccharides from Barley Malt Residue Produced by Microwave-Assisted Enzymatic Hydrolysis and Their Potential Uses as Prebiotics. Plants 2025, 14, 769. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Yang, J.; Ying, S.; Luo, H.; Zha, L.; Li, Q. Xylooligosaccharide and Akkermansia muciniphila synergistically ameliorate insulin resistance by reshaping gut microbiota, improving intestinal barrier and regulating NKG2D/NKG2DL signaling in gestational diabetes mellitus mice. Food Res. Int. 2025, 201, 115634. [Google Scholar] [CrossRef] [PubMed]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Fernández, M.; Porras, D.; Petrov, P.; Román-Sagüillo, S.; García-Mediavilla, M.V.; Soluyanova, P.; Martínez-Flórez, S.; González-Gallego, J.; Nistal, E.; Jover, R.; et al. The synbiotic combination of Akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants 2021, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, H.; Shu, L.; Xing, H.; Wang, C.; Lu, C.; Song, G. Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice. Int. J. Food Sci. Nutr. 2020, 71, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Liudvytska, O. Rheum rhaponticum and Rheum rhabarbarum: A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 2021, 20, 589–607. [Google Scholar] [CrossRef]
- Seydametova, E.; Yu, J.; Shin, J.; Park, Y.; Kim, C.; Kim, H.; Yu, S.H.; Park, Y.; Kweon, D.-H. Search for bacterial α1,2-fucosyltransferases for whole-cell biosynthesis of 2′-fucosyllactose in recombinant Escherichia coli. Microbiol. Res. 2019, 222, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhou, Z.; Zhou, Y.; Zhang, M.; Shuai, Y. 2′-Fucosyllactose promotes the enrichment of Akkermansia muciniphila and the production of short-chain fatty acids in vitro and in vivo. Food Biosci. 2024, 61, 104785. [Google Scholar] [CrossRef]
- Dhillon, S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Kimura, T.; Obata, A.; Shimoda, M.; Kaku, K. Multifaceted mechanisms of action of metformin which have been unraveled one after another in the long history. Int. J. Mol. Sci. 2021, 22, 2596. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Prebiotic capacity of inulin-type fructans. J Nutr. 2007, 137 (Suppl. S11), 2503S–2506S. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
| Bioactive Compounds | Interaction with A. muciniphila | SCFAs | Experimental Model | Reference |
|---|---|---|---|---|
| Non-digestible Fibers | ||||
| Galacto-oligosaccharides (GOS) | ↑ Increase in A. muciniphila abundance compared to the placebo group. | ↑ Acetate and indirectly, butyrate production. | Meta-analysis of 821 human stool samples collected from 451 participants in 4 countries. For the study of GOS, n = 94, 5 g per day were administered for 3 weeks. | [47] |
| Inulin | Inconsistent effect across the three reported studies: A. muciniphila abundance ↑ in one study, but ↓ in the other two. | An analysis of the SCFAs produced is not reported. | Meta-analysis of 821 human stool samples collected from 451 participants in 4 countries. For the inulin intervention, three human studies (n = 303) were included, using doses of 16–20 g/day for 2–12 weeks. | [47] |
| Inulin | ↑ Abundance from 10% to 47% of relative abundance in high-fat diet groups. | Increased activity of the propionate metabolism pathway, associated with inulin consumption (predicted by PICRUSt analysis). | C57BL/6N mice (n = 40) divided into 4 groups (n = 10): control or high-fat diet (HFD) (45% kcal) supplemented with cellulose or inulin (10%) for 8 weeks. Assessed biochemical parameters, liver histology, fecal microbiota (16S rRNA), tight junction proteins (ZO-1, occludin, TLR4), and bacterial metabolic pathways (PICRUSt). | [50] |
| Oligofructose (FOS) | ↑ A. muciniphila abundance from 0.001% to 0.089% in cecal content compared to control. | Not measured; enrichment of SCFAs-producing genera (Butyricimonas, Barnesiella) was observed, but SCFAs levels were not quantified. | C57BL/6 ob/ob mice and HFD-induced obese C57BL/6J mice were treated with oligofructose: 5 weeks in diet (ob/ob) or 8 weeks in drinking water (0.3 g/mouse/day, HFD). Outcomes included microbiota profiling (qPCR, 16S rRNA pyrosequencing, MITChip), glucose tolerance, lipid metabolism, gut barrier integrity (LPS, FITC-dextran, ZO-1/occludin), gene expression (GLP-1, IL-1, LPL, ACC), L-cell number, and leptin sensitivity. | [52] |
| Oligofructose (FOS) | ↑ A. muciniphila from 0.53% to 1.65% (SL) and 0.29% to 0.57% (NL). SL rats showed higher abundance than NL, regardless of diet. | SCFAs were not measured directly; their production is inferred from OFS fermentation. OFS diet ↑ colonic expression of SCFA receptors GPR41 and GPR43 (p < 0.03), regardless of litter size. | Male Sprague–Dawley rats were reared in small (SL, 3 pups) or normal litters (NL, 12 pups), then fed either standard AIN-93 diet or AIN-93 supplemented with 10% oligofructose (w/w) from weaning (P21) to week 19. Outcomes included body composition, glucose tolerance, gut hormones, gene expression, and microbiota. | [53] |
| Polydextrose | ↑ A. muciniphila in relative abundance with polydextrose (no-fiber control: 1.08% → polydextrose: 3.54%; soluble corn fiber: 0.41%). | Fecal SCFAs (acetate, propionate, butyrate) were higher with SCF than with PDX (p < 0.05), despite increased F. prausnitzii with PDX. Authors note that fecal levels do not reflect actual SCFA production due to rapid colonic absorption. | Fecal microbiota composition was an alyzed by 16S rRNA gene pyrosequencing after 21 days of fiber supplementation (21 g/day of polydextrose, soluble corn fiber, or no fiber) in a randomized crossover study with healthy adult men (n = 20). SCFAs were measured in fecal samples using gas chromatography. | [55,56] |
| Resistant starch type 2 (HAM-RS2, high amylose corn) | ↑ Relative abundance after the high-resistant starch diet compared to both the low-RS and baseline diets. Its functions were not analyzed, and no mechanisms were discussed in the study. | SCFAs showed a slight increase in butyrate and propionate after the high-RS diet. This was linked to butyrate-producing genera, but not directly to A. muciniphila, despite its increased abundance. | Crossover study with 39 insulin-resistant adults. Participants consumed high (48–66 g/day) and low (3–4 g/day) RS2 diets for 2 weeks each, with a washout in between. Fecal samples were analyzed by 16S rRNA, metaproteomics, and FT-ICR-MS; SCFAs quantified by UHPLC-MS. | [58] |
| Resistant starch type 2 (HAM-RS2, high amylose maize) | ↑ Relative abundance (35.1-fold) in mice fed 36% HAM-RS2 compared to controls (p < 0.05; control diet contained 0% RS and 100% amylopectin). | Not quantified. Authors hypothesize propionate production by A. muciniphila as a possible mechanism, but no SCFA data were reported. | Aged male C57BL/6J mice (18–20 months; n = 6 per group) were fed diets with 0%, 18%, or 36% HAM-RS2 for 10 weeks. Cecal microbiota analyzed by 16S rRNA gene pyrosequencing (V1–V3 regions). Proglucagon expression measured by RT-qPCR. SCFAs were not measured. | [59] |
| Xylo-oligosaccharides (XOS) | ↑ A. muciniphila abundance only when administered XOS alone, in vivo, and in the presence of a functional gut microbiota. This effect is lost in the pseudo-germ-free (PGF) model and is not enhanced—indeed, it may be reversed—when combined with direct administration of A. muciniphila. | No SCFAs analysis was reported. | C57BL/6J female mice (n = 225) were fed AIN-93 or HFD diets. After 4 weeks, HFD-fed mice were divided into 9 groups (n ≥ 19) with combinations of GDM (induced by STZ), XOS (500 mg/kg), A. muciniphila (4 × 108 CFU), and/or antibiotics (PGF model). Interventions began on gestation day 8. On day 18, fasting blood glucose and body weight were measured. After sacrifice, blood, tissues, feces, and cecal contents were collected for further analysis, including bacterial quantification and DNA extraction. | [62] |
| Plant-Derived Bioactive Compounds | ||||
| Wild blueberry extract (WBE) | The total extract (WBE) did not stimulate A. muciniphila. However, the F2 fraction (PAC oligomers) increased its abundance 2.5-fold, as confirmed by qPCR. | SCFAs were not directly measured in this study. | Male C57BL/6J mice were fed a high-fat, high-sucrose (HFHS) diet for 8 weeks and were orally supplemented by gavage with either the wild blueberry extract (WBE, 200 mg/kg), or one of its polyphenolic fractions: F1 (anthocyanins and phenolic acids, 32 mg/kg), F2 (oligomeric PACs, 53 mg/kg), or F3 (polymeric PACs, 37 mg/kg), in doses equivalent to their natural proportion in the WBE | [64] |
| Grape polyphenol Extract (GPE) | ↑ 468% vs. baseline (day 0); this large increase reflects a proportional rise from a low initial abundance. | No direct changes in SCFAs were reported. Sustained reduction of ROS in the gut. | Lean C57BL/6J mice (n = 5/group) were assigned to four groups: control (vehicle), ascorbic acid, β-carotene, or GPE. All treatments were administered daily by oral gavage (360 mg/kg) for 14 days. Fecal samples were analyzed by qPCR and ABTS assay; gastrointestinal ROS were assessed by NIRF imaging, and metabolic status by OGTT. | [10] |
| Polyphenols (red-fleshed apple andpolyphenols grape-pomace) | No significant change in A. muciniphila abundance was observed in either polyphenol intervention. | No significant changes observed; no SCFA-related pathway alterations reported. | Meta-analysis of 821 human stool samples collected from 451 participants in 4 countries. For polyphenol studies, human intervention trials were conducted: apple supplementation for 5 weeks and grape pomace supplementation for 6 weeks. Fecal microbiota was analyzed by 16S rRNA sequencing and processed with QIIME2 and PICRUSt2. | [47] |
| Quercetin | ↑ Relative abundance of A. muciniphila in rats previously fed a high-fat diet (HFD) and supplemented with the bacterium (alone or with quercetin), compared to non-supplemented groups. | There was no direct effect on SCFAs. The increase in SCFAs was attributed to dietary intervention, not to quercetin. | Twenty-one-day-old male Wistar rats were fed a HFD for 6 weeks to induce early obesity and NAFLD, then received a control diet (10% fat) for 3 weeks, supplemented with or without quercetin (37.5 mg/kg/day in diet), A. muciniphila (2 × 108 CFU/day by oral gavage), or both. | [67] |
| Resveratrol | ↑ A. muciniphila abundance (qPCR; p < 0.001)—restored after resveratrol treatment, following colitis-induced depletion. | In both healthy and colitis mice, it caused a significant ↑ of i-butyrate and acetate, in the TNBS + Resveratrol group. | BALB/c mice with TNBS-induced colitis were administered oral resveratrol (100 mg/kg/day) for 5 days. The microbiota was assessed by 16S rRNA sequencing, SCFAs by chromatography, and T cell subpopulations by flow cytometry. | [69] |
| Resveratrol | ↑ A. muciniphila relative abundance (p < 0.05 vs. HFD and ND). The genus Akkermansia (phylum Verrucomicrobia) was significantly enriched in the HFD + Resveratrol group, as determined by 16S rRNA sequencing and LEfSe analysis. | SCFAs Not directly quantified in this study. | C57BL/6J mice (n = 15) were divided into: ND (normal diet), HFD for 20 weeks), and HFD + RES (HFD plus oral resveratrol, 100 mg/kg/day, for 12 weeks). Metabolic, inflammatory, and intestinal permeability markers (ZO-1, occludin, claudin-1) were assessed. Gut microbiota was analyzed via 16S rRNA sequencing (Illumina MiSeq, QIIME, LEfSe). SCFAs were not directly measured. ANOVA + Tukey test; p < 0.05 was considered significant. | [70] |
| Rhubarb | The abundance of A. muciniphila increased to 38.9% of total fecal microorganisms in DIO mice (vs. 9.4% on standard diet) after 17 days of supplementation. | No effects on SCFAs were specified. A decrease in Firmicutes and improvements in liver inflammation, oxidative stress, and intestinal homeostasis were reported. | DIO mice fed the AIN93M diet were supplemented with rhubarb extract for 17 days. The microbiota was assessed by 16S rRNA pyrosequencing. | [6] |
| Human Milk Oligosaccharides | ||||
| 2′-fucosyllactose (2′-FL) | ↑ abundance between 13 and 18%, significantly greater than in the control group. | ↑ Butyrate, propionate, isovalerate, valerate, caproate, and 4-methylvalerate. | In vitro: A. muciniphila cultured with 2′-FL for 72 h. In vivo: C57BL/6J mice (3 weeks old) treated for 14 days with 2.0 g/kg (low dose) or 6.0 g/kg (high dose) of 2′-FL by gavage. Microbiota analysis by 16S rRNA sequencing. | [73] |
| Drugs | ||||
| Dapagliflozin | Trend towards increased abundance in treated diabetic mice. | No studies reported on 49 s, improved vascular function (↓ arterial stiffness, ↑ endothelial dilation). | C57BLKS mice (type 2 diabetes model), males, 8 weeks of treatment with a diet supplemented with dapagliflozin (60 mg/kg diet; 0.006%). | [75] |
| Metformin | ↑ The abundance of A. muciniphila in HFD-fed mice. This effect is associated with improved glucose tolerance and reduced inflammation. | It improved intestinal barrier integrity, reduced LPS absorption, decreased inflammation, and maintained intestinal homeostasis. No direct effects on SCFA levels were reported. | Mice were fed on a high-fat diet (HFD); metformin was administered orally and validated in fecal cultures in BHI medium. | [6,45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temis-Cortina, J.A.; Prada-Ramírez, H.A.; Ríos-Guerra, H.; Espinosa-Raya, J.; Gómez-Pliego, R. Response of Akkermansia muciniphila to Bioactive Compounds: Effects on Its Abundance and Activity. Fermentation 2025, 11, 427. https://doi.org/10.3390/fermentation11080427
Temis-Cortina JA, Prada-Ramírez HA, Ríos-Guerra H, Espinosa-Raya J, Gómez-Pliego R. Response of Akkermansia muciniphila to Bioactive Compounds: Effects on Its Abundance and Activity. Fermentation. 2025; 11(8):427. https://doi.org/10.3390/fermentation11080427
Chicago/Turabian StyleTemis-Cortina, Jair Alejandro, Harold Alexis Prada-Ramírez, Hulme Ríos-Guerra, Judith Espinosa-Raya, and Raquel Gómez-Pliego. 2025. "Response of Akkermansia muciniphila to Bioactive Compounds: Effects on Its Abundance and Activity" Fermentation 11, no. 8: 427. https://doi.org/10.3390/fermentation11080427
APA StyleTemis-Cortina, J. A., Prada-Ramírez, H. A., Ríos-Guerra, H., Espinosa-Raya, J., & Gómez-Pliego, R. (2025). Response of Akkermansia muciniphila to Bioactive Compounds: Effects on Its Abundance and Activity. Fermentation, 11(8), 427. https://doi.org/10.3390/fermentation11080427







