Elicitation-Induced Enhancement of Lovastatin and Pigment Production in Monascus purpureus C322
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism Maintenance
2.2. Fermentation Setup and Culture Conditions
2.3. Elicitor Preparation and Application
2.4. Analytical Techniques for Biomass, Carbohydrate, Pigment, and Lovastatin Quantification
2.5. Statistical Analysis
3. Results
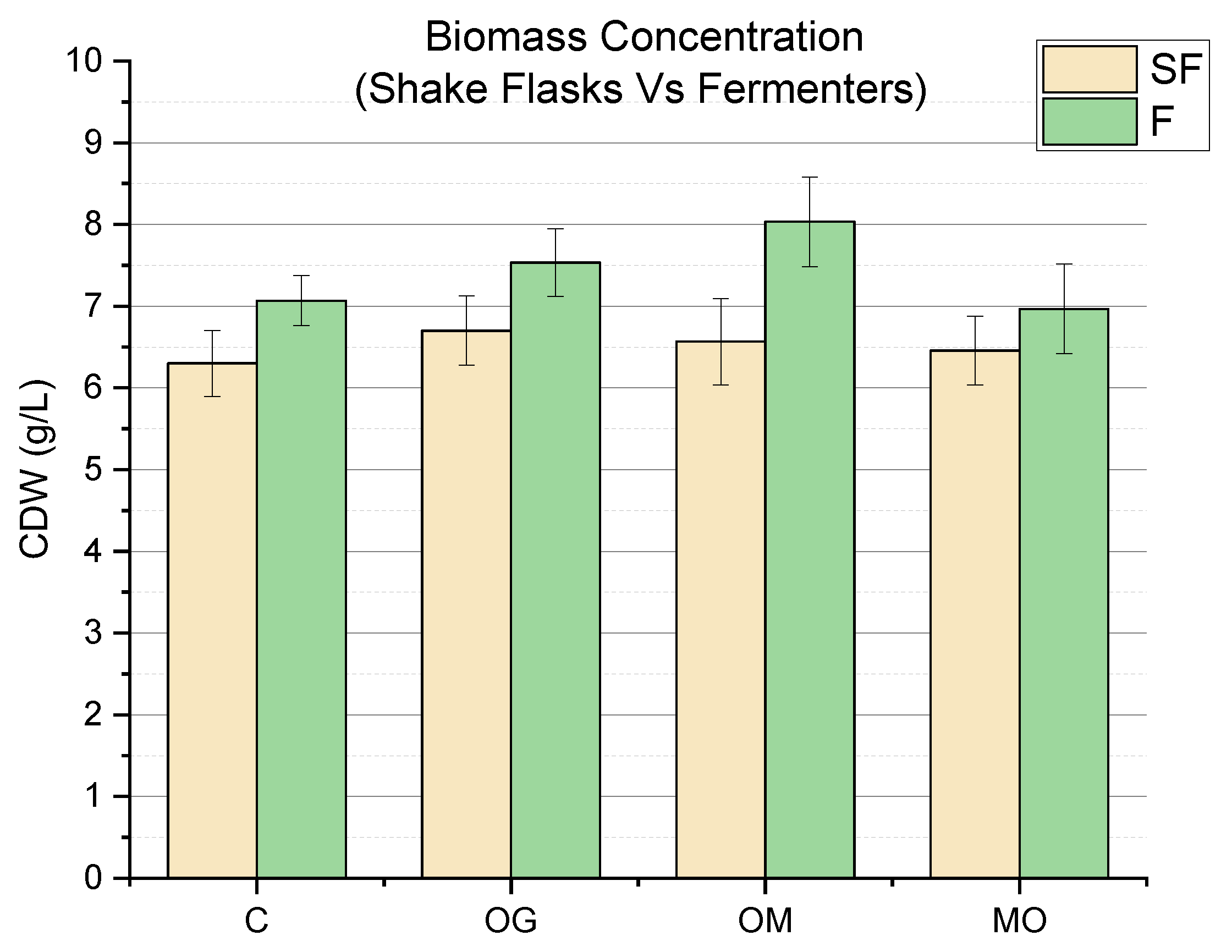
3.1. Impact of Elicitation on Biomass Accumulation
3.2. Elicitor-Driven Enhancement of Pigment Production
3.3. Lovastatin Production in Shake Flasks and Fermenters
3.4. Effect of Elicitors on pH Variation and Carbohydrate Consumption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OG | Oligoguluronate |
| OM | Oligomannuronate |
| MO | Mannan Oligosaccharide |
| OD | Optical Density |
| SF | Shake Flasks |
| F | Fermenters |
| CDW | Cell Dry Weight |
| CHO | Carbohydrate |
| AU | Absorbance Units |
| HPLC | High Performance Liquid Chromatography |
| ANOVA | Analysis of Variance |
References
- Liu, J.; Du, Y.; Ma, H.; Pei, X.; Li, M. Enhancement of Monascus Yellow Pigments Production by Activating the CAMP Signalling Pathway in Monascus purpureus HJ11. Microb. Cell Factories 2020, 19, 224. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.A.; Andhare, P.; Upadhyay, D.; Kumari, S. Microbial Secondary Metabolites. Int. J. Biol. Pharm. Allied Sci. 2021, 10, 488–496. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S. Bin from Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rendon, D.; Passari, A.K.; Ruiz-Villafán, B.; Rodríguez-Sanoja, R.; Sánchez, S.; Demain, A.L. Impact of Novel Microbial Secondary Metabolites on the Pharma Industry. Appl. Microbiol. Biotechnol. 2022, 106, 1855–1878. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Kapoor, N.; Kaur, H.; Abu-Seer, E.A.; Tariq, M.; Siddiqui, S.; Yadav, V.K.; Niazi, P.; Kumar, P.; Alghamdi, S. A Comprehensive Review of the Diversity of Fungal Secondary Metabolites and Their Emerging Applications in Healthcare and Environment. Mycobiology 2024, 52, 335–387. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qin, X.; Zhao, Y.; Sun, X.; Yu, X.; Feng, Y. Strategies to Enhance the Production Efficiency of Monascus Pigments and Control Citrinin Contamination. Process Biochem. 2022, 117, 19–29. [Google Scholar] [CrossRef]
- Mahajan, R.; Sagar, T.; Billowria, P.; Kapoor, N. Elicitation: A Biotechnological Approach for Enhancement of Secondary Metabolites in In Vitro Cultures. In Biotechnology and Crop Improvement; CRC Press: Boca Raton, FL, USA, 2022; pp. 25–47. [Google Scholar] [CrossRef]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In Vitro Strategies for the Enhancement of Secondary Metabolite Production in Plants: A Review. Bull. Natl. Res. Cent. 2022, 46, 35. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.F.M.; de Souza Vandenberghe, L.P.; Herrmann, L.W.; Letti, L.A.J.; Burgos, W.J.M.; Scapini, T.; Manzoki, M.C.; de Oliveira, P.Z.; Soccol, C.R. Strategies and Engineering Aspects on the Scale-Up of Bioreactors for Different Bioprocesses. Syst. Microbiol. Biomanuf. 2023, 4, 365–385. [Google Scholar] [CrossRef]
- Lauer, I.; Philipps, G.; Jennewein, S. Metabolic Engineering of Clostridium Ljungdahlii for the Production of Hexanol and Butanol from CO2 and H2. Microb. Cell Factories 2022, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Thangavelu, K.; Uthandi, S. Lovastatin Production by an Oleaginous Fungus, Aspergillus Terreus KPR12 Using Sago Processing Wastewater (SWW). Microb. Cell Factories 2022, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.B.; Santos, N.H.; de Lima, T.M.; Santana, R.V.; de Oliveira Filho, J.G.; Peres, D.S.; Egea, M.B. Pigment Bioproduction by Monascus purpureus Using Corn Bran, a Byproduct of the Corn Industry. Biocatal. Agric. Biotechnol. 2021, 32, 101931. [Google Scholar] [CrossRef]
- Danuri, H. Optimizing Angkak Pigments and Lovastatin Production by Monascus purpureus. Hayati 2008, 15, 61–66. [Google Scholar] [CrossRef]
- Moradi, S.; Mortazavi, S.A. Evaluation of Monascus purpureus Fermentation in Dairy Sludge-Based Medium for Enhanced Production of Vibrant Red Pigment with Minimal Citrinin Content. PLoS ONE 2024, 19, e0315006. [Google Scholar] [CrossRef] [PubMed]
- Radman, R.; Bucke, C.; Keshavarz, T. Elicitor Effects on Reactive Oxygen Species in Liquid Cultures of Penicillium chrysogenum. Biotechnol. Lett. 2004, 26, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Radman, R.; Bucke, C.; Keshavarz, T. Elicitor Effects on Penicillium chrysogenum Morphology in Submerged Cultures. Biotechnol. Appl. Biochem. 2004, 40, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of Plants and Microbial Cell Systems. Biotechnol. Appl. Biochem. 2003, 37, 91. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Roy, I.; Bucke, C.; Keshavarz, T. Quantitative PCR Study on the Mode of Action of Oligosaccharide Elicitors on Penicillin G Production by Penicillium chrysogenum. J. Appl. Microbiol. 2009, 107, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Roy, I.; Harrop, A.; Dixon, K.; Keshavarz, T. Effect of Oligosaccharide Elicitors on Bacitracin A Production and Evidence of Transcriptional Level Control. J. Biotechnol. 2007, 131, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Parra, R.; Radman, R.; Roy, I.; Harrop, A.; Dixon, K.; Keshavarz, T. Novel Application of Oligosaccharides as Elicitors for the Enhancement of Bacitracin A Production in Cultures of Bacillus licheniformis. Enzym. Microb. Technol. 2007, 40, 1518–1523. [Google Scholar] [CrossRef]
- Nair, R. Elucidation of the Mechanism of Elicitation in Penicillium chrysogenum: Systematic Approach to Study the Effect of Oligosaccharides on Production of Penicillin G. Ph.D. Thesis, University of Westminster, London, UK, 2007. [Google Scholar]
- Reffatti, P.F. Physiological Response of Bacillus licheniformis NCIMB 8874 to Oligosaccharide Elicitors. Ph.D. Thesis, University of Westminster, London, UK, 2012. [Google Scholar]
- Radman, R. Study of the Effects of Oligosaccharides in Liquid Cultures of Penicillium chrysogenum. Ph.D. Thesis, University of Westminster, London, UK, 2002. [Google Scholar]
- Gong, P.; Shi, R.; Liu, Y.; Luo, Q.; Wang, C.; Chen, W. Recent Advances in Monascus Pigments Produced by Monascus purpureus: Biosynthesis, Fermentation, Function, and Application. LWT 2023, 185, 115162. [Google Scholar] [CrossRef]
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, Red, Yellow: Biosynthesis of Azaphilone Pigments in Monascus Fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A Highly Potent Competitive Inhibitor of Hydroxymethylglutaryl-Coenzyme A Reductase and a Cholesterol-Lowering Agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Onuorah, I.; Agrawal, A.; Wenger, N. Management of Cardiovascular Disease in the Elderly. Geriatr. Med. 2024, 343–383. [Google Scholar] [CrossRef]
- Wu, H.; He, H.; Han, T.; Tian, X.; Zhu, Z. Targeting Cholesterol-Dependent Adrenal Steroidogenesis for Management of Primary Aldosteronism. Trends Endocrinol. Metab. 2025. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An Overview on the Biological Activity and Anti-Cancer Mechanism of Lovastatin. Cell. Signal. 2021, 87, 110122. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.; Song, S.M.; Shin, C.S.; Johnston, T.V.; Ahn, H.J.; Kim, D.; Ku, S. Production and Characterization of Anti-Inflammatory Monascus Pigment Derivatives. Foods 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xie, B.; Zong, X.; Yu, X.; Feng, Y. Selective Production, Relationship and Controversy Between Monascus Pigments and Citrinin. Food Biosci. 2023, 56, 103233. [Google Scholar] [CrossRef]
- Agboyibor, C.; Kong, W.B.; Chen, D.; Zhang, A.M.; Niu, S.Q. Monascus Pigments Production, Composition, Bioactivity and its Application: A Review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- Chaudhary, V.; Katyal, P.; Poonia, A.K.; Kaur, J.; Puniya, A.K.; Panwar, H. Natural Pigment from Monascus: The Production and Therapeutic Significance. J. Appl. Microbiol. 2022, 133, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.S.; Adlard, M.W.; Keshavarz, T. Swainsonine production in fed-batch fermentations of Metarhizium anisopliae. Biotechnol. Lett. 1995, 17, 433–438. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; Wu, X.; Li, X. Cost-effective process for the production of Monascus pigments using potato pomace as carbon source by fed-batch submerged fermentation. Food Sci. Nutr. 2001, 9, 5415–5427. [Google Scholar] [CrossRef] [PubMed]
- Krairak, S.; Yamamura, K.; Irie, R.; Nakajima, M.; Shimizu, H.; Chim-Anage, P.; Yongsmith, B.; Shioya, S. Maximizing yellow pigment production in fed-batch culture of Monascus sp. J. Biosci. Bioeng. 2000, 90, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Ajdari, Z.; Ebrahimpour, A.; Abdul Manan, M.; Hamid, M.; Mohamad, R.; Ariff, A.B. Nutritional Requirements for the Improvement of Growth and Sporulation of Several Strains of Monascus purpureus on Solid State Cultivation. BioMed Res. Int. 2011, 2011, 487329. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Maity, S.; Chattopadhyay, P.; Sarkar, A.; Laskar, S.; Sen, S.K. Characterization of Red Pigment from Monascus in Submerged Culture Red Pigment from Monascus purpureus. J. Appl. Sci. Res. 2009, 5, 2102–2108. [Google Scholar]
- Asilonu, E.; Bucke, C.; Keshavarz, T. Enhancement of Chrysogenin Production in Cultures of Penicillium chrysogenum by Uronic Acid Oligosaccharides. Biotechnol. Lett. 2000, 22, 931–936. [Google Scholar] [CrossRef]
- Amache, R. Quorum Sensing for Improved Production of Industrially Useful Products from Filamentous Fungi. Ph.D. Thesis, University of Westminster, London, UK, 2014. [Google Scholar]
- Bühler, R.M.M.; Dutra, A.C.; Vendruscolo, F.; Moritz, D.E.; Ninow, J.L. Monascus Pigment Production in Bioreactor Using a Co-Product of Biodiesel as Substrate. Food Sci. Technol. 2013, 33, 9–13. [Google Scholar] [CrossRef]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis: A Practical Approach; IRL Press: Washington, DC, USA, 1987; 228p. [Google Scholar]
- Merchant, M. Investigating Physical and Chemical Interaction of Aspergillus terreus Spores for Changes in Morphology and Physiology. Ph.D. Thesis, University of Westminster, London, UK, 2020. [Google Scholar] [CrossRef]
- Nichols, L. 4.6: Step-by-Step Procedures for Extractions—Chemistry LibreTexts 2025. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_Lab_Techniques_(Nichols)/04%3A_Extraction/4.06%3A_Step-by-Step_Procedures_For_Extractions (accessed on 23 November 2024).
- Hahn, M.G. Oligosaccharide Elicitors and Elicitor Receptors. In Current Issues in Plant Molecular and Cellular Biology, Proceedings of the 8th International Congress on Plant Tissue and Cell Culture, Florence, Italy, 12–17 June 1994; Springer: Dordrecht, The Netherlands, 1995; pp. 37–58. [Google Scholar] [CrossRef]
- Lebrilla, C.B.; Liu, J.; Widmalm, G.; Prestegard, J.H. Oligosaccharides and Polysaccharides. In Essentials of Glycobiology; The Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2022. [Google Scholar]
- Seletzky, J.M.; Noack, U.; Hahn, S.; Knoll, A.; Amoabediny, G.; Büchs, J. An Experimental Comparison of Respiration Measuring Techniques in Fermenters and Shake Flasks: Exhaust Gas Analyzer vs. RAMOS Device vs. Respirometer. J. Ind. Microbiol. Biotechnol. 2007, 34, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Seletzky, J.M.; Noak, U.; Fricke, F.; Welk, E.; Eberhard, W.; Knocke, C.; Büchs, J. Scale-Up from Shake Flasks to Fermenters in Batch and Continuous Mode with Corynebacterium glutamicum on Lactic Acid Based on Oxygen Transfer and PH. Biotechnol. Bioeng. 2007, 98, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Antonio Rocha-Valadez, J.; Estrada, M.; Galindo, E.; Serrano-Carreón, L. From Shake Flasks to Stirred Fermentors: Scale-Up of an Extractive Fermentation Process for 6-Pentyl-α-Pyrone Production by Trichoderma harzianum Using Volumetric Power Input. Process Biochem. 2006, 41, 1347–1352. [Google Scholar] [CrossRef]
- Kennedy, J.; Auclair, K.; Kendrew, S.G.; Park, C.; Vederas, J.C.; Hutchinson, C.R. Modulation of Polyketide Synthase Activity by Accessory Proteins During Lovastatin Biosynthesis. Science 1999, 284, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; Ijaz, S.S.; Ikram-ul-Haq. Upstream and Downstream Processing of Lovastatin by Aspergillus Terreus. Cell Biochem. Biophys. 2014, 70, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Selwal, N.; Goutam, U.; Akhtar, N.; Sood, M.; Kukreja, S. Elicitation: “A Trump Card” for Enhancing Secondary Metabolites in Plants. J. Plant Growth Regul. 2024, 43, 3027–3047. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular Aspects of the Early Stages of Elicitation of Secondary Metabolites in Plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Mulder, K.C.L.; Mulinari, F.; Franco, O.L.; Soares, M.S.F.; Magalhães, B.S.; Parachin, N.S. Lovastatin Production: From Molecular Basis to Industrial Process Optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.D.; Vederas, J.C. Mini Review: Biosynthesis of Lovastatin and Related Metabolites Formed by Fungal Iterative PKS Enzymes. Biopolymers 2010, 93, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, Y.; Zheng, Y. Production of Lovastatin and its Lipid-Lowering and Anti-Cancer Effects. Highlights Sci. Eng. Technol. 2022, 11, 283–291. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Punt, P.; Visser, J. Production of Organic Acids by Filamentous Fungi. In Industrial Applications; Springer: Berlin, Germany, 2010; Volume 10, pp. 215–234. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic Acids Production from Lactic Acid Bacteria: A Preservation Approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Bate, F.; Amekan, Y.; Pushkin, D.O.; Chong, J.P.J.; Bees, M. Emergent Lag Phase in Flux-Regulation Models of Bacterial Growth. Bull. Math. Biol. 2023, 85, 84. [Google Scholar] [CrossRef] [PubMed]
- Kokoreva, A.S.; Isakova, E.P.; Tereshina, V.M.; Klein, O.I.; Gessler, N.N.; Deryabina, Y.I. The Effect of Different Substrates on the Morphological Features and Polyols Production of Endomyces magnusii Yeast During Long-Lasting Cultivation. Microorganisms 2022, 10, 1709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Ma, Z.; Huang, H.; Zheng, L.; Tian, Y.; Zhong, Q. Microbial Succession and Organic Acid Metabolism during Spontaneous Calamondin Fermentation: The Vital Role of Pichia. Food Res. Int. 2025, 209, 116200. [Google Scholar] [CrossRef] [PubMed]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring Fungal Biodiversity: Organic Acid Production by 66 Strains of Filamentous Fungi. Fungal Biol. Biotechnol. 2014, 1, 1. [Google Scholar] [CrossRef]
- Nie, M.; Li, K.; Li, Z. β-Alanine Metabolism Leads to Increased Extracellular PH during the Heterotrophic Ammonia Oxidation of Pseudomonas Putida Y-9. Microorganisms 2023, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.C.; Lin, Y.C.; Koehler, P.E. Regulation of Growth and Pigmentation of Monascus purpureus by Carbon and Nitrogen Concentrations. Mycologia 1981, 73, 649–654. [Google Scholar] [CrossRef]
- Mata, F.; Valenzuela, P.L.; Gimenez, J.; Tur, C.; Ferreria, D.; Domínguez, R.; Sanchez-Oliver, A.J.; Sanz, J.M.M. Carbohydrate Availability and Physical Performance: Physiological Overview and Practical Recommendations. Nutrients 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Oehlenschläger, K.; Hengsbach, J.N.; Volkmar, M.; Ulber, R. From Pre-Culture to Solvent: Current Trends in Clostridium Acetobutylicum Cultivation. Appl. Microbiol. Biotechnol. 2025, 109, 47. [Google Scholar] [CrossRef] [PubMed]
- Mulyani, H.; Artanti, N.; Yuniati, R.; Yasman, Y. Scaling Up Red Ginger Kombucha Fermentation: Insights into its Chemical Profile and Health-Promoting Properties. Fermentation 2025, 11, 128. [Google Scholar] [CrossRef]



| Group | Yellow | Orange | Red | |||
|---|---|---|---|---|---|---|
| SF | F | SF | F | SF | F | |
| C | 0.61 | 0.71 | 0.54 | 0.55 | 0.52 | 0.59 |
| OG | 1.03 | 1.36 | 1.22 | 1.67 | 1.13 | 1.60 |
| OM | 1.24 | 1.60 | 1.16 | 1.42 | 1.35 | 1.80 |
| MO | 0.87 | 1.04 | 0.89 | 1.12 | 0.98 | 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerramalli, S.; Getting, S.J.; Kyazze, G.; Keshavarz, T. Elicitation-Induced Enhancement of Lovastatin and Pigment Production in Monascus purpureus C322. Fermentation 2025, 11, 422. https://doi.org/10.3390/fermentation11080422
Yerramalli S, Getting SJ, Kyazze G, Keshavarz T. Elicitation-Induced Enhancement of Lovastatin and Pigment Production in Monascus purpureus C322. Fermentation. 2025; 11(8):422. https://doi.org/10.3390/fermentation11080422
Chicago/Turabian StyleYerramalli, Sirisha, Stephen J. Getting, Godfrey Kyazze, and Tajalli Keshavarz. 2025. "Elicitation-Induced Enhancement of Lovastatin and Pigment Production in Monascus purpureus C322" Fermentation 11, no. 8: 422. https://doi.org/10.3390/fermentation11080422
APA StyleYerramalli, S., Getting, S. J., Kyazze, G., & Keshavarz, T. (2025). Elicitation-Induced Enhancement of Lovastatin and Pigment Production in Monascus purpureus C322. Fermentation, 11(8), 422. https://doi.org/10.3390/fermentation11080422







