Diversity and Biological Activity of Secondary Metabolites Produced by the Endophytic Fungus Penicillium ochrochlorae
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Fungal Material
2.3. Fungal Fermentation, Chemical Extraction, and Purification
2.4. Spectroscopic Data of the New Compound
2.5. Antifungal Activity Assay
2.6. Antioxidative Assay
2.7. Cytotoxic Assay
3. Results
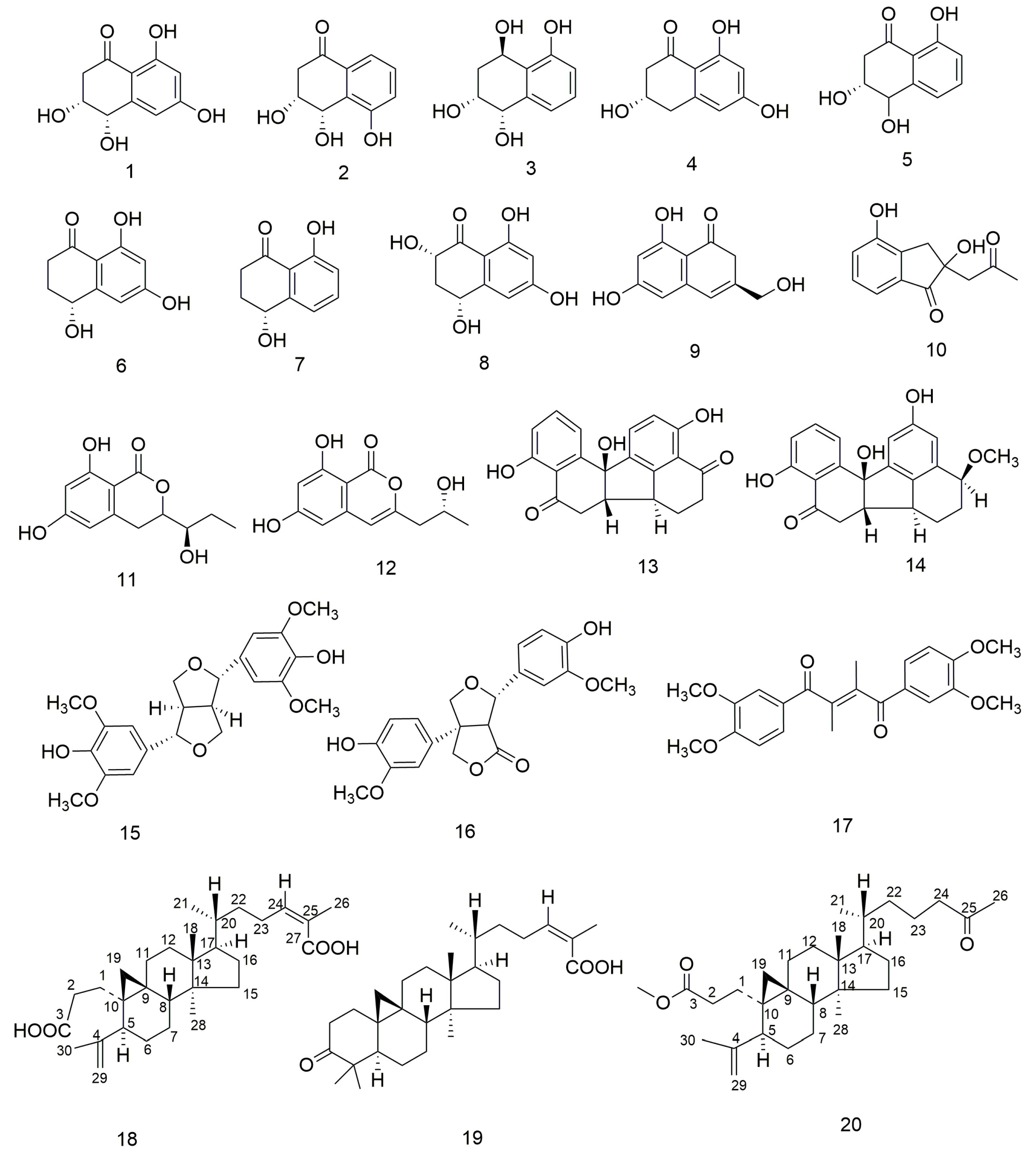
3.1. Structure Elucidation
3.2. Antifungal Assay
3.3. Antioxidant Analysis
3.4. Cytotoxicity Assay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMR | Nuclear magnetic resonance |
| MTT | 3-[4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium] bromide |
| DMSO | Dimethyl sulfoxide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DMEM | Dulbecco’s modified eagle medium |
| TLC | Thin-layer chromatography |
References
- Hoshino, S. Exploring new natural products by utilizing untapped secondary metabolic pathways in actinomycetes. J. Nat. Med. 2025, 79, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Powar, P.V.; Chaudhari, S. Unexploited potentials of endophytic fungi: Patents review on endophytic fungi related to secondary bioactive compounds. Pharmacogn. Res. 2023, 15, 217–225. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tan, Q.; Wu, J.; Chen, T.; Yang, W.; She, Z.; Wang, B. The Polyketides with antimicrobial activities from a mangrove endophytic fungus Trichoderma lentiforme ML-P8-2. Mar. Drugs 2023, 21, 566. [Google Scholar] [CrossRef]
- Jha, P.; Kaur, T.; Chhabra, I.; Panja, A.; Paul, S.; Kumar, V.; Malik, T. Endophytic fungi: Hidden treasure chest of antimicrobial metabolites interrelationship of endophytes and metabolites. Front. Microbiol. 2023, 14, 1227830. [Google Scholar] [CrossRef]
- Jayaweera, S.L.D.; Van, T.T.H.; Dias, D.A. Antifungal natural products originating from endophytic and rhizospheric microbes isolated from coastal vegetation. J. Xenobiotics 2025, 15, 32. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Almoammar, H.; Hashem, A.H.; Abuqamar, S.F. Endophytic Fungi: Exploring Biodiversity and Bioactive Potential; Springer: Singapore, 2025; pp. 1–42. [Google Scholar]
- Ying, Y.M.; Shan, W.G.; Zhan, Z.J. Biotransformation of huperzine a by a fungal endophyte of Huperzia serrata furnished sesquiterpenoid-alkaloid hybrids. J. Nat. Prod. 2014, 77, 2054–2059. [Google Scholar] [CrossRef]
- Ying, Y.M.; Xu, Y.L.; Yu, H.F. Biotransformation of huperzine A by Irpex lacteus-a fungal endophyte of Huperzia serrata. Fitoterapia 2019, 138, 104341. [Google Scholar] [CrossRef]
- Qin, D.; Wang, L.; Han, M.; Wang, J.Q.; Song, H.C.; Yan, X.; Duan, X.X.; Dong, J.Y. Effects of an endophytic fungus Umbelopsis dimorpha on the secondary metabolites of host-Plant Kadsura angustifolia. Front. Microbiol. 2018, 9, 2845. [Google Scholar] [CrossRef]
- Zhou, G.; Cai, J.; Wang, B.; Diao, W.; Zhong, Y.; Pan, S.; Xiong, W.; Huang, G.; Zheng, C. Secondary metabolites from the mangrove ecosystem-derived fungi Penicillium spp.: Chemical diversity and biological activity. Mar. Drugs 2025, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Udayan, E.; Gnanadoss, J.J. Potential of endophytic fungi as therapeutics: Antibiotics, antiviral and anticancer properties. Res. J. Biotechnol. 2023, 18, 132–145. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.C.; Xie, L.; Ran, Y.N.; Hu, Y.C. Endophytic fungi: An effective alternative source of plant-derived bioactive compounds for pharmacological studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef]
- Nielsen, J.; Smedsgaard, J. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A 2003, 1002, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Rancic, A.; Sokovic, M.; Karioti, A.; Vukojevic, J.; Skaltsa, H. Isolation and structural elucidation of two secondary metabolites from the filamentous fungus Penicillium ochrochloron with antimicrobial activity. Environ. Toxicol. Pharmacol. 2006, 22, 80–84. [Google Scholar] [CrossRef]
- Qin, D.; Shen, W.Y.; Wang, J.Q.; Han, M.J.; Chai, F.N.; Duan, X.X.; Guo, J.L.; Gao, T.C.; Zuo, S.H.; Dong, J.Y. Enhanced production of unusual triterpenoids from Kadsura angustifolia fermented by a symbiont endophytic fungus, Penicillium sp. SWUKD4.1850. Phytochemistry 2019, 158, 56–66. [Google Scholar] [CrossRef]
- Qin, D.; Shen, W.Y.; Gao, T.C.; Zuo, S.H.; Song, H.C.; Xu, J.R.; Yu, B.H.; Peng, Y.J.; Guo, J.L.; Tang, W.W.; et al. Kadanguslactones A-E, further oxygenated terpenoids from Kadsura angustifolia fermented by a symbiotic endophytic fungus, Penicillium ochrochloron SWUKD4.1850. Phytochemistry 2020, 174, 112335. [Google Scholar] [CrossRef]
- Huang, Q.; An, H.M.; Song, H.C.; Mao, H.Q.; Shen, W.Y.; Dong, J.Y. Diversity and biotransformative potential of endophytic fungi associated with the medicinal plant Kadsura angustifolia. Res. Microbiol. 2015, 166, 45–55. [Google Scholar] [CrossRef]
- Isaka, M.; Yangchum, A.; Intamas, S.; Kocharin, K.; Gareth Jones, E.B.; Kongsaeree, P.; Prabpai, S. Aigialomycins and related polyketide metabolites from the mangrove fungus Aigialus parvus BCC 5311. Tetrahedron 2009, 65, 4396–4403. [Google Scholar] [CrossRef]
- Isaka, M.; Suyarnsestakorn, C.; Tanticharoen, M. Aigialomycins A-E, New resorcylic macrolides from the marine mangrove fungus Aigialus parvus. J. Org. Chem. 2002, 67, 1561–1566. [Google Scholar] [CrossRef]
- Sugawara, F.; Kim, K.W.; Kobayashi, K.; Uzawa, J.; Strobel, G.A. Zearalenone derivatives produced by the fungus Drechslera portulacae. Phytochemistry 1992, 31, 1987–1990. [Google Scholar] [CrossRef]
- Sloan, A.; Tyler, N.G.; Audrey, F.A.; David, J.K.; Susan, M.; Esperanza, J.C.B.; Shen, Q.; Steven, M.S.; Mansukh, C.W.; Cedric, J.P.; et al. Resorcylic acid lactones with cytotoxic and nf-κb inhibitory activities and their structure-activity relationships. J. Nat. Prod. 2011, 74, 1126–1131. [Google Scholar]
- Song, H.C.; Qin, D.; Liu, H.Y.; Dong, J.Y.; You, C.; Wang, Y.M. Resorcylic acid lactones produced by an endophytic Penicillium ochrochloron strain from Kadsura angustifolia. Planta Med. 2020, 87, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Shi, Y.; Si, H.; Luo, H.; Chen, S.; Wang, Z.; He, H.; Liao, S. Synthesis, antifungal activity and action mechanism of novel citral amide derivatives against. Pest Manag. Sci. 2024, 80, 4482–4494. [Google Scholar] [CrossRef]
- Li, Y.; Mu, Y.; Cao, Y.; Xu, D.; Liu, X.; Xu, G. Synthesis and evaluation of novel 1-methyl-1h-pyrazol-5-amine derivatives with disulfide moieties as potential antimicrobial agents. J. Agric. Food Chem. 2024, 72, 20658–20669. [Google Scholar] [CrossRef]
- Bao, A.L.; Xie, X.S.; Wang, D.Y.; Deng, Z.Q.; Chen, Y.; Liu, D.; Li, W.Y.; Tang, X.R.; Cheng, W.; Yan, Y.K. Design, synthesis and antifungal activity of novel pyrazole-amide -isothiazole derivatives as succinate dehydrogenase inhibitors. Food Chem. 2025, 464, 41465–141478. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Seo, Y.H.; Lee, S.; Shin, E.; Yeon, S.W.; Kim, S.B.; Lee, M.K. Antioxidant and tyrosinase-inhibitory activities and biological bioactivities of flavonoid derivatives from Quercus mongolica pollen. Molecules 2025, 30, 794. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Cimmino, A.; Maddau, L.; Masi, M.; Evidente, M.; Linaldeddu, B.T.; Evidente, A. Further secondary metabolites produced by Diplodia corticola, a fungal pathogen involved in cork oak decline. Tetrahedron 2016, 72, 6788–6793. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Yokoyama, E.; Takahashi, T.; Uzawa, J.; Morooka, N.; Tsunoda, H.; Tatsuno, T. Studies on the metabolites of Penicillium diversum var. aureum. Chem. Pharm. Bull. 1986, 34, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.R.; Qin, X.; Lin, X.P.; Wang, J.F.; Kaliyaperumal, K.; Tian, Y.Q.; Liu, J.; Liu, F.; Tu, Z.; Tu, Z.; et al. New phenyl derivatives from endophytic fungi Botryosphaeria sp. scsio kcf6 derived of mangrove plant Kandelia candel. Nat. Prod. Res. 2016, 30, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.Z.; Varma, A.; Qin, S.; Li, W.J. An endophytic Pseudonocardia species induces the production of artemisinin in Artemisia annua. PLoS ONE 2012, 7, e51410. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.M.; Shang, Z.; Li, C.S.; Wang, B.G. Chemical constituents of Penicillium sp. MA-37, a fungi derived from rhizospheres of mangrove plant Bruguiera gymnorrhiza. Mar. Sci. 2013, 37, 7–11. [Google Scholar]
- Wang, K.; Bao, L.; Ma, K.; Qi, M.; Song, F.; Yao, Y.-J.; Yin, W.-B.; Zhang, L.; Huang, Y.; Han, J.; et al. Bioactive spirobisnaphthalenes and lactones from a cup fungi Plectania sp. collected in the Tibet plateau region. Eur. J. Org. Chem. 2016, 2016, 4338–4346. [Google Scholar] [CrossRef]
- Meng, Z.H.; Xu, L.L.; Zhu, H.J.; Cao, F. Steroids and polyketides from the soil fungi Penicillium janthinellum xl-7. Chem. Nat. Comp. 2020, 56, 1–3. [Google Scholar] [CrossRef]
- Kim, K.H.; Beemelmanns, C.; Murillo, C.; Guillén, A.; Umaña, L.; Tamayo, G.; Kim, S.-N.; Clardy, J.; Cao, S. Naphthalenones and isocoumarins from a costarican fungi Xylariaceae sp. cr1546c. J. Chem. Res. 2014, 12, 722–725. [Google Scholar] [CrossRef]
- El-Elimat, T.; Figueroa, M.; Raja, H.A.; Graf, T.N.; Swanson, S.M.; Falkinham, J.O.; Oberlies, N.H. Biosynthetically distinct cytotoxic polyketides from Setophoma terrestris. Eur. J. Org. Chem. 2014, 2015, 109–121. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.X.; Chen, Y.C.; Sun, Z.H.; Li, H.H.; Li, S.N.; Zhang, W.M. Two new metabolites from the endophytic fungus Alternaria sp. a744 derived from Morinda officinalis. Molecules 2017, 22, 765. [Google Scholar] [CrossRef]
- Harris, J.P.; Mantle, P.G. Biosynthesis of diaporthin and orthosporin by Aspergillus ochraceus. Phytochemistry 2001, 57, 165–169. [Google Scholar] [CrossRef]
- Gu, W.; Ge, H.M.; Song, Y.C.; Ding, H.; Zhu, H.L.; Zhao, X.A.; Tan, R.X. Cytotoxic benzofluoranthene metabolites from Hypoxylon truncatum ifb-18, an endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wu, C.M. Gut microbiota brings a novel way to illuminate mechanisms of natural products in vivo. Chin. Herb. Med. 2017, 9, 301–306. [Google Scholar] [CrossRef]
- Zhang, D.P.; Zhou, X.L.; MA, G.X.; Song, H.L.; Shi, L.L.; Wei, H.Y. A new lignin from stems of Trigonostemon lutescens. Chin. Tradit. Herb. Drugs 2020, 51, 3633–3636. [Google Scholar]
- Moon, S.S.; Rahman, A.A.; Kim, J.Y.; Kee, S.H. Hanultarin, a cytotoxic lignan as an inhibitor of actin cytoskeleton polymerization from the seeds of Trichosanthes kirilowii. Bioorg. Med. Chem. 2008, 16, 7264–7269. [Google Scholar] [CrossRef]
- Xiao, W.L.; Zhu, H.J.; Shen, Y.H.; Li, R.T.; Li, S.H.; Sun, H.D.; Zheng, Y.T.; Wang, R.R.; Lu, Y.; Wang, C.; et al. Lancifodilactone G: A unique nortriterpenoid isolated from Schisandra lancifolia and its anti-HIV activity. Org. Lett. 2005, 7, 2145–2148. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Chen, Y.G.; Song, H.C.; He, Y.P.; Li, L.; Zhong, Y.P.; Zhu, Y.H.; Cao, J.; Wang, L.; Zhang, K.Q. Hydroxylation of nigranoic acid to 6β-hydroxynigranoic acid by Caryospora carllicarpa YMF1.01026. Chin. Chem. Lett. 2007, 18, 165–167. [Google Scholar] [CrossRef]
- Dong, J.Y.; Chen, Y.G.; Song, H.C.; Zhu, Y.H.; Zhou, Y.P.; Li, L.; He, Y.P.; Cao, J.; Zhang, K.Q. Hydroxylation of the triterpenoid nigranoic acid by the fungus Gliocladium roseum YMF1.00133. Chem. Biodivers. 2007, 4, 112–117. [Google Scholar] [CrossRef]
- Sun, H.D.; Qiu, S.X.; Lin, L.Z.; Wang, Z.Y.; Lin, Z.W.; Pengsuparp, T.; Pezzuto, J.M.; Fong, H.H.S.; Cordell, G.A.; Farnsworth, N.R. Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J. Nat. Prod. 1996, 59, 525–527. [Google Scholar] [CrossRef]
- Aziz, B.N.; Abdel-Azeem, A.M. Antifungal activity of secondary metabolites produced by fungi. Microb. Biosyst. J. 2024, 9, 151–159. [Google Scholar] [CrossRef]
- Dahiya, J.S.; Rimmer, S.R. Phytoalexin accumulation in tissues of Brassica napus inoculated with Leptosphaeria maculans. Phytochemistry 1988, 27, 3481. [Google Scholar] [CrossRef]
- Iwasaki, S.; Muro, H.; Sasaki, K.; Nozoe, S.; Okuda, S.; Sato, Z. Isolations of phytotoxic substances produced by Pyricularia oryzae cavara. Tetrahedron Lett. 1973, 14, 3537. [Google Scholar] [CrossRef]
- Pittayakhajonwut, P.; Sohsomboon, P.; Dramae, A.; Suvannakad, R.; Lapanun, S.; Tantichareon, M. Antimycobacterial substances from Phaeosphaeria sp BCC8292. Planta Med. 2008, 74, 281. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Chen, X.; Mei, J.; Xin, L.; Yan, G. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba. J. Nanjing Agric. Univ. 2008, 105, 548–554. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Azaphilone and isocoumarin derivatives from the endophytic fungus Penicillium sclerotiorum PSU-A13. Nat. Prod. Res. 2016, 30, 46. [Google Scholar] [CrossRef]
- Galindo-Solis, J.M.; Fernandez, F.J. Endophytic fungal terpenoids: Natural role and bioactivities. Microorganisms 2022, 10, 339. [Google Scholar] [CrossRef]
- Sonowal, S.; Gogoi, U.; Buragohain, K.; Nath, R. Endophytic fungi as a potential source of anti-cancer drug. Arch. Microbiol. 2024, 206, 122. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. Review on anti-inflammatory activity of natural products. Chemistryselect 2025, 10, e05885. [Google Scholar] [CrossRef]
- Tamam, E.E.; Mario, F.; Huzefa, A.R.; Tyler, N.G.; Audrey, F.A.; David, J.K.; Cynthia, S.D.; Mansukh, C.W.; Cedric, J.P.; Nicholas, H.O. Benzoquinones and terphenyl compounds as phosphodiesterase-4B inhibitors from a fungus of the order Chaetothyriales (MSX 47445). J. Nat. Prod. 2013, 76, 382–387. [Google Scholar]
- Devi, R.; Verma, R.; Dhalaria, R.; Kumar, A.; Kumar, D.; Puri, S.; Thakur, M.; Chauhan, S.; Chauhan, P.P.; Nepovimova, E.; et al. A systematic review on endophytic fungi and its role in the commercial applications. Planta 2023, 257, 70. [Google Scholar] [CrossRef]
- An, S.; An, J.; Lee, D.; Kang, H.N.; Kang, S.; Ahn, C.H.; Syahputra, R.A.; Ribeiro, R.I.M.A.; Kim, B. Natural products for melanoma therapy: From traditional medicine to modern drug discovery. Plants 2025, 14, 951. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhang, P.L.; Liu, J.S.; Wang, G.K.; Xu, F.Q.; Chen, L.; Yu, Y.; Wang, G. Aspergilates A to E, second metabolites from Aspergillus sp. isolated from Paeonia, ostii. Fitoterapia 2018, 131, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906–920. [Google Scholar] [CrossRef]
- Kusari, S.; Verma, V.C.; Lamshoft, M.; Spiteller, M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J. Microbiol. Biotechnol. 2012, 28, 1287–1294. [Google Scholar] [CrossRef]

| No. | Compound 20 a | ||||
|---|---|---|---|---|---|
| δH | δC | No. | δH | δC | |
| 1 | 1.01 (m); 1.04 (m) | 35.6 t | 16 | 1.86 (m); 1.26 (m) | 26.9 t |
| 2 | 2.48 (m); 2.24 (m) | 31.4 t | 17 | 1.56 (m) | 52.0 d |
| 3 | 174.5 s | 18 | 0.95 (d, 6.1) | 18.0 q | |
| 4 | 149.5 s | 19 | 0.72 (d, 4.4, βH); 0.40 (d, 4.4, αH) | 29.7 t | |
| 5 | 2.41 (m) | 45.8 d | 20 | 1.36 (m) | 35.9 d |
| 6 | 1.88 (m); 1.52 (m) | 27.7 t | 21 | 0.88 (d, 6.3) | 18.2 q |
| 7 | 2.02 (m); 1.08 (m) | 28.0 t | 22 | 1.28 (m); 1.07 (m) | 24.9 t |
| 8 | 1.54 (m) | 47.7 d | 23 | 2.01 (m); 1.34 (m) | 28.9 t |
| 9 | 21.3 s | 24 | 2.38 (m) | 44.3 d | |
| 10 | 27.0 s | 25 | 209.6 s | ||
| 11 | 2.10 (m); 2.02 (m) | 29.9 t | 26 | 2.14 (s) | 19.8 q |
| 12 | 1.26 (m); 1.05 (m) | 35.5 t | 27 | ||
| 13 | 45.1 s | 28 | 4.81 (s); 4.73 (s) | 111.5 t | |
| 14 | 48.9 s | 29 | 1.67 (s) | 19.7 q | |
| 15 | 1.64 (m); 1.36 (m) | 33.0 t | 30 | 0.92 (s) | 19.3 q |
| 3-OCH3 | 3.64 (s) | 51.5 q | |||
| Compounds | Average Inhibition Rate ± SD (%) b | |||
|---|---|---|---|---|
| V. dahliae | B. cinerea | R. solani | F. oxysporum | |
| 1 | 44.6 ± 0.5 | 14.1 ± 0.2 | 9.3 ± 0.9 | 29.1 ± 1.2 |
| 2 | 30.4 ± 0.8 | 29.5 ± 1.2 | 11.9 ± 1.1 | 35.2 ± 0.5 |
| 3 | 34.8 ± 1.0 | 7.2 ± 0.8 | 10.3 ± 0.4 | 17.6 ± 0.5 |
| 4 | 70.3 ± 0.7 | 87.4 ± 1.0 | 47.2 ± 1.1 | 50.6 ± 0.3 |
| 5 | 36.2 ± 0.3 | 10.3 ± 0 | 6.3 ± 0.7 | 15.7 ± 1.4 |
| 6 | 23.4 ± 1.1 | 47.1 ± 0.3 | 36.3 ± 1.0 | 28.1 ± 0.1 |
| 7 | 33.6 ± 0.6 | 65.2 ± 0.2 | 55.1 ± 0.5 | 32.6 ± 1.8 |
| 8 | 52.1 ± 0.7 | 13.9 ± 0 | 21.7 ± 1.2 | 7.4 ± 1.9 |
| 9 | 63.6 ± 0 | 60.2 ± 1.6 | 58.3 ± 1.3 | 35.2 ± 0.2 |
| 10 | 74.9 ± 1.2 | 78.4 ± 1.4 | 85.3 ± 0.9 | 82.5 ± 0.1 |
| 11 | 46.8 ± 1.2 | 74.8 ± 0.7 | 24.5 ± 0.1 | 87.9 ± 1.5 |
| 12 | 37.2 ± 0.5 | 70.3 ± 0.9 | 30.4 ± 0.4 | 82.6 ± 1.2 |
| 13 | 12.5 ± 0.8 | 35.4 ± 0.4 | 42.8 ± 1.6 | 7.2 ± 2.0 |
| 14 | 20.0 ± 0.8 | 23.8 ± 0 | 10.1 ± 0.2 | 22.7 ± 0.1 |
| 15 | 4.72 ± 0.51 | 3.58 ± 0.21 | 0.0 ± 0.0 | 2.04 ± 0.3 |
| 16 | 2.31 ± 0.33 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 17 | 0.0 ± 0.0 | 7.34 ± 0.3 | 13.5 ± 1.2 | 0.0 ± 0.0 |
| 18 | 0.0 ± 0.0 | 0.0 ± 0.0 | 6.1 ± 0.8 | 0.0 ± 0.0 |
| 19 | 0.0 ± 0.0 | 5.6 ± 0.8 | 0.0 ± 0.0 | 7.6 ± 0.1 |
| 20 | 9.1 ± 1.3 | 7.2 ± 1.2 | 0.0 ± 0.0 | 3.6 ± 0.8 |
| carbendazim c | 98.5 ± 0.3 | 100 ± 0.0 | 100 ± 0.0 | 93.1 ± 1.3 |
| boscalid c | 72.6 ± 0.4 | 86.3 ± 0.1 | 95.1 ± 0.6 | 76.8 ± 0.6 |
| Compounds | 100 μM | Compounds | 100 μM |
|---|---|---|---|
| 1 | 30.1 ± 0.2 | 12 | 59.5 ± 0.9 |
| 2 | 53.9 ± 0.8 | 13 | 60.4 ± 0.2 |
| 3 | 33.5 ± 1.2 | 14 | 62.1 ± 2.3 |
| 4 | 42.9 ± 0.2 | 15 | 25.7 ± 1.6 |
| 5 | 43.1 ± 1.6 | 16 | 21.8 ± 2.1 |
| 6 | 50.9 ± 2.3 | 17 | 62.1 ± 3.4 |
| 7 | 68.4 ± 0.8 | 18 | >100 |
| 8 | 29.7 ± 0.5 | 19 | >100 |
| 9 | 52.1 ± 1.9 | 20 | >100 |
| 10 | 80.2 ± 1.8 | Vitamin E b | 58.8 ± 0.6 |
| 11 | 60.6 ± 0.7 | Vitamin C b | 35.7 ± 0.8 |
| Compounds | HL-60 | SMMC-7721 | A549 | MCF-7 |
|---|---|---|---|---|
| 1 | 35.1 ± 0.6 | 39.2 ± 0.5 | 38.7 ± 0.1 | 38.7 ± 0.8 |
| 2 | >40 | >40 | >40 | >40 |
| 3 | 30.1 ± 1.2 | 35.8 ± 3.8 | >40 | 37.2 ± 0.5 |
| 4 | >40 | >40 | >40 | >40 |
| 5 | >40 | >40 | >40 | >40 |
| 6 | >40 | >40 | >40 | >40 |
| 7 | >40 | >40 | >40 | >40 |
| 8 | 25.8 ± 2.6 | 38.2 ± 2.1 | 33.7 ± 1.5 | 36.4 ± 0.6 |
| 9 | >40 | >40 | >40 | >40 |
| 10 | >40 | >40 | >40 | >40 |
| 11 | >40 | >40 | >40 | >40 |
| 12 | >40 | >40 | >40 | >40 |
| 13 | >40 | >40 | >40 | >40 |
| 14 | >40 | >40 | >40 | >40 |
| 15 | 37.2 ± 0.93 | 26.5 ± 0.63 | 34.6 ± 0.37 | 38.1 ± 0.39 |
| 16 | 32.4 ± 0.25 | 23.4 ± 3.01 | 30.8 ± 1.45 | 31.2 ± 0.45 |
| 17 | >40 | >40 | >40 | >40 |
| 18 | >40 | >40 | >40 | >40 |
| 19 | >40 | >40 | >40 | >40 |
| 20 | 9.31 ± 0.04 | 6.50 ± 0.16 | >40 | 17.83 ± 0.01 |
| cisplatin b | 6.84 ± 0.01 | 5.80 ± 0.21 | 4.72 ± 0.19 | 3.66 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Qin, D. Diversity and Biological Activity of Secondary Metabolites Produced by the Endophytic Fungus Penicillium ochrochlorae. Fermentation 2025, 11, 394. https://doi.org/10.3390/fermentation11070394
Hu J, Qin D. Diversity and Biological Activity of Secondary Metabolites Produced by the Endophytic Fungus Penicillium ochrochlorae. Fermentation. 2025; 11(7):394. https://doi.org/10.3390/fermentation11070394
Chicago/Turabian StyleHu, Jian, and Dan Qin. 2025. "Diversity and Biological Activity of Secondary Metabolites Produced by the Endophytic Fungus Penicillium ochrochlorae" Fermentation 11, no. 7: 394. https://doi.org/10.3390/fermentation11070394
APA StyleHu, J., & Qin, D. (2025). Diversity and Biological Activity of Secondary Metabolites Produced by the Endophytic Fungus Penicillium ochrochlorae. Fermentation, 11(7), 394. https://doi.org/10.3390/fermentation11070394






