Valorization of Fruit Pomace by Enzymatic Treatment and Microbial Fermentation
Abstract
1. Introduction
2. The Main Components of Fruit Pomace
3. Valorization Strategies for Fruit and Vegetable Pomaces
3.1. Fruit Pomace as a Source of Fiber in Food Products
3.2. Fruit Pomace as a Source of Phenolic Compounds
3.3. Fruit Pomace as a Source of Prebiotics
4. Enzymatic Treatment and Fermentation of Fruit Pomace
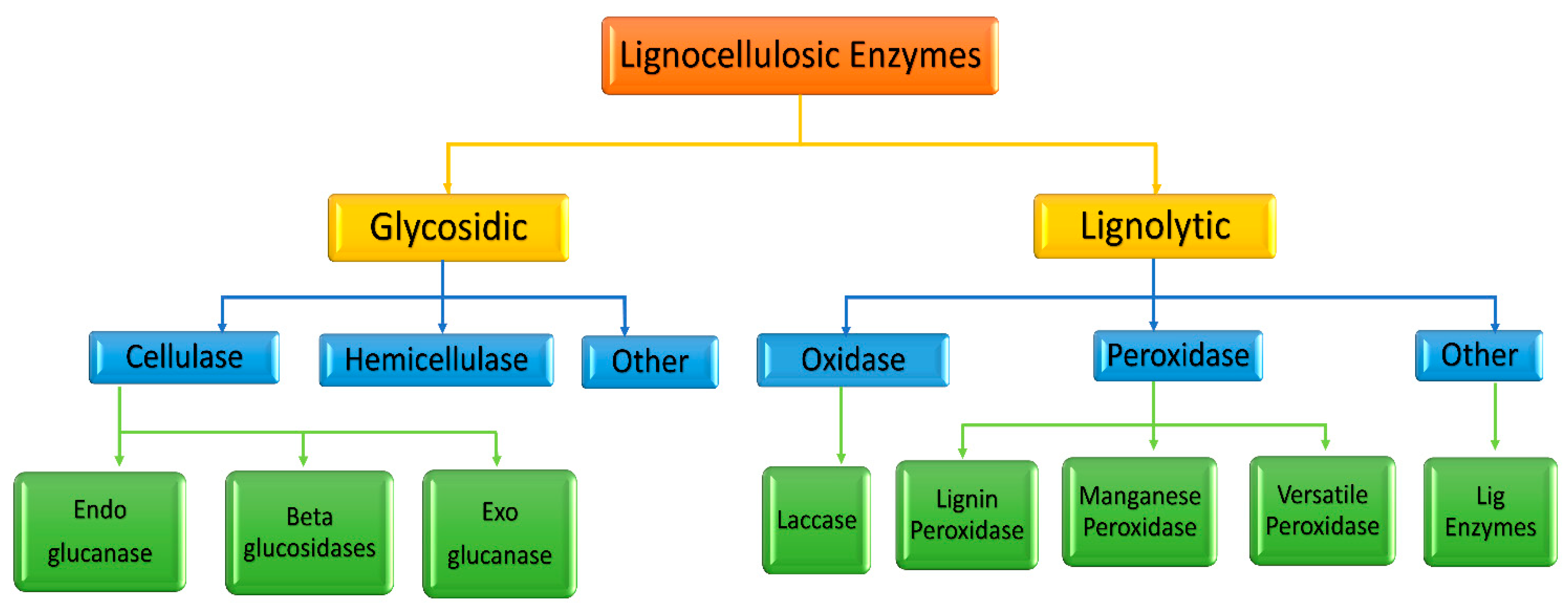
4.1. Enzymatic Valorization of Fruit Pomace
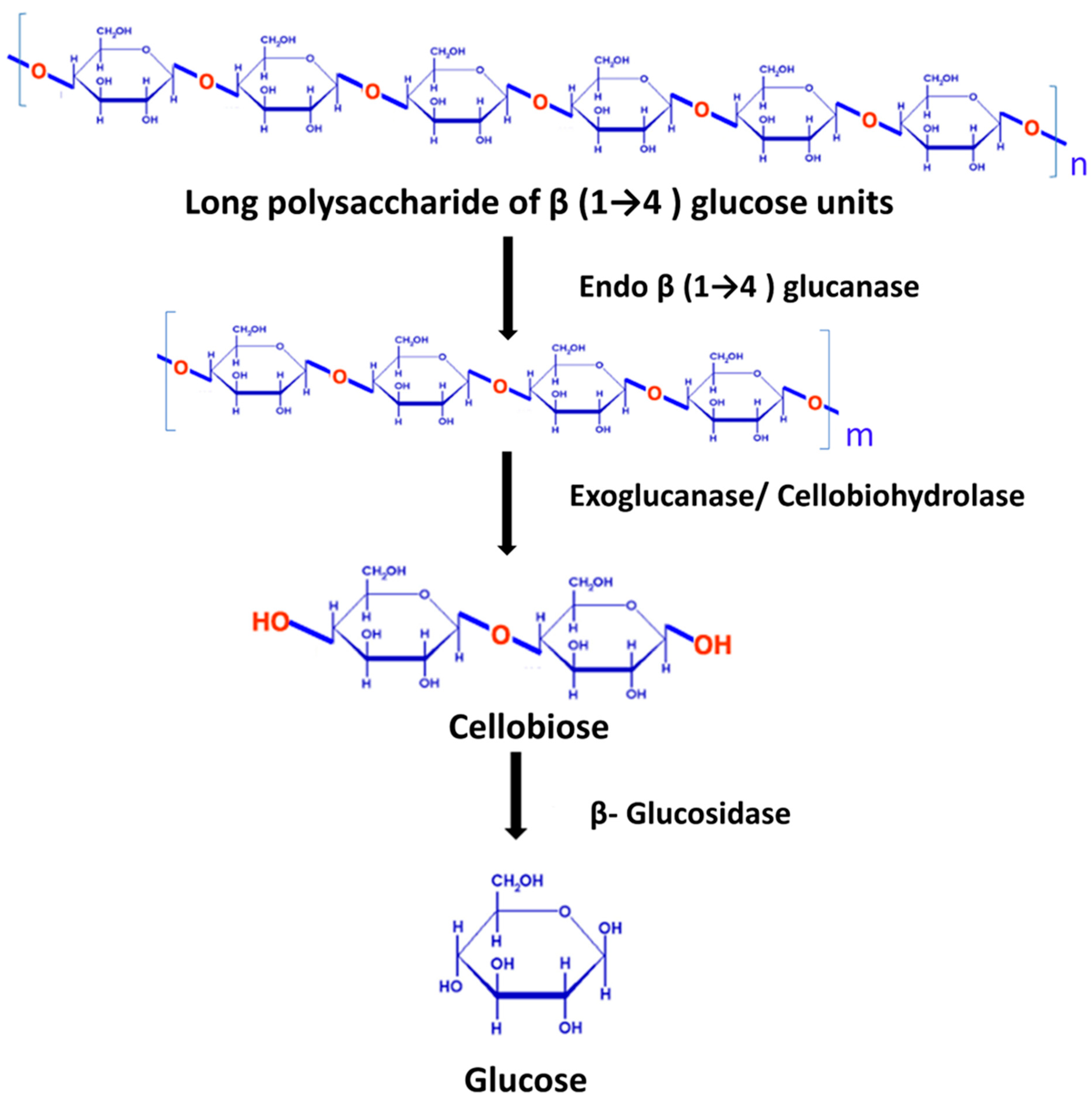
4.1.1. Cellulases
4.1.2. Hemicellulases
4.1.3. Pectinases
4.1.4. Laccases
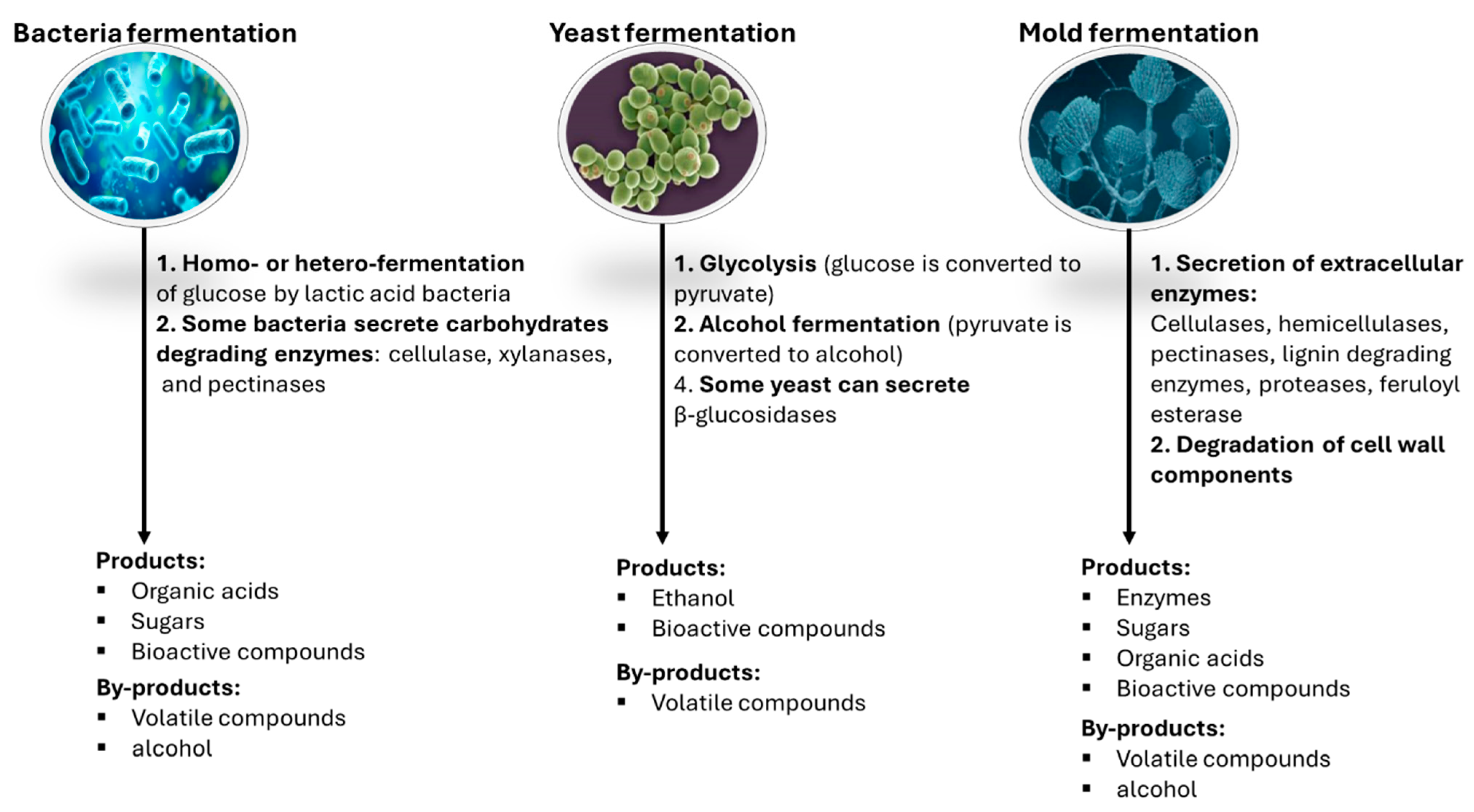
4.2. Valorization of Fruit Pomace by Microbial Fermentation
4.2.1. Bacterial Fermentation and Fermented Products
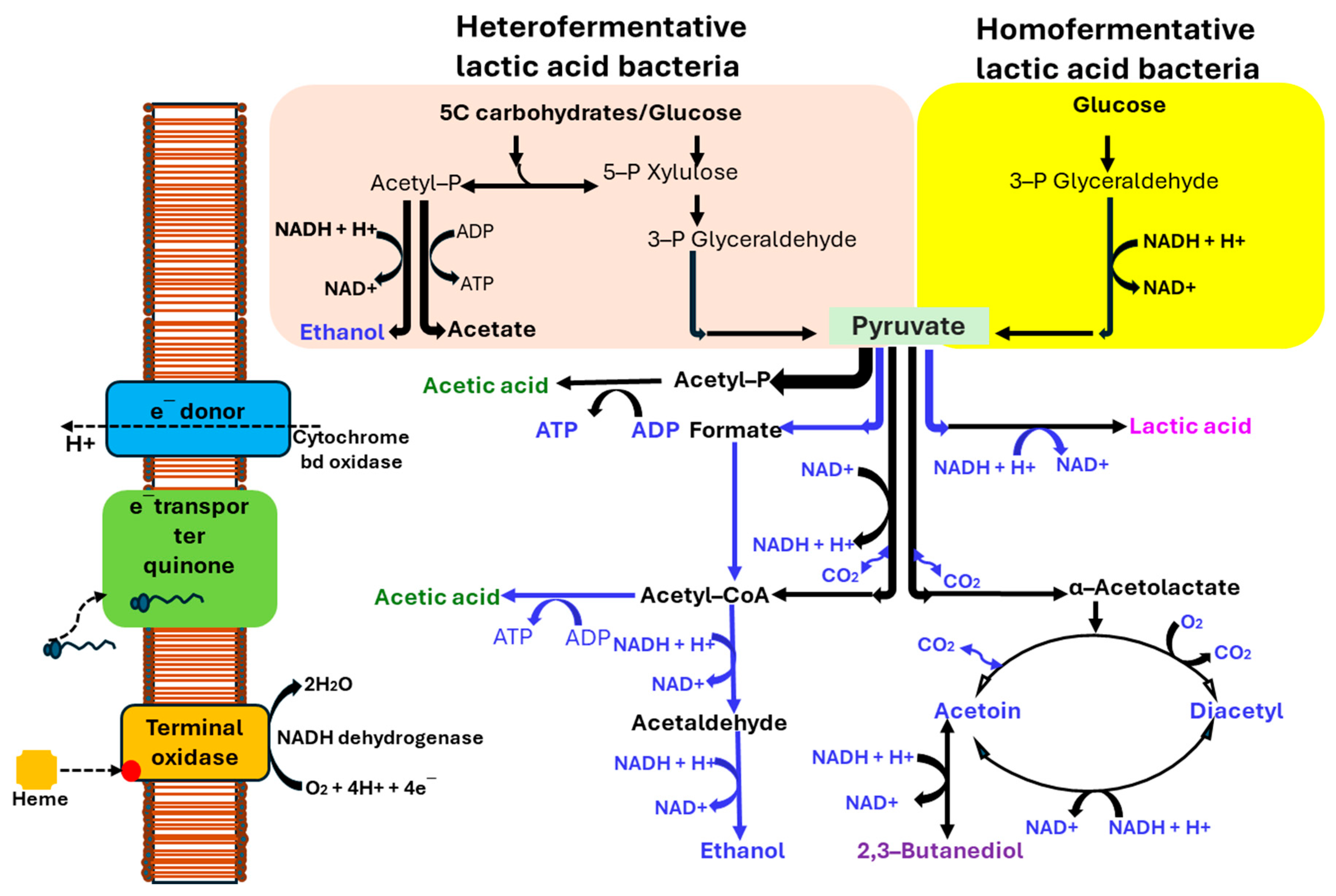
4.2.2. Yeast Fermentation and Fermented Products
4.2.3. Mold Fermentation and Fermented Products
| Pomace | Strains | Findings | References |
|---|---|---|---|
| Apple | Aspergillus aculeatus, A. japonicus, A. niger, A. tubingensis, and A. niger | Reduced quercetin and its glycosides. Increased phenolic and flavonoid content. Maximum citric acid yield (4.6 g/100 g pomace) was achieved at 5 days of fermentation, 30 °C, and with 4% methanol. | [202,204] |
| Orange | Sporotrichum thermophile Apinis, A. niger, and Paecilomyces variotii | Increased the production of polygalacturonase, pectinase, tannase, and phytase. Increased antioxidant activity. | [205,206] |
| Grape | A. awamori, Actinomucor elegans, and Umbelopsis isabellina | Substrate particle size did not affect enzyme production. High moisture substrate had lower enzyme activity. Phenolics, flavonoids, and antioxidant activity were higher in A. elegans fermented pomace. | [198,207] |
| Olive | Pleurotus ostreatus, P. pulmonarius, A. niger, and A. ibericus | Improved protein content and reduced polyphenols and hemicellulose. Increased enzymes (xylanase and cellulase) production. | [193,208,209] |
| Tomato | A. awamori, P. ostreatus, and Phanerochaete chrysosporium | Produced higher xylanase compared to exo-polygalacturonase, cellulase, and α-amylase. Aeration during fermentation enhanced xylanase and cellulase production but inhibited exo-polygalacturonase and α-amylase. | [189,210] |
| Dates | A. niger | Efficiently produced endopectinase and increased phenolics and flavonoids. | [211] |
| Pear | A. niger and A. oryzae sp. | Enriched the bioactive compounds, particularly tannins. | [212] |
5. Technologies Used in the Enzymatic Treatment and Microbial Fermentation of Pomace
6. Challenges and Concerns of Utilization of Fruit Pomaces in Food Industries and Possible Solutions
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Agricultural Production Statistics 2000–2022; FAOSTAT Analytical Briefs, No. 79; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. 15-Agricultural and Food Industry By-Products: Source of Bioactive Components for Functional Beverages. Nutr. Bev. 2019, 12, 543–589. [Google Scholar]
- Mateus, A.R.S.; Pena, A.; Sanches-Silva, A. Unveiling the potential of bioactive compounds in vegetable and fruit by-products: Exploring phytochemical properties, health benefits, and industrial opportunities. Curr. Opin. Green. Sustain. Chem. 2024, 48, 100938. [Google Scholar] [CrossRef]
- Areti, H.A.; Muleta, M.D.; Abo, L.D.; Hamda, A.S.; Adugna, A.A.; Edae, I.T.; Daba, B.J.; Gudeta, R.L. Innovative uses of agricultural by-products in the food and beverage sector: A review. Food Chem. Adv. 2024, 5, 100838. [Google Scholar] [CrossRef]
- Rodríguez García, S.L.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Ignatia, F.; Meivira, K.; Kartawiria, I.S.; Gunawan-Puteri, M.D. Review: Nutrient, Fiber, and Bioactive Content of Fruit Pomace, Major By-product of Juice Industry. Adv. Biol. Sci. Res. 2022, 16, 214–220. [Google Scholar]
- Quiles, A.; Campbell, G.M.; Struck, S.; Rohm, H.; Hernando, I. Fiber from fruit pomace: A review of applications in cereal-based products. Food Rev. Int. 2018, 34, 162–181. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Kithama, M.; Hassan, Y.I.; Guo, K.; Kiarie, E.; Diarra, M.S. The enzymatic digestion of pomaces from some fruits for value-added feed applications in animal production. Front. Sustain. Food Syst. 2021, 5, 611259. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Y.; Wang, X.; Huang, J. Biohydrogen production from apple pomace by anaerobic fermentation with river sludge. Int. J. Hydrogen Energy 2010, 35, 3058–3064. [Google Scholar] [CrossRef]
- Malhi, N.; Carragher, J.; Saarela, M.; Pahl, S. Fight Food Waste, C.R.C. A Review of Opportunities to Recover Value from Apple and Pear Pomace; Fight Food Waste Cooperative Research Centre: Adelaide, Australia, 2021; pp. 4–29. [Google Scholar]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Nawirska, A.; Uklańska, C. Waste products from fruit and vegetable processing as potential sources for food enrichment in dietary fibre. Acta Sci. Pol. Technol. Aliment. 2008, 7, 35–42. [Google Scholar]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Rodriguez-Uribe, A.; Misra, M.; Khalil, H.; Thimmanagari, M.; Mohanty, A.K. Physicochemical Analysis of Apple and Grape Pomaces. BioResources 2019, 14, 3210–3230. [Google Scholar] [CrossRef]
- Singla, G.; Panesar, P.S.; Sangwan, R.S.; Krishania, M. Enzymatic processing of Citrus reticulata (Kinnow) pomace using naringinase and its valorization through preparation of nutritionally enriched pasta. J. Food Sci. Technol. 2021, 58, 3853–3860. [Google Scholar] [CrossRef]
- Arcia, P.; Curutchet, A.; Pérez-Pirotto, C.; Hernando, I. Upcycling fruit pomaces (orange, apple, and grape-wine): The impact of particle size on phenolic compounds’ bioaccessibility. Heliyon 2024, 10, 19. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.; Jia, X.; Zhang, N.; Fan, G.; Pan, S.; Zhang, Z.; Cao, S. Valorization of citrus pomace for cellulose extraction and double-crosslinked cellulose hydrogel fabrication. Food Hydrocoll. 2025, 167, 111442. [Google Scholar] [CrossRef]
- O’shea, N.; Ktenioudaki, A.; Smyth, T.P.; McLoughlin, P.; Doran, L.; Auty, M.A.E.; Arendt, E.; Gallagher, E. Physicochemical assessment of two fruit by-products as functional ingredients: Apple and orange pomace. J. Food Eng. 2015, 153, 89–95. [Google Scholar] [CrossRef]
- John, I.; Muthukumar, K.; Arunagiri, A. A review on the potential of citrus waste for D-Limonene, pectin, and bioethanol production. Int. J. Green. Energy 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Teles, A.S.; Chávez, D.W.; Oliveira, R.A.; Bon, E.P.; Terzi, S.C.; Souza, E.F.; Gottschalk, L.M.; Tonon, R.V. Use of grape pomace for the production of hydrolytic enzymes by solid-state fermentation and recovery of its bioactive compounds. Food Res. Int. 2019, 120, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional characterization of different industrial white and red grape pomaces in Virginia and the potential valorization of the major components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Valiente, C.; Arrigoni, E.; Esteban, R.M.; Amado, R. Grape pomace as a potential food fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and sustainable valorisation of olive pomace using a fractionation approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Oliveira, B.C.; Barbosa, C.; Costa, A.S.; Nunes, M.A.; Oliveira, M.B.P.; Alves, R.C. Pasta Incorporating Olive Pomace: Impact on Nutritional Composition and Consumer Acceptance of a Prototype. Foods 2024, 13, 2933. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Lama-Muñoz, A.; García, A.; Fernández-Bolaños, J. Properties of lignin, cellulose, and hemicelluloses isolated from olive cake and olive stones: Binding of water, oil, bile acids, and glucose. J. Agric. Food Chem. 2014, 62, 8973–8981. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; del Mar Contreras, M.; Romero, I.; Castro, E. A biorefinery approach to obtain antioxidants, lignin and sugars from exhausted olive pomace. J. Ind. Eng. Chem. 2021, 96, 356–363. [Google Scholar] [CrossRef]
- Jamaleddine, A.; De Caro, P.; Bouajila, J.; Evon, P.; Haddad, J.G.; El-Kalamouni, C.; Hijazi, A.; Merah, O. In vitro bioactivities of extracts from tomato pomace. Front. Biosci. 2022, 27, 259. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, M.; Cámara, M.; Torija, M.E. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Eslami, E.; Carpentieri, S.; Pataro, G.; Ferrari, G. A comprehensive overview of tomato processing by-product valorization by conventional methods versus emerging technologies. Foods 2022, 12, 166. [Google Scholar] [CrossRef]
- Shao, D.; Atungulu, G.G.; Pan, Z.; Yue, T.; Zhang, A.; Chen, X. Separation methods and chemical and nutritional characteristics of tomato pomace. Trans. ASABE 2013, 56, 261–268. [Google Scholar] [CrossRef]
- Haris, S.; Alam, M.; Galiwango, E.; Mohamed, M.M.; Kamal-Eldin, A.; Al-Marzouqi, A.H. Characterization analysis of date fruit pomace: An underutilized waste bioresource rich in dietary fiber and phenolic antioxidants. Wast. Manag. 2023, 163, 34–42. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Oladzad, S.; Fallah, N.; Mahboubi, A.; Afsham, N.; Taherzadeh, M.J.; Toghyani, J. Comparison of acid and hydrothermal pretreatments of date waste for value creation. Sci. Rep. 2024, 14, 18056. [Google Scholar] [CrossRef]
- Almoumen, A.; Mohamed, H.; Ayyash, M.; Yuliarti, O.; Kamleh, R.; Al-Marzouqi, A.H.; Kamal-Eldin, A. Harnessing date fruit pomace: Extraction of high fibre dietary ingredient and its impact on high fibre wheat flour dough. NFS J. 2024, 35, 100178. [Google Scholar] [CrossRef]
- Stojanovska, L.; Ali, H.I.; Kamal-Eldin, A.; Souka, U.; Al Dhaheri, A.S.; Cheikh Ismail, L.; Hilary, S. Soluble and insoluble dietary fibre in date fruit varieties: An evaluation of methods and their implications for human health. Foods 2023, 12, 1231. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, H.; Wang, H.; Yu, H. Coproduction of bacterial cellulose and pear vinegar by fermentation of pear peel and pomace. Bioproc. Biosyst. Eng. 2021, 44, 2231–2244. [Google Scholar] [CrossRef]
- Tsegay, Z.T.; Sathyanarayana, C.B.; Lemma, S.M. Optimization of cactus pear fruit fermentation process for wine production. Foods 2018, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Sahni, P.; Shere, D.M. Utilization of fruit and vegetable pomace as functional ingredient in bakery products: A review. Asia. J. Dairy Food Res. 2018, 37, 202–211. [Google Scholar]
- Venkidasamy, B.; Samynathan, R.; Ramasamy, P.; Kumar, M.S.; Thiruvengadam, M.; Khayrullin, M.; Shariati, M.A.; Nile, A.S.; Nile, S.H. Unveiling novel applications of fruit pomace for sustainable production of value-added products and health benefits: A review. Food Biosci. 2024, 61, 104533. [Google Scholar] [CrossRef]
- Ikusika, O.O.; Akinmoladun, O.F.; Mpendulo, C.T. Enhancement of the Nutritional Composition and Antioxidant Activities of Fruit Pomaces and Agro-Industrial Byproducts through Solid-State Fermentation for Livestock Nutrition: A Review. Fermentation 2024, 10, 227. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, T.; Sun, L.; Qiao, Z.; Pan, H.; Zhong, Y.; Zhuang, Y. Recent advances of fermented fruits: A review on strains, fermentation strategies, and functional activities. Food Chem. X 2024, 22, 101482. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of Freeze-Drying on Apple Pomace and Pomegranate Peel Powders Used as a Source of Bioactive Ingredients for the Development of Functional Yogurt. J. Food Qual. 2022, 2022, 3327401. [Google Scholar] [CrossRef]
- Granucci, N.; Harris, P.J.; Villas-Boas, S.G. Chemical compositions of fruit and vegetable pomaces from the beverage industries. Waste Biomass Valorization 2023, 14, 3841–3856. [Google Scholar] [CrossRef]
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.R.; Jones, C.L.; Huhnke, R.L. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour. Technol. 2012, 114, 663–669. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple pomace as a potential ingredient for the development of new functional foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Alves Cayres, C.; Luis Ramírez Ascheri, J.; Antonieta Peixoto Gimenes Couto, M.; Lopes Almeida, E. Whole-grain sorghum, orange pomace, and whey blends as a novel gluten-free pregelatinized ingredient: Assessment of physicochemical and pasting properties (sorghum-based pregelatinized flour). J. Food Process. Preserv. 2021, 45, e15014. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid-and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Panaite, T.D.; Ropota, M.; Turcu, R.; Trandafir, I.; Corbu, A.R. Nutritional and bioactive compounds in dried tomato processing waste. CyTA-J. Food 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Almeida, P.V.; Gando-Ferreira, L.M.; Quina, M.J. Tomato Residue Management from a Biorefinery Perspective and towards a Circular Economy. Foods 2024, 13, 1873. [Google Scholar] [CrossRef]
- Alhuthayli, H.F.; Ahmed, M.; Al Kharashi, N.A.; Al-Jasass, F.M.; Osman, M.A. Correlation of in vitro digestibility with chemical composition and bioaccessibility of phenolic compounds in date pomace and antioxidant potential. CyTA-J. Food. 2024, 22, 2405631. [Google Scholar] [CrossRef]
- Fernandes, A.; Simões, S.; Ferreira, I.M.; Alegria, M.J.; Mateus, N.; Raymundo, A.; de Freitas, V. Upcycling rocha do oeste pear pomace as a sustainable food ingredient: Composition, rheological behavior and microstructure alone and combined with yeast protein extract. Molecules 2022, 28, 179. [Google Scholar] [CrossRef]
- Krajewska, A.; Dziki, D. Utilization of pear pomace as a functional additive in biscuit production: Physicochemical and sensory evaluation. Int. Agrophys. 2025, 39, 53–60. [Google Scholar] [CrossRef]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Brodowska, A.J. Raspberry pomace-composition, properties and application. Eur. J. Biol. Res. 2017, 7, 86–96. [Google Scholar]
- Čechovičienė, I.; Šlepetienė, A.; Gumbytė, M.; Paulauskienė, A.; Tarasevičienė, Ž. Composition and Physicochemical Properties of Pomace of Various Cultivars of Blackberry (Rubus fruticosus L.). Horticulturae 2023, 10, 38. [Google Scholar] [CrossRef]
- Pakulska, A.; Kawecka, L.; Galus, S. Physical properties of selected fruit fibre and pomace in the context of their sustainable use for food applications. Appl. Sci. 2024, 14, 9051. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Fidriyanto, R.; Singh, B.P.; Manju, K.M.; Widyastuti, Y.; Goel, G. Multivariate analysis of structural and functional properties of fibres from apple pomace using different extraction methods. Food Prod. Process. Nutr. 2023, 5, 6. [Google Scholar] [CrossRef]
- Ye, H.; Shen, J.; Deng, S.; Yu, W. Mechanistic Insights into Lignin’s Inhibition of Gluten Digestibility: Evidence from In Vitro Static Digestion. J. Agric. Food Chem. 2024, 72, 28084–28092. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Paquot, M.; Wathelet, B. Comparative study of alkaline extraction process of hemicelluloses from pear pomace. Biomass Bioenerg. 2014, 61, 254–264. [Google Scholar] [CrossRef]
- Lochab, B.; Shukla, S.; Varma, I.K. Naturally occurring phenolic sources: Monomers and polymers. RSC Adv. 2014, 4, 21712–21752. [Google Scholar] [CrossRef]
- Vergara-Valencia, N.; Granados-Pérez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Pérez, L.A. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT Food Sci. Technol. 2007, 40, 722–729. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Chen, L.; Yu, Z.; Yang, Y. Structure, physicochemical property, and functional activity of dietary fiber obtained from pear fruit pomace (Pyrus ussuriensis Maxim) via different extraction methods. Foods 2022, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tan, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Nagarajan, J.; Pui Kay, H.; Krishnamurthy, N.P.; Ramakrishnan, N.R.; Aldawoud, T.M.; Galanakis, C.M.; Wei, O.C. Extraction of carotenoids from tomato pomace via water-induced hydrocolloidal complexation. Biomolecules 2020, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods–a review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Alam, M.Z.; Ramachandran, T.; Antony, A.; Hamed, F.; Ayyash, M.; Kamal-Eldin, A. Melanin is a plenteous bioactive phenolic compound in date fruits (Phoenix dactylifera L.). Sci. Rep. 2022, 12, 6614. [Google Scholar] [CrossRef] [PubMed]
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Jeevitha, G.C.; Ramamoorthy, S.; Ahmad, F.; Saravanan, R.; Haque, S.; Capanoglu, E. Recent advances in extraction methodologies for the valorization of mango peel wastes. Int. J. Food Prop. 2023, 26, 3492–3511. [Google Scholar] [CrossRef]
- Pradhan, A.; Sharma, L.; Bhutia, S.G.; Sherpa, N.D. Characterization of essential oil from the peel of three citrus species grown in Sikkim Himalaya. J. Appl. Hortic. 2019, 21, 157–163. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Shrestha, S.; Rahman, M.S.; Qin, W. New insights in pectinase production development and industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 9069–9087. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zeng, J.; Shen, F.; Xia, X.; Tian, X.; Wu, Z. Citrus pomace fermentation with autochthonous probiotics improves its nutrient composition and antioxidant activities. LWT 2022, 157, 113076. [Google Scholar] [CrossRef]
- Vlad, C.C.; Păcularu-Burada, B.; Vasile, A.M.; Milea, Ș.A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Upgrading the functional potential of apple pomace in value-added ingredients with probiotics. Antioxidants 2022, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and functional properties of water-soluble dietary fiber from apple pomace. J. Food Process Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Almoumen, A.; Mohamed, H.; Sobti, B.; Ayyash, M.; Kamleh, R.; Al-Marzouqi, A.H.; Kamal-Eldin, A. Quality of bread rolls fortified with date fruit pomace: Structure, proximate composition, staling, and sensory evaluation. NFS J. 2025, 38, 100214. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Havrlentová, M.; Ivanišová, E.; Mezey, J.; Tóthová, Z.; Gabriny, L.; Kačániová, M. Selected physico-chemical, nutritional, antioxidant and sensory properties of wheat bread supplemented with apple pomace powder as a by-product from juice production. Plants 2022, 11, 1256. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef]
- Bchir, B.; Rabetafika, H.N.; Paquot, M.; Blecker, C. Effect of Pear, Apple and Date Fibres from Cooked Fruit By-products on Dough Performance and Bread Quality. Food Bioprocess Technol. 2014, 7, 1114–1127. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Górnaś, P.; Segliņa, D.; Pilarska, A.; Jesionowski, T. Physical and bioactive properties of muffins enriched with raspberry and cranberry pomace powder: A promising application of fruit by-products rich in biocompounds. Plant Foods Hum. Nutr. 2016, 71, 165–173. [Google Scholar] [CrossRef]
- Antoniolli, A.; Becerra, L.; Piccoli, P.; Fontana, A. Phenolic, Nutritional and Sensory Characteristics of Bakery Foods Formulated with Grape Pomace. Plants 2024, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.A.; Nguyen, H.H.; Ton, N.M.N.; Tran, T.T.T.; Le, V.V.M. Enzymatic treatment of pineapple pomace and its application into fiber-rich biscuit making. J. Agric. Food Res. 2024, 15, 100936. [Google Scholar] [CrossRef]
- Nguyen, T.P.T.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Use of cashew apple pomace powder in pasta making: Effects of powder ratio on the product quality. Pol. J. Food Nutr. Sci. 2023, 73, 50–58. [Google Scholar] [CrossRef]
- dos Santos, S.S.; Paraíso, C.M.; Romanini, E.B.; Correa, V.G.; Peralta, R.M.; da Costa, S.C.; Junior, O.D.O.S.; Visentainer, J.V.; Reis, M.H.M.; Madrona, G.S. Bioavailability of blackberry pomace microcapsules by using different techniques: An approach for yogurt application. Innov. Food Sci. Emerg. Technol. 2022, 81, 103111. [Google Scholar] [CrossRef]
- Richards, J.; Lammert, A.; Madden, J.; Cahn, A.; Kang, I.; Amin, S. Addition of Carrot Pomace to Enhance the Physical, Sensory, and Functional Properties of Beef Patties. Foods 2024, 13, 3910. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, Y.; Wang, Y.; Shi, T.; Zhang, L.; Chen, Y.; Xie, M. Comparison of (poly) phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019, 271, 425–432. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbosa, P.D.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids—An application to orange peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Özkan, G.; Sagdiç, O.; Göktürk Baydar, N.; Kurumahmutoglu, Z. Antibacterial activities and total phenolic contents of grape pomace extracts. J. Sci. Food Agric. 2004, 84, 1807–1811. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of phenolic compounds from fresh apple pomace by different non-conventional techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef]
- Krajewska, A.; Dziki, D.; Yilmaz, M.A.; Özdemir, F.A. Physicochemical properties of dried and powdered pear pomace. Molecules 2024, 29, 742. [Google Scholar] [CrossRef] [PubMed]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an extract rich in phenolic compounds from olive pomace by pressurized liquid extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of apple pomace extracts as a source of bioactive compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Natural deep eutectic solvents and ultrasound-assisted extraction for the recovery of antioxidant phenolic compounds from orange pomace. Microchem. J. 2025, 212, 113366. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel processes for the extraction of phenolic compounds from olive pomace and their protection by encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.; Vasyliev, G. Extraction of phenolic compounds from tomato pomace using choline chloride–based deep eutectic solvents. J. Food Meas. Charact. 2022, 16, 1087–1104. [Google Scholar] [CrossRef]
- Vasyliev, G.; Lyudmyla, K.; Hladun, K.; Skiba, M.; Vorobyova, V. Valorization of tomato pomace: Extraction of value-added components by deep eutectic solvents and their application in the formulation of cosmetic emulsions. Biomass Convers. Biorefinery 2022, 12, 95–111. [Google Scholar] [CrossRef]
- Ferreira, J.; Tkacz, K.; Turkiewicz, I.P.; Santos, M.I.; Belas, A.; Lima, A.; Wojdyło, A.; Sousa, I. Influence of particle size and extraction methods on phenolic content and biological activities of pear pomace. Foods 2023, 12, 4325. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Tkacz, K.; Turkiewicz, I.P.; Santos, I.; Camoesase Silva, M.; Lima, A.; Sousa, I. Exploring the Bioactive Properties and Therapeutic Benefits of Pear Pomace. Antioxidants 2024, 13, 784. [Google Scholar] [CrossRef] [PubMed]
- Petrov Ivanković, A.; Ćorović, M.; Milivojević, A.; Simović, M.; Banjanac, K.; Veljković, M.; Bezbradica, D. Berries pomace valorization: From waste to potent antioxidants and emerging skin prebiotics. Int. J. Fruit Sci. 2024, 24, 85–101. [Google Scholar] [CrossRef]
- Pop, C.; Suharoschi, R.; Pop, O.L. Dietary fiber and prebiotic compounds in fruits and vegetables food waste. Sustainability 2021, 13, 7219. [Google Scholar] [CrossRef]
- de Oliveira, F.L.; Arruda, T.Y.P.; Morzelle, M.C.; Pereira, A.P.A.; Casarotti, S.N. Fruit by-products as potential prebiotics and promising functional ingredients to produce fermented milk. Food Res. Int. 2022, 161, 111841. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Gehlot, R.; Singh, D.; Chaudhary, T. Probiotics and prebiotics from fruit waste. In Adding Value to Fruit Wastes; Academic Press: Cambridge, MA, USA, 2024; pp. 261–290. [Google Scholar]
- Mall, U.P.; Patel, V.H. Carrot pomace powder: A promising source of polyphenols and prebiotics for improving gut health. Nutrire 2024, 49, 9. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of mango, apple and banana fruit peels as prebiotics and functional ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Hao, C.L.; Esah, E.M.; Tajarudin, H.A.; Akter, B.; Salleh, R.M. Effect of potential prebiotics from selected fruits peel on the growth of probiotics. J. Food Process. Preserv. 2021, 45, e15581. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Li, L.; Liu, H. Comparison the structural, physicochemical, and prebiotic properties of litchi pomace dietary fibers before and after modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Antón, M.J.; Moreno, F.J.; Riestra, S.; Margolles, A.; Ruiz, L. Prebiotic potential of apple pomace and pectins from different apple varieties: Modulatory effects on key target commensal microbial populations. Food Hydrocoll. 2022, 133, 107958. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Huang, Q.; Liu, G.; Li, C. Phytochemical profile, bioactivity and prebiotic potential of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber during in vitro digestion and fermentation. Food Funct. 2022, 13, 8880–8891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Yu, C.; Lu, S.; Xiong, S.; Yuan, Y. Continuous self-cycling fermentation leads to economical lycopene production by Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, M.C.; Ramos, C.L.; Kuddus, M.; Rodriguez-Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic potential for the valorization of agro-industrial by-products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-waste technology through the enzymatic hydrolysis of agro-industrial by-products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-assisted extraction of plants for sustainable and functional applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Comp. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Thitiratsakul, B.; Anprung, P. Prebiotic activity score and bioactive compounds in longan (Dimocarpus longan Lour.): Influence of pectinase in enzyme-assisted extraction. J. Food Sci. Technol. 2014, 51, 1947–1955. [Google Scholar]
- Wolski, P.; Blankenship, B.W.; Umar, A.; Cabrera, M.; Simmons, B.A.; Sale, K.L.; Achinivu, E.C. Factors that influence the activity of biomass-degrading enzymes in the presence of ionic liquids—A review. Front. Energy Res. 2023, 11, 1212719. [Google Scholar] [CrossRef]
- Will, F.; Bauckhage, K.; Dietrich, H. Apple pomace liquefaction with pectinases and cellulases: Analytical data of the corresponding juices. Eur. Food Res. Technol. 2000, 211, 291–297. [Google Scholar] [CrossRef]
- Alonso, J.L.; Garrote, G.; Domínguez, H.; Santos, V.; Parajó, J.C. Lactic acid from apple pomace: A laboratory experiment for teaching valorisation of wastes. CyTA-J. Food. 2009, 7, 83–88. [Google Scholar] [CrossRef]
- Parmar, I.; Rupasinghe, H.V. Bio-conversion of apple pomace into ethanol and acetic acid: Enzymatic hydrolysis and fermentation. Bioresour. Technol. 2013, 130, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Sultana, M.S.; Kurnianta, H. Polyphenolic contents and antioxidant activities of underutilized grape (Vitis vinifera L.) pomace extracts. Prev. Nutr. Food Sci. 2015, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Costoya, N.; Sineiro, J.; Pinelo, M.; Rubilar, M.; Nuñez, M.J. Enzyme-aided extraction of polyphenols from grape pomace. Electron. J. Env. Agric. Food Chem. 2010, 9, 696–705. [Google Scholar]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of apple pomace by extraction of valuable compounds. Comp. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Application of Celluclast 1.5 L in apple pectin extraction. Carbohydr. Polym. 2015, 134, 251–257. [Google Scholar] [CrossRef]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Cellulose degradation: A therapeutic strategy in the improved treatment of Acanthamoeba infections. Parasit. Vectors 2015, 8, 23. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef]
- Pedrolli, D.B.; Monteiro, A.C.; Gomes, E.; Carmona, E.C. Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 2009, 3, 9–18. [Google Scholar] [CrossRef]
- Renard, C.M.; Voragen, A.G.J.; Thibault, J.F.; Pilnik, W. Comparison between enzymatically and chemically extracted pectins from apple cell walls. Anim. Feed. Sci. Technol. 1991, 32, 69–75. [Google Scholar] [CrossRef]
- Laroze, L.; Soto, C.; Zúñiga, M.E. Phenolic antioxidants extraction from raspberry wastes assisted by-enzymes. Electron. J. Biotechnol. 2010, 13, 11–12. [Google Scholar] [CrossRef]
- Wang, S.S.; Ning, Y.J.; Wang, S.N.; Zhang, J.; Zhang, G.Q.; Chen, Q.J. Purification, characterization, and cloning of an extracellular laccase with potent dye decolorizing ability from white rot fungus Cerrena unicolor GSM-01. Int. J. Biol. Macromol. 2017, 95, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Utilization of agroindustrial wastes for the production of laccase by Achromobacter xylosoxidans HWN16 and Bordetella bronchiseptica HSO16. J. Environ. Manag. 2019, 231, 222–231. [Google Scholar] [CrossRef]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Nazar, M.; Xu, L.; Ullah, M.W.; Moradian, J.M.; Wang, Y.; Sethupathy, S.; Iqbal, B.; Nawaz, M.Z.; Zhu, D. Biological delignification of rice straw using laccase from Bacillus ligniniphilus L1 for bioethanol production: A clean approach for agro-biomass utilization. J. Clean. Prod. 2022, 360, 132171. [Google Scholar] [CrossRef]
- De La Torre, M.; Martín-Sampedro, R.; Fillat, Ú.; Eugenio, M.E.; Blánquez, A.; Hernández, M.; Ibarra, D. Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J. Ind. Microbiol. Biotechnol. 2017, 44, 1561–1573. [Google Scholar] [CrossRef]
- Saikia, K.; Vishnu, D.; Rathankumar, A.K.; Palanisamy Athiyaman, B.; Batista-García, R.A.; Folch-Mallol, J.L.; Kumar, V.V. Development of a magnetically separable co-immobilized laccase and versatile peroxidase system for the conversion of lignocellulosic biomass to vanillin. J. Air Waste Manag. Assoc. 2020, 70, 1252–1259. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, Z.; Bao, J.; Zhao, M.; Si, Q.; Sun, P.; Ge, G.; Jia, Y. Effects of different types of lab on dynamic fermentation quality and microbial community of native grass silage during anaerobic fermentation and aerobic exposure. Microorganisms 2023, 11, 513. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.H.; Dong, C.D. Lignin valorisation via enzymes: A sustainable approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Aron, N.S.M.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R.; Chew, K.W.; Show, P.L. A review on bioconversion processes for hydrogen production from agro-industrial residues. Int. J. Hydrogen Energy 2022, 47, 37302–37320. [Google Scholar] [CrossRef]
- Hu, K.; Zhao, H.; Kang, X.; Ge, X.; Zheng, M.; Hu, Z.; Tao, Y. Fruity aroma modifications in Merlot wines during simultaneous alcoholic and malolactic fermentations through mixed culture of S. cerevisiae, P. fermentans, and L. brevis. LWT 2022, 154, 112711. [Google Scholar]
- Kim, K.H.; Yun, Y.S.; Chun, S.Y.; Yook, H.S. Antioxidant and antibacterial activities of grape pomace fermented by various microorganisms. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1049–1056. [Google Scholar] [CrossRef]
- Kurt, A.S.; Cekmecelioglu, D. Bacterial cellulase production using grape pomace hydrolysate by shake-flask submerged fermentation. Biomass Convers. Biorefinery 2021, 13, 6981–6988. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhou, X.; Du, B.; Chen, Y. Release of bound phenol from Rosa roxburghii pomace via solid-state fermentation with Trichoderma viride: Mechanisms of change. Food Biosci. 2024, 61, 105011. [Google Scholar] [CrossRef]
- Paniagua-García, A.I.; Garita-Cambronero, J.; González-Rojo, S.; Díez-Antolínez, R. Optimization of lactic acid production from apple and tomato pomaces by thermotolerant bacteria. J. Environ. Manag. 2024, 366, 121806. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; De Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, T.; Chu, X.; Tang, S.; Cao, W.; Liang, F.; Fang, Y.; Pan, S.; Xu, X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT 2020, 125, 109260. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołebiewska, E.; Zawadzka, M.; Choińska, R.; Koronkiewicz, K.; Piasecka-Jóźwiak, K.; Bujak, M. Sustainable extraction of bioactive compound from apple pomace through lactic acid bacteria (LAB) fermentation. Sci. Rep. 2023, 13, 19310. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of polyphenols in apple pomace fermented by β-glucosidase-producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef]
- Uzun, D.E.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Tomas, M.; Capanoglu, E. Exploring the impact of fermentation on bioactive compounds in two different types of carrot pomace. Food Biosci. 2024, 61, 104646. [Google Scholar] [CrossRef]
- Chai, Z.; Yan, Y.; Zan, S.; Meng, X.; Zhang, F. Probiotic-fermented blueberry pomace alleviates obesity and hyperlipidemia in high-fat diet C57BL/6J mice. Food Res. Int. 2022, 157, 111396. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, J.; Yue, Y.; Feng, Z.; Chen, J.; Ye, X. Influence of mixed probiotics on the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT 2020, 133, 110076. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Ye, X.; Zhou, Z. Mixed fermentation of Chinese bayberry pomace using yeast, lactic acid bacteria and acetic acid bacteria: Effects on color, phenolics and antioxidant ingredients. LWT 2022, 163, 113503. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, S.; Wu, T.; Pan, S.; Xu, X. Lactobacillus casei-fermented blueberry pomace ameliorates colonic barrier function in high fat diet mice through MAPK-NF-κB-MLCK signaling pathway. J. Funct. Foods 2022, 95, 105139. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef]
- Dikmetas, D.N.; Nemli, E.; Karbancioglu-Guler, F.; Apak, R.; Bener, M.; Zhang, W.; Jia, N.; Zhao, C.; Tomas, M.; Capanoglu, E. Lactic Acid Bacterial Culture Selection for Orange Pomace Fermentation and Its Potential Use in Functional Orange Juice. ACS Omega 2025, 10, 11038–11053. [Google Scholar] [CrossRef] [PubMed]
- Crugeira, P.; Khelifa, H.; Barreira, L.; Halla, N.; Peres, A.; Schreiner, T.; Barreiro, M.; Rodrigues, P. Bacterial cellulose biosynthesis in the presence of raw moist olive pomace: A green sustainable approach that enhances biopolymer production and properties. Biomass Bioenergy 2025, 197, 107789. [Google Scholar] [CrossRef]
- Haddadin, M.; Abu-Reesh, I.; Haddadin, F.; Robinson, R. Utilisation of tomato pomace as a substrate for the production of vitamin B12–a preliminary appraisal. Bioresour. Technol. 2001, 78, 225–230. [Google Scholar] [CrossRef]
- Singh, R.; Katyal, M.; Mahajan, R.; Gupta, R.; Aggarwal, N.K.; Yadav, A. Valorization of Pomace Waste for the Production of Cellulose by Komagataeibacter diospyri RSA4. Biotechnol. Appl. Biochem. 2025, e2757. [Google Scholar] [CrossRef]
- Sunte, J. Effect of Fungus, Yeast, Microorganisms on Decomposition of Human Body and Foods. Res. Rev. J. Environ. Sci. 2023, 5, 1–8. [Google Scholar]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Vamvakas, S.S.; Kapolos, J. Factors affecting yeast ethanol tolerance and fermentation efficiency. World J. Microbiol. Biotechnol. 2020, 36, 114. [Google Scholar] [CrossRef]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Enhancement of the nutritional properties of apple pomace by fermentation with autochthonous yeasts. LWT Food Sci. Technol. 2017, 79, 27–33. [Google Scholar] [CrossRef]
- Kanwar, S.; Kumar, G.; Sahgal, M.; Singh, A. Ethanol production through Saccharomyces based fermentation using apple pomace amended with molasses. Sugar Tech. 2012, 14, 304–311. [Google Scholar] [CrossRef]
- Lalou, S.; Mantzouridou, F.; Paraskevopoulou, A.; Bugarski, B.; Levic, S.; Nedovic, V. Bioflavour production from orange peel hydrolysate using immobilized Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 9397–9407. [Google Scholar] [CrossRef]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Genisheva, Z.; Macedo, S.; Mussatto, S.I.; Teixeira, J.A.; Oliveira, J.M. Production of white wine by Saccharomyces cerevisiae immobilized on grape pomace. J. Inst. Brew. 2012, 118, 163–173. [Google Scholar] [CrossRef]
- Williams, D.L.; Schueckel, J.; Vivier, M.A.; Buffetto, F.; Zietsman, A.J. Grape pomace fermentation and cell wall degradation by Kluyveromyces marxianus Y885. Biochem. Eng. J. 2019, 150, 107282. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, S.; Waterhouse, G.I.; Zhou, T.; Du, Y.; Sun-Waterhouse, D.; Wu, P. Yeast fermentation of apple and grape pomaces affects subsequent aqueous pectin extraction: Composition, structure, functional and antioxidant properties of pectins. Food Hydrocoll. 2022, 133, 107945. [Google Scholar] [CrossRef]
- Haddadin, M.S.; Abdulrahim, S.M.; Al-Khawaldeh, G.Y.; Robinson, R.K. Solid state fermentation of waste pomace from olive processing. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1999, 74, 613–618. [Google Scholar] [CrossRef]
- Mu, Y.; Maimaitiyiming, R.; Hong, J.; Wang, Y.; Zhao, Y.; Liu, R.; Wang, L.; Chen, K.; Aihaiti, A. Study on Fermentation Preparation, Stability, and Angiotensin-Converting Enzyme Inhibitory Activity of Tomato Pomace Peptide. Foods 2025, 14, 145. [Google Scholar] [CrossRef]
- Ayyash, M.; Tarique, M.; Alaryani, M.; Al-Sbiei, A.; Masad, R.; Al-Saafeen, B.; Fernandez-Cabezudo, M.; Al-Ramadi, B.; Kizhakkayil, J.; Kamal-Eldin, A. Bioactive properties and untargeted metabolomics analysis of bioaccessible fractions of non-fermented and fermented date fruit pomace by novel yeast isolates. Food Chem. 2022, 396, 133666. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Alam, M.Z.; Al-Nabulsi, A.; Osaili, T.M.; Olaimat, A.; Liu, S.Q.; Kamal-Eldin, A.; Ayyash, M. Fermentation of date pulp residues using Saccharomyces cerevisiae and Pichia Kudriavzevii—Insights into biological activities, phenolic and volatile compounds, untargeted metabolomics, and carbohydrate analysis post in vitro digestion. Fermentation 2023, 9, 561. [Google Scholar] [CrossRef]
- He, W.; Tian, Y.; Liu, S.; Vaateri, L.; Ma, X.; Haikonen, T.; Yang, B.; Laaksonen, O. Comparison of phenolic composition and sensory quality among pear beverages made using Saccharomyces cerevisiae and Torulaspora delbrueckii. Food Chem. 2023, 422, 136184. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Tosun, R. Improving nutritional qualities of tomato pomace by Pleurotus ostreatus and Phanerochaete chrysosporium fermentation. KSU J. Agric. Nat. 2020, 23, 527–535. [Google Scholar] [CrossRef]
- Takenaka, S.; Ogawa, C.; Uemura, M.; Umeki, T.; Kimura, Y.; Yokota, S.; Doi, M. Identification and characterization of extracellular enzymes secreted by Aspergillus spp. involved in lipolysis and lipid-antioxidation during katsuobushi fermentation and ripening. Int. J. Food Microbiol. 2021, 353, 109299. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Papanikolaou, S.; Philippoussis, A.; Diamantopoulou, P. Upgrading grape pomace through Pleurotus spp. cultivation for the production of enzymes and fruiting bodies. Microorganisms 2019, 7, 207. [Google Scholar] [CrossRef]
- Thapa, S.; Mishra, J.; Arora, N.; Mishra, P.; Li, H.; O′Hair, J.; Bhatti, S.; Zhou, S. Microbial cellulolytic enzymes: Diversity and biotechnology with reference to lignocellulosic biomass degradation. Rev. Environ. Sci. Bio/Technol. 2020, 19, 621–648. [Google Scholar] [CrossRef]
- Leite, P.; Salgado, J.M.; Venâncio, A.; Domínguez, J.M.; Belo, I. Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour. Technol. 2016, 214, 737–746. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Patil, N.N.; Shinde, S.S.; Gunjal, A.B.; Patil, N.N.; Shinde, S.S. Pectinase in degradation of lignocellulosic wastes. In Enzymes in Degradation of the Lignocellulosic Wastes; Springer: Cham, Switzerland, 2020; pp. 71–103. [Google Scholar] [CrossRef]
- Giacobbe, S.; Pezzella, C.; Lettera, V.; Sannia, G.; Piscitelli, A. Laccase pretreatment for agrofood wastes valorization. Bioresour. Technol. 2018, 265, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Knuf, F.; Caspers-Weiffenbach, R.; Schieber, A.; Fontana, A. Peptidomics profiling and biological activities of grape pomace protein hydrolysates. Food Chem. 2025, 463, 141032. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.R.; Cabezudo, I.; Galetto, C.S.; Romanini, D. Production of grape pomace extracts with enhanced antioxidant and prebiotic activities through solid-state fermentation by Aspergillus niger and Aspergillus oryzae. Food Biosci. 2021, 42, 101168. [Google Scholar] [CrossRef]
- Botella, C.; Diaz, A.; De Ory, I.; Webb, C.; Blandino, A. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem. 2007, 42, 98–101. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Solid state production of polygalacturonase by Lentinus edodes using fruit processing wastes. Process Biochem. 2000, 35, 825–830. [Google Scholar] [CrossRef]
- Romo Sánchez, S.; Gil Sánchez, I.; Arévalo-Villena, M.; Briones Pérez, A. Production and immobilization of enzymes by solid-state fermentation of agroindustrial waste. Bioprocess Biosyst. Eng. 2015, 38, 587–593. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, S.; Satyanarayana, T. Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresour. Technol. 2004, 94, 239–243. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, R.; Bhalla, T.C. Citric acid production by Aspergillus niger van. Tieghem MTCC 281 using waste apple pomace as a substrate. J. Food Sci. Technol. 2010, 47, 458–460. [Google Scholar]
- Villas-Bôas, S.G.; Esposito, E.; Mendonca, M.M. Bioconversion of apple pomace into a nutritionally enriched substrate by Candida utilis and Pleurotus ostreatus. World J. Microbiol. Biotechnol. 2003, 19, 461–467. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar]
- Madeira, J.V.; Macedo, J.A.; Macedo, G.A. A new process for simultaneous production of tannase and phytase by Paecilomyces variotii in solid-state fermentation of orange pomace. Bioprocess Biosyst. Eng. 2012, 35, 477–482. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Najafpour, G.D.; Mohammadi, M. Production of pectinases for quality apple juice through fermentation of orange pomace. J. Food Sci. Technol. 2017, 54, 4123–4128. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Brozzoli, V.; Bartocci, S.; Terramoccia, S.; Contò, G.; Federici, F.; D’Annibale, A.; Petruccioli, M. Stoned olive pomace fermentation with Pleurotus species and its evaluation as a possible animal feed. Enzym. Microb. Technol. 2010, 46, 223–228. [Google Scholar] [CrossRef]
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J.M. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod. Biorefining 2020, 14, 78–91. [Google Scholar] [CrossRef]
- Umsza-Guez, M.A.; Díaz, A.B.; Ory, I.D.; Blandino, A.; Gomes, E.; Caro, I. Xylanase production by Aspergillus awamori under solid state fermentation conditions on tomato pomace. Braz. J. Microbiol. 2011, 42, 1585–1597. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H.; Castangia, I.; Manca, M.L. Valorization of Date By-Products: Enhancement of Antioxidant and Antimicrobial Potentials through Fermentation. Antioxidants 2024, 13, 1102. [Google Scholar] [CrossRef]
- Coronado-Contreras, A.; Ruelas-Chacón, X.; Reyes-Acosta, Y.K.; Dávila-Medina, M.D.; Ascacio-Valdés, J.A.; Sepúlveda, L. Valorization of Prickly Pear Peel Residues (Opuntia ficus-indica) Using Solid-State Fermentation. Foods 2023, 12, 4213. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.; Ribeiro, M.H. Enzyme immobilization and co-immobilization: Main framework, advances and some applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Talekar, S.; Joshi, A.; Kambale, S.; Jadhav, S.; Nadar, S.; Ladole, M. A tri-enzyme magnetic nanobiocatalyst with one pot starch hydrolytic activity. Chem. Eng. J. 2017, 325, 80–90. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Gupta, K.; Bardhan, P.; Saikia, D.; Rather, M.A.; Loying, S.; Mandal, M.; Kataki, R. Microbial fermentation: Basic fundamentals and its dynamic prospect in various industrial applications. In Industrial Microbiology and Biotechnology; Springer: Singapore, 2022; pp. 107–128. [Google Scholar] [CrossRef]
- Chen, Q.; Su, J.; Zhang, Y.; Li, C.; Zhu, S. Phytochemical profile and bioactivity of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber by solid-state fermentation with Aspergillus niger. Molecules 2024, 29, 1689. [Google Scholar] [CrossRef]
- Siller-Sánchez, A.; Aguilar, C.N.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Kumar Verma, D.; Aguilar-González, M. Solid-State Fermentation-Assisted Extraction of Flavonoids from Grape Pomace Using Co-Cultures. Processes 2024, 12, 2027. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Arely Prado-Barragán, L. Improved extraction of high value-added polyphenols from pomegranate peel by solid-state fermentation. Fermentation 2023, 9, 530. [Google Scholar] [CrossRef]
- Ramesh, C.; Prasastha, V.R.; Venkatachalam, M.; Dufossé, L. Natural substrates and culture conditions to produce pigments from potential microbes in submerged fermentation. Fermentation 2022, 8, 460. [Google Scholar] [CrossRef]
- Chuah, H.Q.; Tang, P.L.; Ang, N.J.; Tan, H.Y. Submerged fermentation improves bioactivity of mulberry fruits and leaves. Chin. Herb. Med. 2021, 13, 565–572. [Google Scholar] [CrossRef]
- Meng, Q.; Chuai, S.; Chen, L.; Wang, L.; Cai, G.; Mao, J.; Gu, Z.; Shi, G.; Ding, Z. Effect of surfactants on the production of polysaccharides from Schizophyllum commune through submerged fermentation. Int. J. Biol. Macromol. 2021, 192, 210–218. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, S.; Liu, J. Recent advances in chitin biosynthesis associated with the morphology and secondary metabolite synthesis of filamentous fungi in submerged fermentation. J. Fungi. 2023, 9, 205. [Google Scholar] [CrossRef]
- Batbayar, B.; Kryachko, Y.; Nickerson, M.T.; Korber, D.R.; Tanaka, T. Solid-state and submerged fermentation effects on functional properties of pea protein-enriched flour. Cereal Chem. 2023, 100, 1092–1105. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Prasada Rao, U.J.S. Sustainable solutions for agro processing waste management: An overview. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012; pp. 65–109. [Google Scholar] [CrossRef]
- Taghian Dinani, S.; Van Der Goot, A.J. Challenges and solutions of extracting value-added ingredients from fruit and vegetable by-products: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7749–7771. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace—A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Bizzo, H.R.; Doria Chaves, A.C.S.; Faria-Machado, A.F.; Gomes Soares, A.; de Oliveira Fonseca, M.J.; Kidmose, U.; Rosenthal, A. Sustainable use of tropical fruits? Challenges and opportunities of applying the waste-to-value concept to international value chains. Crit. Rev. Food Sci. Nutr. 2023, 63, 1339–1351. [Google Scholar] [CrossRef]
- Hasan, M.M.; Islam, M.R.; Haque, A.R.; Kabir, M.R.; Khushe, K.J.; Hasan, S.K. Trends and challenges of fruit by-products utilization: Insights into safety, sensory, and benefits of the use for the development of innovative healthy food: A review. Bioresour. Bioprocess 2024, 11, 10. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maciel, G.M.; Lima, N.P.; Lima, N.F.; Ribeiro, I.S.; Pinheiro, D.F.; Haminiuk, C.W.I. Valorization of bioactive compounds from juice industry waste: Applications, challenges, and future prospects. Trends Food Sci. Technol. 2024, 152, 104693. [Google Scholar] [CrossRef]



| Pomace | Soluble Sugars | Dietary Fibre | Protein | Fat | Ash | References |
|---|---|---|---|---|---|---|
| Apple | 1.1 | 26.8–82.0 | 1.2–6.0 | 0.3–8.5 | 0.5–4.3 | [12,52,53] |
| Orange | - | 33.8–46.9 | 5.0–6.6 | 1.0–1.5 | 2.9–3.6 | [18,54,55] |
| Grape | 1.3–77.5 | 17.3–88.70 | 3.57–14.2 | 8.2–10.1 | 4.6–4.7 | [24,25,56] |
| Olive | 21–32 | 53–59 | 7.4–9.0 | 5.8–16.6 | 1.9–6.46 | [29,30,57] |
| Tomato | 25.7–26.7 | 39–74.8 | 15–30 | 2.2–16.2 | 3.1–4.2 | [34,35,36,58,59] |
| Dates | 35.3 | 45.5–77.2 | 2.1–10.6 | 0.7–2.0 | 1.9–4.1 | [37,40,60] |
| Pear | 0.3 | 57.9–90.7 | 1.8–5.7 | 0.9–3.7 | 0.9–2.0 | [12,61,62] |
| Pomace | Cellulose | Hemicellulose | Pectin | Lignin | References |
|---|---|---|---|---|---|
| Apple | 3.6–43.6 | 4.3–24.4 | 11.7 | 3.0–29.2 | [13,14,15,16] |
| Orange | 14.3–21.5 | 6.3–14.3 | - | 3.3–10.7 | [19,20] |
| Grape | 3.2–14.0 | 1.7–8.3 | 4.1–8.8 | 7.9–53.6 | [16,25,26] |
| Olive | 8.6–9.7 | 8.1–11.3 | 6.0–6.5 | 21.8–44.0 | [29,31,32] |
| Tomato | 8.6–34.0 | 5.3–14.4 | 7.6–8.8 | 5.9–37.3 | [13,36,59] |
| Dates | 13.0–18.0 | 17.4 | 1.4–3.7 | 52.9 | [38,39] |
| Pear | 32.4–34.5 | 18.6–20.2 | 2.1–13.4 | 26.0–33.5 | [15,70] |
| Target | Pomace | References |
|---|---|---|
| Insoluble fiber | Apple, grapes, peach, orange, pear | [7,73] |
| Soluble fiber | Apple, mango, pear | [7,73] |
| Pectin | Apple, orange, tomato | [12,74,75,76] |
| Phenolic antioxidants | Olive, dates, mango, plum, berry, grape, apple | [7,77,78] |
| Carotenoids | Mango, tomato, carrot | [75,76,79,80] |
| Essential oils | Citrus fruits | [81] |
| Industrial enzymes (Polygalacturonase, tannase, pectinases) | Grape, apple, olive | [82,83] |
| Prebiotics | Citrus fruit, apple, grapes | [84,85] |
| Pomace | Total Phenolic Content (mg GAE/g) | Identified Compounds | References |
|---|---|---|---|
| Apple | 1.1 | Phloridzin, chlorogenic acid, gallic acid, catechin, rutin, ursolic acid, oleanolic acid, etc. | [105,108] |
| Orange | 18–21.8 | Hesperidin, naringenin, hesperitin, apigenin, quercetin, isorhamnetin, p-coumaric acid, sinapic acid, etc. | [101,103,109] |
| Grape | 68.8–96.3 | Quercetin, peonidin, cyanidin, catechin, epicatechin, procyanidin, etc. | [100,104] |
| Olive | 20.1–34.1 | Quinic acid, oleuropein, hydroxytyrosol, caffeic acid, vanillin, rutin, luteolin, apigenin, etc. | [102,110] |
| Tomato | 0.9–1.0 | Chlorogenic acid, gallic acid, caffeic acid, rutin, quercetin, ferulic acid, naringenin, etc. | [111,112,113] |
| Dates | 0.2–2.6 | Nil | [37] |
| Pear | 3.8–6.6 | Chlorogenic acid, quinic acid, protocatechuic acid, gallic acid, isoquercitrin, rutin, epicatechin, etc. | [106,114,115] |
| Pomace | Strains | Findings | References |
|---|---|---|---|
| Apple | Lactiplantibacillus plantarum KKP 1527, L. rhamnosus | Increased phenolic and flavonoid content, especially gallic acid, procyanidin A2, protocatechuic acid, and procyanidin B2. Increased volatile compounds by 80%. | [162,163,169] |
| Orange | L. plantarum P10, L. plantarum M14, Bacillus subtilis BF2 and L. acidophilus LA-5 | Co-fermentation with B. subtilis and L. plantarum raised the phenolic content by 133%. Improved dietary fiber and organic acid by 47% and 1429%, respectively. | [155,170] |
| Grape | L. plantarum, L. casei, and Bacillus subtilis natto DSM 17766 | Increased antioxidant and antibacterial activities of the pomace. Bacillus subtilis fermentation efficiently produces cellulase. | [156,157] |
| Olive | Komagataeibacter intermedius | Bacterial cellulose was successfully synthesized. The bacterial cellulose has improved antioxidant activity. | [171] |
| Tomato | Propionibacterium shermanii | Fermentation produced vitamin B12 (11.1 mg/L). | [172] |
| Date fruit | Lactobacillus casei 431 | Produced a high concentration of lactic acid. | [37] |
| Pear | Komagataeibacter rhaeticus | Produced bacterial cellulose and vinegar simultaneously. | [173] |
| Pomace | Strains | Findings | References |
|---|---|---|---|
| Apple | Saccharomyces cerevisiae, S. bayanus, Saccharomycodes ludwigii, Hanseniaspora uvarum, H. valbyensis | Increased protein, fat, dietary fiber, and phenolic compounds. Hanseniaspora showed lower ethanol production than Saccharomyces. Increased the aromatic richness of the apple pomace. | [175,177] |
| Orange | S. cerevisiae | Sterilized orange pomace supported yeast fermentation and produced greater flavor compounds. Immobilized cell technology protected yeast cells from hydrolysate inhibitors. | [179,180] |
| Grape | S. cerevisiae, Kluyveromyces marxianus | Produce white wine with better sensory characteristics. The yeast immobilized system had higher ethanol levels and volatile compounds. | [181,182,183] |
| Olive | S. cerevisiae | Increased crude protein content by 85%. | [184] |
| Tomato | S. cerevisiae | Increased soluble protein concentration. | [185] |
| Dates | S. cerevisiae and Pichia kudriavzevii | Increased malic acid production and volatile compounds. Increased antimicrobial activity and reduced antioxidant activity. | [186,187] |
| Pear | S. cerevisiae | Increased fruit pomace protein content and phenolic compounds. | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, N.; Okonkwo, C.E.; Ayyash, M.; Al-Marzouqi, A.H.; Yuliarti, O.; Kamal-Eldin, A. Valorization of Fruit Pomace by Enzymatic Treatment and Microbial Fermentation. Fermentation 2025, 11, 376. https://doi.org/10.3390/fermentation11070376
Samad N, Okonkwo CE, Ayyash M, Al-Marzouqi AH, Yuliarti O, Kamal-Eldin A. Valorization of Fruit Pomace by Enzymatic Treatment and Microbial Fermentation. Fermentation. 2025; 11(7):376. https://doi.org/10.3390/fermentation11070376
Chicago/Turabian StyleSamad, Nadiya, Clinton E. Okonkwo, Mutamed Ayyash, Ali H. Al-Marzouqi, Oni Yuliarti, and Afaf Kamal-Eldin. 2025. "Valorization of Fruit Pomace by Enzymatic Treatment and Microbial Fermentation" Fermentation 11, no. 7: 376. https://doi.org/10.3390/fermentation11070376
APA StyleSamad, N., Okonkwo, C. E., Ayyash, M., Al-Marzouqi, A. H., Yuliarti, O., & Kamal-Eldin, A. (2025). Valorization of Fruit Pomace by Enzymatic Treatment and Microbial Fermentation. Fermentation, 11(7), 376. https://doi.org/10.3390/fermentation11070376







