Production, Purification, and Application of a Biomolecule with Herbicidal Activity Produced by Fusarium fujikuroi in Submerged Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Storage and Maintenance of the Strain
2.2. Submerged Cultivation
2.3. Extraction and Purification
2.4. Bioassays
2.4.1. Seed Preparation
2.4.2. Phytotoxic Potential of Crude Extract (EXT) and Purified Compound (PC) in 96-Well Microplates
2.4.3. Phytotoxic Potential of the Purified Compound (PC) in the Pre-Emergence of R. sativus and T. aestivum
2.4.4. Effect of the Purified Compound (PC) on Soil Infested with Weed Seeds
2.4.5. Residual Effect of the Purified Compound (PC) on Soil and Its Influence on the Emergence and Development of R. sativus Seedlings
3. Results
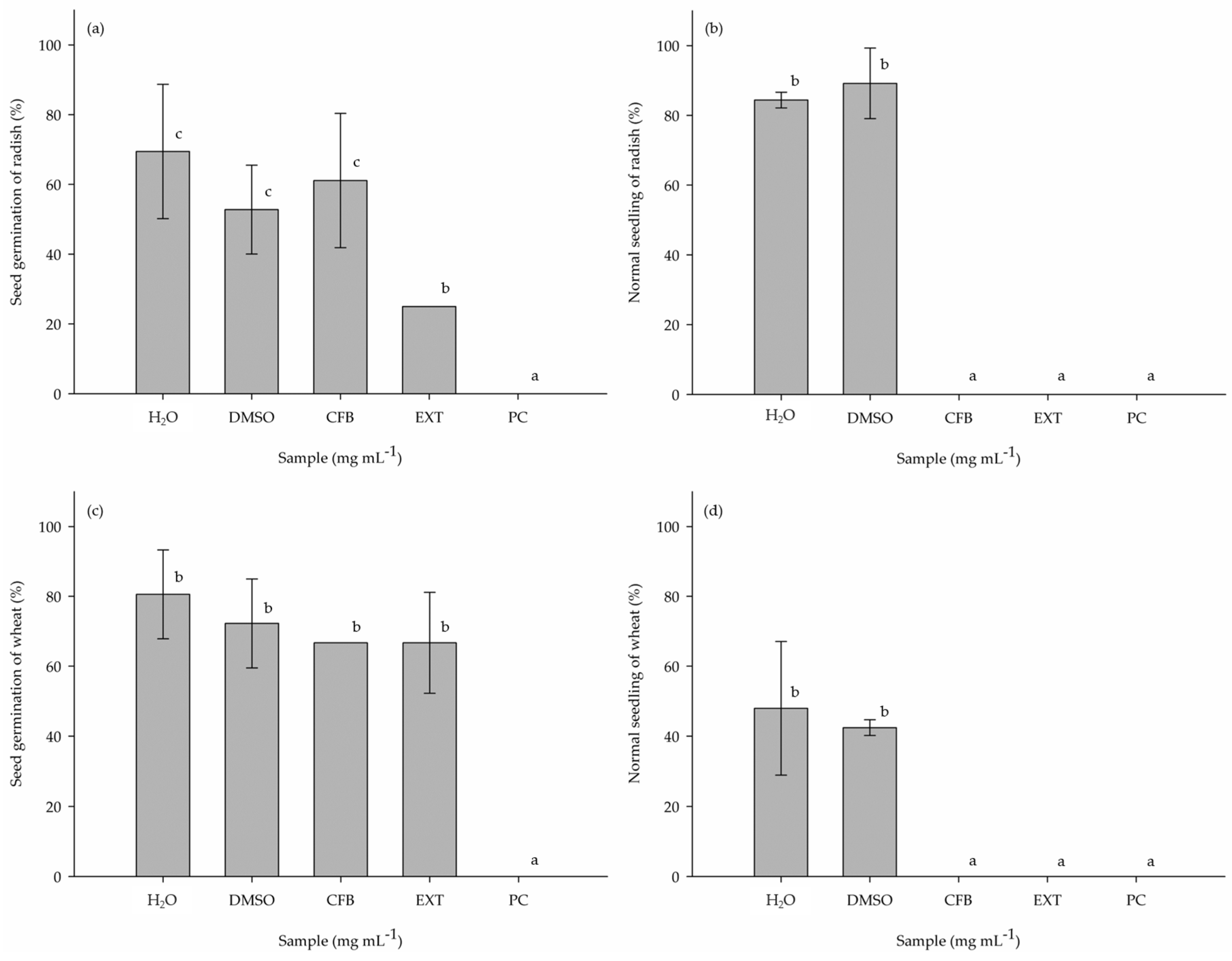
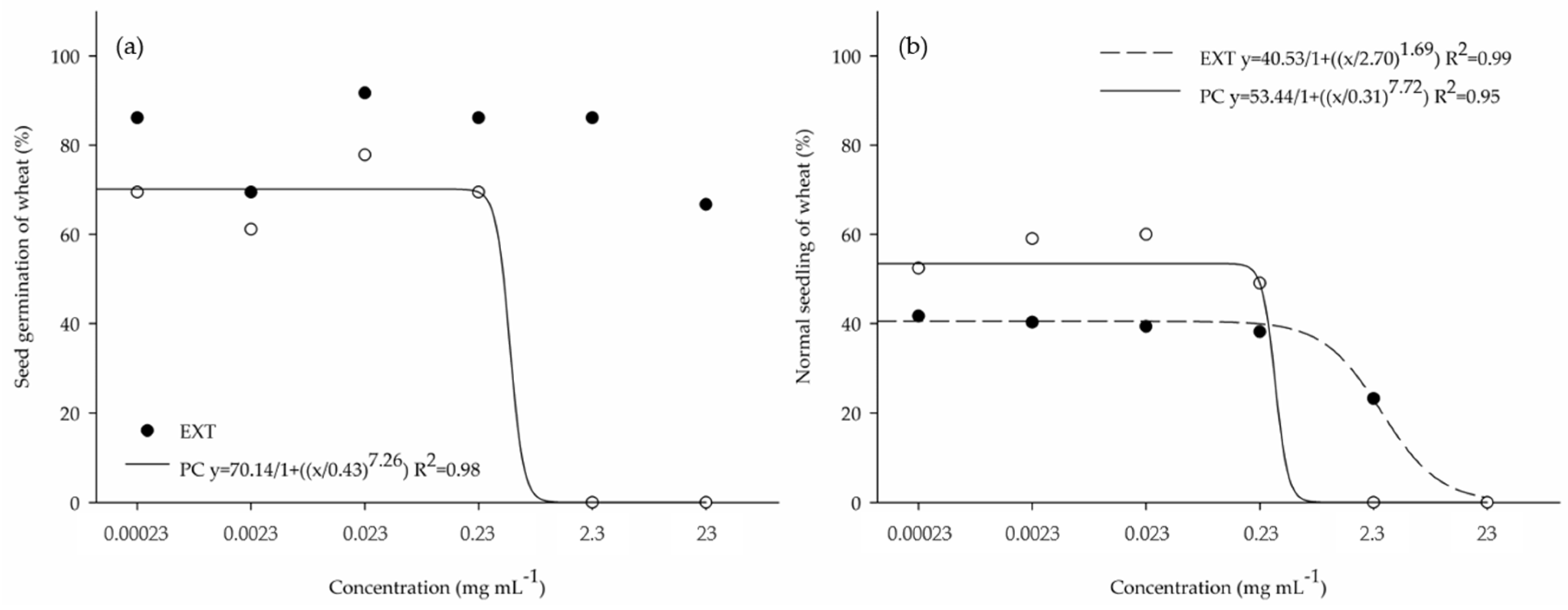
3.1. Phytotoxic Potential of the Crude Extract (EXT) and Purified Compound (PC) in 96-Well Microplates
3.2. Phytotoxic Potential of the Purified Compound (PC) on the Pre-Emergence of R. sativus and T. aestivum
3.3. Effect of the Purified Compound (PC) on Soil Infested with Weed Seeds
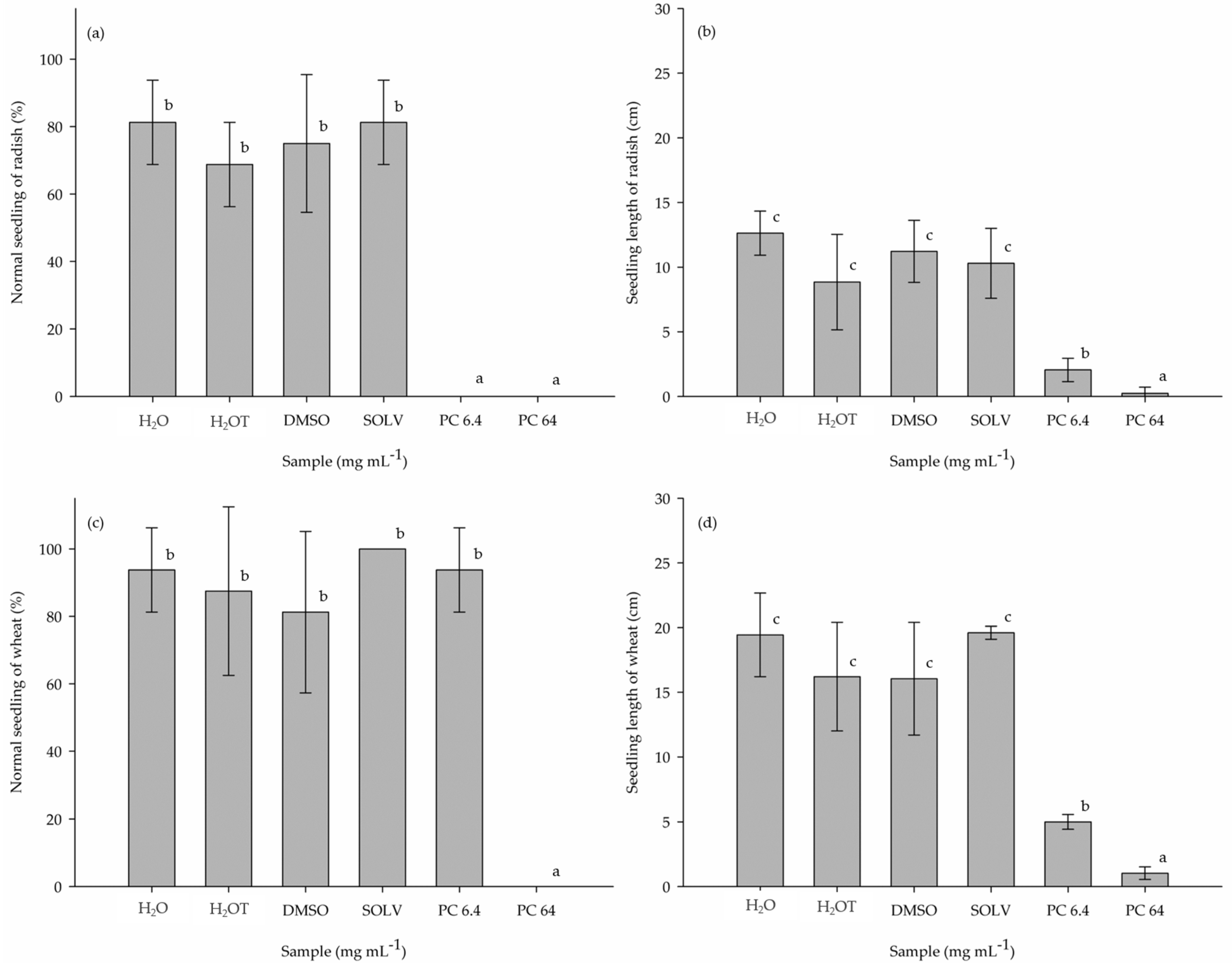
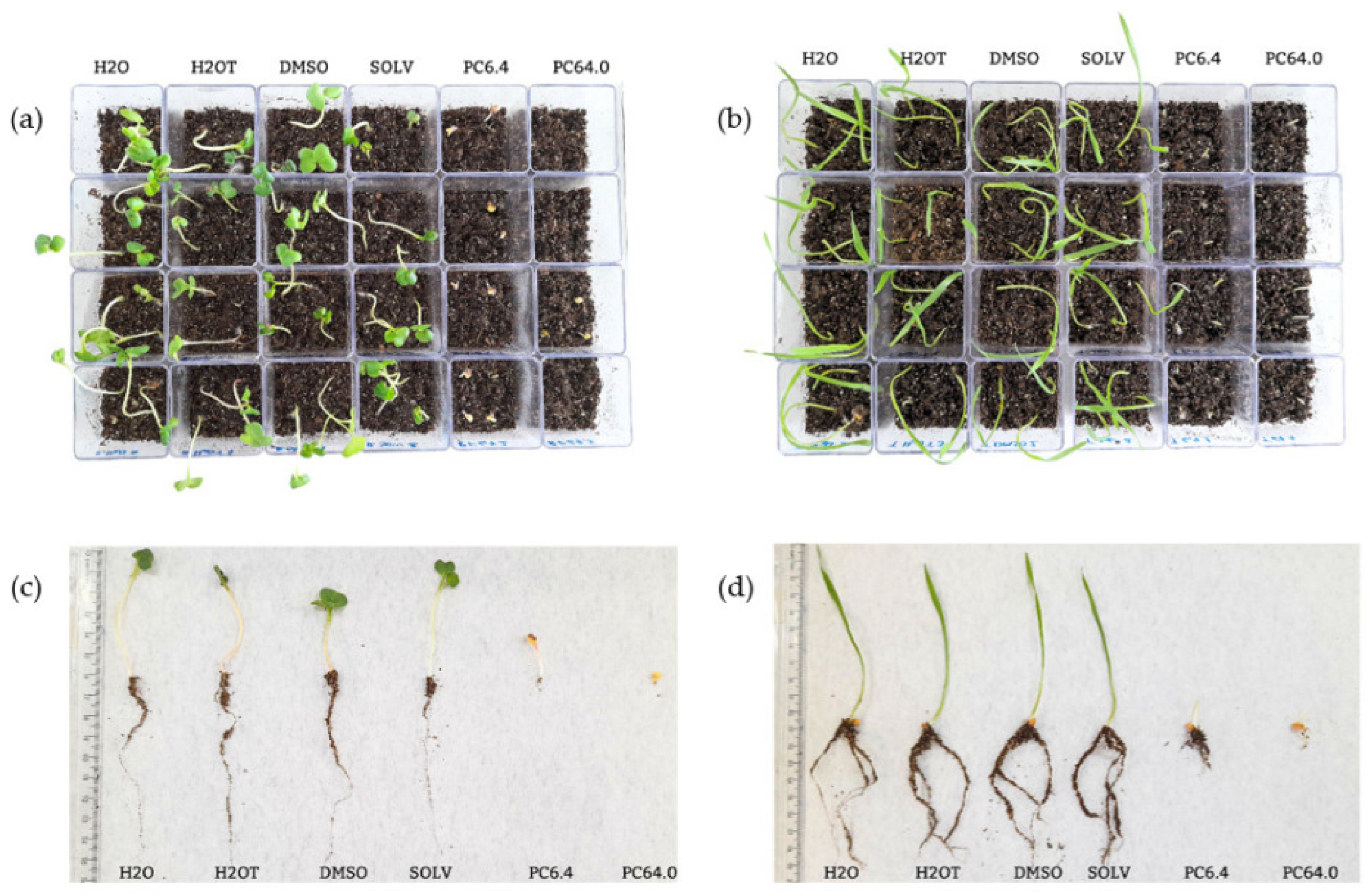
3.4. Residual Effect of the Purified Compound (PC) in Soil and Its Influence on the Emergence and Development of R. sativus Seedlings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef]
- Moura, M.S.; Lacerda, J.W.F.; Siqueira, K.A.; Bellete, B.S.; Sousa, P.T.; Dall’Óglio, E.L.; Soares, M.A.; Vieira, L.C.C.; Sampaio, O.M. Endophytic fungal extracts: Evaluation as photosynthesis and weed growth inhibitors. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 470–476. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Ocán-Torres, D.; Martínez-Burgos, W.J.; Manzoki, M.C.; Soccol, V.T.; Neto, C.J.D.; Soccol, C.R. Microbial Bioherbicides Based on Cell-Free Phytotoxic Metabolites: Analysis and Perspectives on Their Application in Weed Control as an Innovative Sustainable Solution. Plants 2024, 13, 1996. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential Corn Yield Losses from Weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Soltani, N.; DIlle, J.A.; Burke, I.C.; Everman, W.J.; Vangessel, M.J.; Davis, V.M.; Sikkema, P.H. Perspectives on Potential Soybean Yield Losses from Weeds in North America. Weed Technol. 2017, 31, 148–154. [Google Scholar] [CrossRef]
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 1–18. [Google Scholar] [CrossRef]
- Triolet, M.; Guillemin, J.P.; Andre, O.; Steinberg, C. Fungal-based bioherbicides for weed control: A myth or a reality? Weed Res. 2019, 60, 60–77. [Google Scholar] [CrossRef]
- Heap. International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org/ (accessed on 15 September 2024).
- Duke, S.O.; Pan, Z.; Bajsa-Hirschel, J.; Boyette, C.D. The potential future roles of natural compounds and microbial bioherbicides in weed management in crops. Adv. Weed Sci. 2022, 40, e020210054. [Google Scholar] [CrossRef]
- Berestetskiy, A.O. A review of fungal phytotoxins: From basic studies to practical use. Appl. Biochem. Microbiol. 2008, 44, 453–465. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.; Hynes, R.K.; Erlandson, M.; Bailey, K.L. The biochemistry behind biopesticide efficacy. Sustain. Chem. Process. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Varejão, E.V.V.; Demuner, A.J.; Barbosa, L.C.A.; Barreto, R.W. The search for new natural herbicides—Strategic approaches for discovering fungal phytotoxins. Crop Prot. 2013, 48, 41–50. [Google Scholar] [CrossRef]
- Schmaltz, S.; Silva, M.A.; Ninaus, R.G.; Guedes, J.V.C.; Zabot, G.L.; Tres, M.V.; Mazutti, M.A. Biomolecules in modern and sustainable agriculture. 3 Biotech 2023, 13, 1–20. [Google Scholar] [CrossRef]
- Daniel, J.J.; Zabot, G.L.; Tres, M.V.; Harakava, R.; Kuhn, R.C.; Mazutti, M.A. Fusarium fujikuroi: A novel source of metabolites with herbicidal activity. Biocatal. Agric. Biotechnol. 2018, 14, 314–320. [Google Scholar] [CrossRef]
- Daniel, J.J.; Brun, T.; Todero, I.; Almeida, T.; Confortin, T.; Schmaltz, S.; Kuhn, K.R.; Tres, M.; Zabot, G.L.; Kuhn, R.C.; et al. Association of adjuvants with culture filtrate from Fusarium Fujikuroi for increasing the control of Conyza sp. Biointerface Res. Appl. Chem. 2020, 10, 6481–6487. [Google Scholar] [CrossRef]
- Todero, I.; Confortin, T.C.; Luft, L.; Seibel, J.; Kuhn, R.C.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Concentration of exopolysaccharides produced by Fusarium fujikuroi and application of bioproduct as an effective bioherbicide. Environ. Technol. 2020, 41, 2742–2749. [Google Scholar] [CrossRef]
- Brasil. Regras Para Análise de Sementes (RAS). In Revista Brasileira de Botânica; Ministério da Agricultura, Pecuária e Abastecimento (MAPA): Brasília, DF, Brazil, 2009; Volume 27. Available online: https://www.gov.br/agricultura/pt-br/assuntos/lfda/arquivos-publicacoes-laboratorio/regras-para-analise-de-sementes.pdf/view (accessed on 15 September 2024).
- Ranucci, E.; Treccani, S.; Ferruti, P.; Alongi, J. The Seed Germination Test as a Valuable Tool for the Short-Term Phytotoxicity Screening of Water-Soluble Polyamidoamines. Polymers 2024, 16, 1744. [Google Scholar] [CrossRef]
- Wolny, E.; Betekhtin, A.; Rojek, M.; Braszewska-Zalewska, A.; Lusinska, J.; Hasterok, R. Germination and the early stages of seedling development in brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 2916. [Google Scholar] [CrossRef]
- Haywood, J.; Breese, K.J.; Zhang, J.; Waters, M.T.; Bond, C.S.; Stubbs, K.A.; Mylne, J.S. A fungal tolerance trait and selective inhibitors proffer HMG-CoA reductase as a herbicide mode-of-action. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Bello, O.M.; Pradeep, M.; Jolly, R.S. Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ. Technol. Innov. 2019, 13, 304–317. [Google Scholar] [CrossRef]
- Berestetskiy, A.O.; Poluektova, E.V.; Sabashuk, Y.A.; Pervushin, A.L. Development of Chromatography Techniques for Analysis and Preparative Isolation of Phytotoxic Metabolites Produced by Stagonospora cirsii. Appl. Biochem. Microbiol. 2019, 55, 684–690. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Chiara Zonno, M.; Avolio, F.; Berestetskiy, A.; Vurro, M.; Evidente, A. Chenopodolans A-C: Phytotoxic furopyrans produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2013, 96, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bo, A.B.; Kim, J.D.; Kim, Y.S.; Khaitov, B.; Ko, Y.K.; Cho, K.M.; Jang, K.S.; Park, K.W.; Choi, J.S. Herbicidal Characteristics and Structural Identification of the Potential Active Compounds from Streptomyces sp. KRA17-580. J. Agric. Food Chem. 2020, 68, 15373–15380. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Niu, Y.-C.; Deng, H.; Luo, D.-Q. Characterization and phytotoxicity of ophiobolins produced by Bipolaris setariae. Mycoscience 2021, 62, 64–70. [Google Scholar] [CrossRef]
- Sukhoverkov, K.V.; Corral, M.G.; Leroux, J.; Haywood, J.; Johnen, P.; Newton, T.; Stubbs, K.A.; Mylne, J.S. Refining physico-chemical rules for herbicides using an antimalarial library. BioRxiv 2020, 10, 356576. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Cousens, R.; Mortimer, M. Dynamics of Weed Populations; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Bailey, K.L. The Bioherbicide Approach to Weed Control Using Plant Pathogens. In Integrated Pest Management: Current Concepts and Ecological Perspective; Dharam, P.A., Ed.; Academic Press: New York, NY, USA, 2014; pp. 245–266. [Google Scholar] [CrossRef]
- Maia, L.O.R.; Piveta, L.B.; Johnson, W.G. Residual Herbicide in Cover Cropping Systems. Agriculture 2024, 14, 2089. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmaltz, S.; Walker, C.; Silva, K.S.d.; Ninaus, R.G.; Dutra, C.B.; Schmidt, L.A.; Zeni, G.; Mazutti, M.A. Production, Purification, and Application of a Biomolecule with Herbicidal Activity Produced by Fusarium fujikuroi in Submerged Cultivation. Fermentation 2025, 11, 375. https://doi.org/10.3390/fermentation11070375
Schmaltz S, Walker C, Silva KSd, Ninaus RG, Dutra CB, Schmidt LA, Zeni G, Mazutti MA. Production, Purification, and Application of a Biomolecule with Herbicidal Activity Produced by Fusarium fujikuroi in Submerged Cultivation. Fermentation. 2025; 11(7):375. https://doi.org/10.3390/fermentation11070375
Chicago/Turabian StyleSchmaltz, Silvana, Clair Walker, Keli Souza da Silva, Renata Gulart Ninaus, Cláudia Braga Dutra, Luiza Andrea Schmidt, Gilson Zeni, and Marcio Antonio Mazutti. 2025. "Production, Purification, and Application of a Biomolecule with Herbicidal Activity Produced by Fusarium fujikuroi in Submerged Cultivation" Fermentation 11, no. 7: 375. https://doi.org/10.3390/fermentation11070375
APA StyleSchmaltz, S., Walker, C., Silva, K. S. d., Ninaus, R. G., Dutra, C. B., Schmidt, L. A., Zeni, G., & Mazutti, M. A. (2025). Production, Purification, and Application of a Biomolecule with Herbicidal Activity Produced by Fusarium fujikuroi in Submerged Cultivation. Fermentation, 11(7), 375. https://doi.org/10.3390/fermentation11070375





