Nutritional Enhancement of Plant-Based Fermented Foods: Microbial Innovations for a Sustainable Future
Abstract
1. Introduction
2. Microbial Diversity and Functional Role in Plant-Based Fermentation
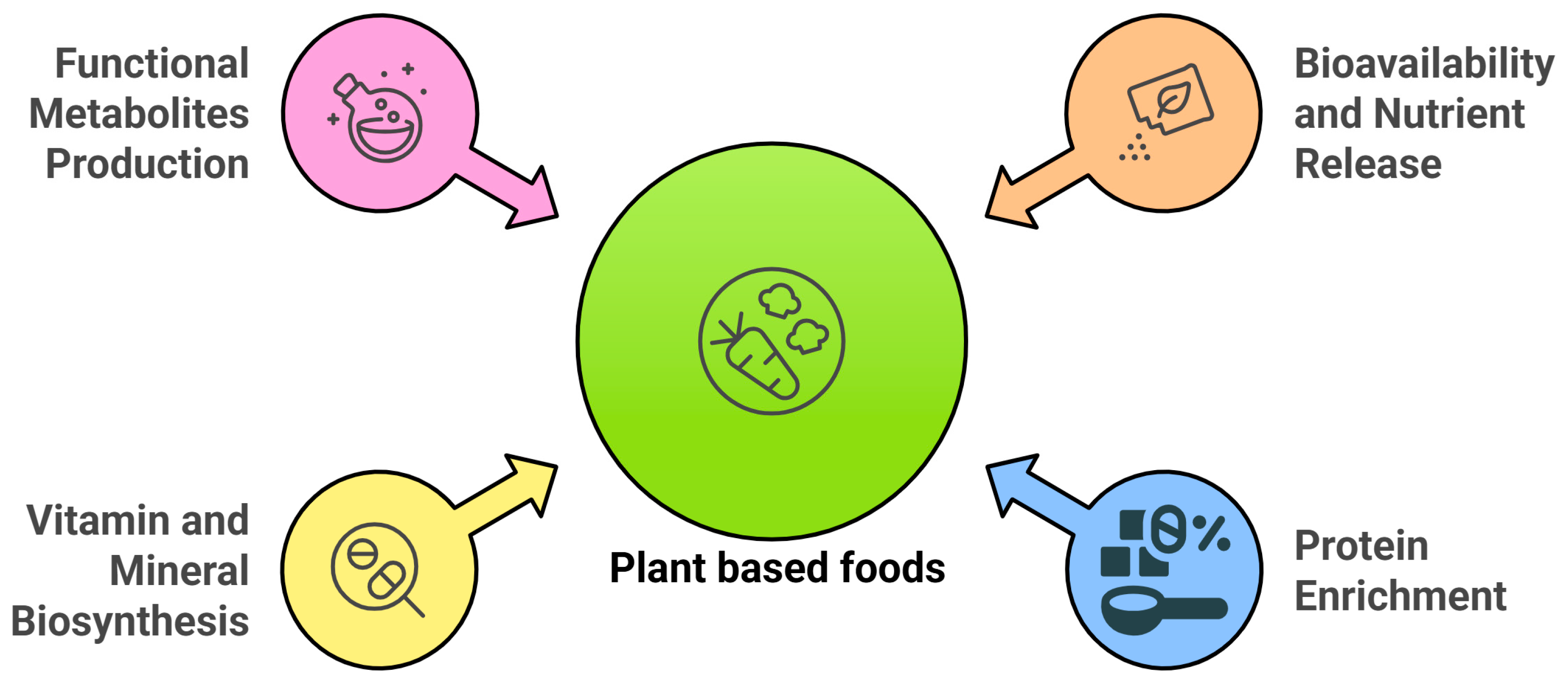
3. Nutritional Enhancement of Plant-Based Foods Through Fermentation
3.1. Bioavailability and Nutrient Release
3.2. Protein Enrichment and Quality Improvement
3.3. Enhanced Vitamin Synthesis
3.4. Production of Functional Metabolites
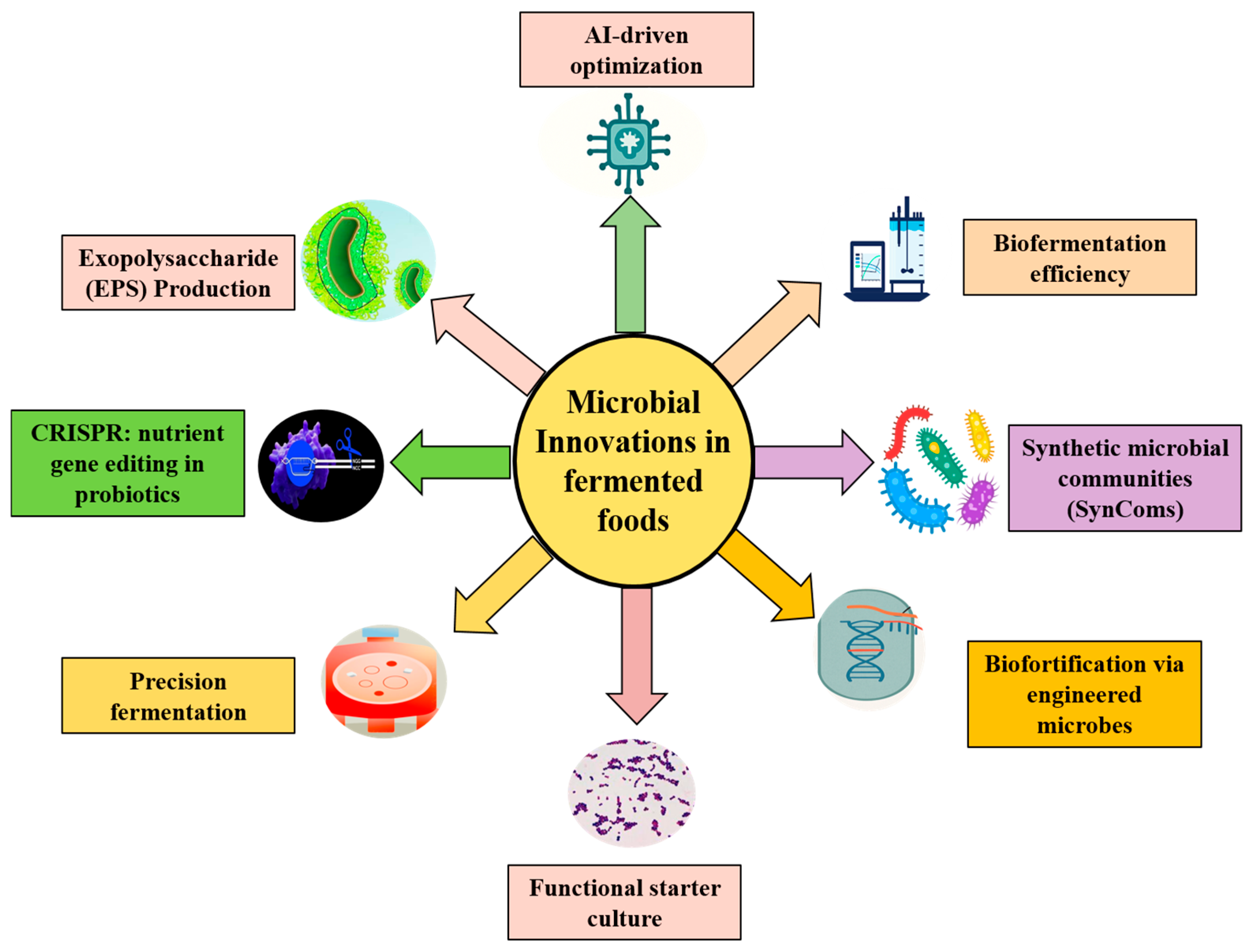
4. Microbial Innovations for Next-Gen Plant-Based Fermented Foods
4.1. CRISPR and Synthetic Biology Approaches: Engineering Microbes for Targeted Nutrient Synthesis
4.1.1. CRISPR-Cas Approaches
Precision Genome Editing with CRISPR-Cas9
Engineering Microbes for Amino Acid and Fatty Acid Production
Addressing Nutritional Deficiencies in Plant-Based Diets
4.2. Precision Fermentation and AI-Driven Optimization
4.3. Microbial Exopolysaccharides and Textural Modifications
5. Health Benefits and Functional Properties
5.1. Fermented Foods and Gut Microbiota
5.1.1. Mechanisms of Action: Host-Microbiota Interactions
5.1.2. Prebiotics: Fuel for Beneficial Bacteria
5.1.3. Synergistic Effects of Fermented Foods and Prebiotics
5.2. Metabolic and Anti-Inflammatory Benefits: Role in Obesity, Diabetes, and Cardiovascular Health
5.3. Cognitive and Neurological Impact: Neuroprotective Metabolites from Fermented Foods
5.4. Allergen Reduction and Digestibility Improvements: Mitigation of Food Intolerance Risks
6. Sustainability and Future Trends in Plant-Based Fermentation
6.1. Fermentation for Food Waste Valorization
| Fermentation Approach | Microorganism/s Involved | Substrate | Key Products | Yield/Key Characteristics | Reference |
|---|---|---|---|---|---|
| SSF | Starmerella bombicola | oil cake and molasses | Sophorolipids | 0.2 g SL g−1 | [191] |
| Submerged Fermentation (SmF) | Aspergillus niger | Pineapple waste | Single-cell proteins (SCPs) | Protein content-9.79 ± 0.11 g/L after 10 days | [192] |
| Mixed Solid-State Fermentation (M-SDF) | Trichoderma reesei and A. niger | Orange peel by-products | Soluble dietary fiber | The water holding capacity and oil holding capacity of M-SDF were 5.68 ± 0.36 g/g and 5.04 ± 0.04 g/g, respectively, approximately six times and two times greater than those of Untreated soluble dietary fiber. | [193] |
| SSF | Trichoderma harzianum | Grass clippings and pruning waste | Indole-3-acetic acid (IAA) and Conidial spore | 101.46 µg g−1 dry matter IAA and 3.03 × 109 spore g−1 dry matter | [194] |
| Lactic Acid Fermentation | L. plantarum | cocoa bean shell | Lactic acid | 19.00 g/100 g of lactic acid after 24 h of fermentation | [195] |
| SSF | Pseudomonas aeruginosa PTCC 1074s | Soyabean meal | Rhamnolipid | 14.63 g/kg substrate | [196] |
| SSF | Clostridium tyrobutyricum | Wheat bran, rice polishings and molasses | Butyric acid | 5.63 mg/100 g from rice polishing | [197] |
| Yeast fermentation | Saccharomyces cerevisiae | Pineapple crumbs by-product | Fermented pineapple juice | Alcohol content: 5–6 %v, Soluble solids concentration: 13–14 °Bx. | [198] |
| SSF | A. fumigatus TXD105 | Sugarcane bagasse | Paclitaxel | 145.61 mg/kg | [199] |
| Fungal Fermentation (SSF) | A. terreus | mixed food waste | Glucose, free amino nitrogen, inorganic phosphate | Glucose-0.57 g/g, free amino nitrogen-185 mg/L, inorganic phosphate-195 mg/L | [200] |
| SSF | Bacillus subtilis D19 | Wheat bran | Amylase | 1239 U/g | [201] |
| Fungal Fermentation (SSF) | Neurospora intermedia | Okara | Fermented food | Increased protein content from 25% to 28% and lipid content from 12% to 14%. | [202] |
6.2. Eco-Friendly Fermentation Technologies: Bioreactor Innovations and Low-Energy Processes
6.3. Personalized Fermented Foods: AI-Driven Formulation Based on Microbiome Profiling
6.4. Space and Extreme Environment Fermentation: Potential Applications in Space Travel and Food Security
7. Challenges and Limitations
7.1. Regulatory Challenges
7.2. Scalability and Commercialization Challenges
7.3. Consumer Perception and Market Acceptance
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goksen, G.; Sugra Altaf, Q.; Farooq, S.; Bashir, I.; Capozzi, V.; Guruk, M.; Bavaro, S.L.; Sarangi, P.K. A glimpse into plant-based fermented products alternative to animal-based products: Formulation, processing, health benefits. Food Res. Int. 2023, 173, 113344. [Google Scholar] [CrossRef]
- Venter de Villiers, M.; Cheng, J.; Truter, L. The Shift Towards Plant-Based Lifestyles: Factors Driving Young Consumers’ Decisions to Choose Plant-Based Food Products. Sustainability 2024, 16, 9022. [Google Scholar] [CrossRef]
- Mallari, P.; Brogi, S. Microbial Fermentation in Food and Beverage Industries: Innovations, Challenges, and Opportunities. Foods 2025, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.S.; Anjum, R.; Yang, Y.; Tu, M.; Zhang, T.; Pan, D.; Sun, Y.; Wu, Z. Recent Trends in Fermented Plant-Based Analogues and Products, Bioactive Peptides, and Novel Technologies-Assisted Fermentation. Trends Food Sci. Technol. 2024, 149, 104529. [Google Scholar] [CrossRef]
- Khayatan, D.; Nouri, K.; Momtaz, S.; Roufogalis, B.D.; Alidadi, M.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. Plant-derived fermented products: An interesting concept for human health. Curr. Dev. Nutr. 2024, 8, 102162. [Google Scholar] [CrossRef] [PubMed]
- Machneva, I.A. The biological value of fermented plant-based food products. Fruit Grow. Vitic. South Russ. 2024, 3, 154–192. [Google Scholar] [CrossRef]
- Plant-Based Food Market—Global Opportunity Analysis and Industry Forecast (2024–2031). (n.d.). Available online: https://www.meticulousresearch.com/product/plant-based-food-market-5108 (accessed on 21 April 2025).
- Singh, A.K.; Elango, D.; Raigne, J.; der Laan, L.V.; Rairdin, A.; Soregaon, C.D.; Singh, A. Plant-based protein crops and their improvement: Current status and future perspectives. Crop Sci. 2024, 65, e21389. [Google Scholar] [CrossRef]
- Veniranda, V.; Surya, R. Consumer Analysis of Commercial Plant-Based Jerky. IOP Conf. Ser. Earth Environ. Sci. 2022, 998, 012059. [Google Scholar] [CrossRef]
- Yang, C. Rsesearch on Plant-Based Food Market Implications and Opportunities. Adv. Econ. Manag. Political Sci. 2023, 6, 398–402. [Google Scholar] [CrossRef]
- European Plant-Based Foods Sales Data 2017–2020 (Nielsen Market Track). 2022. Available online: https://zenodo.org/records/6411841 (accessed on 21 April 2025).
- Abbaspour, N. Fermentation’s pivotal role in shaping the future of plant-based foods: An integrative review of fermentation processes and their impact on sensory and health benefits. Appl. Food Res. 2024, 4, 100468. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Hassoun, A.; Zouari, A.; Tülbek, M.; Mefleh, M.; Aït-Kaddour, A.; Castellari, M. Fermentation for designing innovative plant-based meat and dairy alternatives. Foods 2023, 12, 1005. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Sela, D.A.; Nolden, A.A. Sucrose Concentration and Fermentation Temperature Impact the Sensory Characteristics and Liking of Kombucha. Foods 2023, 12, 3116. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Dai, T.; Huang, S.; Wu, K.; Wang, M.; Tan, C.; Zhang, F.; Sheng, J.; Zhao, C. Physical and Chemical Properties, Flavor and Organoleptic Characteristics of a Walnut and Purple Rice Fermented Plant Drink. Foods 2024, 13, 400. [Google Scholar] [CrossRef]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of lactobacillus rhamnosus on physicochemical properties of fermented plant-based raw materials. Foods 2021, 10, 573. [Google Scholar] [CrossRef]
- Bayazitov, K.R.; Ivanov, M.S.; Gelazov, R.K.; Barua, S.; Lavrentev, F.V.; Antsyperova, M.A.; Fedorov, A.A.; Iakovchenko, N.V. Enhancing nutritional and organoleptic properties of jerusalem artichoke tuber through probiotic fermentation. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Liu, Z.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility and movement of phenolic compounds from tomato (Solanum lycopersicum) during in vitro gastrointestinal digestion and colonic fermentation. Food Funct. 2022, 13, 4954–4966. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef]
- Thierry, A.; Madec, M.; Chuat, V.; Bage, A.; Picard, O.; Grondin, C.; Rué, O.; Mariadassou, M.; Marché, L.; Valence, F. Microbial communities of a variety of 75 homemade fermented vegetables. Front. Microbiol. 2023, 14, 1323424. [Google Scholar] [CrossRef]
- Gautam, A.C.; Poopalarajah, R.; Ahmad, A.; Rana, B.S.; Denekew, T.W.; Ngọc Anh, N.T.; Utenova, L.; Kunwar, Y.S.; Bhandari, N.S.; Jha, A.R. Ecological factors that drive microbial communities in culturally diverse fermented foods. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ghatani, K.; Sha, S.P.; Thapa, S.; Sarkar, I.; Sen, G.; Sen, A. Investigating bacterial diversity involved in the production of vegetable-based ethnic fermented food of North Bengal and their metabolic pathways with reverse ecology approach. Front. Sustain. Food Syst. 2024, 8, 1322192. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, Z.; Huang, T.; Qianqian, G.; Li, J.; Xie, M.; Xiong, T. Bacterial community dynamics and physicochemical characteristics in natural fermentation of jiang-shui, a traditional food made in northwest China. J. Sci. Food Agric. 2019, 99, 3391–3397. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.S.; Zhang, C.; Kim, Y.B.; Roh, S.W.; Liu, D. Culture-independent analysis of the bacterial community in Chinese fermented vegetables and genomic analysis of lactic acid bacteria. Arch. Microbiol. 2021, 203, 4693–4703. [Google Scholar] [CrossRef]
- Miller, E.R.; Kearns, P.J.; Niccum, B.A.; O’Mara Schwartz, J.; Ornstein, A.; Wolfe, B.E. Establishment limitation constrains the abundance of lactic acid bacteria in the napa cabbage phyllosphere. Appl. Environ. Microbiol. 2019, 85, e00269-19. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Ozdal, T.; Abu-khalil, F. Sourdough-based microbiota, fermentations, and development of taste and aroma active compounds and their impact on the final products and in the global sensorial perception and preferences by the consumers. In Handbook of Sourdough Microbiota and Fermentation; Elsevier: Amsterdam, The Netherlands, 2025; pp. 229–245. [Google Scholar] [CrossRef]
- Alrosan, M.; Tan, T.-C.; Easa, A.M.; Gammoh, S.; Alu’datt, M.H.; Kubow, S.; Almajwal, A.M.; Razzak Mahmood, A.A.; Al-Qaisi, A.; Bawadi, H. Enhancing the quality of lentil proteins via combination with whey proteins based on a dual process: A novel strategy through the incorporation of complexation and fermentation. Food Sci. Biotechnol. 2025, 34, 65–78. [Google Scholar] [CrossRef]
- Pasaribu, T.; Laconi, E.; Kompiang, I. Evaluation of the nutrient contents of palm kernel cake fermented by microbial cocktails as a potential feedstuff for poultry. J. Indones. Trop. Anim. Agric. 2019, 44, 295–302. [Google Scholar] [CrossRef]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Deveci, G.; Çelik, E.; Ağagündüz, D.; Bartkiene, E.; Rocha, J.M.F.; Özogul, F. Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms. Fermentation 2023, 9, 923. [Google Scholar] [CrossRef]
- Chen, A.; Yuan, J. Fermentation Utilizing Engineered Microbes: Revolutionizing the Production of Commercial Products from Plant-Derived Bioactive Compounds; Elsevier BV: Amsterdam, The Netherlands, 2024; pp. 153–201. [Google Scholar] [CrossRef]
- Fernández-Varela, R.; Hansen, A.H.; Svendsen, B.A.; Moghadam, E.G.; Bas, A.; Kračun, S.K.; Harlé, O.; Kuzina, V. Harnessing Fermentation by Bacillus and Lactic Acid Bacteria for Enhanced Texture, Flavor, and Nutritional Value in Plant-Based Matrices. Fermentation 2024, 10, 411. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional Applications of Lactic Acid Bacteria: Enhancing Safety, Quality, and Nutritional Value in Foods and Fermented Beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.; Fabritius, M.; Yang, B. Determination of vitamin K composition of fermented food. Food Chem. 2019, 275, 515–522. [Google Scholar] [CrossRef]
- Liu, L.; Li, G.; Cui, L.; Cai, R.; Yuan, Y.; Gao, Z.; Yue, T.; Wang, Z. The health benefits of fermented fruits and vegetables and their underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70072. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic production: Harnessing the power of microbial metabolites for health applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Caffrey, E.B.; Olm, M.R.; Kothe, C.I.; Evans, J.; Sonnenburg, J.L. MiFoDB, a workflow for microbial food metagenomic characterization, enables high-resolution analysis of fermented food microbial dynamics. bioRxiv 2024. [Google Scholar] [CrossRef]
- Jin, R.; Song, J.; Liu, C.; Lin, R.; Liang, D.; Aweya, J.J.; Weng, W.; Zhu, L.; Shang, J.; Yang, S. Synthetic microbial communities: Novel strategies to enhance the quality of traditional fermented foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13388. [Google Scholar] [CrossRef]
- Tang, N.; Xing, X.; Li, H.; Suo, B.; Wang, Y.; Ai, Z.; Yang, Y. Co-culture fermentation by Saccharomycopsisfibuligera and lactic acid bacteria improves bioactivity and aroma profile of wheat bran and the bran-containing Chinese steamed bread. Food Res. Int. 2024, 182, 114179. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, T.; Huang, C.; Hardie, J.; Peng, Z.; Xie, M.; Xiong, T. The microbial communities and flavour compounds of Jiangxi yancai, Sichuan paocai and Dongbeisuancai: Three major types of traditional Chinese fermented vegetables. Lwt-Food Sci. Technol. 2020, 121, 108865. [Google Scholar] [CrossRef]
- de Jong, M.F.; Alekseeva, A.Y.; Miraji, K.F.; Phiri, S.; Linnemann, A.R.; Schoustra, S.E. Environmental Selection Shapes Bacterial Community Composition in Traditionally Fermented Maize-Based Foods from Benin, Tanzania and Zambia. Microorganisms 2022, 10, 1354. [Google Scholar] [CrossRef]
- Casciano, F.; Mayr, H.L.; Nissen, L.; Putti, A.; Zoli, F.; Gianotti, A.; Conterno, L. Red Beetroot Fermentation with Different Microbial Consortia to Develop Foods with Improved Aromatic Features. Foods 2022, 11, 3055. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Saint-Eve, A.; Irlinger, F.; Souchon, I.; Bonnarme, P. Modulation of Metabolome and Overall Perception of Pea Protein-Based Gels Fermented with Various Synthetic Microbial Consortia. Foods 2022, 11, 1146. [Google Scholar] [CrossRef]
- Kothe, C.I.; Carøe, C.; Mazel, F.; Zilber, D.; Cruz-Morales, P.; Mohellibi, N.; Evans, J.P. Novel misos shape distinct microbial ecologies: Opportunities for flavourful sustainable food innovation. Food Res. Int. 2024, 189, 114490. [Google Scholar] [CrossRef]
- Siroli, L.; Giordani, B.; Rossi, S.; Gottardi, D.; McMahon, H.; Augustyniak, A.; Menon, A.; Vannini, L.; Vitali, B.; Patrignani, F.; et al. Antioxidant and Functional Features of Pre-Fermented Ingredients Obtained by the Fermentation of Milling By-Products. Fermentation 2022, 8, 722. [Google Scholar] [CrossRef]
- Bergsveinson, J.; Kajala, I.; Ziola, B.; Ziola, B. Next-generation sequencing approaches for improvement of lactic acid bacteria-fermented plant-based beverages. AIMS Microbiol. 2017, 3, 8–24. [Google Scholar] [CrossRef]
- Janavi, R.; Punia, D.; Soumya, C. Fermentation technology. In Futuristic Trends in Biotechnology Volume 3 Book 4; Iterative International Publishers: Chikmagalur, India, 2024; pp. 61–73. [Google Scholar] [CrossRef]
- Adebo, J.A.; Njobeh, P.B.; Gbashi, S.; Oyedeji, A.B.; Ogundele, O.M.; Oyeyinka, S.A.; Adebo, O.A. Fermentation of Cereals and Legumes: Impact on Nutritional Constituents and Nutrient Bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Akanni, G.B.; Adebo, O.A. Metabolite perturbations in fermented legumes as elucidated using metabolomics: A review. Int. J. Food Sci. Technol. 2024, 59, 4234–4250. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.-J.; Zhao, Y.; Zhu, Y.; Bai, J.; Fan, S.; Zhu, L.; Song, C.; Xiao, X. Recent Developments in Fermented Cereals on Nutritional Constituents and Potential Health Benefits. Foods 2022, 11, 2243. [Google Scholar] [CrossRef]
- Melhem, M.; Omri, G.; Limongelli, R.; Minervini, F.; Santamaria, M.; Faccia, M. Enhancing nutritional and sensory properties of plant-based beverages: A study on chickpea and Kamut® flours fermentation using Lactococcus lactis. Front. Nutr. 2024, 11, 1269154. [Google Scholar] [CrossRef]
- Sivakumar, K.; Adebo, O.A.; Gieng, J.; Feng, X. Exploring Fermented Rice-Based Foods: A Review of Nutritional Enhancement, Microbial Analysis, and Global Health Implications; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Boukid, F.; Fanari, F.; Mefleh, M. Plant-Based Fermented Foods and Microbial Ingredients in Meat Analogs; Elsevier BV: Amsterdam, The Netherlands, 2024; pp. 169–186. [Google Scholar] [CrossRef]
- Onyeka, O.B.; Nyerhovwo, T.J.; Oghenetega, A.J.; Eferhire, A. Effect of fermentation on sensory, nutritional and antioxidant properties of mixtures of aqueous extracts of hibiscus sabdariffa (zobo) and raphia hookeri (raffia) wine. Niger. J. Sci. Environ. 2017, 15, 66–74. [Google Scholar]
- Aleman, R.S.; Montero Fernández, I.; Marcía, J.; Saravia-Maldonado, S.A.; Martín-Vertedor, D. Application of Fermentation as a Strategy for the Transformation and Valorization of Vegetable Matrices. Fermentation 2024, 10, 124. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, N.; Tan, B. Effects of Germination, Fermentation and Extrusion on the Nutritional, Cooking and Sensory Properties of Brown Rice Products: A Comparative Study. Foods 2023, 12, 1542. [Google Scholar] [CrossRef]
- Irakoze, M.L.; Wafula, E.N.; Owaga, E. Effect of Lactic Acid Fermentation on Phytochemical Content, Antioxidant Capacity, Sensory Acceptability and Microbial Safety of African Black Nightshade and African Spider Plant Vegetables. Bacteria 2023, 2, 48–59. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; Yu, B.; Zheng, Z.; Xu, Y. Fermentation improves flavors, bioactive substances, and antioxidant capacity of Bian-Que Triple-Bean Soup by lactic acid bacteria. Front. Microbiol. 2023, 14, 1152654. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Soni, B.K.; Sharkey, B.; Acree, T.E.; Lavin, E.H.; Bailey, H.M.; Stein, H.-H.; Han, A.; Elie, M.R.; Nadal, M. Shiitake mycelium fermentation improves digestibility, nutritional value, flavor and functionality of plant proteins. Lebensm.-Wiss. Technol. 2022, 156, 113065. [Google Scholar] [CrossRef]
- Nath, H.; Samtiya, M.; Dhewa, T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: A review. Hum. Nutr. Metab. 2022, 28, 200147. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Puniya, A.K.; Dhewa, T. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. [Google Scholar] [CrossRef]
- Qi, N.; Zhan, X.; Milmine, J.; Sahar, M.; Chang, K.-H.; Li, J. Isolation and characterization of a novel hydrolase-producing probiotic Bacillus licheniformis and its application in the fermentation of soybean meal. Front. Nutr. 2023, 10, 1123422. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Life 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Sarıtaş, S.; Duman, H.; Karav, S. Nutritional and functional aspects of fermented algae. Int. J. Food Sci. Technol. 2024, 59, 5270–5284. [Google Scholar] [CrossRef]
- Naseem, A.; Akhtar, S.; Ismail, T.; Qamar, M.; Sattar, D.-e.-s.; Saeed, W.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Growth Stages and Lactic Acid Fermentation on Anti-Nutrients and Nutritional Attributes of Spinach (Spinacia oleracea). Microorganisms 2023, 11, 2343. [Google Scholar] [CrossRef]
- Zayed, A.; Adly, G.M.; Farag, M.A. Management strategies for the anti-nutrient oxalic acid in foods: A comprehensive overview of its dietary sources, roles, metabolism, and processing. Food Bioprocess Technol. 2025, 18, 4280–4300. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Mottaghitalab, M.; Hashemi, M. Influence of microbial fermentation processing of sesame meal and enzyme supplementation on broiler performances. Ital. J. Anim. Sci. 2020, 19, 712–722. [Google Scholar] [CrossRef]
- Olawoye, B.T.; Gbadamosi, S.O. Effect of different treatments on in vitro protein digestibility, antinutrients, antioxidant properties and mineral composition of Amaranthus viridis seed. Cogent Food Agric. 2017, 3, 1296402. [Google Scholar] [CrossRef]
- Subedi, U.; Raychaudhuri, S.; Fan, S.; Ogedengbe, O.; Obanda, D.N. Fermenting kale (Brassica oleracea L.) enhances its functional food properties by increasing accessibility of key phytochemicals and reducing antinutritional factors. Food Sci. Nutr. 2024, 12, 5480–5496. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast: Biotransformation of isoflavones. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef]
- Bahaciu, G.V.; Nicolae, C.G.; Șuler, A.D.; Segal, R. Germinated and lactic fermented soybean seeds, a natural alternative for healthy bones. A scientific approach. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2018, 75, 8. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, J.; Cai, G. Quality improvement of soybean meal by yeast fermentation based on the degradation of anti-nutritional factors and accumulation of beneficial metabolites. J. Sci. Food Agric. 2024, 104, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Badgujar, P.C.; Chandratre, G.A.; Aluko, R.E.; Kumar, A.; Bhushan, B.; Dhewa, T. Effect of selective fermentation on nutritional parameters and techno-functional characteristics of fermented millet-based probiotic dairy product. Food Chem. X 2024, 22, 101483. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; Cabrera-Hidalgo, A.d.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toala, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef]
- Rangaswamaiah, R. Use of Microorganisms for the Enrichment of Zn and Se by Using Solid-State Fermentation. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2023. [Google Scholar]
- Christensen, L.F.; García-Béjar, B.; Bang-Berthelsen, C.H.; Hansen, E.B. Extracellular microbial proteases with specificity for plant proteins in food fermentation. Int. J. Food Microbiol. 2022, 381, 109889. [Google Scholar] [CrossRef]
- Moore, J.F.; DuVivier, R.; Johanningsmeier, S.D. Changes in the free amino acid profile of pickling cucumber during lactic acid fermentation. J. Food Sci. 2022, 87, 599–611. [Google Scholar] [CrossRef]
- Gientka, I.; Synowiec, A.; Pobiega, K.; Staniszewska, P.; Perkowska, J.; Procyk, M.; Pokrywczyński, B.I.; Janowicz, M. The Nutritional Profile of Root Vegetables Through Spontaneous Fermentation with Apples: Amino Acid Composition and Microbial Dynamics. Fermentation 2025, 11, 110. [Google Scholar] [CrossRef]
- Filipe, D.; Vieira, L.; Ferreira, M.; Oliva-Teles, A.; Salgado, J.; Belo, I.; Peres, H. Enrichment of a Plant Feedstuff Mixture’s Nutritional Value through Solid-State Fermentation. Animals 2023, 13, 2883. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Yu, S.-H.; Cheng, K.-W.; Liou, Y.-W.; Hsu, C.-C.; Hsieh, C.-W.; Kuo, C.-H.; Cheng, K.-C. Production and analysis of metabolites from solid-state fermentation of Chenopodium formosanum (Djulis) sprouts in a bioreactor. Food Res. Int. 2023, 168, 112707. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- Ranjan, A.; Sahu, N.P.; Deo, A.D.; Kumar, S. Solid state fermentation of de-oiled rice bran: Effect on in vitro protein digestibility, fatty acid profile and anti-nutritional factors. Food Res. Int. 2019, 119, 1–5. [Google Scholar] [CrossRef]
- Terefe, Z.K.; Omwamba, M.N.; Nduko, J.M. Effect of solid state fermentation on proximate composition, antinutritional factors and in vitro protein digestibility of maize flour. Food Sci. Nutr. 2021, 9, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Wikiera, A. Proteolysis in tempeh-type products obtained with Rhizopus and Aspergillus strains from grass pea (Lathyrus sativus) seeds. Acta Sci. Aliment. 2015, 14, 125–132. [Google Scholar] [CrossRef]
- Marttinen, M.; Anjum, M.; Saarinen, M.T.; Ahonen, I.; Lehtinen, M.J.; Nurminen, P.; Laitila, A. Enhancing Bioaccessibility of Plant Protein Using Probiotics: An In Vitro Study. Nutrients 2023, 15, 3905. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, P.; Chen, X. Bioactive peptides derived from fermented foods: Preparation and biological activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- He, M.T.; Howell, K.S. Vitamin-B12 enrichment in tempeh by co-culture with Propionibacterium freudenreichii during fermentation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lim, M.-J.; Barathikannan, K.; Jeong, Y.-J.; Chelliah, R.; Vijayalakshmi, S.; Park, S.-J.; Oh, D.-H. Exploring the impact of fermentation on brown rice: Health benefits and value-added foods—A comprehensive meta-analysis. Fermentation 2023, 10, 3. [Google Scholar] [CrossRef]
- Van Bokhorst-van De Veen, H.; Berendsen, L.; Helmond, M.; Nierop Groot, M. In situ fortification of protein-enriched brewer’s spent grain with vitamin B12 by fermentation with Priestia megaterium and Propionibacterium freudenreichii. LWT 2024, 205, 116520. [Google Scholar] [CrossRef]
- Rana, A.; Taneja, N.K.; Singh, A.; Dhewa, T.; Kumar, V.; Kumar, A.; Chauhan, K.; Juneja, V.; Oberoi, H.S. Synergistic fermentation of vitamin B2 (Riboflavin) bio-enriched soy milk: Optimization and techno-functional characterization of next generation functional vegan foods. Discov. Food 2025, 5, 10. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Berasarte, I.; Zeid, A.F.; Fernández, M.; Russo, P.; López, P.; Dueñas, M.T.; Mohedano, M.L. Functional characterization of the riboflavin-overproducing and dextran-producing Weissellacibaria BAL3C-5 C120T strain for the development of biofortified plant-based beverages. Int. J. Food Microbiol. 2025, 426, 110908. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Thumthanaruk, B.; Shetty, K. Changes in physico-chemical, astringency, volatile compounds and antioxidant activity of fresh and concentrated cashew apple juice fermented with Lactobacillus plantarum. J. Food Sci. Technol. 2018, 55, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Sooklim, C.; Paemanee, A.; Ratanakhanokchai, K.; Wiwatratana, D.; Soontorngun, N. Integrated omic analysis of a new flavor yeast strain in fermented rice milk. FEMS Yeast Res. 2025, 25, foaf017. [Google Scholar] [CrossRef] [PubMed]
- Słowik-Borowiec, M.; Potocki, L.; Oklejewicz, B.; Broda, D.; Podbielska, M.; Szpyrka, E. Preparation of vitamin k2 mk-7 in a process of fermentation of different seeds and cereals by bacteria bacillus subtilis. Acta Universitatis Cibiniensis. Ser. E Food Technol. 2021, 25, 93–104. [Google Scholar] [CrossRef]
- Horlacher, N.; Oey, I.; Agyei, D. Learning from Tradition: Health-Promoting Potential of Traditional Lactic Acid Fermentation to Drive Innovation in Fermented Plant-Based Dairy Alternatives. Fermentation 2023, 9, 452. [Google Scholar] [CrossRef]
- Sawant, S.S.; Park, H.-Y.; Sim, E.-Y.; Kim, H.-S.; Choi, H.-S. Microbial fermentation in food: Impact on functional properties and nutritional enhancement—A review of recent developments. Fermentation 2025, 11, 15. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Jalili, M.; Nazari, M.; Magkos, F. Fermented Foods in the Management of Obesity: Mechanisms of Action and Future Challenges. Int. J. Mol. Sci. 2023, 24, 2665. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Sutheeworapong, S.; Jenjaroenpun, P.; Charoensiddhi, S.; Khoiri, A.N.; Topanurak, S.; Sutthikornchai, C.; Jintaridth, P. Microbiome analysis of thai traditional fermented soybeans reveals short-chain fatty acid-associated bacterial taxa. Sci. Rep. 2023, 13, 7573. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Q.; Zhao, Z.; Shen, N.; Qin, Y.; Lin, W.; Xiao, Y.; Yuan, M.; Chen, H.; Chen, H.; et al. Evaluation of fermentation properties, antioxidant capacity in vitro and in vivo, and metabolic profile of a fermented beverage made from apple and cantaloupe. LWT 2023, 179, 114661. [Google Scholar] [CrossRef]
- Yaqoob, S.; Imtiaz, A.; Khalifa, I.; Maqsood, S.; Ullah, R.; Shahat, A.A.; Al-Asmari, F.; Murtaza, M.S.; Qian, J.-Y.; Ma, Y. Multi-frequency sono-fermentation with mono and co-cultures of LAB synergistically enhance mulberry juice: Evidence from metabolic, micromorphological, sensorial, and computational approaches. Ultrason. Sonochem. 2024, 111, 107117. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Abdelhedi, O.; Nasri, M.; Jridi, M. Fermented protein hydrolysates: Biological activities and applications. Curr. Opin. Food Sci. 2022, 43, 120–127. [Google Scholar] [CrossRef]
- Tonini, S.; Zein AlabidenTlais, A.; Galli, B.D.; Helal, A.; Tagliazucchi, D.; Filannino, P.; Zannini, E.; Gobbetti, M.; Di Cagno, R. Lentils protein isolate as a fermenting substrate for the production of bioactive peptides by lactic acid bacteria and neglected yeast species. Microb. Biotechnol. 2024, 17, e14387. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Lin, H.-W.; Huang, H.-C.; Khumsupan, D.; Shen, S.-C.; Lin, S.-P.; Hsieh, C.-W.; Tsai, T.-Y.; Jantama, S.S.; Kuo, H.-C.; et al. Peptide from tempeh-like fermented Chenopodium formosanum counters senescence while enhancing antioxidant ability in non-replicative aging. LWT 2025, 222, 117641. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Tong, B.; Cui, Y.; Vonesch, S.C.; Dong, H.; Zhang, D. An Efficient CRISPR/Cas12e System for Genome Editing in Sinorhizobiummeliloti. ACS Synth. Biol. 2023, 12, 898–903. [Google Scholar] [CrossRef]
- Amore, A.; Philip, S. Artificial intelligence in food biotechnology: Trends and perspectives. Front. Ind. Microbiol. 2023, 1, 1255505. [Google Scholar] [CrossRef]
- Demarinis, C.; Verni, M.; Koirala, P.; Cera, S.; Rizzello, C.G.; Coda, R. Effect of LAB starters on technological and functional properties of composite carob and chickpea flour plant-based gurt. Future Foods 2024, 9, 100289. [Google Scholar] [CrossRef]
- Erdoğan, İ.; Cevher-Keskin, B.; Bilir, Ö.; Hong, Y.; Tör, M. Recent Developments in CRISPR/Cas9 Genome-Editing Technology Related to Plant Disease Resistance and Abiotic Stress Tolerance. Biology 2023, 12, 1037. [Google Scholar] [CrossRef]
- Fang, H.; Li, D.; Kang, J.; Jiang, P.; Sun, J.; Zhang, D. Metabolic engineering of Escherichia coli for de novo biosynthesis of vitamin B12. Nat. Commun. 2018, 9, 4917. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, B.Y.; Bae, J.M.; Wang, Y.; Jin, Y.S. Genome-edited Saccharomyces cerevisiae strains for improving quality, safety, and flavor of fermented foods. Food Microbiol. 2022, 104, 103971. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Y.-S.; Miller, M.J. Exploiting endogenous cas9-based genome editing of Lacticaseibacillus rhamnosus gg (Lgg) for precision food fermentation and live biotherapeutics. bioRxiv 2025. [Google Scholar] [CrossRef]
- Han, C.; Xia, K.; Ma, J.; Liang, X.; Wang, W. CRISPR-Cas9 mediated construction of a food-grade pyrroloquinoline quinone high-yielding Acetobacter pasteurianus Ab3 strain. Food Biosci. 2025, 68, 106452. [Google Scholar] [CrossRef]
- Haryani, Y.; Abdul Halid, N.; Goh, S.G.; Nor-Khaizura, M.A.R.; Md Hatta, M.A.; Sabri, S.; Radu, S.; Hasan, H. Efficient metabolic pathway modification in various strains of lactic acid bacteria using CRISPR/Cas9 system for elevated synthesis of antimicrobial compounds. J. Biotechnol. 2024, 395, 53–63. [Google Scholar] [CrossRef]
- Hussain, M.I.; Raziq, A.; Ahmed, A.; Iqbal, M.W.; Tian, R.; Li, J.; Liu, L.; Liu, Y. Recent progress in CRISPR-based bioengineering of microbial cell factories for important nutraceuticals synthesis. J. Appl. Microbiol. 2023, 134, lxad114. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Sharma, J.; Khare, P. Recent advancements and strategies for omega-3 fatty acid production in yeast. J. Basic Microbiol. 2025, 65, e2400491. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, Z.; Zhang, Q.; Wang, H. Enhanced lysine production in Escherichia coli via CRISPR-mediated metabolic rewiring. ACS Synth. Biol. 2024, 13, 287–298. [Google Scholar] [CrossRef]
- Wang, F.; Cai, N.; Leng, Y.; Wu, C.; Wang, Y.; Tian, S.; Zhang, C.; Xu, Q.; Peng, H.; Chen, N.; et al. Metabolic Engineering of Corynebacterium glutamicum for the High-Level Production of l-Valine under Aerobic Conditions. ACS Synth. Biol. 2024, 13, 2861–2872. [Google Scholar] [CrossRef]
- Cleto, S.; Jensen, J.V.; Wendisch, V.F.; Lu, T.K. Corynebacterium glutamicum Metabolic Engineering with CRISPR Interference (CRISPRi). ACS Synth. Biol. 2016, 5, 375–385. [Google Scholar] [CrossRef]
- Han, X.; Li, Z.; Wen, Y.; Chen, Z. Overproduction of docosahexaenoic acid in Schizochytrium sp. through genetic engineering of oxidative stress defense pathways. Biotechnol. Biofuels 2021, 14, 70. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, T.; Qiao, M. Current application and future prospects of CRISPR-Cas in lactic acid Bacteria: A review. Food Res. Int. 2025, 209, 116315. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pudhuvai, B.; Shrestha, A.; Mishra, A.K.; Shah, M.P.; Koul, B.; Dey, N. CRISPR-mediated iron and folate biofortification in crops: Advances and perspectives. Biotechnol. Genet. Eng. Rev. 2024, 40, 4138–4168. [Google Scholar] [CrossRef] [PubMed]
- Deokar, G.S.; Pathak, V.A.; Kshirsagar, S.J.; Al-Asmari, F.; Nirmal, N. Enhancement of vitamin B12 in plant-based food through microbial fermentation-a sustainable food system. Food Chem. 2025, 484, 144437. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, A.; Ahmad, R.; Dwivedi, U.N.; Yadav, K. CRISPR-Based Genome Editing for Nutrient Enrichment in Crops: A Promising Approach Toward Global Food Security. Front. Genet. 2022, 13, 932859. [Google Scholar] [CrossRef]
- Cui, Y.; Qu, X. CRISPR-Cas systems of lactic acid bacteria and applications in food science. Biotechnol. Adv. 2024, 71, 108323. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M. Advances in genetically engineered microorganisms: Transforming food production through precision fermentation and synthetic biology. Future Foods 2025, 11, 100601. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, A.; Partap, M.; Thakur, M.; Bhargava, B. CRISPR-Cas: A robust technology for enhancing consumer-preferred commercial traits in crops. Front. Plant Sci. 2023, 14, 1122940. [Google Scholar] [CrossRef] [PubMed]
- GurubelTun, K.J.; León-Becerril, E.; García-Depraect, O. Optimal control strategy based on artificial intelligence applied to a continuous dark fermentation reactor for energy recovery from organic wastes. Green Energy Resour. 2025, 3, 100112. [Google Scholar] [CrossRef]
- Chai, W.Y.; Tan, M.K.; Teo, K.T.K.; Tham, H.J. Optimization of fed-batch baker’s yeast fermentation using deep reinforcement learning. Process Integr. Optim. Sustain. 2024, 8, 395–411. [Google Scholar] [CrossRef]
- Li, D.; Zhu, F.; Wang, X.; Jin, Q. Multi-objective reinforcement learning for fed-batch fermentation process control. J. Process Control 2022, 115, 89–99. [Google Scholar] [CrossRef]
- Simethy, G. Implementation and Assessment of Advanced Reinforcement Learning Methods for Control of Chemical Processes. Doctoral Dissertation, Hochschule für AngewandteWissenschaften Hamburg, Hamburg, Germany, 2024. [Google Scholar]
- Reza, A.; Chen, L.; Mao, X. Response surface methodology for process optimization in livestock wastewater treatment: A review. Heliyon 2024, 10, e30326. [Google Scholar] [CrossRef] [PubMed]
- Rabiya, R.; Sen, R. Artificial intelligence driven advanced optimization strategy vis-à-vis response surface optimization of production medium: Bacterial exopolysaccharide production as a case-study. Biochem. Eng. J. 2022, 178, 108271. [Google Scholar] [CrossRef]
- Dan, T.; Hu, H.; Tian, J.; He, B.; Tai, J.; He, Y. Influence of Different Ratios of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus on Fermentation Characteristics of Yogurt. Molecules 2023, 28, 2123. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Zhang, J.; Li, X.; Wang, J.; Sun, B. Oat milk analogue versus traditional milk: Comprehensive evaluation of scientific evidence for processing techniques and health effects. Food Chem. X 2023, 19, 100859. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Wätjen, A.P.; Gumulya, Y.; Fernández-Pacheco, P.; Marcellin, E.; Prakash, S.; Bang-Berthelsen, C.H.; Turner, M.S. Isolation of an exopolysaccharide-producing Weissellaconfusa strain from lettuce and exploring its application as a texture modifying adjunct culture in a soy milk alternative. Int. J. Food Microbiol. 2025, 428, 110992. [Google Scholar] [CrossRef] [PubMed]
- Păcularu-Burada, B.; Georgescu, L.A.; Bahrim, G.-E. Current approaches in sourdough production with valuable characteristics for technological and functional applications. Ann. Univ. Dunarea De Jos Galati Fascicle VI–Food Technol. 2020, 44, 132–148. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Li, C.; Liu, L. Biological Activity of Lactic Acid Bacteria Exopolysaccharides and Their Applications in the Food and Pharmaceutical Industries. Foods 2024, 13, 1621. [Google Scholar] [CrossRef]
- Dhakal, D.; Kumar, G.; Devkota, L.; Subedi, D.; Dhital, S. The choice of probiotics affects the rheological, structural, and sensory attributes of lupin-oat-based yoghurt. Food Hydrocoll. 2024, 156, 110353. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pham, T.T.; Nguyen, P.T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid Bacteria Exopolysaccharides Producers: A Sustainable Tool for Functional Foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature reviews. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Kaur, S.; Rani, N.; Kaur, R.; Upadhyay, S.K.; Tripathi, M. Exploring Microbial Contributions to Nutraceutical Production: From Natural to Designed Foods. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Probiotics, prebiotics, synbiotics, and fermented foods as potential biotics in nutrition improving health via microbiome-gut-brain axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Obayomi, O.V.; Olaniran, A.F.; Owa, S.O. Unveiling the role of functional foods with emphasis on prebiotics and probiotics in human health: A review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Gill, P.A.; Van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, E.; Boakye, F.; Zielińska, M.; Dereń, K.; Bartosiewicz, A.; Oleksy, Ł.; Stolarczyk, A. Vegan diet: Nutritional components, implementation, and effects on adults’ health. Front. Nutr. 2023, 10, 1294497. [Google Scholar] [CrossRef] [PubMed]
- Mantri, A.; Klümpen, L.; Seel, W.; Krawitz, P.; Stehle, P.; Weber, B.; Simon, M.C. Beneficial effects of Synbiotics on the gut microbiome in individuals with low Fiber intake: Secondary analysis of a double-blind, randomized controlled trial. Nutrients 2024, 16, 2082. [Google Scholar] [CrossRef] [PubMed]
- Nithya, A.; Misra, S.; Panigrahi, C.; Dalbhagat, C.G.; Mishra, H.N. Probiotic potential of fermented foods and their role in non-communicable diseases management: An understanding through recent clinical evidences. Food Chem. Adv. 2023, 3, 100381. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Xiong, Z.; Xia, Y.; Song, X.; Zhang, H.; Ai, L. Probiotics interact with lipids metabolism and affect gut health. Front. Nutr. 2022, 9, 917043. [Google Scholar] [CrossRef]
- Buendia, J.R.; Li, Y.; Hu, F.B.; Cabral, H.J.; Bradlee, M.L.; Quatromoni, P.A.; Singer, M.R.; Curhan, G.C.; Moore, L.L. Regular yogurt intake and risk of cardiovascular disease among hypertensive adults. Am. J. Hypertens. 2018, 31, 557–565. [Google Scholar] [CrossRef]
- Porras-García, E.; Fernández-Espada Calderón, I.; Gavala-González, J.; Fernández-García, J.C. Potential neuroprotective effects of fermented foods and beverages in old age: A systematic review. Front. Nutr. 2023, 10, 1170841. [Google Scholar] [CrossRef]
- Icer, M.A.; Sarikaya, B.; Kocyigit, E.; Atabilen, B.; Çelik, M.N.; Capasso, R.; Ağagündüz, D.; Budán, F. Contributions of gamma-aminobutyric acid (GABA) produced by lactic acid bacteria on food quality and human health: Current applications and future prospects. Foods 2024, 13, 2437. [Google Scholar] [CrossRef]
- Park, G.; Johnson, K.; Miller, K.; Kadyan, S.; Singar, S.; Patoine, C.; Hao, F.; Lee, Y.; Patterson, A.D.; Arjmandi, B.; et al. Almond snacking modulates gut microbiome and metabolome in association with improved cardiometabolic and inflammatory markers. Npj Sci. Food 2025, 9, 35. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Schneider, E.; Gunnigle, E.; Cotter, P.D.; Cryan, J.F. Fermented foods: Harnessing their potential to modulate the microbiota-gut-brain axis for mental health. Neurosci. Biobehav. Rev. 2024, 158, 105562. [Google Scholar] [CrossRef]
- Galland, F.; de Espindola, J.S.; Lopes, D.S.; Taccola, M.F.; Pacheco, M.T.B. Food-derived bioactive peptides: Mechanisms of action underlying inflammation and oxidative stress in the central nervous system. Food Chem. Adv. 2022, 1, 100087. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- El Mecherfi, K.E.; Todorov, S.D.; Cavalcanti de Albuquerque, M.A.; Denery-Papini, S.; Lupi, R.; Haertlé, T.; Franco, B.D.G.d.M.; Larré, C. Allergenicity of fermented foods: Emphasis on seeds protein-based products. Foods 2020, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Sun, Y.; Fu, G.; Wu, Z.; Cheng, J. Effect of processing on soybean allergens and their allergenicity. Trends Food Sci. Technol. 2021, 118, 316–327. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; Gobbetti, M. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Castro-Muñoz, R. An overview of fermentation in the food industry-looking back from a new perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Tarchi, I.; Loudiyi, M.; Boukria, O.; Cahyana, Y.; Ozogul, F.; Khwaldia, K. Transforming plant-based waste and by-products into valuable products using various “Food Industry 4.0” enabling technologies: A literature review. Sci. Total Environ. 2024, 955, 176872. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Peres Fabbri, L.; Cavallero, A.; Vidotto, F.; Gabriele, M. Bioactive peptides from fermented foods: Production approaches, sources, and potential health benefits. Foods 2024, 13, 3369. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular bioeconomy in action: Transforming food wastes into renewable food resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gea, T.; Font, X. Sophorolipids production from oil cake by solid-state fermentation. Inventory for economic and environmental assessment. Front. Chem. Eng. 2021, 3, 632752. [Google Scholar] [CrossRef]
- Gouda, S.A.; Donia, S.; Hassanein, N.M. Production of single cell protein by fungi from different food wastes. Biomass Convers. Biorefinery 2024, 15, 5447–5462. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, P.; Chen, Y.; Xie, J.; Peng, G.; Tian, S.; Chang, X.; Yu, Q. Effect of soluble dietary fiber of navel orange peel prepared by mixed solid-state fermentation on the quality of jelly. Foods 2023, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, G.; Barrena, R.; Font, X. Using green waste as substrate to produce biostimulant and biopesticide products through solid-state fermentation. Waste Manag. 2023, 159, 84–92. [Google Scholar] [CrossRef]
- Chiarini, E.; Alessandria, V.; Buzzanca, D.; Giordano, M.; Seif Zadeh, N.; Mancuso, F.M.; Zeppa, G. Valorization of Fruit By-Products Through Lactic Acid Fermentation for Innovative Beverage Formulation: Microbiological and Physiochemical Effects. Foods 2024, 13, 3715. [Google Scholar] [CrossRef]
- Dabaghi, S.; Ataei, S.A.; Taheri, A. Production of rhamnolipid biosurfactants in solid-state fermentation: Process optimization and characterization studies. BMC Biotechnol. 2023, 23, 2. [Google Scholar] [CrossRef]
- Akhtar, T.; Hashmi, A.S.; Tayyab, M.; Anjum, A.A.; Saeed, S.; Ali, S. Bioconversion of agricultural waste to butyric acid through solid state fermentation by clostridium tyrobutyricum. Waste Biomass Valoriz. 2020, 11, 2067–2073. [Google Scholar] [CrossRef]
- Hien, T.T.; Khang, V.C.; Muoi, N.V.; Truc, T.T. Production of a fermented beverage from pineapple (Ananas comosus) byproduct crumbs. Mater. Today Proc. 2022, 60, 2034–2042. [Google Scholar] [CrossRef]
- El-Sayed, E.R.; Ahmed, A.S.; Al-Hagar, O.E.A. Agro-industrial wastes for production of paclitaxel by irradiated Aspergillus fumigatus under solid-state fermentation. J. Appl. Microbiol. 2020, 128, 1427–1439. [Google Scholar] [CrossRef]
- Chandukishore, T.; Das, S.; Narasimhulu, K.; Prabhu, A.A. Biovalorisation of mixed food waste through newly isolated thermo tolerant fungal cell factories: A step toward transforming waste to wealth. Sustain. Chem. Pharm. 2023, 35, 101230. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Vijayaraghavan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alyahya, S.A. Solid state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. J. King Saud Univ.-Sci. 2020, 32, 1555–1561. [Google Scholar] [CrossRef]
- Maini Rekdal, V.; Villalobos-Escobedo, J.M.; Rodriguez-Valeron, N.; Olaizola Garcia, M.; Prado Vásquez, D.; Rosales, A.; Sörensen, P.M.; Baidoo, E.E.K.; Calheiros De Carvalho, A.; Riley, R.; et al. Neurospora intermedia from a traditional fermented food enables waste-to-food conversion. Nat. Microbiol. 2024, 9, 2666–2683. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, B.; Jalilnejad, E.; Ghasemzadeh, K.; Iulianelli, A. Recent progresses in application of membrane bioreactors in production of biohydrogen. Membranes 2019, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Adeleke, I.; Nwulu, N.; Adebo, O.A. Internet of Things (IoT) in the food fermentation process: A bibliometric review. J. Food Process Eng. 2023, 46, e14321. [Google Scholar] [CrossRef]
- D’Urso, F.; Broccolo, F. Applications of Artificial Intelligence in Microbiome Analysis and Probiotic Interventions—An Overview and Perspective Based on the Current State of the Art. Appl. Sci. 2024, 14, 8627. [Google Scholar] [CrossRef]
- Walsh, A.M.; Leech, J.; Huttenhower, C.; Delhomme-Nguyen, H.; Crispie, F.; Chervaux, C.; Cotter, P.D. Integrated molecular approaches for fermented food microbiome research. FEMS Microbiol. Rev. 2023, 47, fuad001. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Gibson, G.R. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Elinav, E. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328. [Google Scholar] [CrossRef]
- Tesei, D.; Jewczynko, A.; Lynch, A.M.; Urbaniak, C. Understanding the complexities and changes of the astronaut microbiome for successful long-duration space missions. Life 2022, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Eldridge, A.L.; Hartmann, C.; Klassen, P.; Ingram, J.; Meijer, G.W. Benefits and challenges of food processing in the context of food systems, value chains and sustainable development goals. Trends Food Sci. Technol. 2024, 153, 104703. [Google Scholar] [CrossRef]
- Lietzow, J.; Luckert, C.; Schafer, B. Novel and Traditional Foods: Novel Food Regulation in the EU; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Crevel, R. Novel Foods and Ingredients: Laws and Regulations Europe; Elsevier: Amsterdam, The Netherlands, 2023; pp. 65–74. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pentieva, K.; et al. Guidance on the scientific requirements for an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2024, 22, e8961. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Godefroy, S.B. Novel foods and ingredients: Laws and regulations in USA and Canada. In Sustainable Food Science—A Comprehensive Approach; Elsevier: Amsterdam, The Netherlands, 2023; pp. 59–64. [Google Scholar] [CrossRef]
- Kedar, O.; Golberg, A.; Obolski, U.; Confino-Cohen, R. Allergic to bureaucracy? Regulatory allergenicity assessments of novel food: Motivations, challenges, compromises, and possibilities. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13300. [Google Scholar] [CrossRef] [PubMed]
- Garud, S.R.; Lamdande, A.G.; Gholap, S.R. Regulations on Functional Foods and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2023; pp. 785–823. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, M.M.; Sharma, D. Nutraceuticals Regulation: An Overview of the Regulatory Frameworks in USA, EU, and Japan; Elsevier BV: Amsterdam, The Netherlands, 2024; pp. 421–440. [Google Scholar] [CrossRef]
- Bourdichon, F.; Fontana, A.; Patrone, V.; Morelli, L. Safety demonstration of food and feed cultures. In Microbial Fermentations in Nature and as Designed Processes; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 261–280. [Google Scholar] [CrossRef]
- Sundh, I.; Del Giudice, T.; Cembalo, L. Reaping the Benefits of Microorganisms in Cropping Systems: Is the Regulatory Policy Adequate? Microorganisms 2021, 9, 1437. [Google Scholar] [CrossRef]
- Kallscheuer, N. Engineered Microorganisms for the Production of Food Additives Approved by the European Union—A Systematic Analysis. Front. Microbiol. 2018, 9, 1746. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; den Besten, H.M.W.; Rietjens, I.M.C.M.; Widjaja, F. Chemical and Microbiological Hazards Arising from New Plant-Based Foods, Including Precision Fermentation–Produced Food Ingredients. Annu. Rev. Food Sci. Technol. 2025, 16, 171–194. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef]
- Maurya, N.K. Regulating Innovation: A Review of Product Development and Regulatory Frameworks in the Food Industry. Nutr. Food Process. 2024, 7, 01–08. [Google Scholar] [CrossRef]
- Ong, H.C.; Yong, A.M.H.; Fattori, V.; Mukherjee, K. Addressing the safety of new food sources and production systems. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13341. [Google Scholar] [CrossRef]
- Samarasiri, M.H.; Chai, K.F. Forward-looking risk assessment framework for novel foods. Food Humanit. 2023, 1, 500–513. [Google Scholar] [CrossRef]
- Molitorisová, A.; Monaco, A. Innovating Food Law with Mycelium: EU Regulations. Social Science Research Network. 2023. Available online: https://ssrn.com/abstract=4530242 (accessed on 28 April 2025).
- Cámara, V.; Fernández-Ruiz, V.; Domínguez Díaz, L.; Cámara Hurtado, R.M.; Sánchez Mata, M.C. Global Concepts and Regulations in Functional Foods. In Functional Foods; Wiley: Hoboken, NJ, USA, 2022; pp. 511–554. [Google Scholar] [CrossRef]
- Intrasook, J. Trends and current food safety regulations and policies for functional foods and beverages containing botanicals. J. Food Drug Anal. 2024, 32, 118–145. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, D.; Fanelli, F.; Fusco, V. Legislation of probiotic foods and supplements. In Probiotics for Human Nutrition in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 25–44. [Google Scholar] [CrossRef]
- Aswathy, M.R.; Pandikkadan Sundaran, S.P.S.; Sangari, T.; Samuel, S. Fermentation technology: Empowering biotechnology for a sustainable future. In Futuristic Trends in Biotechnology Volume 3 Book 6; Iterative International Publishers: Chikmagalur, India, 2024; pp. 87–116. [Google Scholar] [CrossRef]
- Jung, M.; Lee, Y.; Han, S.O.; Hyeon, J.E. Advancements in Sustainable Plant-Based Alternatives: Exploring Proteins, Fats, and Manufacturing Challenges in Alternative Meat Production. J. Microbiol. Biotechnol. 2024, 34, 994–1002. [Google Scholar] [CrossRef]
- Yılmaz Tuncel, N.; Polat Kaya, H.; Andaç, A.E.; Korkmaz, F.; Tuncel, N.B. A comprehensive review of antinutrients in plant-based foods and their key ingredients. Nutr. Bull. 2025, 50, 171–205. [Google Scholar] [CrossRef]
- Mishra, T.; Machireddy, J.; Vuppu, S. Comprehensive Study on Hygiene and Quality Assessment Practices in the Production of Drinkable Dairy-Based and Plant-Based Fermented Products. Fermentation 2024, 10, 489. [Google Scholar] [CrossRef]
- Pascall, M.A.; DeAngelo, K.; Richards, J.; Arensberg, M.B. Role and Importance of Functional Food Packaging in Specialized Products for Vulnerable Populations: Implications for Innovation and Policy Development for Sustainability. Foods 2022, 11, 3043. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hartley, C.J.; Maloney, G.R.; Tyndall, S.M. Innovation in precision fermentation for food ingredients. Crit. Rev. Food Sci. Nutr. 2023, 64, 6218–6238. [Google Scholar] [CrossRef]
- Giacalone, D.; Clausen, M.P.; Jaeger, S.R. Understanding barriers to consumption of plant-based foods and beverages: Insights from sensory and consumer science. Curr. Opin. Food Sci. 2022, 48, 100919. [Google Scholar] [CrossRef]
- Appiani, M.; Cattaneo, C.; Laureati, M. Sensory properties and consumer acceptance of plant-based meat, dairy, fish and eggs analogs: A systematic review. Front. Sustain. Food Syst. 2023, 7, 1268068. [Google Scholar] [CrossRef]
- Amyoony, J.; Moss, R.; Dabas, T.; Gorman, M.; Ritchie, C.; LeBlanc, J.; McSweeney, M.B. An investigation into consumer perception of the aftertaste of plant-based dairy alternatives using a word association task. Appl. Food Res. 2023, 3, 100320. [Google Scholar] [CrossRef]
- Tahseen, A.; Muthukumar, M.; Sathu, T.; Vasudevan, V.N.; Irshad, A.; John, P. Navigating Plant-based Meat Analogues: A Review of Challenges and Strategies for Consumer Acceptance. Eur. J. Nutr. Food Saf. 2024, 16, 139–155. [Google Scholar] [CrossRef]
- Geng, W. Navigating the Future of Food: Plant-Based Meats as Sustainable Answer to Global Challenges. Highlights Sci. Eng. Technol. 2024, 123, 61–65. [Google Scholar] [CrossRef]
- Erfanian, S.; Qin, S.; Waseem, L.A.; Dayo, M.A. Cultivating a greener plate: Understanding consumer choices in the plant-based meat revolution for sustainable diets. Front. Sustain. Food Syst. 2024, 7, 1315448. [Google Scholar] [CrossRef]
- Gonçalves, G.C.; Weis, C.M.S.C.; Wolff, É.R.; Alves, V.; Sanches, F.L.; Tormen, L.; Treichel, H.; Bertan, L.C. Vegan Fermented Drinks as an Alternative to Milk: Trend or Challenge? Food Sci. Eng. 2024, 6, 1–26. [Google Scholar] [CrossRef]
- Laureati, M.; De Boni, A.; Saba, A.; Lamy, E.; Minervini, F.; Delgado, A.M.; Sinesio, F. Determinants of Consumers’ Acceptance and Adoption of Novel Food in View of More Resilient and Sustainable Food Systems in the EU: A Systematic Literature Review. Foods 2024, 13, 1534. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.-S. Kimchi and other widely consumed traditional fermented foods of korea: A review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; González-Domenech, C.M.; Borrego, J.J. The Role of Fermented Vegetables as a Sustainable and Health-Promoting Nutritional Resource. Appl. Sci. 2024, 14, 10853. [Google Scholar] [CrossRef]
- Filho, E.R.; Silva, R.; Campelo, P.H.; Platz, V.H.C.B.; Spers, E.E.; Freitas, M.Q.; Cruz, A.G. Think and Choose! The Dual Impact of Label Information and Consumer Attitudes on the Choice of a Plant-Based Analog. Foods 2024, 13, 2269. [Google Scholar] [CrossRef]
- Kühl, S.; Schäfer, A.; Kircher, C.; Mehlhose, C. Beyond the Cow: Consumer Perceptions and Information Impact on Acceptance of Precision Fermentation-Produced Cheese in Germany. Future Foods 2024, 10, 100411. [Google Scholar] [CrossRef]
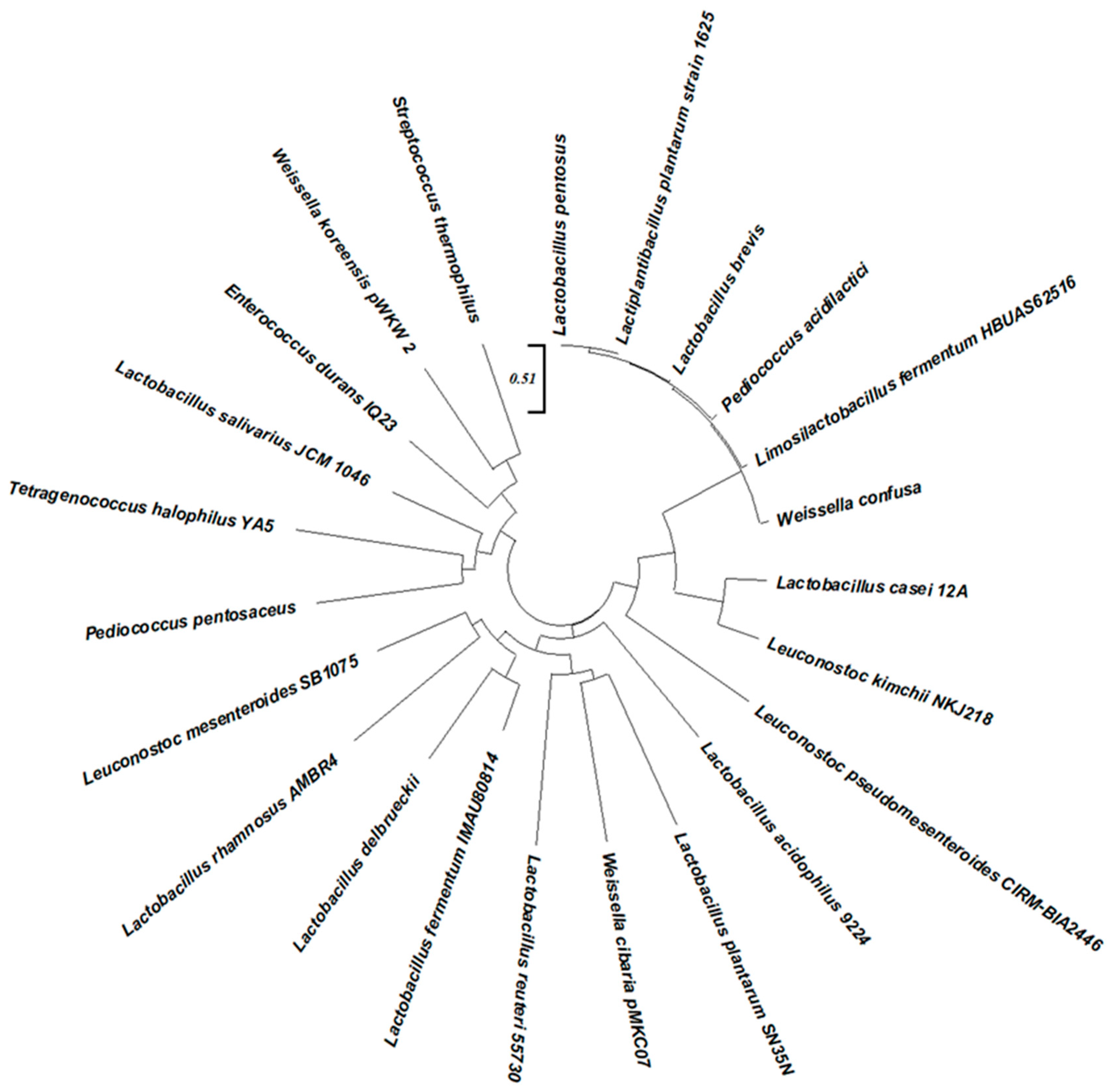


| Food Type | Dominant Microbes | Key Characteristics | Reference |
|---|---|---|---|
| Chinese Fermented Vegetables | Lactobacillus sakei, Lactobacillus acetotolerans, Pediococcus pentosaceus | Flavor development through organic acids and volatile compounds; inhibition of spoilage microorganisms | [44] |
| Vegetable Ferments | Lactobacillus, Pediococcus, Leuconostoc | High LAB diversity, pathways for carbohydrate degradation | [23,24] |
| Legume Ferments | Bacillales, Enterobacterales | Enriched with protein and lipid degradation pathways | [23,45] |
| Red Beetroot Ferments | Lactobacillus plantarum, Weissella cibaria | Complex volatilome, lactic acid fermentation | [46] |
| French Fermented Vegetables | Lactiplantibacillus pentosus, Levilactobacillus brevis | Dominance of LAB, no pathogenic bacteria detected | [22] |
| Pea Protein Gels | Geotrichum candidum, Lactococcus lactis, Lactobacillus rhamnosus | Decreased pea note intensity in pea gels, bitterness increased after fermentation, enhanced cheesy perception | [47] |
| African Fermented Maize | Lactobacillus, Weisella, Curvibacter | Environmental selection shapes microbial composition | [45] |
| Novel Miso Varieties | Lactococcus lactis, Lactobacillus rhamnosus | Substrate-dependent microbial composition, carotenoid biosynthesis genes | [48] |
| Plant-Based Matrix | Nutritional Enhancement | Sensory Improvement | Reference |
|---|---|---|---|
| Legumes | Increased vitamins (folate, riboflavin), minerals (iron, zinc) | Reduced beany off-flavor, improved texture | [52,53] |
| Cereals | Enhanced bioactive compounds, antioxidants | Improved palatability, aroma | [54] |
| Chickpea-Based Beverages | Enhanced phosphorus (478 vs. 331 mg/kg), calcium (165 vs. 117 mg/kg), reduced raffinose | Increased viscosity, consumer acceptability | [55] |
| Rice-Based Foods | Increased antioxidants, reduced antinutrients | Enhanced flavor, reduced off-flavors | [56] |
| Meat Analogues | Tailored carotenoid production for color | Mimicked meat-like texture and flavor | [14,57] |
| Legume Matrices | Carotenoid production | Improved texture and flavor | [35] |
| Plant-based beverages (mixtures of Hibiscus sabdariffa (zobo) and Raphia hookeri wine) | Increased antioxidant activity | Improved color and taste | [58] |
| Vegetable Matrices | Increased bioavailability of nutrients | Pleasant sensory characteristics | [59] |
| Fermented brown rice | Increased soluble dietary fiber, total phenolic content, and antioxidant capacity | Improved texture | [60] |
| Vegetable Matrices (African black nightshade and African spiderplant) | Increased phenolic compounds and flavonoid contents | Improved sensory acceptability | [61] |
| Bian-Que Triple-Bean Soup | Increased total flavone contents and phenol contents | Improvedflavors | [62] |
| Pea and rice protein concentrate blend | Increase in protein’s Digestible Indispensable Amino Acid Score (DIAAS) to an “excellent source” for humans Reduced antinutrients like phytates and protease inhibitors | Reduction in off-note compounds substantially improving its organoleptic performance | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhiman, S.; Kaur, S.; Thakur, B.; Singh, P.; Tripathi, M. Nutritional Enhancement of Plant-Based Fermented Foods: Microbial Innovations for a Sustainable Future. Fermentation 2025, 11, 346. https://doi.org/10.3390/fermentation11060346
Dhiman S, Kaur S, Thakur B, Singh P, Tripathi M. Nutritional Enhancement of Plant-Based Fermented Foods: Microbial Innovations for a Sustainable Future. Fermentation. 2025; 11(6):346. https://doi.org/10.3390/fermentation11060346
Chicago/Turabian StyleDhiman, Sunny, Sukhminderjit Kaur, Babita Thakur, Pankaj Singh, and Manikant Tripathi. 2025. "Nutritional Enhancement of Plant-Based Fermented Foods: Microbial Innovations for a Sustainable Future" Fermentation 11, no. 6: 346. https://doi.org/10.3390/fermentation11060346
APA StyleDhiman, S., Kaur, S., Thakur, B., Singh, P., & Tripathi, M. (2025). Nutritional Enhancement of Plant-Based Fermented Foods: Microbial Innovations for a Sustainable Future. Fermentation, 11(6), 346. https://doi.org/10.3390/fermentation11060346









