CRISPRa-Mediated Triple-Gene Activation of ARO10, ARO80, and ADH2 for Enhancing 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae
Abstract
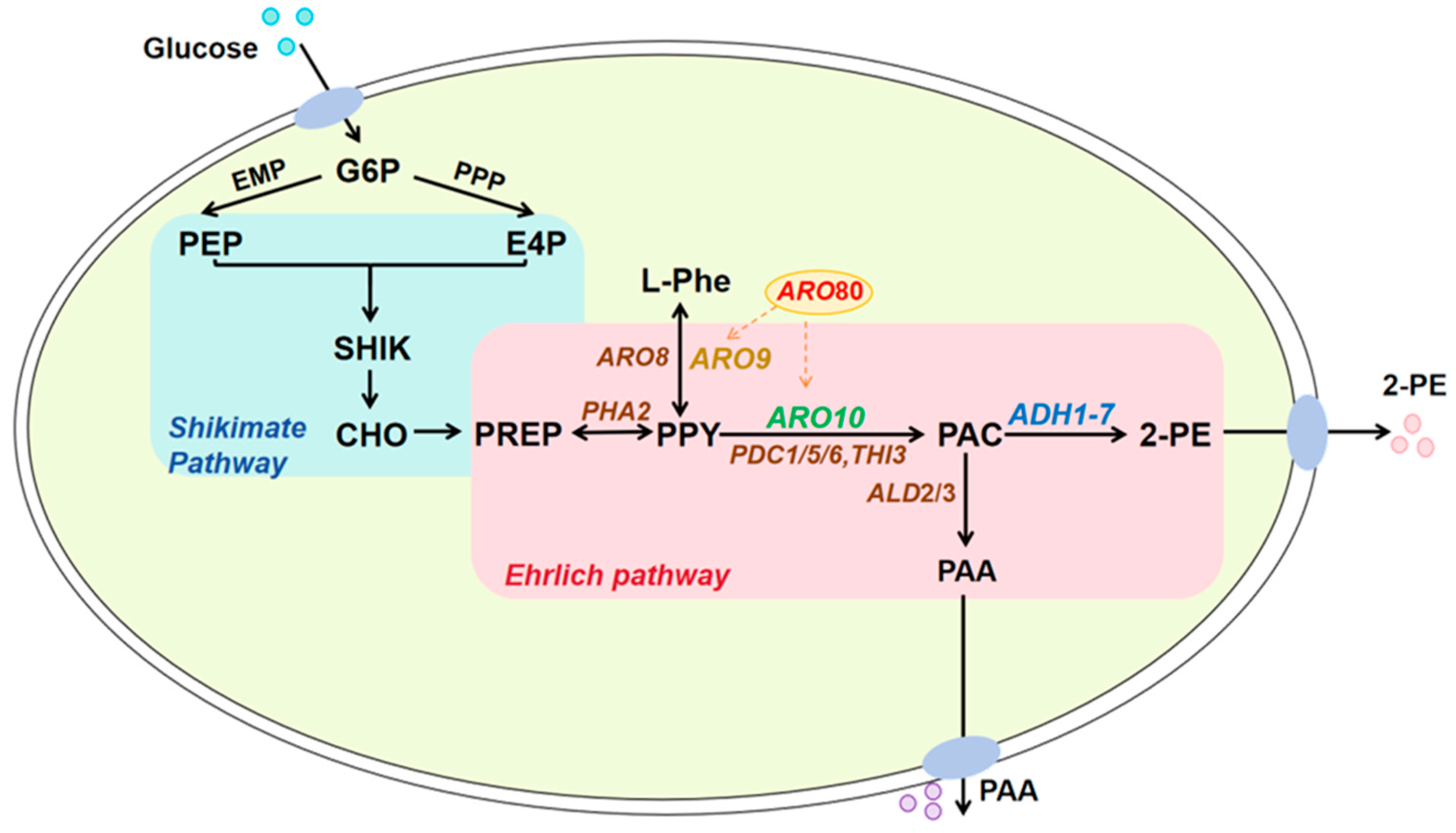
1. Introduction
2. Materials and Methods
2.1. Plasmids, Strains, Primers, and GS Oligonucleotides
2.2. Media and Cultivation Conditions
- Media for cultivation
- Media for selection
- Media for fermentation
- Cultivation conditions
2.3. Reagents and Instruments
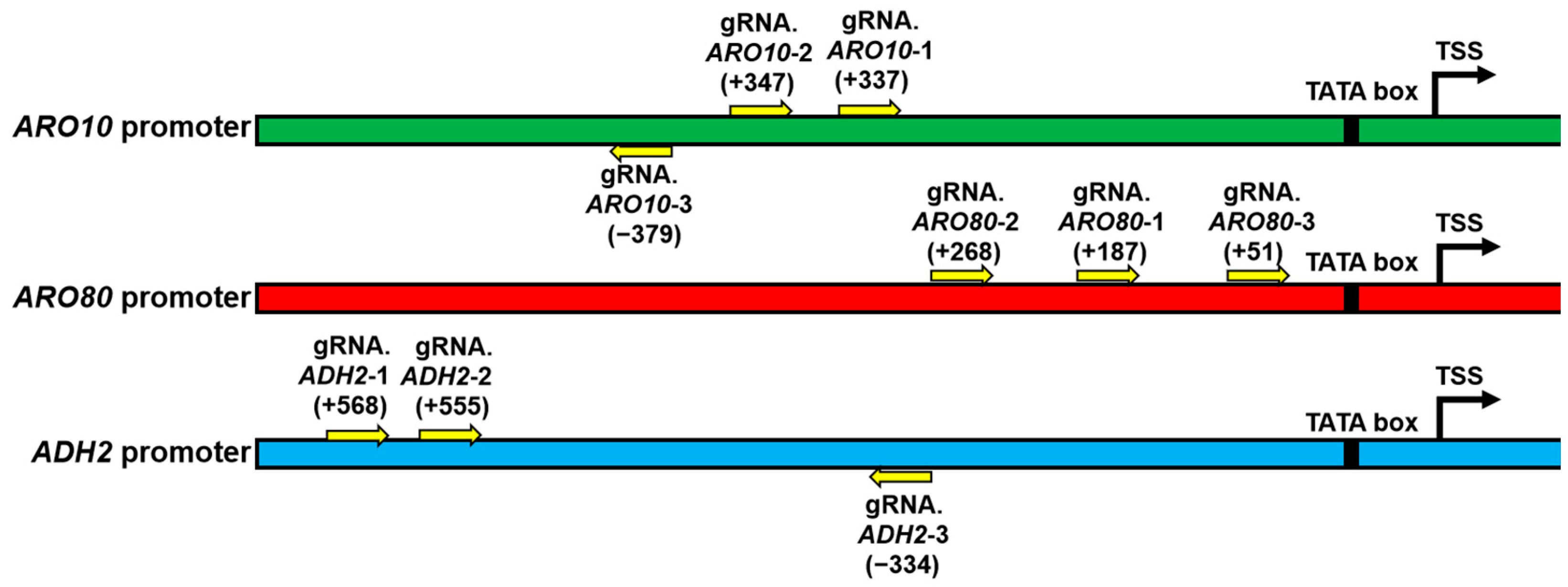
2.4. GS Design for ARO10/ARO80/ADH2
2.5. Construction of ARO10, ARO80, and ADH2 Single-Gene Activation Series Engineered Strains
2.6. Optimal GS Screening of ARO10, ARO80, and ADH2
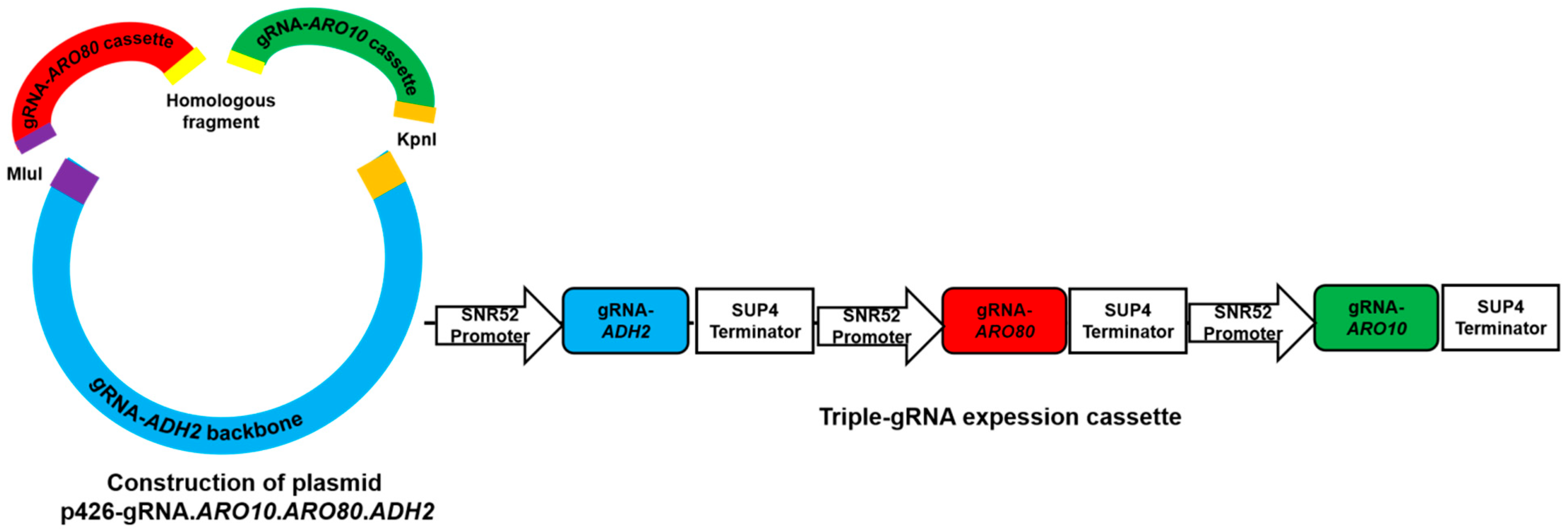
2.7. Construction of INVScI-ARO10-ARO80-ADH2 Engineered Strain
2.8. Analytical Methods
2.8.1. Quantitative Real-Time PCR (qPCR) for Gene Expression Analysis
2.8.2. High-Performance Liquid Chromatography (HPLC) for 2-PE Quantification
2.8.3. Cell Density (OD600) Measurement
2.9. Statistical Analysis
3. Results
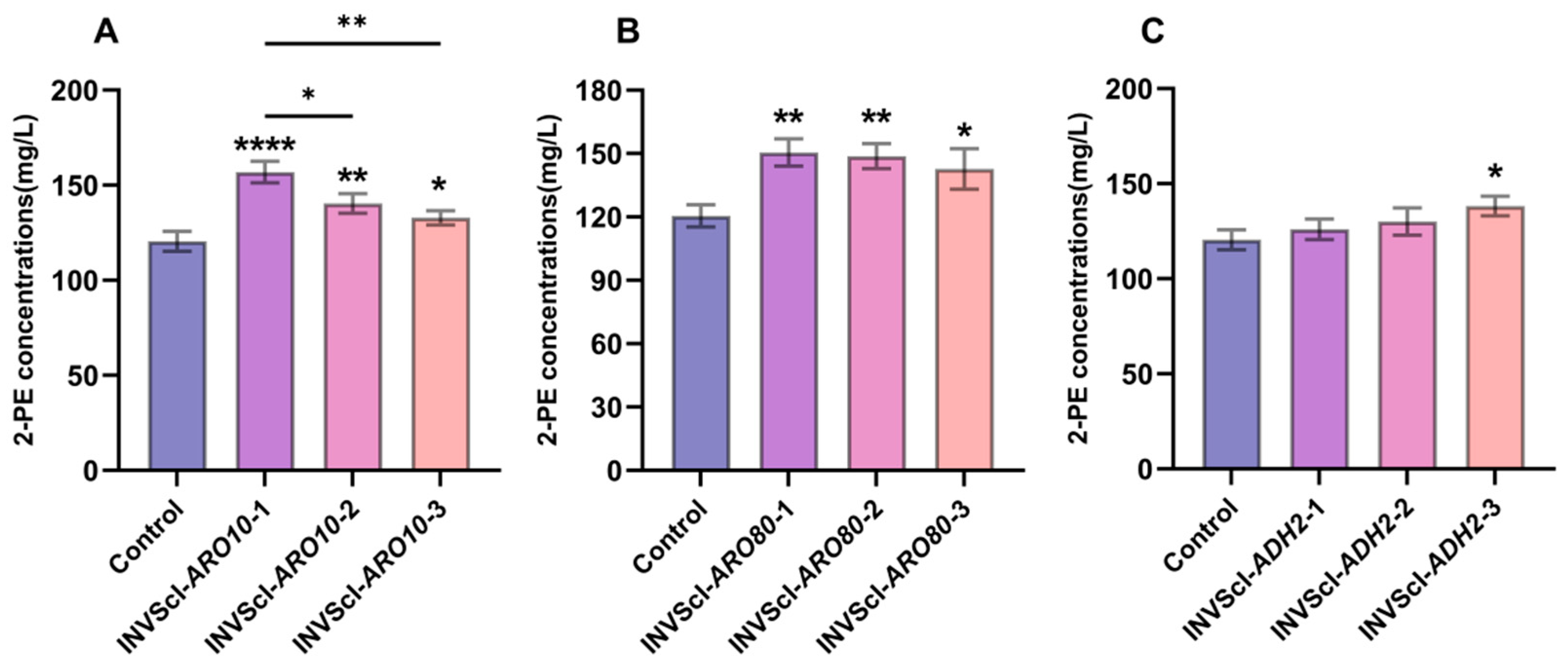
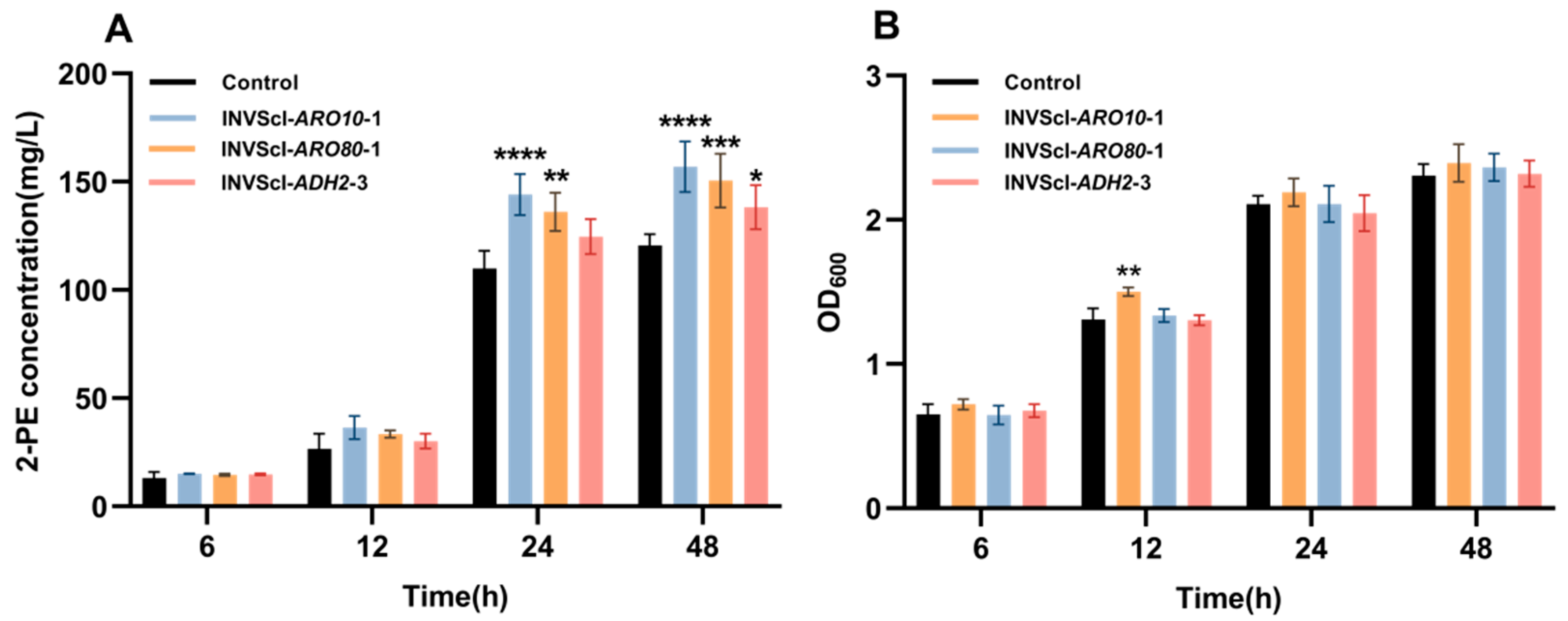
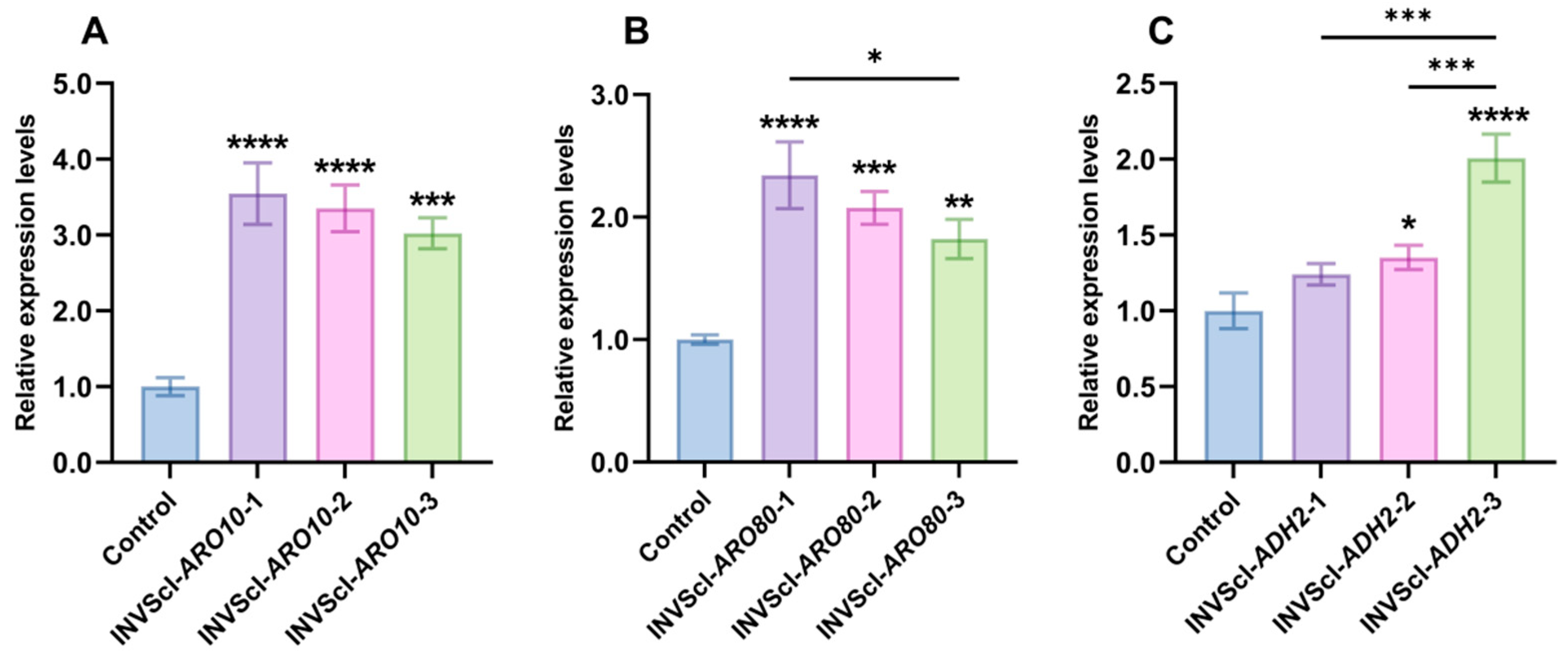
3.1. Construction, 2-PE Concentrations, Gene Expression Levels, and Growth Analysis of Single-Gene Activation Engineered Strains
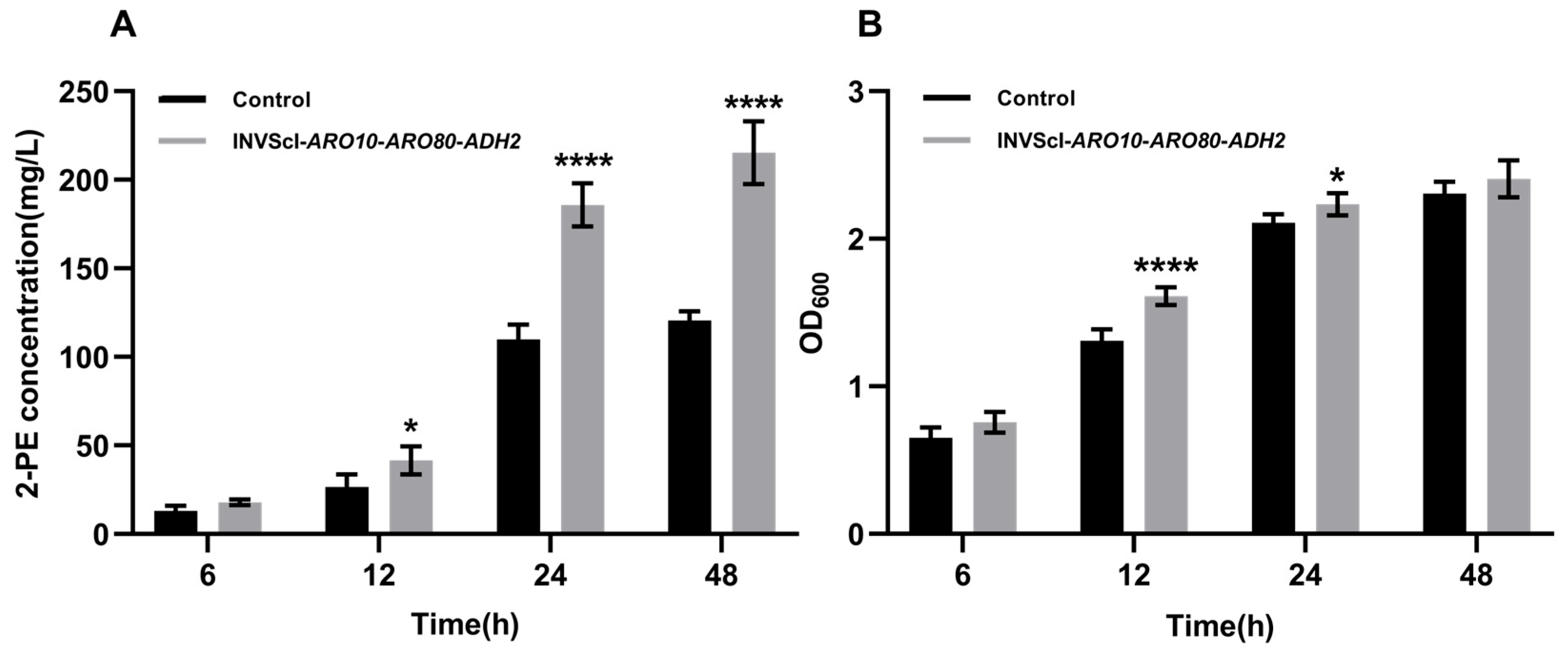
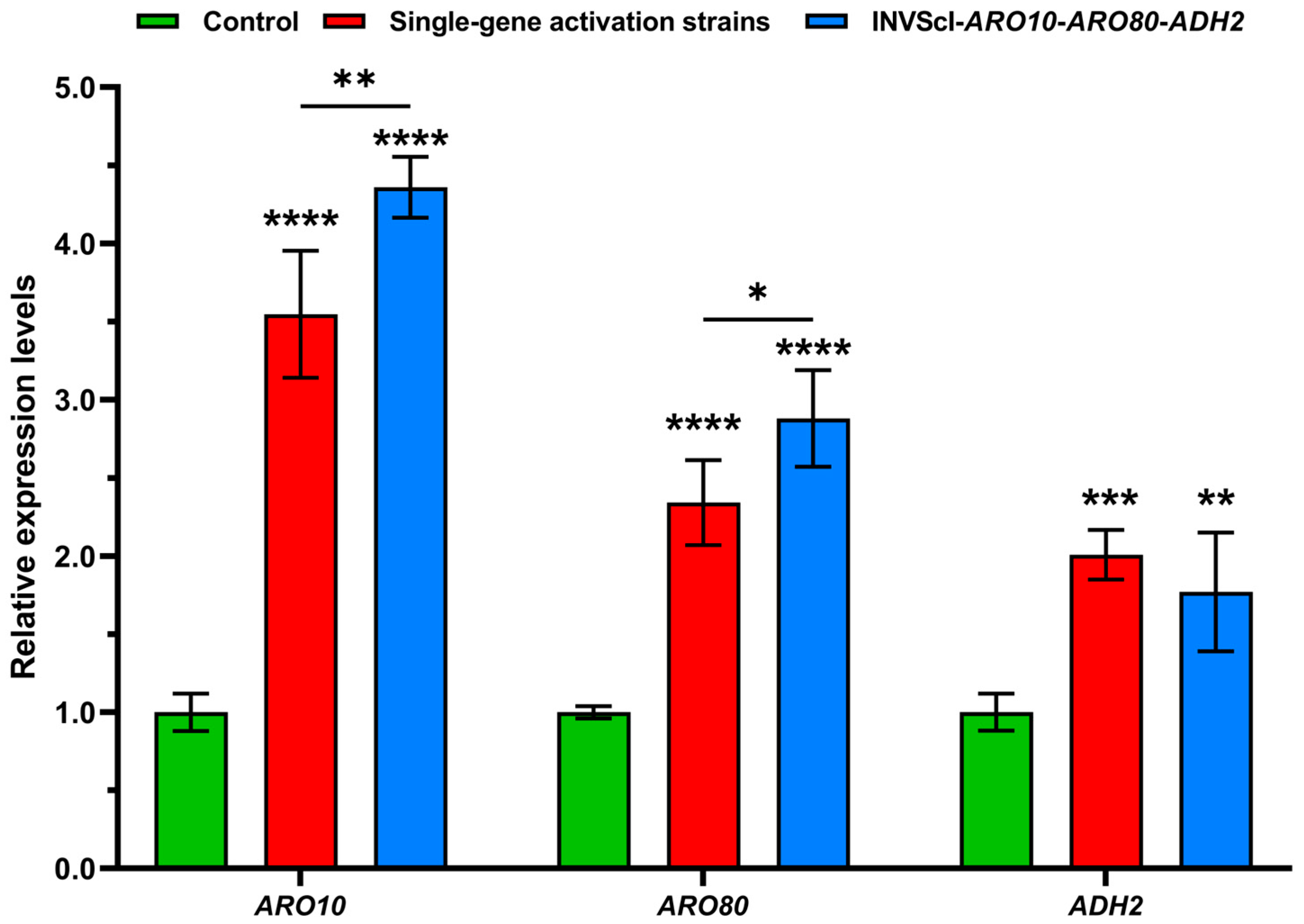
3.2. Construction, 2-PE Concentration Measurement, Gene Expression Level Assay, and Growth Analysis of Triple-Gene Activation Engineered Strain INVScI-ARO10-ARO80-ADH2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-PE | 2-Phenylethanol |
| 3D | Three dimensional |
| ANOVA | Analysis of variance |
| CAGR | Compound annual growth rate |
| CRISPRa | Clustered regularly interspaced short palindromic repeats activation |
| GS | Guide sequence |
| HPLC | High-performance liquid chromatography |
| OD | Optical density |
| OE PCR | Overlap extension polymerase chain reaction |
| PAM | Protospacer adjacent motif |
| PCR | Polymerase chain reaction |
| qPCR | Quantitative real-time polymerase chain reaction |
| TSS | Transcription start site |
| WT | Wild type |
References
- Hua, D.; Xu, P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Majdabadi, N.; Falahati, M.; Heidarie-Kohan, F.; Farahyar, S.; Rahimi-Moghaddam, P.; Ashrafi-Khozani, M.; Razavi, T.; Mohammadnejad, S. Effect of 2-phenylethanol as antifungal agent and common antifungals (amphotericin B, fluconazole, and itraconazole) on Candida species isolated from chronic and recurrent cases of candidal vulvovaginitis. Assay. Drug Dev. Technol. 2018, 16, 141–149. [Google Scholar] [CrossRef]
- Martinez-Avila, O.; Sanchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-phenylethanol and 2-phenylethyl acetate production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef]
- 2-Phenylethanol Market to Hit $350 Million by 2027, Says Global Market Insights Inc. Available online: https://www.globenewswire.com/en/news-release/2021/06/03/2241082/0/en/2-Phenylethanol-Market-to-hit-350-million-by-2027-SaysGlobal-Market-Insights-Inc.htm (accessed on 6 March 2021).
- Bernardino, A.R.S.; Torres, C.A.V.; Crespo, J.G.; Reis, M.A.M. Biotechnological 2-phenylethanol production: Recent developments. Molecules 2024, 29, 5761. [Google Scholar] [CrossRef]
- Qian, X.J.; Yan, W.; Zhang, W.M.; Dong, W.L.; Ma, J.F.; Ochsenreither, K.; Jiang, M.; Xin, F.X. Current status and perspectives of 2-phenylethanol production through biological processes. Crit. Rev. Biotechnol. 2019, 39, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.-M.; Louka, N. Bioproduction of 2-phenylethanol through yeast fermentation on synthetic media and on agro-industrial waste and by-products: A review. Foods 2022, 11, 109. [Google Scholar] [CrossRef]
- Chen, A.; Qu, T.; Smith, J.R.; Li, J.; Du, G.; Chen, J. Osmotic tolerance in Saccharomyces cerevisiae: Implications for food and bioethanol industries. Food Biosci. 2024, 60, 104451. [Google Scholar] [CrossRef]
- Braus, G.H. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: A model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol. Rev. 1991, 55, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Etschmann, M.M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef]
- Iraqui, I.; Vissers, S.; André, B.; Urrestarazu, A. Transcriptional induction by aromatic amino acids in Saccharomyces cerevisiae. Mol. Cell Biol. 1999, 19, 3360–3371. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, X.; Guo, X.; He, X. Regulation of crucial enzymes and transcription factors on 2-phenylethanol biosynthesis via Ehrlich pathway in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, B.R.; Hahn, J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014, 111, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Lu, X.; Zong, H.; Zhuge, B. Genetic engineering of an industrial yeast Candida glycerinogenes for efficient production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2020, 104, 10481–10491. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ma, J.; Zhu, Y.; Xu, P. Refactoring Ehrlich pathway for high-yield 2-phenylethanol production in Yarrowia lipolytica. ACS Synth. Biol. 2020, 9, 623–633. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, S.W.; Oh, M.K. Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzym. Microb. Technol. 2014, 61–62, 44–47. [Google Scholar] [CrossRef]
- Noda, S.; Mori, Y.; Ogawa, Y.; Fujiwara, R.; Dainin, M.; Shirai, T.; Kondo, A. Metabolic and enzymatic engineering approach for the production of 2-phenylethanol in engineered Escherichia coli. Bioresour. Technol. 2024, 406, 130927. [Google Scholar] [CrossRef]
- Kong, S.; Pan, H.; Liu, X.; Li, X.; Guo, D. De novo biosynthesis of 2-phenylethanol in engineered Pichia pastoris. Enzym. Microb. Technol. 2020, 133, 109459. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, L.; Chen, Z.; Wang, K.; Sun, J.; Zhu, T. The same genetic regulation strategy produces inconsistent effects in different Saccharomyces cerevisiae strains for 2-phenylethanol production. Appl. Microbiol. Biotechnol. 2022, 106, 4041–4052. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Geng, Y.; Li, Y.; Wang, Q.; Liang, Q.; Qi, Q. A strategy of gene overexpression based on tandem repetitive promoters in Escherichia coli. Microb. Cell Factories 2012, 11, 19. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 2014, 159, 647. [Google Scholar] [CrossRef]
- Laughery, M.F.; Hunter, T.; Brown, A. New vectors for simple and streamlined CRISPR–Cas9 genome editing in Saccharomyces cerevisiae. Yeast 2015, 32, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Fan, X.; Li, J.; Zhu, Z.; Huang, P.; Qi, X. Triple-CRISPRi-mediated down-regulation of the shikimate pathway branch genes for enhancing 2-PE biosynthesis in Saccharomyces cerevisiae. Eur. Food Res. Technol. 2024, 250, 1881–1890. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002, 350, 87–96. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823. [Google Scholar] [CrossRef]
- Deaner, M.; Mejia, J.; Alper, H.S. Enabling graded and large-scale multiplex of desired genes using a dual-mode dCas9 activator in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Ferreira, R.; Jakociunas, T.; Arsovska, D.; Zhang, J.; Ding, L.; Smith, J.D.; David, F.; Nielsen, J.; Jensen, M.K.; et al. Transcriptional reprogramming in yeast using dCas9 and combinatorial gRNA strategies. Microb. Cell Fact. 2017, 16, 46. [Google Scholar] [CrossRef]
- Ferreira, R.; Skrekas, C.; Hedin, A.; Sánchez, B.J.; Siewers, V.; Nielsen, J.; David, F. Model-assisted fine-tuning of central carbon metabolism in yeast through dCas9-based regulation. ACS Synth. Biol. 2019, 8, 2457–2463. [Google Scholar] [CrossRef]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; Iyer, E.P.R.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J.; et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 2015, 12, 326–328. [Google Scholar] [CrossRef]
- Lian, J.Z.; Carl, S.; Cao, M.F. Multi-functional genome-wide CRISPR system for high throughput genotype–phenotype mapping. Nat. Commun. 2019, 10, 5794. [Google Scholar] [CrossRef] [PubMed]
- Zalatan, J.G.; Lee, M.E.; Almeida, R.; Gilbert, L.A.; Whitehead, E.H.; La Russa, M.; Tsai, J.C.; Weissman, J.S.; Dueber, J.E.; Qi, L.S.; et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 2015, 160, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, G.; Qin, L.; Li, J.; Li, C. Simultaneously down-regulation of multiplex branch pathways using CRISPRi and fermentation optimization for enhancing β-amyrin production in Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2019, 4, 79–85. [Google Scholar] [CrossRef]
- Löbs, A.-K.; Schwartz, C.; Thorwall, S.; Wheeldon, I. Highly multiplexed CRISPRi repression of respiratory functions enhances mitochondrial localized ethyl acetate biosynthesis in Kluyveromyces marxianus. ACS Synth. Biol. 2018, 7, 2647–2655. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Graf, R.; Li, X.; Chu, V.; Rajewsky, K. sgRNA sequence motifs blocking efficient CRISPR/Cas9-Mediated gene editing. Cell Rep. 2019, 26, 1098–1103. [Google Scholar] [CrossRef]
- Fontana, J.; Dong, C.; Kiattisewee, C.; Chavali, V.P.; Tickman, B.I.; Carothers, J.M.; Zalatan, J.G. Effective CRISPRa-mediated control of gene expression in bacteria must overcome strict target site requirements. Nat. Commun. 2020, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Fløe, L.; Petersen, T.; Huang, J.; Xu, F.; Bolund, L.; Luo, Y.; Lin, L. Chromatin accessibility and guide sequence secondary structure affect CRISPR-Cas9 gene editing efficiency. FEBS Lett. 2017, 591, 1892–1901. [Google Scholar] [CrossRef]
- Thyme, S.B.; Akhmetova, L.; Montague, T.G.; Valen, E.; Schier, A.F. Internal guide RNA interactions interfere with Cas9-mediated cleavage. Nat. Commun. 2016, 7, 11750. [Google Scholar] [CrossRef]
- Xu, J.; Lian, W.; Jia, Y.; Li, L.; Huang, Z. Optimized guide RNA structure for genome editing via Cas9. Oncotarget 2017, 8, 94166–94171. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.; Wang, X.; Huang, Q.; Yang, S. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Chavez, A.; Tuttle, M.; Hal, R.N.; Chari, R.; Ter-Ovanesyan, D.; Qian, J.; Pruitt, B.W.; Beal, J.; Vora, S.; et al. Cas9 gRNA engineering for genome editing, activation and repression. Nat. Methods 2015, 12, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- La Russa, M.F.; Qi, L.S. The new state of the art: Cas9 for gene activation and repression. Mol. Cell Biol. 2015, 35, 3800–3809. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Chen, X.; Liu, M.; Zhan, C.; Liu, X.; Bai, Z. High efficiency CRISPR/Cas9 genome editing system with an eliminable episomal sgRNA plasmid in Pichia pastoris. Enzyme Microb. Technol. 2020, 138, 109556. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, X.; Li, S.; Ma, C.; An, Y.; Cheng, T.; Liang, Y.; Sun, S.; Cheng, T.; Zhao, Y.; et al. Dynamic properties of transcriptional condensates modulate CRISPRa-mediated gene activation. Nat. Commun. 2025, 16, 1640. [Google Scholar] [CrossRef]
- Chardon, F.M.; McDiarmid, T.A.; Page, N.F.; Daza, R.M.; Martin, B.K.; Domcke, S.; Regalado, S.G.; Lalanne, J.-B.; Calderon, D.; Li, X.; et al. Multiplex, single-cell CRISPRa screening for cell type specific regulatory elements. Nat. Commun. 2024, 15, 8209. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, Z.; Soccio, R.E.; Nakadai, T.; Hankey, W.; Zhao, Y.; Huang, F.; Yuan, F.; Wang, H.; Cui, Z.; et al. Phosphorylated MED1 links transcription recycling and cancer growth. Nucleic Acids Res. 2022, 50, 4450–4463. [Google Scholar] [CrossRef]
- Rubtsov, M.A.; Polikanov, Y.S.; Bondarenko, V.A.; Wang, Y.-H.; Studitsky, V.M. Chromatin structure can strongly facilitate enhancer action over a distance. Proc. Natl. Acad. Sci. USA. 2006, 103, 17690–17695. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Wang, G.; Becker, C.; Zaidem, M.; Weigel, D. Genome-wide analysis of chromatin packing in Arabidopsis thaliana at single-gene resolution. Genome Res. 2016, 26, 1057–1068. [Google Scholar] [CrossRef]
- Farzadfard, F.; Perli, S.D.; Lu, T.K. Tunable and multifunctional eukaryotic transcription factors based on CRISPR/Cas. ACS Synth. Biol. 2013, 2, 604–613. [Google Scholar] [CrossRef]
- Smith, J.D.; Suresh, S.; Schlecht, U.; Wu, M.; Wagih, O.; Peltz, G.; Davis, R.W.; Steinmetz, L.M.; Parts, L.; St. Onge, R.P. Quantitative CRISPR interference screens in yeast identify chemical-genetic interactions and new rules for guide RNA design. Genome Biol. 2016, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Zhang, J.Z.; Si, T. Development and applications of CRISPR/dCas9-based gene transcriptional regulation tool in Saccharomyces cerevisiae. Biotechnol. Bull. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Romagnoli, G.; Knijnenburg, T.A.; Liti, G. Deletion of the Saccharomyces cerevisiae ARO8 gene, encoding an aromatic amino acid transaminase, enhances phenylethanol production from glucose. Yeast 2015, 32, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Hahn, J.S. Interplay of Aro80 and GATA activators in regulation of genes for catabolism of aromatic amino acids in Saccharomyces cerevisiae. Mol. Microbiol. 2013, 88, 1120–1134. [Google Scholar] [CrossRef]
- He, Q.; Yang, Y.; Yang, S.; Donohoe, B.S.; Van Wychen, S.; Zhang, M.; Himmel, M.E.; Knoshaug, E.P. Oleaginicity of the yeast strain Saccharomyces cerevisiae D5A. Biotechnol. Biofuels 2018, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Verdone, L.; Camilloni, G.; Mauro, E.D.; Caserta, M. Chromatin Remodeling during Saccharomyces cerevisiae ADH2 Gene activation. Mol. Cell Biol. 1996, 16, 1978–1988. [Google Scholar] [CrossRef]
- Ghosh, S.; Kebaara, B.W.; Atkin, A.L.; Nickerson, K.W. Regulation of Aromatic Alcohol Production in Candida albicans. Appl. Environ. Microbiol. 2008, 74, 7211–7218. [Google Scholar] [CrossRef]
- Kavšček, M.; Stražar, M.; Curk, T.; Natter, K.; Petrovič, U. Yeast as a cell factory: Current state and perspectives. Microb. Cell Fact. 2015, 14, 94. [Google Scholar] [CrossRef]
- Chen, B.; Lee, H.L.; Heng, Y.C.; Chua, N.; Teo, W.S.; Choi, W.J.; Leong, S.S.J.; Foo, J.L.; Chang, M.W. Synthetic biology toolkits and applications in Saccharomyces cerevisiae. Biotechnol. Adv. 2018, 36, 1870–1881. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.; Xu, S.; Shi, G. Improved aromatic alcohol production by strengthening the shikimate pathway in Saccharomyces cerevisiae. Process Biochem. 2021, 103, 18–30. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, M.; Guo, X.-N.; Liu, Z.; He, X. Reconstruction of metabolic module with improved promoter strength increases the productivity of 2-phenylethanol in Saccharomyces cerevisiae. Microb. Cell Fact. 2018, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Shi, J.; Xiao, Y.; Zhou, F.; Wang, H.; Xu, H.; Li, Z.; Yang, S.; Cai, D.; Chen, S. Multilevel metabolic engineering of Bacillus licheniformis for de novo biosynthesis of 2-phenylethanol. Metab. Eng. 2022, 70, 43–54. [Google Scholar] [CrossRef]
- Shen, L.; Nishimura, Y.Y.; Matsuda, F. Overexpressing enzymes of the Ehrlich pathway and deleting genes of the competing pathway in Saccharomyces cerevisiae for increasing 2-phenylethanol production from glucose. J. Biosci. Bioeng. 2016, 122, 34–39. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, X.; Shen, Q.; Wang, K.; Zhu, T. Optimisation of biotransformation conditions for production of 2-phenylethanol by a Saccharomyces cerevisiae CWY132 mutant. Nat. Prod. Res. 2011, 25, 754–759. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Muñoz-Torrero, P.; Sánchez, A.; Font, X.; Barrena, R. Valorization of agro-industrial wastes by producing 2-phenylethanol via solid-state fermentation: Influence of substrate selection on the process. Waste Manag. 2021, 121, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Kang, Y.; Ma, Z.; Hu, C.; Yang, Q.; Zhang, X.; Yang, S.; Dai, J.; Chen, X. Evolutionary and reverse engineering in Saccharomyces cerevisiae reveals a Pdr1p mutation-dependent mechanism for 2-phenylethanol tolerance. Microb. Cell Fact. 2022, 21, 269. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Mierzejewska, J. Enhanced bioproduction of 2-phenylethanol in a biphasic system with rapeseed oil. New Biotechnol. 2018, 42, 56–61. [Google Scholar] [CrossRef]
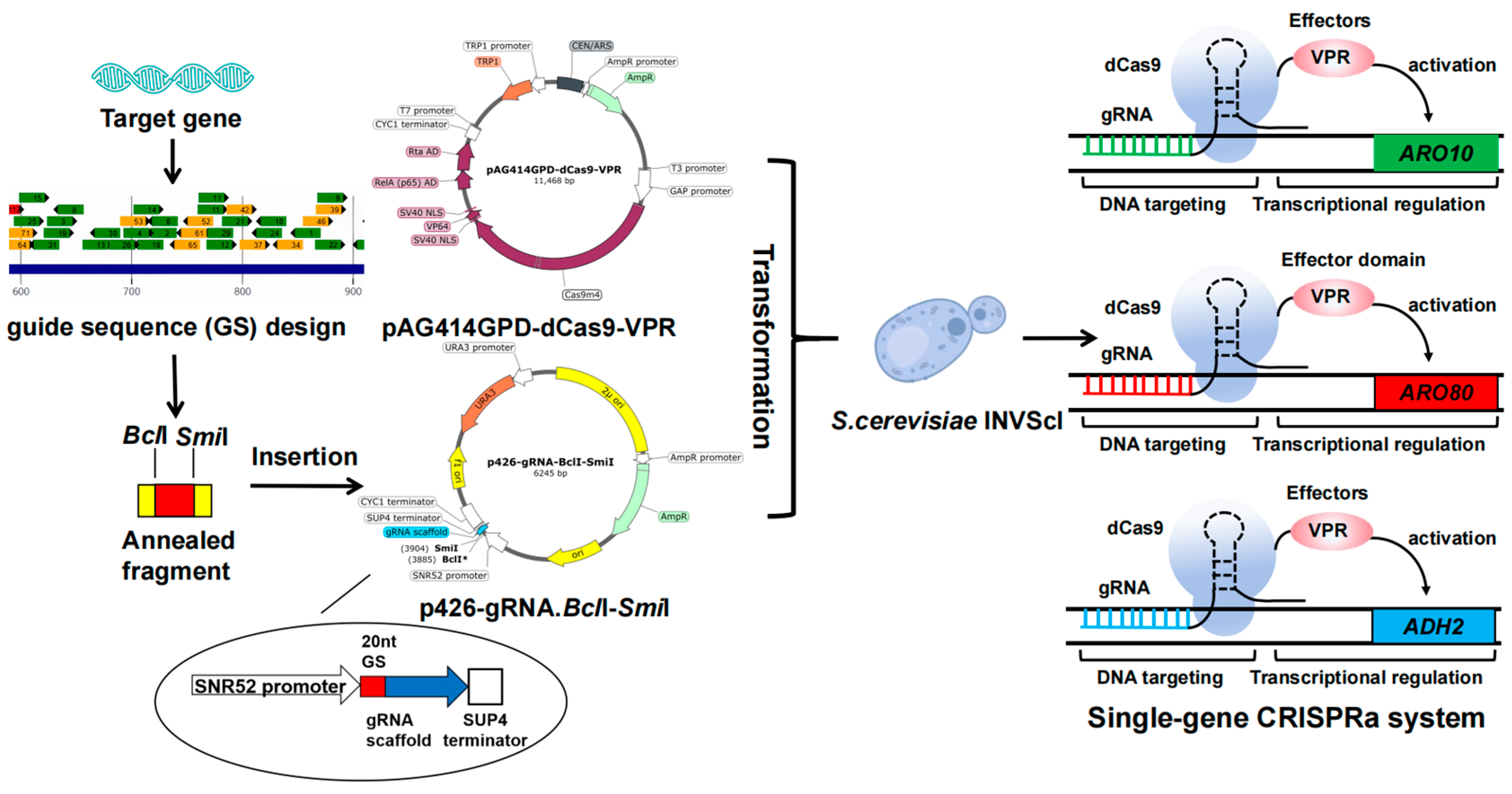
| Plasmid Name | Description | Source |
|---|---|---|
| p426-gRNA-BclⅠ-SmiⅠ | optimization of p426-SNR52p-gRNA.CAN1.Y-SUP4t | Lab stock |
| pAG414GPD-dCas9-VPR | pAG414 series plasmid with GPD promoter driving expression of dCas9-VPR; carrying TRP1 marker | Add gene |
| P426-gRNA.ARO10-1 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ARO10-1 | This study |
| P426-gRNA.ARO10-2 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ARO10-2 | This study |
| P426-gRNA.ARO10-3 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ARO10-3 | This study |
| P426-gRNA.ARO80-1 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ARO80-1 | This study |
| P426-gRNA.ARO80-2 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ARO80-2 | This study |
| P426-gRNA.ARO80-3 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ARO80-3 | This study |
| P426-gRNA.ADH2-1 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ADH2-1 | This study |
| P426-gRNA.ADH2-2 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ADH2-2 | This study |
| P426-gRNA.ADH2-3 | p426-gRNA-BclⅠ-SmiⅠ,expressing gRNA.ADH2-3 | This study |
| p426-gRNA.ARO10-ARO80-ADH2 | p426-gRNA-BclⅠ-SmiⅠ,integrating gRNA cassettes of ARO10, ARO80 and ADH2 | This study |
| Strain Name | Description | Source |
|---|---|---|
| S.cerevisiae INVScⅠ | MATa his3Δ1 leu2 TRP1-289 ura3-52/MATα his3Δ1 leu2 TRP1-289 ura3-52 | ZOMANBIO, Beijing, China |
| E. coli DH5α | Φ80 lacZΔM15 ΔlacU169 recA1 endA1 hsdR17 supE44 thi-1 gyrA relA1 | ZOMANBIO, Beijing, China |
| INVScⅠ-ARO10-1 | INVScⅠ, p426-gRNA.ARO10-1 | This study |
| INVScⅠ-ARO10-2 | INVScⅠ, p426-gRNA.ARO10-2 | This study |
| INVScⅠ-ARO10-3 | INVScⅠ, p426-gRNA.ARO10-3 | This study |
| INVScⅠ-ARO80-1 | INVScⅠ, p426-gRNA.ARO80-1 | This study |
| INVScⅠ-ARO80-2 | INVScⅠ, p426-gRNA.ARO80-2 | This study |
| INVScⅠ-ARO80-3 | INVScⅠ, p426-gRNA.ARO80-3 | This study |
| INVScⅠ-ADH2-1 | INVScⅠ, p426-gRNA.ADH2-1 | This study |
| INVScⅠ-ADH2-2 | INVScⅠ, p426-gRNA.ADH2-2 | This study |
| INVScI-ADH2-3 | INVScⅠ, p426-gRNA.ADH2-3 | This study |
| INVScⅠ-ARO10-ARO80-ADH2 | INVScⅠ, p426-gRNA.ARO10-ARO80-ADH2 | This study |
| GSs Name | Sequence (5′-3′) | PAM |
|---|---|---|
| gRNA.ARO10-1 | CCTACCGGGAGGGATAACCG | +337 |
| gRNA.ARO10-2 | AGTGTCGGTTACCTACCGGG | +347 |
| gRNA.ARO10-3 | CTCCAAAGTGTCGGTTACCT | −379 |
| gRNA.ARO80-1 | TCCTTCTTAGTAATACATGA | +187 |
| gRNA.ARO80-2 | TGATACCCATTAATACACAT | +268 |
| gRNA.ARO80-3 | ATAATATCAAGTTAGTCGT | +51 |
| gRNA.ADH2-1 | AACTGATAGTTTGATCAAAG | +568 |
| gRNA.ADH2-2 | ATCAAAGGGGCAAAACGTAG | +555 |
| gRNA.ADH2-3 | GATCAGTCTCGTGAAGTGGA | −334 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Fang, S.; Huang, P.; Luo, D.; Qi, X. CRISPRa-Mediated Triple-Gene Activation of ARO10, ARO80, and ADH2 for Enhancing 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae. Fermentation 2025, 11, 345. https://doi.org/10.3390/fermentation11060345
Zhu Z, Fang S, Huang P, Luo D, Qi X. CRISPRa-Mediated Triple-Gene Activation of ARO10, ARO80, and ADH2 for Enhancing 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae. Fermentation. 2025; 11(6):345. https://doi.org/10.3390/fermentation11060345
Chicago/Turabian StyleZhu, Zijing, Shuaihu Fang, Pingping Huang, Dianqiang Luo, and Xiaobao Qi. 2025. "CRISPRa-Mediated Triple-Gene Activation of ARO10, ARO80, and ADH2 for Enhancing 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae" Fermentation 11, no. 6: 345. https://doi.org/10.3390/fermentation11060345
APA StyleZhu, Z., Fang, S., Huang, P., Luo, D., & Qi, X. (2025). CRISPRa-Mediated Triple-Gene Activation of ARO10, ARO80, and ADH2 for Enhancing 2-Phenylethanol Biosynthesis via the Ehrlich Pathway in Saccharomyces cerevisiae. Fermentation, 11(6), 345. https://doi.org/10.3390/fermentation11060345






