Seaweed (Laminaria digitata) and Honey Kombucha: A Fermented Antioxidant-Rich Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Analytical Equipment
2.2. Method Description
2.2.1. Preparation of Kombucha Samples
2.2.2. Incorporation of Adjuncts
2.2.3. pH Measurement, Total Soluble Solids (TSS), and Alcohol
2.2.4. Titratable Acidity (TA)
2.2.5. Sensory Analysis
2.2.6. Colorimeter Analysis
2.2.7. Antioxidant Potential Measurements
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. pH Monitoring
3.2. Total Soluble Solids (TSS) and SCOBY Growth
3.3. Titratable Acidity (TA)
3.4. Alcohol Content
3.5. Sensory Analysis
3.6. Colorimeter Analysis
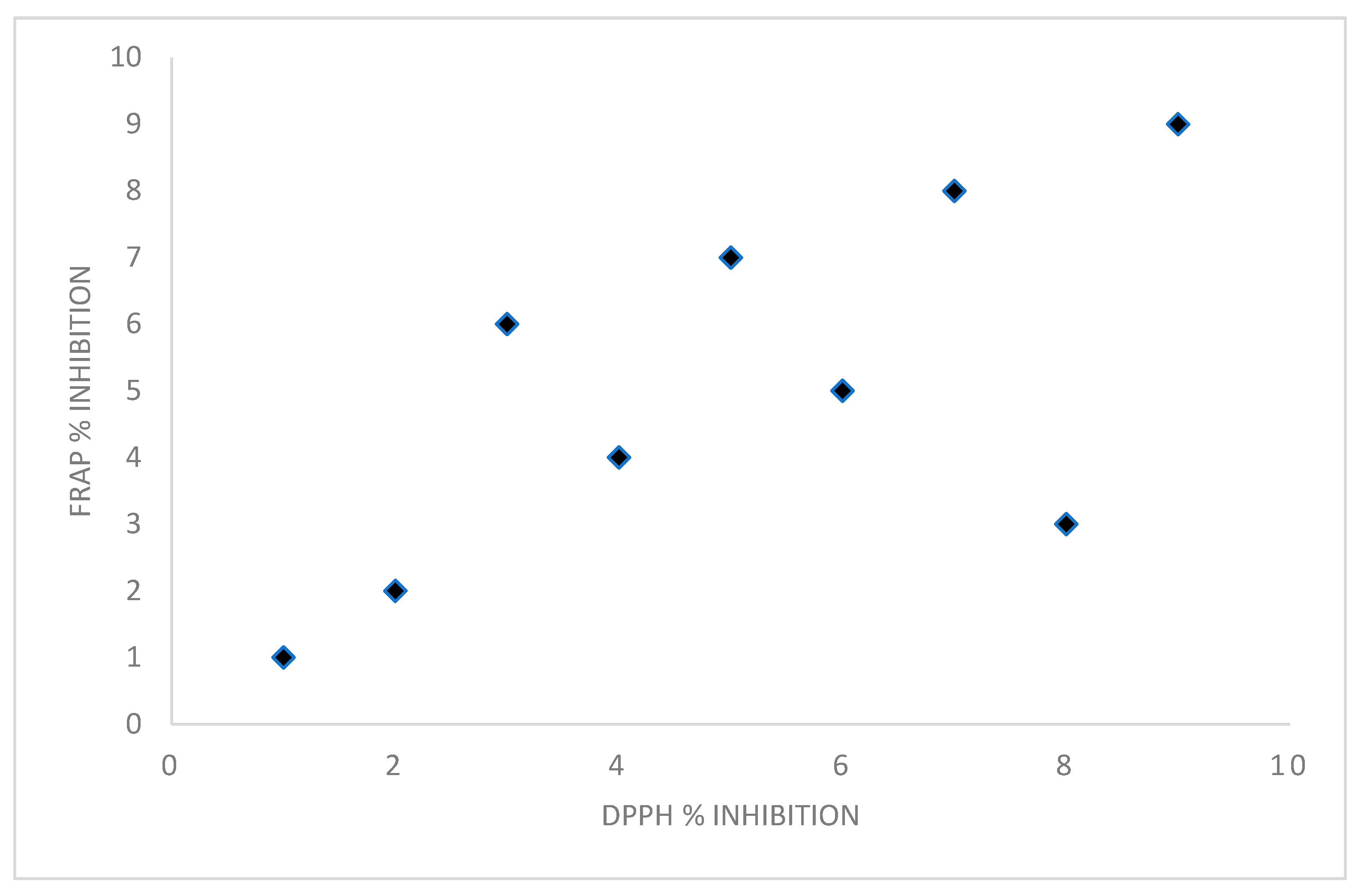
3.7. Antioxidant Potential
3.8. Shelf-Life Analysis
4. Discussion
5. Conclusions, Recommendations, and Limitations
- Conducting comprehensive shelf-life analyses to elucidate the characteristics and functional efficacy of microorganisms present in kombucha.
- Investigating varying concentrations of adjuncts like seaweed and ginger to assess their impact on the biochemical properties of kombucha.
- Engaging a larger and more diverse sensory panel to improve the accuracy and representativeness of consumer acceptance data.
- Further optimizing sugar and honey concentrations to enhance both the flavor profile and health benefits across all kombucha variants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and Chemical Profiles of Commercial Kombucha Products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. Food Sci. Technol. 2022, 168, 113931. [Google Scholar] [CrossRef]
- Góis, M.; Batista, P.; Araújo, M.; Oliveira-Silva, P. Perceptions of Probiotics and Kombucha Consumption in Relation to Emotion Regulation: An Exploratory Study Comparing Portugal and Brazil. Beverages 2023, 9, 61. [Google Scholar] [CrossRef]
- Amarasinghe, H.; Weerakkody, N.S.; Waisundara, V.Y. Evaluation of Physicochemical Properties and Antioxidant Activities of Kombucha “Tea Fungus” During Extended Periods of Fermentation. Food Sci. Nutr. 2018, 6, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Vargas, B.K.; Fabricio, M.F.; Záchia Ayub, M.A. Health effects and probiotic and prebiotic potential of Kombucha: A bibliometric and systematic review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and Microbiological Impacts on the Chemical and Flavor Profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- La Torre, C.; Fazio, A.; Caputo, P.; Plastina, P.; Caroleo, M.C.; Cannataro, R.; Cione, E. Effects of Long-Term Storage on Radical Scavenging Properties and Phenolic Content of Kombucha from Black Tea. Molecules 2021, 26, 5474. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. Kombuchas from Green and Black Teas Have Different Phenolic Profiles, Which Impact Their Antioxidant Capacities, Antibacterial, and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Fabricio, M.F.; Vargas, B.K.; Tischer, B.; Wagner, R.; Ribeiro, S.R.; Cordeiro, N.; Flôres, S.H.; Záchia Ayub, M.A. Revamping Kombucha Production: Achieving Consistency and Probiotic Potential Through a Tailor-Made Microbial Consortium. Int. J. Gastron. Food Sci. 2023, 34, 100844. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and Technological Parameters Impacting the Chemical Composition and Sensory Quality of Kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef]
- Yuliana, N.; Nurainy, F.; Sari, G.W.; Sumardi, S.; Widiastuti, E.L. Total Microbe, Physicochemical Property, and Antioxidative Activity During Fermentation of Cocoa Honey into Kombucha Functional Drink. Appl. Food Res. 2023, 3, 100297. [Google Scholar] [CrossRef]
- Shen, S.; Yang, W.; Li, L.; Zhu, Y.; Yang, Y.; Ni, H.; Jiang, Z.; Zheng, M. In vitro fermentation of seaweed polysaccharides and tea polyphenol blends by human intestinal flora and their effects on intestinal inflammation. Food Funct. 2023, 14, 1133–1147. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef] [PubMed]
- Cubas, A.L.V.; Provin, A.P.; Dutra, A.R.A.; Mouro, C.; Gouveia, I.C. Advances in the Production of Biomaterials through Kombucha Using Food Waste: Concepts, Challenges, and Potential. Polymers 2023, 15, 1701. [Google Scholar] [CrossRef]
- Vitas, J.; Malbasa, R.; Grahovac, J.; Loncar, E. The Antioxidant Activity of Kombucha Fermented Milk Products with Stinging Nettle and Winter Savory. Chem. Ind. Chem. Eng. Q. 2013, 19, 129–139. [Google Scholar] [CrossRef]
- Lončar, E.; Djurić, M.; Malbaša, R.; Kolarov, L.J.; Klašnja, M. Influence of Working Conditions upon Kombucha Conducted Fermentation of Black Tea. Food Bioprod. Process. 2006, 84, 186–192. [Google Scholar] [CrossRef]
- Nyhan, L.M.; Lynch, K.M.; Sahin, A.W.; Arendt, E.K. Advances in Kombucha Tea Fermentation: A Review. Appl. Microbiol. 2022, 2, 73–103. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Candra, K.; Yusof, N.A.; Daud, N.H.M. Health Benefits and Sensory Acceptance of Kombucha Prepared from Different Substrates. Foods 2023, 12, 1354. [Google Scholar] [CrossRef]
- Njieukam, J.A.; Ciccone, M.; Gottardi, D.; Ricci, A.; Parpinello, G.P.; Siroli, L.; Lanciotti, R.; Patrignani, F. Microbiological, Functional, and Chemico-Physical Characterization of Artisanal Kombucha: An Interesting Reservoir of Microbial Diversity. Foods 2024, 13, 1947. [Google Scholar] [CrossRef]
- Sinamo, K.N.; Ginting, S.; Pratama, S. Effect of sugar concentration and fermentation time on secang kombucha drink. IOP Conf. Ser. Earth Environ. Sci. 2022, 977, 012080. [Google Scholar] [CrossRef]
- Hur, J.; Choi, Y.; Kim, H.; Lee, J. The Antioxidant Activity of Kombucha Fermented with Different Tea Types. Food Chem. 2014, 43, 3565–3571. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Değirmencioğlu, N.; Yıldız, E.; Sahan, Y.; Gulda, M.; Gurbuz, O. Phenolics Antioxidant Capacity and Bioaccessibility of Kombucha Tea. In Proceedings of the ACS Spring 2019 National Meeting, Orlando, FL, USA, 31 March–4 April; 2019. [Google Scholar]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- FSAI. Guidance Note 37: Good Manufacturing Practices for the Production of Ready-to-Eat Unpasteurized Fermented Products; Food Safety Authority of Ireland: Dublin, Ireland, 2021; Available online: https://www.fsai.ie/publications/guidance_note_37.html (accessed on 12 June 2023).
- Jang, S.S.; McIntyre, L.; Chan, M.; Brown, P.N.; Finley, J.; Chen, S.X. Ethanol Concentration of Kombucha Teas in British Columbia, Canada. J. Food Prot. 2021, 84, 1771–1780. [Google Scholar] [CrossRef]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory Effects of Edible Seaweeds, Polyphenolics, and Alginates on the Activities of Porcine Pancreatic α-Amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Carlos, J.; Boldori, J.R.; Paulo, L.; Lunardi, A.G.; Aguiar, T.A.; Sigal, R.; Roehrs, R.; Denardin, C.C. Toxicity and Antioxidant Activity of Black Tea Kombucha in Wistar Rats: A 28-Day Repeated Dose Oral Study. Chem. Biodivers. 2025, e202500046. [Google Scholar] [CrossRef]
- Pagliari, S.; Forcella, M.; Lonati, E.; Sacco, G.; Romaniello, F.; Rovellini, P.; Fusi, P.; Palestini, P.; Campone, L.; Labra, M.; et al. Antioxidant and Anti-Inflammatory Effect of Cinnamon (Cinnamomum verum J. Presl) Bark Extract After In Vitro Digestion Simulation. Foods 2023, 12, 452. [Google Scholar] [CrossRef]
- Salafzoon, S.; Hosseini, H.M.; Halabian, R. Evaluation of the antioxidant impact of ginger-based kombucha on the murine breast cancer model. J. Complement. Integr. Med. 2018, 15, 20170071. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Cui, X.; Yao, Z.; Javed, B.; Naik, A.S.; Tian, F. Evaluating the Health Implications of Kombucha Fermented with Gardenia jasminoides Teas: A Comprehensive Analysis of Antioxidant, Antimicrobial, and Cytotoxic Properties. BioChem 2024, 4, 350–370. [Google Scholar] [CrossRef]
- Joshi, V.K.; Kumar, V. Kombucha: Technology, Microbiology, Production, Composition, and Therapeutic Value. Int. J. Food Ferment. Technol. 2016, 6, 13–21. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, H.J.; Yi, S.H.; Jung, Y.H. Antioxidant Properties of Kombucha Made with Tartary Buckwheat Tea and Burdock Tea. Prev. Nutr. Food Sci. 2023, 28, 347–352. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Lin, H.-W.; Wang, C.-Y.; Hsieh, C.-C.; Santoso, S.P.; Lin, S.-P.; Cheng, K.-C. Enhancing Antioxidant Benefits of Kombucha Through Optimized Glucuronic Acid by Selected Symbiotic Fermentation Culture. Antioxidants 2024, 13, 1323. [Google Scholar] [CrossRef] [PubMed]

| pH Values | TSS(°Bx) | Total Acidity (TA) | SCOBY (g) | Alcohol (%) | ||
|---|---|---|---|---|---|---|
| Day 0 | PT | 5.94 ± 0.01 | 80.05 | |||
| ST | 5.80 ± 0.01 | 10 ± 0.00 | 80.55 | |||
| HT | 5.44 ± 0.01 | 6 ± 0.00 | ||||
| Day 3 | SK | 2.9 ± 0.01 | 9.5 ± 0.00 | |||
| HK | 2.9 ± 0.01 | 6 ± 0.00 | ||||
| PT | ||||||
| ST | ||||||
| HT | ||||||
| Day 7 | SK | 2.8 ± 0.10 | 9.4 ± 0.00 | 0.69 ± 0.00 | 103.64 | 0.45 |
| HK | 2.7 ± 0.01 | 5.4 ± 0.06 | 0.75 ± 0.07 | 126.67 | 0.3 | |
| PT | ||||||
| ST | ||||||
| HT | ||||||
| Day 9 | SK | 3.0 ± 0.01 | 9 ± 0.00 | 0.55 ± 0.00 | ||
| HK | 3.0 ± 0.00 | 5.4 ± 0.06 | 0.56 ± 0.00 | |||
| SKS | 2.8 ± 0.00 | 9 ± 0.00 | 0.61 ± 0.00 | |||
| HKS | 2.9 ± 0.00 | 5 ± 0.00 | 0.83 ± 0.00 | |||
| SKG | 2.87 ± 0.00 | 9 ± 0.00 | 0.67 ± 0.00 | |||
| HKG | 2.87 ± 0.00 | 5 ± 0.00 | 0.71 ± 0.00 | |||
| SKC | 2.9 ± 0.00 | 9.5 ± 0.00 | 0.66 ± 0.00 | |||
| HKC | 2.9 ± 0.01 | 4.5 ± 0.00 | 0.64 ± 0.00 | |||
| SKL | 2.9 ± 0.00 | 9.5 ± 0.00 | 0.64 ± 0.00 | |||
| HKL | 2.9 ± 0.01 | 5.5 ± 0.00 | 0.66 ± 0.00 | |||
| V | 0.44 ± 0.00 | |||||
| Vshot | 0.45 ± 0.00 | |||||
| Day 11 | SK | 3.1 ± 0.04 | 9 ± 0.00 | 0.53 | ||
| HK | 3.1 ± 0.00 | 5.4 ± 0.06 | 0.39 | |||
| SKS | 2.87 ± 0.06 | 8.6 ± 0.00 | 0.85 | |||
| HKS | 2.97 ± 0.03 | 5 ± 0.00 | 0.62 | |||
| SKG | 2.91 ± 0.04 | 8.6 ± 0.00 | 0.98 | |||
| HKG | 2.98 ± 0.07 | 5 ± 0.00 | 0.68 | |||
| SKC | 2.8 ± 0.00 | 9.0 ± 0.00 | 0.22 | |||
| HKC | 2.8 ± 0.01 | 4.0 ± 0.00 | 0.32 | |||
| SKL | 2.8 ± 0.00 | 9.0 ± 0.00 | 0.32 | |||
| HKL | 2.8 ± 0.01 | 4.0 ± 0.00 | 0.44 |
| Colorimeter Readings | |||
|---|---|---|---|
| L* | a* | b* | |
| Day 0 | |||
| PT | 0.12 ± 0.02 | 0.22 ± 0.08 | 0.06 ± 0.03 |
| ST | 0.12 ± 0.01 | 0.18 ± 0.08 | 0.05 ± 0.02 |
| HT | 0.12 ± 0.01 | 0.20 ± 0.04 | 0.05 ± 0.01 |
| Day 7 | |||
| SK | 0.32 ± 0.06 | 0.08 ± 0.10 | 0.19 ± 0.13 |
| HK | 0.13 ± 0.02 | 0.11 ± 0.02 | 0.06 ± 0.04 |
| Day 11 | |||
| SK | 1.17 ± 0.06 | 0.37 ± 0.21 | 0.31 ± 0.09 |
| HK | 0.13 ± 0.02 | 0.23 ± 0.06 | 0.06 ± 0.02 |
| SKS | 0.44 ± 0.04 | 0.35 ± 0.27 | 0.12 ± 0.14 |
| HKS | 0.34 ± 0.05 | 0.43 ± 0.10 | 0.08 ± 0.11 |
| SKG | 0.31 ± 0.04 | 0.09 ± 0.07 | 0.15 ± 0.05 |
| HKG | 0.35 ± 0.03 | 0.66 ± 0.10 | 0.12 ± 0.14 |
| SKC | 0.26 ± 0.06 | 0.04 ± 0.08 | 0.45 ± 0.01 |
| SKL | 1.91 ± 0.04 | −1.6 ± 0.04 | 8.9 ± 0.48 |
| HKC | 3.72 ± 0.06 | −6.7 ± 0.04 | 1.52 ± 0.10 |
| HKL | 3.43 ± 0.03 | 1.14 ± 0.12 | 1.45 ± 0.10 |
| FRAP antioxidant activity | |||||
| TE (µg/mL) | |||||
| Day | 0 | 3 | 7 | 9 | 11 |
| PT | 87.14 ± 6.34 | 78.24 ± 3.10 | 83.33 ± 2.29 | 89.76 ± 1.57 | 73.29 ± 1.10 |
| ST | 91.75 ± 6.38 | 95.97 ± 2.62 | 95.81 ± 1.52 | 94.32 ± 4.42 | 105 ± 3.19 |
| HT | 99.37 ± 5.12 | 110.14 ± 4.52 | 98.83 ± 3.12 | 104.33 ± 2.62 | 109.33 ± 3.05 |
| SK | 144.47 ± 1.95 | 111 ± 5.67 | 121.48 ± 1.48 | 108.19 ± 3.39 | |
| HK | 155.48 ± 2.14 | 155.29 ± 4.43 | 120 ± 1.24 | 141.52 ± 2.20 | |
| SKS | 105.24 ± 4.76 | 96.48 ± 2.19 | |||
| HKS | 136.35 ± 2.02 | 104.67 ± 4.10 | |||
| SKG | 101.29 ± 1.19 | 101.02 ± 2.05 | |||
| HKG | 107.68 ± 3.57 | 125.43 ± 3.62 | |||
| DPPH assay results | |||||
| % Inhibition | |||||
| Day | 0 | 3 | 7 | 9 | 11 |
| PT | 140.78 ± 0.95 | 140.41 ± 3.25 | 140.51 ± 2.75 | 146.52 ± 2.14 | 133.12 ± 3.07 |
| ST | 145.74 ± 2.64 | 141.37 ± 5.51 | 142.50 ± 4.45 | 146.74 ± 4.49 | 145.95 ± 1.32 |
| HT | 137.52 ± 1.84 | 138.50 ± 2.17 | 144.81 ± 1.11 | 146.54 ± 4.41 | 146.50 ± 0.17 |
| SK | 164.27 ± 0.41 | 163.74 ± 0.93 | 162.51 ± 0.58 | 159.62 ± 1.29 | |
| HK | 164.78 ± 0.41 | 142.50 ± 4.45 | 163.00 ± 0.39 | ||
| SKS | 162.94 ± 1.32 | 160.60 ± 2.59 | |||
| HKS | 163.74 ± 0.93 | 159.25 ± 0.39 | |||
| SKG | 163.86 ± 0.32 | 141.55 ± 0.98 | |||
| HKG | 146.54 ± 4.14 | 162.67 ± 0.68 | |||
| Sample | DPPH (%) | Sample | FRAP (µg/mL) |
|---|---|---|---|
| SK | 162.54 ± 2.08 a | HK | 143.07 ± 16.71 a |
| SKS | 161.77 ± 1.66 a | SK | 121.29 ± 16.48 ab |
| HKS | 161.50 ± 3.17 a | HKS | 120.51 ± 22.40 abcd |
| HK | 158.68 ± 10.81 a | HKG | 116.56 ± 12.55 abd |
| HKG | 154.61 ± 11.40 a | SKS | 104.4 ± 5.33 bcd |
| SKG | 152.70 ± 15.78 a | HT | 101.16 ± 0.19 abcd |
| ST | 144.46 ± 2.37 b | SKG | 100.86 ± 22.40 bcd |
| HT | 142.78 ± 4.42 b | ST | 96.57 ± 5.01 bcd |
| PT | 140.27 ± 4.76 b | PT | 82.35 ± 6.67 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpova, A.; Adesina, D.; Tian, F.; Naik, A.S. Seaweed (Laminaria digitata) and Honey Kombucha: A Fermented Antioxidant-Rich Beverage. Fermentation 2025, 11, 379. https://doi.org/10.3390/fermentation11070379
Karpova A, Adesina D, Tian F, Naik AS. Seaweed (Laminaria digitata) and Honey Kombucha: A Fermented Antioxidant-Rich Beverage. Fermentation. 2025; 11(7):379. https://doi.org/10.3390/fermentation11070379
Chicago/Turabian StyleKarpova, Anastasia, Deborah Adesina, Furong Tian, and Azza Silotry Naik. 2025. "Seaweed (Laminaria digitata) and Honey Kombucha: A Fermented Antioxidant-Rich Beverage" Fermentation 11, no. 7: 379. https://doi.org/10.3390/fermentation11070379
APA StyleKarpova, A., Adesina, D., Tian, F., & Naik, A. S. (2025). Seaweed (Laminaria digitata) and Honey Kombucha: A Fermented Antioxidant-Rich Beverage. Fermentation, 11(7), 379. https://doi.org/10.3390/fermentation11070379







