Indigenous Malolactic Starter Cultures as Innovative Tools to Modify the Sensory Profile of a Wine: An Oenological Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Starter Culture Preparation
2.2. Acclimation and Inoculation Conditions
2.3. Sample Preparation for Freeze-Drying
2.4. Winemaking Process
2.5. Monitoring of LAB Viability
2.6. L-Malic Acid Consumption
2.7. Wine Physicochemical Analysis
2.8. Implantation Capacity
2.9. Sensorial Analysis
2.10. Statistical Analysis and Reproducibility Assay
3. Results
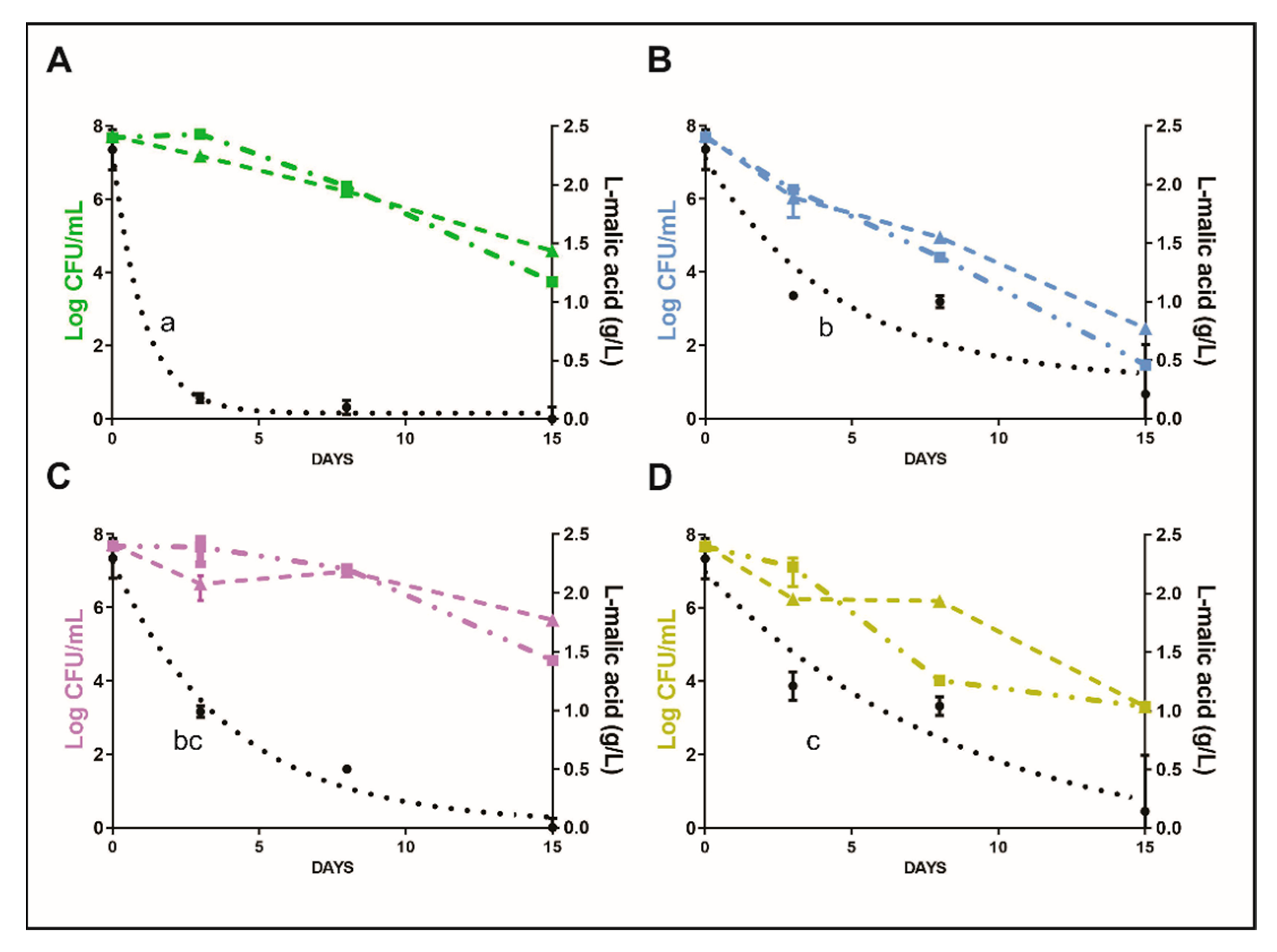
3.1. Pilot-Scale Winemaking
3.2. Evaluation of MAC and Cell Survival
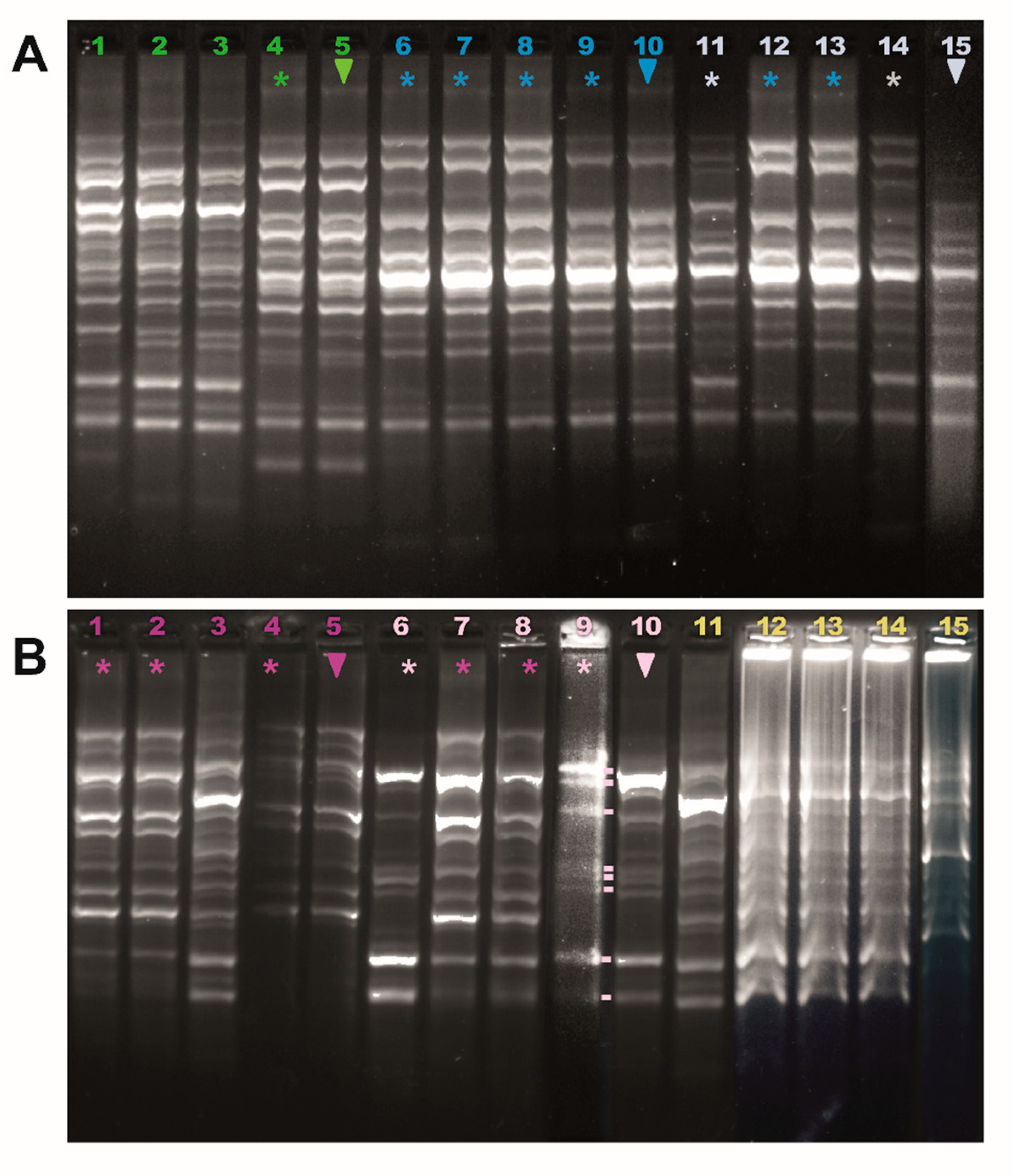
3.3. Implantation of LAB
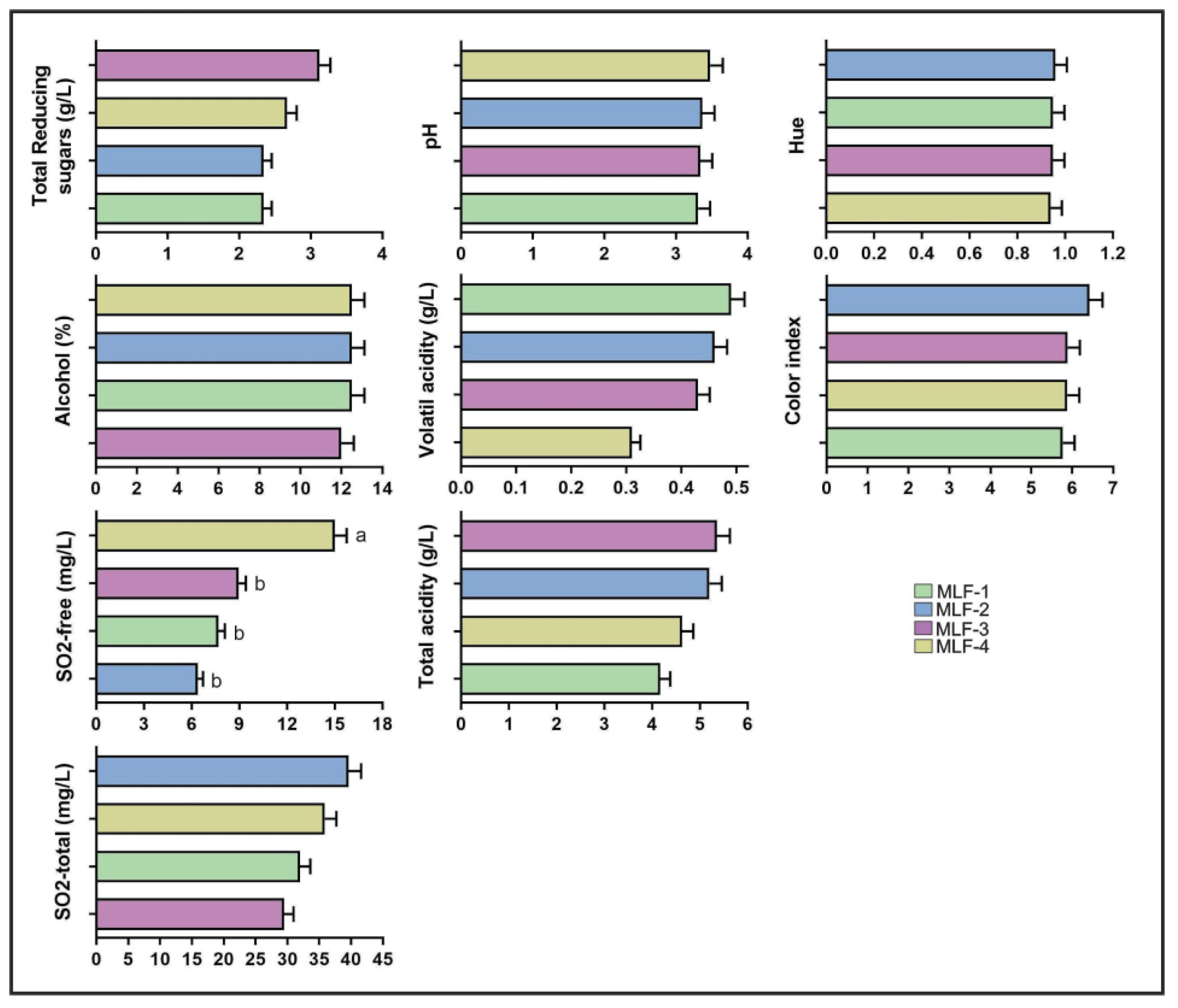
3.4. Physicochemical Analysis of Wines
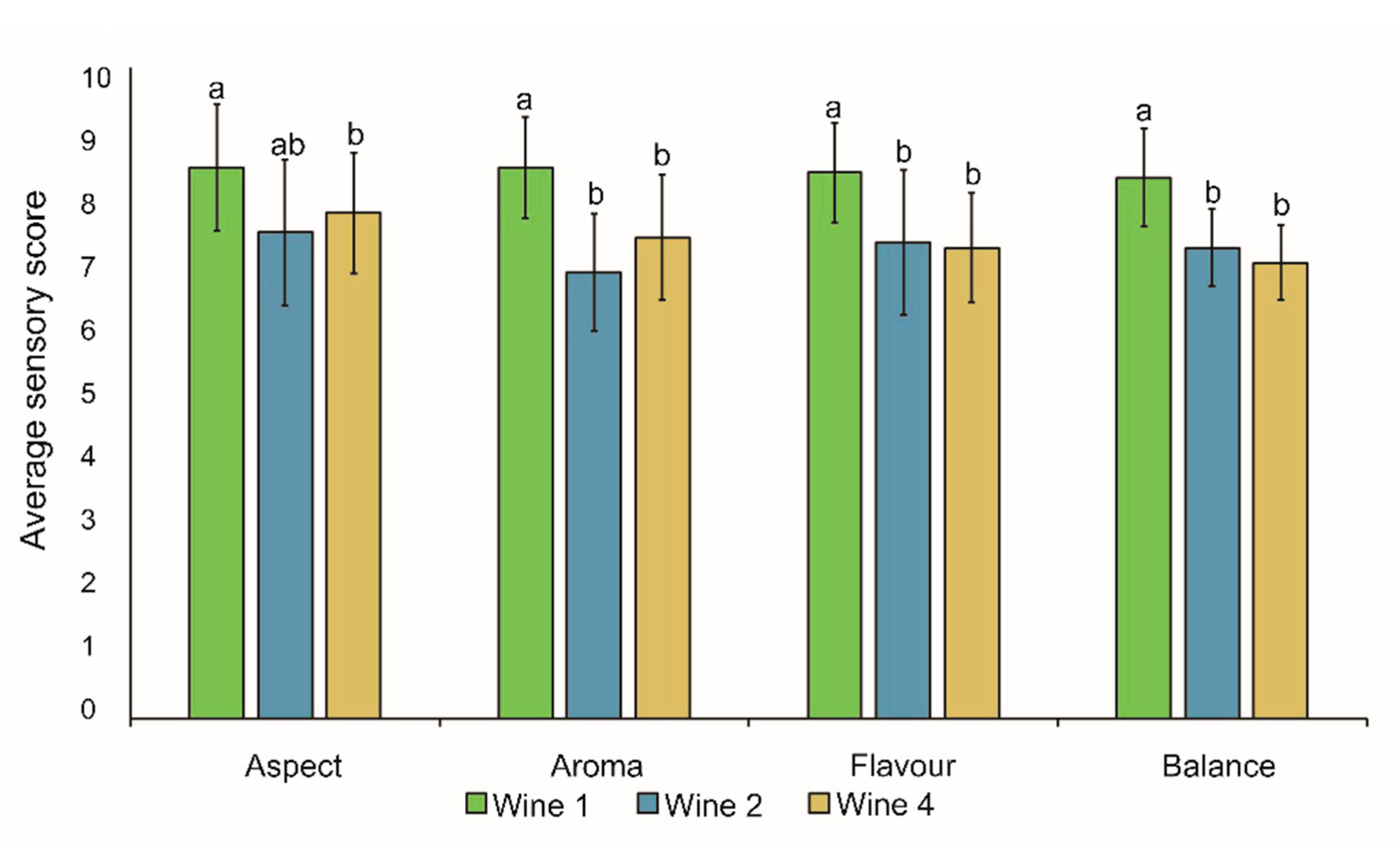
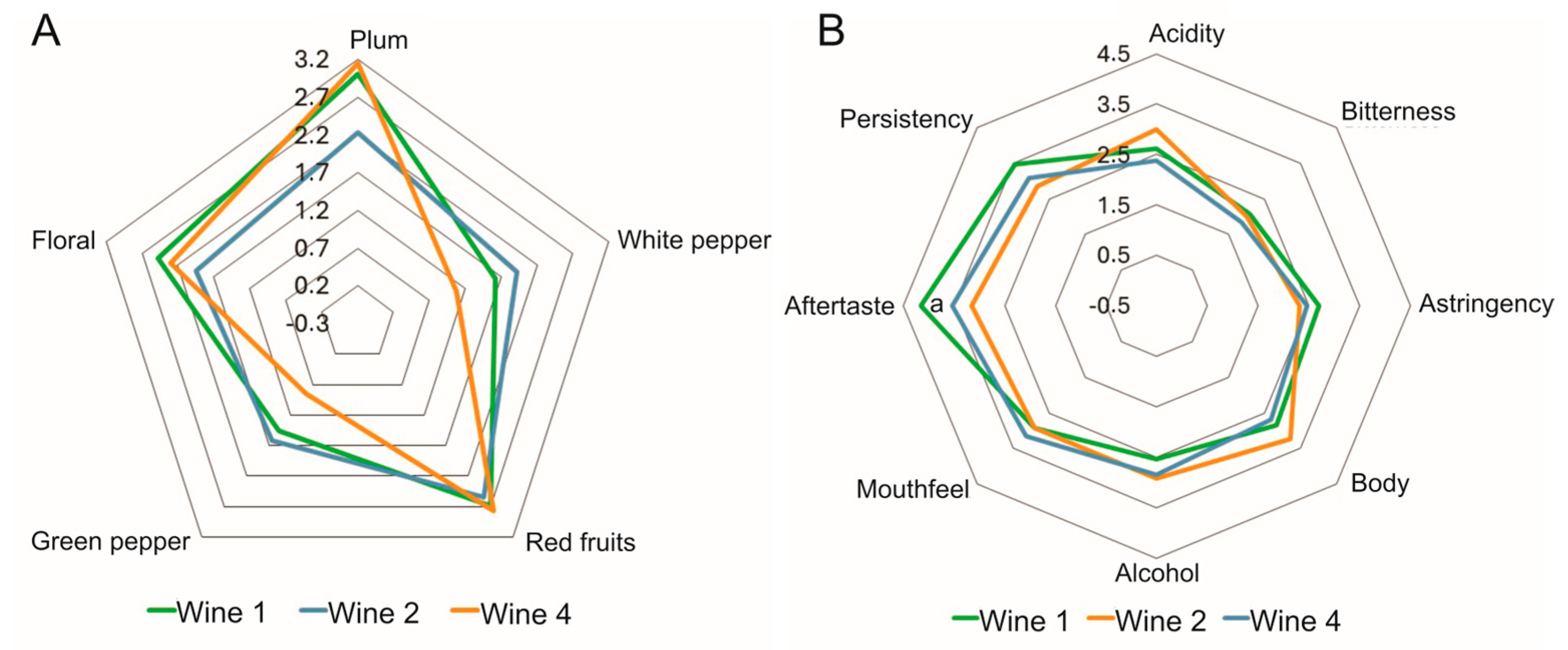
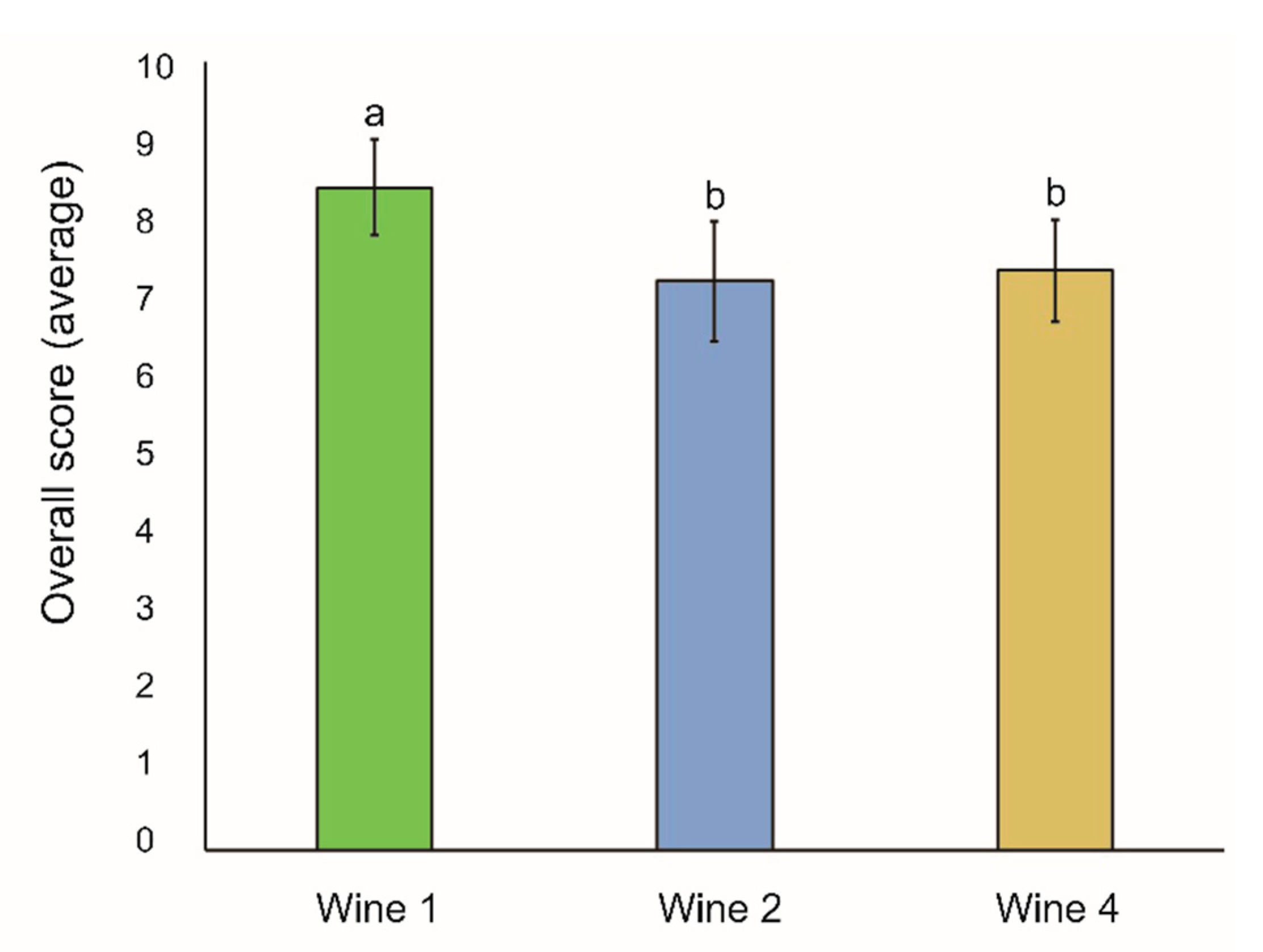
3.5. Sensory Analysis
4. Discussion
4.1. Effect of Native MLFS on Wine Properties
4.2. Starters Implantation Capacity
4.3. Sensory Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Alcoholic Fermentation |
| MLF | Malolactic Fermentation |
| LAB | Lactic Acid Bacteria |
| IMBA | Instituto de Microbiología Básica y Aplicada |
| DCyT | Departamento de Ciencia y Tecnología |
| UNQ | Universidad Nacional de Quilmes |
| CIC | Comisión de Investigaciones Científicas |
| RGPRN | Registro Provincial de Recursos Naturales |
References
- OIV 2024 World Wine Production Outlook. Available online: https://www.oiv.int/sites/default/files/documents/OIV_2024_World_Wine_Production_Outlook_1.pdf (accessed on 10 April 2025).
- Ciezack, G.; Hazo, L.; Chambat, G.; Heyraud, A.; Lonvaud-Funel, A.; Dols-Lafargue, M. Evidence for exopolysaccharide production by Oenococcus oeni strains isolated from non-ropy wines. J. Appl. Microbiol. 2010, 108, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Maturano, C.; Saguir, F.M. Influence of glycosides on behavior of Oenococcus oeni in wine conditions: Growth, substrates and aroma compounds. World J. Microbiol. Biotechnol. 2017, 33, 151. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.S.; Franco-Luesma, E.; Bravo-Ferrada, B.M.; Perez-Jimenez, M.; Semorile, L.; Tymczyszyn, E.E.; Pozo-Bayon, M. Influence of Patagonian Lactiplantibacillus plantarum and Oenococcus oeni strains on sensory perception of Pinot Noir wine after malolactic fermentation. Aust. J. Grape Wine Res. 2021, 27, 118–127. [Google Scholar] [CrossRef]
- Bai, X.F.; Jin, G.; Liu, S.; Ma, W.; Zhang, Z.; Wang, H.Q.; Zhang, J.X. Malolactic fermentation characteristics of Lactobacillus hilgardii Q19 at low temperature and its effect on aroma components in wine. Food Sci. 2020, 41, 146–152. [Google Scholar]
- Wang, L.; Huang, G.; Ma, W.; Jin, G. Preparation and application of directed vat set indigenous freeze-drying Lentilactobacillus hilgardii Q19 starter in winemaking. Foods 2023, 12, 1053. [Google Scholar] [CrossRef]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Selection and Characterisation of Oenococcus oeni and Lactobacillus plantarum South African Wine Isolates for Use as Malolactic Fermentation Starter Cultures; Stellenbosch University: Stellenbosch, South Africa, 2011. [Google Scholar]
- Berbegal, C.; Peña, N.; Russo, P.; Grieco, F.; Pardo, I.; Ferrer, S.; Spano, G.; Capozzi, V. Technological properties of Lactobacillus plantarum strains isolated from grape must fermentation. Food Microbiol. 2016, 57, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.S.; Zapparoli, G.; Logrieco, A.; Bartowsky, E.J. Linking wine lactic acid bacteria diversity with wine aroma and flavour. Int. J. Food Microbiol. 2017, 243, 16–27. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef] [PubMed]
- Manera, C.; Rivas, G.A.; Flores, N.E.; Brizuela, N.S.; Caballero, A.C.; Semorile, L.C.; Valdes La Hens, D. Prevalence of Lentilacobacillus hilgardii over Lactiplantibacillus plantarum in low-temperature spontaneous malolactic fermentation of a Patagonian Pinot Noir. Fermentation 2023, 9, 809. [Google Scholar] [CrossRef]
- Rivas, G.A.; Flores, N.E.; Brizuela, N.S.; Guillade, A.C.; Semorile, L.C.; Delfederico, L. Novel malolactic fermentation starter formulated using native lactic acid bacteria strains from a re-emerging wine-growing region of Argentina—A pilot scale vinification. Fermentation 2025, 11, 140. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Delfederico, L.; Valdés La Hens, D.; Caballero, A.; Semorile, L. Patagonian red wines: Selection of Lactobacillus plantarum starter cultures for malolactic fermentation. World J. Microbiol. Biotechnol. 2013, 29, 1537–1549. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; La Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative vinification assays with selected Patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT Food Sci. Technol. 2017, 77, 348–355. [Google Scholar] [CrossRef]
- Manera, C.; Olguin, N.T.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Delfederico, L.; Bibiloni, H.; Caballero, A.C.; Semorile, L.C.; Valdes La Hens, D.V. Survival and implantation of indigenous psychrotrophic Oenococcus oeni strains during malolactic fermentation in a Patagonian Pinot noir wine. LWT 2019, 108, 353–360. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Semorile, L. Effect of acclimation medium on cell viability, membrane integrity and ability to consume malic acid in synthetic wine by oenological Lactobacillus plantarum strains. J. Appl. Microbiol. 2014, 116, 360–367. [Google Scholar] [CrossRef]
- Maicas, S.; Pardo, I.; Ferrer, S. The effects of freezing and freeze-drying of Oenococcus oeni upon induction of malolactic fermentation in red wine. Int. J. Food Sci. Technol. 2000, 35, 75–79. [Google Scholar] [CrossRef]
- Corral-Lugo, A.; Morales-García, Y.E.; Pazos-Rojas, L.A.; Ramírez-Valverde, A.; Martínez-Contreras, R.D.; Muñoz-Rojas, J. Cuantificación de bacterias cultivables mediante el método de Goteo en Placa por Sellado (o estampado). Masivo. Ann. Microbiol. 2012, 14, 147–156. [Google Scholar]
- Glories, Y. The color of red wines. Connaiss. Vigne Vin 1984, 18, 195–217. [Google Scholar]
- Stendid, J.; Karlsson, J.O.; Hogberg, N. Intraspecific genetic variation in Heterobasidium annosum revealed by amplification of minisatellite DNA. Mycol. Res. 1994, 98, 57–63. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Chapman & Hall: New York, NY, USA, 1998; p. 827. [Google Scholar]
- Kessi-Pérez, E.I.; Gómez, M.; Farías, W.; García, V.; Ganga, M.A.; Querol, A.; Martínez, C. Genetically improved yeast strains with lower ethanol yield for the wine industry generated through a two-round breeding program. J. Fungi 2025, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Goldner, M.C.; Zamora, M.C. Sensory characterization of Vitis vinifera cv. Malbec wines from seven viticulture regions of Argentina. J. Sens. Stud. 2007, 22, 520–532. [Google Scholar] [CrossRef]
- Veríssimo, C.M.; de Macêdo Morais, S.; Leite de Andrade Lima, L.; Pereira, G.E.; Sucupira Maciel, M.I. A short training as an enhancer of sensory ability: The case of red wine consumers. J. Sens. Stud. 2021, 36, e12629. [Google Scholar] [CrossRef]
- Aruani, A.C.; Quini, C.I.; Ortiz, H.; Videla, R.; Murgo, M.; Prieto, S. Argentinean commercial Malbec wines: Regional sensory profiles. J. Life Sci. 2014, 8, 134–141. [Google Scholar]
- Barroso, R.; Carbajal, H.; Ortiz, H.; Malaniuk, M.; Quénol, H.; Murgo, M.; Coria, C.; Videla, R.; Prieto, S.; Manzano, H.; et al. Vinos de altura del noroeste Argentino—Características físico-químicas y sensoriales. BIO Web Conf. 2019, 15, 01002. [Google Scholar] [CrossRef]
- La Cata Del Vino Tinto. Available online: http://catast.com/Documentos/Fullsperfil/FFPPvinegre.pdf (accessed on 1 February 2025).
- Manca, E. Investigating the language of red wine tasting notes across the us, the Australian and the Italian cultures. Lingue Linguaggi 2020, 39, 247–269. [Google Scholar] [CrossRef]
- ISO 3591:1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. ISO: Geneva, Switzerland, 1977.
- Ares, G.; Antúnez, L.; Bruzzone, F.; Vidal, L.; Giménez, A.; Pineau, B.; Beresford, M.K.; Jin, D.; Paisley, A.G.; Chheang, S.L.; et al. Comparison of sensory product profiles generated by trained assessors and consumers using CATA questions: Four case studies with complex and/or similar samples. Food Qual. Prefer. 2015, 45, 75–86. [Google Scholar] [CrossRef]
- Rinaldi, A.; Vecchio, R.; Moio, L. Differences in astringency subqualities evaluated by consumers and trained assessors on Sangiovese wine using check-all-that-apply (CATA). Foods 2021, 10, 218. [Google Scholar] [CrossRef]
- Graph Pad Prism, version 6.01; Graph Pad Software Inc.: San Diego, CA, USA, 2007.
- Statistix, version 8.0; Analytical Software: Tallahassee, FL, USA, 2008.
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic fermentation—Theoretical advances and practical considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Inês, A.; Falco, V. Lactic Acid Bacteria Contribution to Wine Quality and Safety. In Generation of Aromas and Flavours; Vilela, A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Russo, P.; Fragasso, M.; Berbegal, C.; Grieco, F.; Spano, G.; Capozzi, V. Microorganisms able to produce biogenic amines and factors affecting their activity. In Biogenic Amines in Food: Analysis, Occurrence and Toxicity; Saad, B., Tofalo, R., Eds.; The Royal Society of Chemistry: London, UK, 2019; pp. 18–40. [Google Scholar]
- Lorentzen, M.P.; Lucas, P.M. Distribution of Oenococcus oeni populations in natural habitats. AMBB 2019, 103, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a new biological tool to control malolactic fermentation: A review and an outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef]
- López-Seijas, J.; García-Fraga, B.; da Silva, A.F.; Zas-García, X.; Lois, L.C.; Gago-Martínez, A.; Leão-Martins, J.M.; Sieiro, C. Evaluation of malolactic bacteria associated with wines from Albariño variety as potential starters: Screening for quality and safety. Foods 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jiang, D.; Fan, M.; Li, H.; Jin, C.; Liu, W. Selection of a versatile Lactobacillus plantarum for wine production and identification and preliminary characterisation of a novel histamine-degrading enzyme. IJFST 2020, 55, 2608–2618. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Huang, L.; Ma, W.; Jin, G. Physiological and transcriptomic insights into ethanol stress tolerance in Lentilactobacillus hilgardii Q19. Food Sci. Hum. Well. 2024. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Gonçalves, S.; Semorile, L.; Santos, N.C.; Brizuela, N.S.; Tymczyszyn, E.E.; Hollmann, A. Cell surface damage and morphological changes in Oenococcus oeni after freeze-drying and incubation in synthetic wine. Cryobiology 2018, 82, 15–21. [Google Scholar] [CrossRef]
- Nikolaou, A.; Nelios, G.; Kanellaki, M.; Kourkoutas, Y. Freeze-dried immobilized kefir culture in cider-making. J. Sci. Food Agric. 2020, 100, 3319–3327. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, H.T. Environmental stress for improving the functionality of lactic acid bacteria in malolactic fermentation. Microbe 2024, 100138. [Google Scholar] [CrossRef]
- Costanigro, M.; Appleby, C.; Menke, S.D. The wine headache: Consumer perceptions of sulfites and willingness to pay for non-sulfited wines. Food Qual. 2014, 31, 81–89. [Google Scholar] [CrossRef]
- Reguant, C.; Carreté, R.; Constantí, M.; Bordons, A. Population dynamics of Oenococcus oeni strains in a new winery and the effect of SO2 and yeast strain. FEMS Microbiol. Lett. 2005, 246, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Costello, P.J.; Chambers, P.J. Emerging trends in the application of malolactic fermentation. Aust. J. Grape Wine Res. 2015, 21, 663–669. [Google Scholar] [CrossRef]
- Jin, G.; Jiranek, V.; Hayes, A.M.; Grbin, P.R. Isolation and characterization of high-ethanol-tolerance lactic acid bacteria from Australian wine. Foods 2022, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Urvieta, R.; Jones, G.; Buscema, F.; Bottini, R.; Fontana, A. Terroir and vintage discrimination of Malbec wines based on phenolic composition across multiple sites in Mendoza, Argentina. Sci. Rep. 2021, 11, 2863. [Google Scholar] [CrossRef]
- King, E.S.; Stoumen, M.; Buscema, F.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H.; Boulton, R.B. Regional sensory and chemical characteristics of Malbec wines from Mendoza and California. Food Chem. 2014, 143, 256–267. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Fu, J.; Wang, L.; Sun, J.; Ju, N.; Jin, G. Malolactic fermentation: New approaches to old problems. Microorganisms 2022, 10, 2363. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, E.N.; Rivas, G.A.; Guillade, A.C.; Brizuela, N.S.; Navarro, M.E.; Tymczyszyn, E.E.; Delfederico, L.; Perez, C.F.; Semorile, L.C.; Valdes La Hens, D.; et al. Indigenous Malolactic Starter Cultures as Innovative Tools to Modify the Sensory Profile of a Wine: An Oenological Challenge. Fermentation 2025, 11, 337. https://doi.org/10.3390/fermentation11060337
Flores EN, Rivas GA, Guillade AC, Brizuela NS, Navarro ME, Tymczyszyn EE, Delfederico L, Perez CF, Semorile LC, Valdes La Hens D, et al. Indigenous Malolactic Starter Cultures as Innovative Tools to Modify the Sensory Profile of a Wine: An Oenological Challenge. Fermentation. 2025; 11(6):337. https://doi.org/10.3390/fermentation11060337
Chicago/Turabian StyleFlores, Elizabeth Naiquen, Gabriel Alejandro Rivas, Andrea Cecilia Guillade, Natalia Soledad Brizuela, Marina Edith Navarro, Emma Elizabeth Tymczyszyn, Lucrecia Delfederico, Carolina Fabiana Perez, Liliana Carmen Semorile, Danay Valdes La Hens, and et al. 2025. "Indigenous Malolactic Starter Cultures as Innovative Tools to Modify the Sensory Profile of a Wine: An Oenological Challenge" Fermentation 11, no. 6: 337. https://doi.org/10.3390/fermentation11060337
APA StyleFlores, E. N., Rivas, G. A., Guillade, A. C., Brizuela, N. S., Navarro, M. E., Tymczyszyn, E. E., Delfederico, L., Perez, C. F., Semorile, L. C., Valdes La Hens, D., & Bravo-Ferrada, B. M. (2025). Indigenous Malolactic Starter Cultures as Innovative Tools to Modify the Sensory Profile of a Wine: An Oenological Challenge. Fermentation, 11(6), 337. https://doi.org/10.3390/fermentation11060337






