Unlocking the Potential of Pomelo Albedo: A Novel Substrate for Alpha-Amylase Production Using Bacillus licheniformis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strain and Materials
2.2. Amylase Assay
2.3. Fermentation Conditions
2.4. Enzyme Purification
2.5. Enzyme Characterization
2.6. HPLC Analysis
3. Results and Discussion
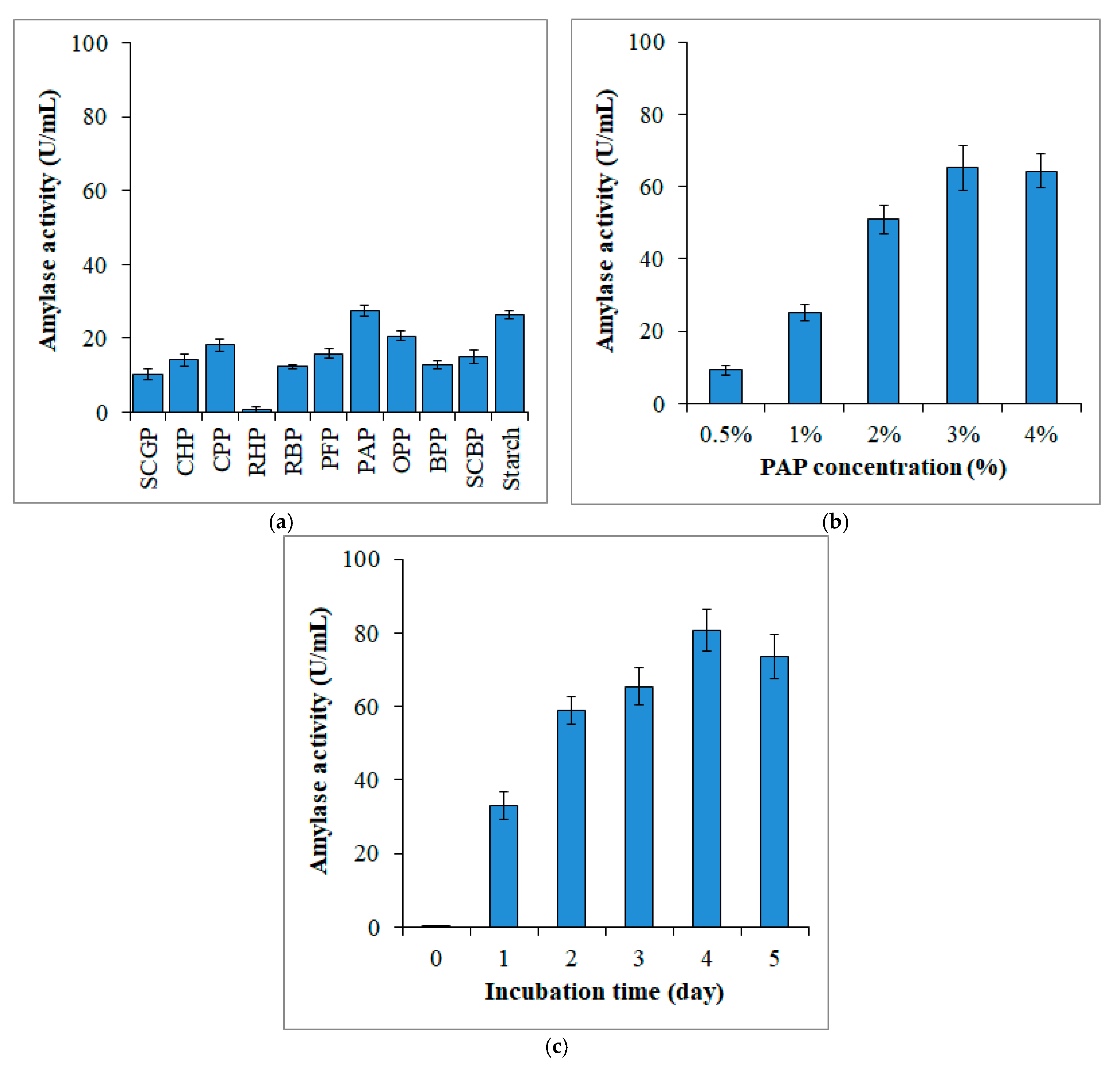
3.1. Enzyme Production
3.2. Enzyme Purification and Identification
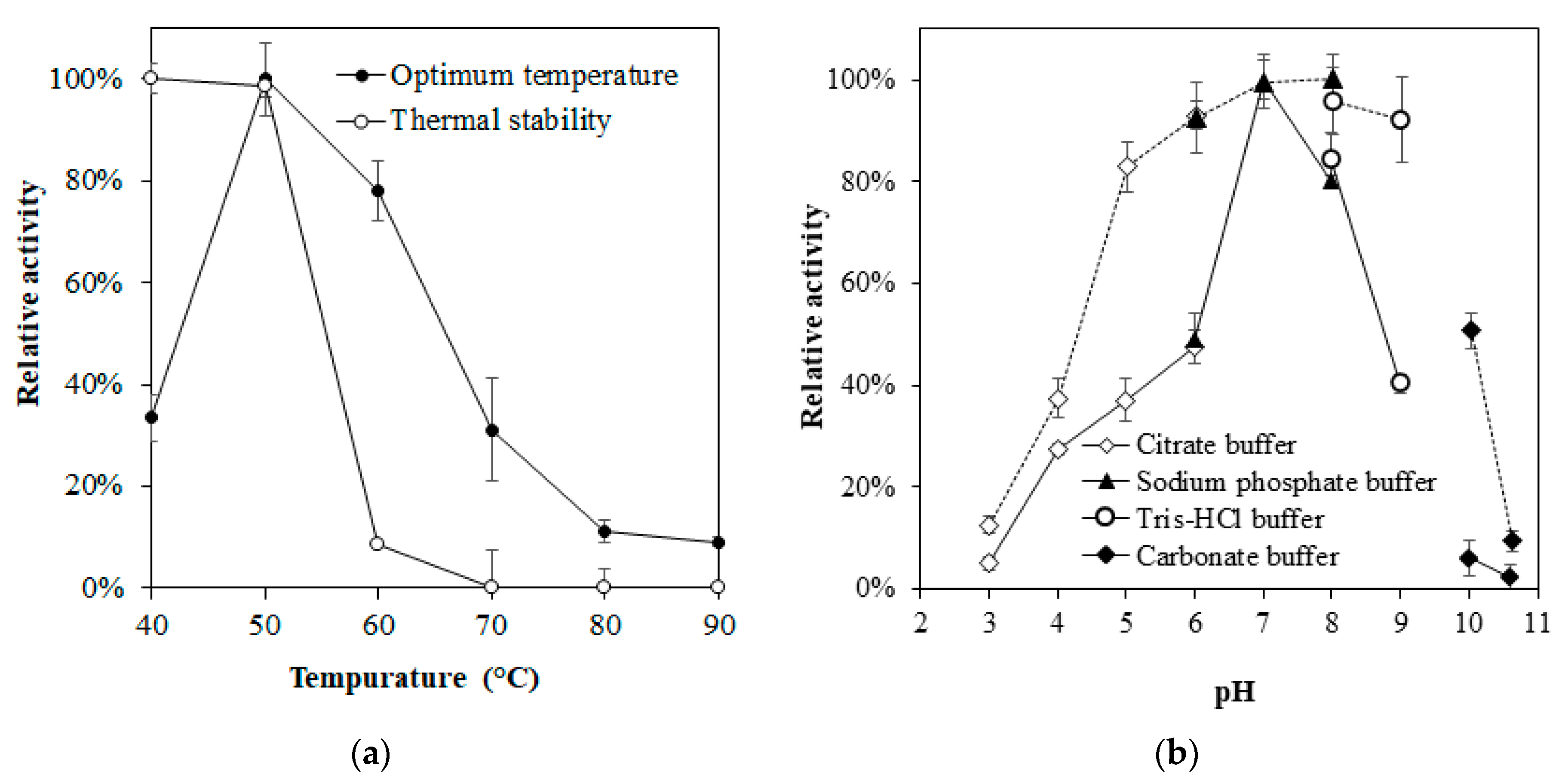
3.3. Enzyme Characterization
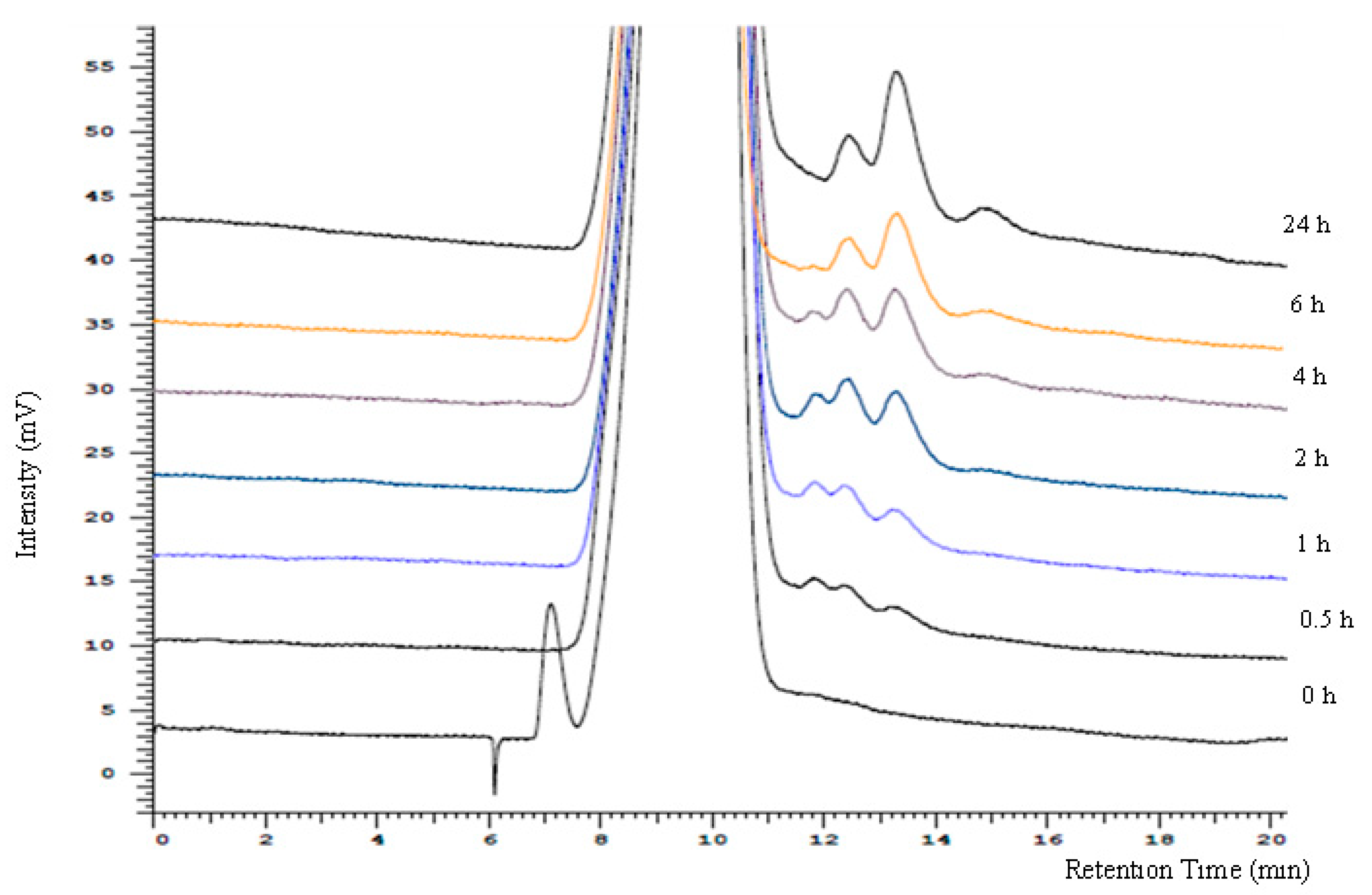
3.4. HPLC Analysis of the Hydrolysis Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondal, S.; Mondal, K.; Halder, S.K.; Thakur, N.; Mondal, K.C. Microbial amylase: Old but still at the forefront of all major industrial enzymes. Biocatal. Agric. Biotechnol. 2022, 45, 102509. [Google Scholar] [CrossRef]
- Zinck, S.S.; Christensen, S.J.; Sørensen, O.B.; Svensson, B.; Meyer, A.S. Importance of inactivation methodology in enzymatic processing of raw potato starch: NaOCl as efficient α-amylase inactivation agent. Molecules 2023, 28, 2947. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.M.; De Magalhães, P.O. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, B.; Prajapati, V.; Patel, K.; Trivedi, U. Kitchen waste for economical amylase production using Bacillus amyloliquefaciens KCP2. Biocatal. Agric. Biotechnol. 2020, 26, 101654. [Google Scholar] [CrossRef]
- Vaikundamoorthy, R.; Rajendran, R.; Selvaraju, A.; Moorthy, K.; Perumal, S. Development of thermostable amylase enzyme from Bacillus cereus for potential antibiofilm activity. Bioorg. Chem. 2018, 77, 494–506. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Ben-Mahmoud, A.; Al-Barazie, R. Bacillus amyloliquefaciens: Harnessing its potential for industrial, medical, and agricultural applications—A comprehensive review. Microorganisms 2023, 11, 2215. [Google Scholar] [CrossRef]
- Yao, D.; Su, L.; Li, N.; Wu, J. Enhanced extracellular expression of Bacillus stearothermophilus α-amylase in Bacillus subtilis through signal peptide optimization, chaperone overexpression and α-amylase mutant selection. Microb. Cell Fact. 2019, 18, 69. [Google Scholar] [CrossRef]
- Rafanomezantsoa, P.; Gharbi, S.; Karkachi, N.; Kihal, M. Optimization of amylase production by the biological control agent Bacillus halotolerans RFP74 using response surface methodology. J. Genet. Eng. Biotechnol. 2023, 21, 63. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, W.; Zhou, D.; Huang, J. Bacillus subtilis contributes to amylase production in the honey sac of Apis mellifera. Insects 2025, 16, 221. [Google Scholar] [CrossRef]
- Zhang, A.; Ma, Y.; Deng, Y.; Zhou, Z.; Cao, Y.; Yang, B.; Bai, J.; Sun, Q. Enhancing protease and amylase activities in Bacillus licheniformis XS-4 for traditional soy sauce fermentation using ARTP mutagenesis. Foods 2023, 12, 2381. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, T.T.; Tran, T.P.H.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.-L. Production of sucrolytic enzyme by Bacillus licheniformis by the bioconversion of pomelo albedo as a carbon source. Polymers 2021, 13, 1959. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Xiang, L.; Byambabaatar, S.; Kim, H.; Jin, K.S.; Ree, M. Bacillus licheniformis α-amylase: Structural feature in a biomimetic solution and structural changes in extrinsic conditions. Int. J. Biol. Macromol. 2019, 127, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Mamo, J.; Kangwa, M.; Fernandez-Lahore, H.M.; Assefa, F. Optimization of media composition and growth conditions for production of milk-clotting protease (MCP) from Aspergillus oryzae DRDFS13 under solid-state fermentation. Braz. J. Microbiol. 2020, 51, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Tran, T.P.H.; Nguyen, T.T.; Nguyen, H.K.; Tran, T.K.T.; Vu, B.T.; Trinh, T.H.T.; Nguyen, A.D.; Wang, S.-L. Chitosanase production from the liquid fermentation of squid pens waste by Paenibacillus elgii. Polymers 2023, 15, 3724. [Google Scholar] [CrossRef]
- Khaswal, A.; Mishra, S.K.; Chaturvedi, N.; Saini, S.; Pletschke, B.; Kuhad, R.C. Microbial enzyme production: Unlocking the potential of agricultural and food waste through solid-state fermentation. Bioresour. Technol. Rep. 2024, 27, 101880. [Google Scholar] [CrossRef]
- Lima, C.A.; Contato, A.G.; de Oliveira, F.; da Silva, S.S.; Hidalgo, V.B.; Irfan, M.; Gambarato, B.C.; Carvalho, A.K.F.; Bento, H.B.S. Trends in enzyme production from Citrus by-products. Processes 2025, 13, 766. [Google Scholar] [CrossRef]
- Huang, R.L.; Cao, M.; Guo, H.; Qi, W.; Su, R.X.; He, Z.M. Enhanced ethanol production from pomelo peel waste by integrated hydrothermal treatment, multienzyme formulation, and fed-batch operation. J. Agric. Food Chem. 2014, 62, 4643–4651. [Google Scholar] [CrossRef]
- Xiao, L.; Ye, F.Y.; Zhou, Y.; Zhao, G.H. Utilization of pomelo peels to manufacture value-added products: A review. Food Chem. 2021, 351, 129247. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, R.; Wang, Y.X.; Ma, L.Y.; Nie, S.P.; Xie, M.Y.; Yin, J.Y. Physicochemical and rheological properties of pomelo albedo pectin and its interaction with konjac glucomannan. Int. J. Biol. Macromol. 2020, 151, 1205–1212. [Google Scholar] [CrossRef]
- Zain, N.F.M.; Yusop, S.M.; Ahmad, I. Preparation and characterization of cellulose and nanocellulose from pomelo (Citrus grandis) albedo. J. Nutr. Food Sci. 2014, 5, 1000334. [Google Scholar]
- Wang, S.L.; Kao, T.Y.; Wang, C.L.; Yen, Y.H.; Chern, M.K.; Chen, Y.H. A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for β-chitin preparation. Enzym. Microb. Technol. 2006, 39, 724–731. [Google Scholar] [CrossRef]
- Xie, F.; Quan, S.; Liu, D.; Ma, H.; Li, F.; Zhou, F.; Chen, G. Purification and characterization of a novel α-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem. 2014, 49, 47–53. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Abo-Kamer, A.M.; Abd-El-Salam, I.S.; Mostafa, F.A.; Mustafa, A.-E.A.; Al-Madboly, L.A. A promising microbial α-amylase production, and purification from Bacillus cereus and its assessment as antibiofilm agent against Pseudomonas aeruginosa pathogen. Microb. Cell Factories 2023, 22, 141. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Dinh, T.K.L.; Duong, T.H.N.; Phan, T.T.U.; Le, T.T.L.; Tran, T.D.; Hoang, P.H.Q.; Nguyen, A.D.; Wang, S.-L. Optimization production of an endo-β-1,4-xylanase from Streptomyces thermocarboxydus using wheat bran as sole carbon source. Recycling 2024, 9, 50. [Google Scholar] [CrossRef]
- Iram, N.; Shakir, H.A.; Irfan, M.; Khan, M.; Ali, S.; Anwer, A.; Saeed, S.; Qazi, J.I. Statistical optimization of amylase production using grape fruit peels in submerged fermentation. Acta Sci. Technol. 2020, 43, e50538. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Krishnan, C. α-Amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour. Technol. 2008, 99, 3044–3050. [Google Scholar] [CrossRef]
- Leloup, L.; Haddaoui, E.A.; Chambert, R.; Petit-Glatron, M.F. Characterization of the rate-limiting step of the secretion of Bacillus subtilis alpha-amylase overproduced during the exponential phase of growth. Microbiology 1997, 143, 3295–3303. [Google Scholar] [CrossRef][Green Version]
- Özdemir, S.; Matpan, F.; Güven, K.; Baysal, Z. Production and characterization of partially purified extracellular thermostable α-amylase by Bacillus subtilis in submerged fermentation (SmF). Prep. Biochem. Biotechnol. 2011, 41, 365–381. [Google Scholar] [CrossRef]
- Singh, R.; Langyan, S.; Sangwan, S.; Gaur, P.; Khan, F.N.; Yadava, P.; Rohatgi, B.; Shrivastava, M.; Khandelwal, A.; Darjee, S.; et al. Optimization and production of alpha-amylase using Bacillus subtilis from apple peel: Comparison with alternate feedstock. Food Biosci. 2022, 49, 101978. [Google Scholar] [CrossRef]
- Mabrouk, S.B.; Hmida, B.B.H.; Sebii, H.; Fendri, A.; Sayari, A. Production of an amylase from newly Bacillus strain: Optimization by response-surface methodology, characterization and application with a fungal lipase in bread making. Int. J. Biol. Macromol. 2025, 285, 138147. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bai, H.; Wang, Z.; Peng, L.; Li, L.; Yan, H.; Zhu, L.; Wang, Y.; Shao, J.; Liu, J. Characterization of newly isolated Bacillus cereus D3 co-producing α-amylase and protease and its application in food raw materials. Food Biosci. 2024, 62, 105255. [Google Scholar] [CrossRef]
- Saad, W.F.; Othman, A.M.; Abdel-Fattah, M.; Ahmad, M.S. Response surface methodology as an approach for optimization of α-amylase production by the newly isolated thermotolerant Bacillus licheniformis WF67 strain in submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 32, 101944. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Abdella, M.A.; El-Sherbiny, G.M.; Ibrahim, A.M.; El-Shamy, A.R.; Atalla, S.M. Application of one-factor-at-a-time and statistical designs to enhance α-amylase production by a newly isolate Bacillus subtilis strain-MK1. Biocatal. Agric. Biotechnol. 2019, 22, 101397. [Google Scholar] [CrossRef]
- Silva-Salinas, A.; Rodríguez-Delgado, M.; Gómez-Treviño, J.; López-Chuken, U.; Olvera-Carranza, C.; Blanco-Gámez, E.A. Novel thermotolerant amylase from Bacillus licheniformis strain LB04: Purification, characterization and agar-Agarose. Microorganisms 2021, 9, 1857. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Tong, B.; Chen, X.; Chen, J. Purification and biochemical characterization of a thermostable and acid-stable alpha-amylase from Bacillus licheniformis B4-423. Int. J. Biol. Macromol. 2018, 109, 329–337. [Google Scholar] [CrossRef]
- Fincan, S.A.; Özdemir, S.; Karakaya, A.; Enez, B.; Mustafov, S.D.; Ulutaş, M.S.; Şen, F. Purification and characterization of thermostable α-amylase produced from Bacillus licheniformis So-B3 and its potential in hydrolyzing raw starch. Life Sci. 2021, 264, 118639. [Google Scholar] [CrossRef]
- Božić, N.; Ruiz, J.; López-Santín, J.; Vujčić, Z. Production and properties of the highly efficient raw starch digesting α-amylase from a Bacillus licheniformis ATCC 9945a. Biochem. Eng. J. 2011, 53, 203–209. [Google Scholar] [CrossRef]
- Sumrin, A.; Ahmad, W.; Ijaz, B.; Sarwar, M.T.; Gull, S.; Kausar, H.; Shahid, I.; Jahan, S.; Asad, S.; Hussain, M. Purification and medium optimization of α-amylase from Bacillus subtilis 168. Afr. J. Biotechnol. 2011, 10, 2119–2129. [Google Scholar]
- Rakaz, M.A.; Hussien, M.O.; Ibrahim, H.M. Isolation, extraction, purification, and molecular characterization for thermostable α-Amylase from locally isolated Bacillus Species in Sudan. Biochem. Res. Int. 2021, 2021, 6670380. [Google Scholar] [CrossRef]
- Mahfudz, M.K.; Jaikhan, S.; Phirom-on, K.; Apiraksakorn, J. Cost-effective strategy and feasibility for amylase production from okara by Bacillus subtilis J12. Fermentation 2024, 10, 561. [Google Scholar] [CrossRef]
- Bandal, J.N.; Tile, V.A.; Sayyed, R.Z.; Jadhav, H.P.; Azelee, N.I.W.; Danish, S.; Datta, R. statistical based bioprocess design for improved production of amylase from halophilic Bacillus sp. H7 isolated from marine water. Molecules 2021, 26, 2833. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Song, Q.; Zhang, Q.; Zhao, F.; Kim, R.C.; Zhou, Z.; Han, Y. Purification and characterization of novel thermostable and Ca-independent α-amylase produced by Bacillus amyloliquefaciens BH072. Int. J. Biol. Macromol. 2018, 115, 1151–1156. [Google Scholar] [CrossRef]
- Kikani, B.; Singh, S. Single step purification and characterization of a thermostable and calcium independent α-amylase from Bacillus amyloliquifaciens TSWK1-1 isolated from Tulsi Shyam hot spring reservoir, Gujarat (India). Int. J. Biol. Macromol. 2011, 48, 676–681. [Google Scholar] [CrossRef]
- Abd-Elaziz, A.M.; Karam, E.A.; Ghanem, M.M.; Moharam, M.E.; Kansoh, A.L. Production of a novel α-Amylase by Bacillus atrophaeus NRC1 isolated from honey: Purification and characterization. Int. J. Biol. Macromol. 2020, 148, 292–301. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial alpha-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Hagihara, H.; Igarashi, K.; Hayashi, Y.; Endo, K.; Ikawa-Kitayama, K.; Ozaki, K.; Kawai, S.; Ito, S. Novel α-amylase that is highly resistant to chelating reagents and chemical oxidants from the Alkaliphilic Bacillus isolate KSM-K38. Appl. Environ. Microbiol. 2001, 67, 1744–1750. [Google Scholar] [CrossRef]
- Kumar, N.M.; Karthikeyan, S.; Jayaraman, G. Thermostable alpha-amylase enzyme production from Bacillus laterosporus: Statistical optimization, purification, and characterization. Biocatal. Agric. Biotechnol. 2013, 2, 38–44. [Google Scholar] [CrossRef]
- Sharma, A.; Satyanarayana, T. Cloning and expression of acid-stable, high maltose-forming, Ca2+-independent α-amylase from an acidophile Bacillus acidicola and its applicability in starch hydrolysis. Extremophiles 2012, 16, 515–522. [Google Scholar] [CrossRef]
- Sharma, A.; Satyanarayana, T. Microbial acid-stable α-amylases: Characteristics, genetic engineering and applications. Process Biochem. 2013, 48, 201–211. [Google Scholar] [CrossRef]
- Ben Hadj Hmida, B.; Ben Mabrouk, S.; Fendri, A.; Hmida-Sayari, A.; Sayari, A. Optimization of newly isolated Bacillus cereus α-amylase production using orange peels and crab shells and application in wastewater treatment. 3 Biotech 2024, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Nagasaki, K.; Nishimoto, H.; Shigematu, R.; Umesaki, J.; Takenaka, S.; Kaulpiboon, J.; Prousoontorn, M.; Limpaseni, T.; Pongsawasdi, P.; et al. Purification and characterization of five alkaline, thermotolerant, and maltotetraose-producing α-amylases from Bacillus halodurans MS-2-5, and production of recombinant enzymes in Escherichia coli. Enzym. Microb. Technol. 2008, 43, 321–328. [Google Scholar] [CrossRef]
- Alonazi, M.; Karray, A.; Badjah-Hadj-Ahmed, A.Y.; Ben Bacha, A. Alpha amylase from Bacillus pacificus associated with brown algae Turbinaria ornata: Cultural conditions, purification, and biochemical characterization. Processes 2021, 9, 16. [Google Scholar] [CrossRef]
- Morgan, F.J.; Priest, F.G. Characterization of a thermostable α-amylase from Bacillus licheniformis NCIB 6346. J. Appl. Bacteriol. 1981, 50, 107–114. [Google Scholar] [CrossRef]

| Purification Step | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Purification Fold | Recovery (%) |
|---|---|---|---|---|---|
| Liquid supernatant | 11361.584 | 20558.271 | 1.809 | 1 | 100 |
| (NH4)2SO4 precipitation | 866.582 | 16552.180 | 19.101 | 10.556 | 80.513 |
| High Q column | 5.528 | 3441.206 | 622.547 | 344.052 | 16.739 |
| Peptide Sequence | Identified Protein and Coverage Rate | Strain |
|---|---|---|
| 69DIHDAGYTAIQTSPINQVK87 114YLGTEQEFKEMCAAAEEYGIK134 157SIPNWTHGNTQIK169 206ALNDGADGFR215 284NLGVSNISHYASDVSADK301 329LGWAVIASRSGSTPLFFSRPEGGGNGVR356 367GSALFEDQAITAVNRFHNVMAGQPEELSNPNGNNQIFMNQR407 439AGAGSFQVNDGKLTGTINARSVAVLYPDDIAKAPHVFLENYKTGVTHSFNDQLTITLR496 504AVYQINNGPETAFKDGDQFTIGK526 533TYTIMLK539 603NADGIYTLTLPADTDTTNAK622 | α-amylase 39% | B. subtilis strain 168 |
| Relative Activity (%) (Mean ± SD) | |
|---|---|
| Control | 100.000 ± 3.016 |
| KCl | 99.465 ± 3.000 |
| FeCl2 | 97.600 ± 6.350 |
| CaCl2 | 79.158 ± 0.400 |
| BaCl2 | 80.263 ± 0.900 |
| MgCl2 | 78.260 ± 1.150 |
| CuCl2 | 8.634 ± 0.950 |
| NaCl | 91.176 ± 3.000 |
| ZnCl2 | 2.141± 0.350 |
| SDS | 68.313 ± 2.650 |
| Tween 20 | 76.533 ± 0.900 |
| Tween 40 | 40.684 ± 3.650 |
| Triton X-100 | 44.414 ± 3.850 |
| EDTA | 123.986 ± 0.850 |
| PMSF | 97.048 ± 5.150 |
| β-mercaptoethanol | 96.495 ± 3.350 |
| Relative Activity (%) (Mean ± SD) | |
|---|---|
| Starch powder | 3.266 ± 1.755 |
| Gelatinized starch | 100.000 ± 1.194 |
| CMC | N.A. |
| Pectin | 16.439 ± 1.849 |
| Gum arabic | 22.061 ± 2.285 |
| Dextran | 4.120 ± 1.587 |
| Glycogen | 79.055 ± 3.270 |
| β-1,3-glucan | 14.675 ± 1.972 |
| Stachyose | N.A. |
| Raffinose | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Chen, S.-C.; Doan, C.T.; Wang, S.-L. Unlocking the Potential of Pomelo Albedo: A Novel Substrate for Alpha-Amylase Production Using Bacillus licheniformis. Fermentation 2025, 11, 336. https://doi.org/10.3390/fermentation11060336
Tran TN, Chen S-C, Doan CT, Wang S-L. Unlocking the Potential of Pomelo Albedo: A Novel Substrate for Alpha-Amylase Production Using Bacillus licheniformis. Fermentation. 2025; 11(6):336. https://doi.org/10.3390/fermentation11060336
Chicago/Turabian StyleTran, Thi Ngoc, Si-Chun Chen, Chien Thang Doan, and San-Lang Wang. 2025. "Unlocking the Potential of Pomelo Albedo: A Novel Substrate for Alpha-Amylase Production Using Bacillus licheniformis" Fermentation 11, no. 6: 336. https://doi.org/10.3390/fermentation11060336
APA StyleTran, T. N., Chen, S.-C., Doan, C. T., & Wang, S.-L. (2025). Unlocking the Potential of Pomelo Albedo: A Novel Substrate for Alpha-Amylase Production Using Bacillus licheniformis. Fermentation, 11(6), 336. https://doi.org/10.3390/fermentation11060336










