Development of a Functional Yogurt Containing Probiotics and Phenolic Compounds of Coffee Encapsulated in Alginate Beads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Extraction of Phenolic Compounds
2.3. Encapsulation of Bioactive Compounds
2.4. Determination of the Viability of Lactiplantibacillus fabifermentans BAL-27 ITTG
2.5. Gastrointestinal Simulation
2.6. Quantification of Phenolic Compounds in the Beads
2.7. Preparation of Yogurt
2.7.1. Viability of Lactiplantibacillus fabifermentans BAL-27 ITTG in Yogurt
2.7.2. Physicochemical Analysis of Yogurt
Syneresis
pH
Titratable Acidity
Sensory Analysis
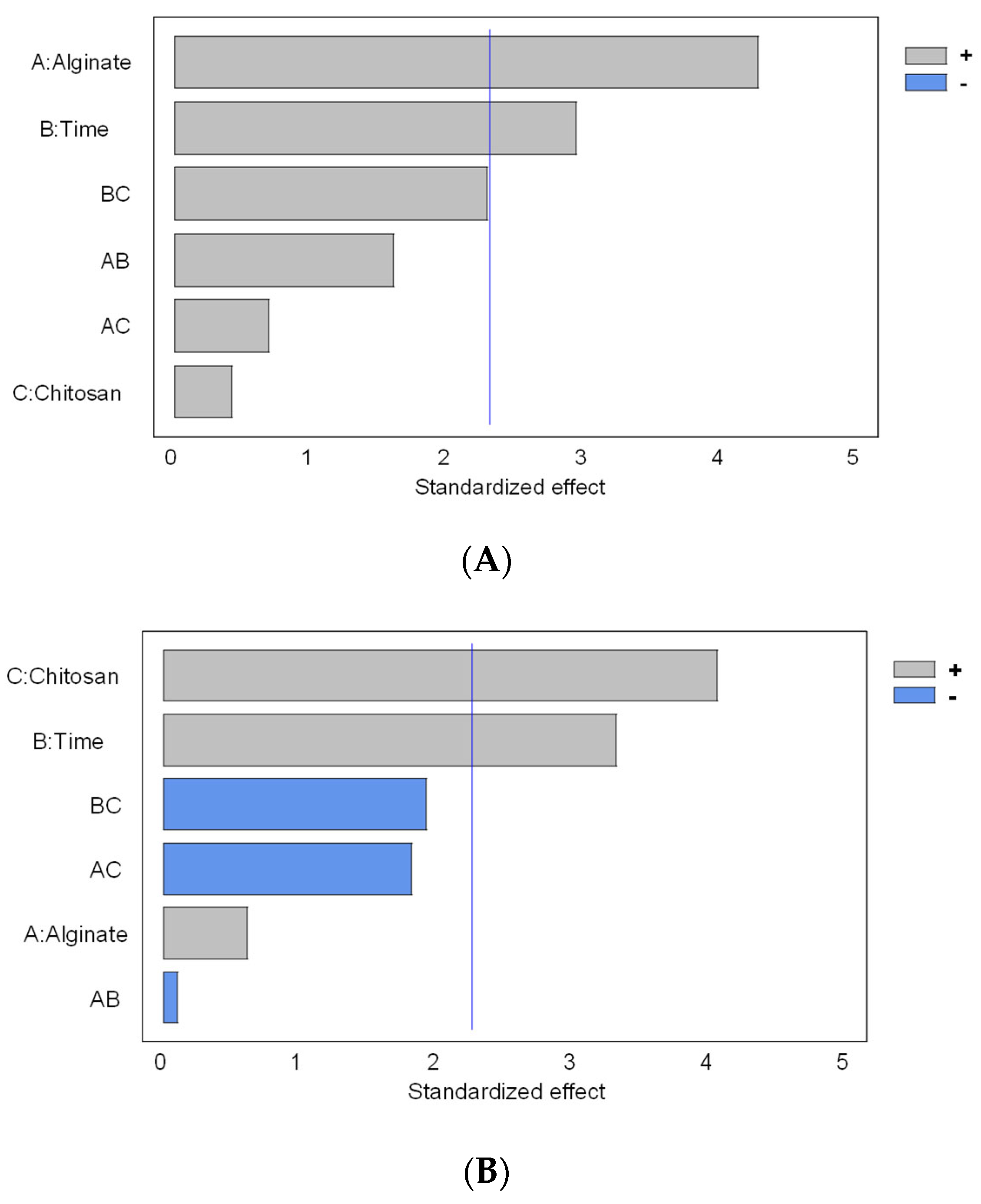
2.8. Experimental Design and Statistical Analysis
3. Results
3.1. Encapsulation of Lactiplantibacillus fabifermentans BAL-27 ITTG and Phenolic Compounds
3.1.1. Viability of Encapsulated Lactiplantibacillus fabifermentans BAL-27 ITTG
3.1.2. Encapsulated Phenolic Compounds
3.1.3. Viability of Lactiplantibacillus fabifermentans BAL-27 ITTG During Gastrointestinal Simulation
3.2. Evaluation of the Quality of Alginate Beads During Yogurt Storage
3.2.1. Viability of Lactiplantibacillus fabifermentans BAL-27 ITTG
3.2.2. Phenolic Compounds in Beads During Storage
3.2.3. Viability of Lactiplantibacillus fabifermentans BAL-27 ITTG from Alginate Beads During Gastrointestinal Simulation after Four Weeks of Yogurt Storage
3.3. Effects of the Addition of Lactiplantibacillus fabifermentans BAL-27 ITTG and Encapsulated Phenolic Compounds on the Physicochemical and Sensory Properties of Yogurt
3.3.1. Determination of the Physicochemical and Microbiological Properties of Yogurt
Yogurt Syneresis
Titratable pH and Acidity
Sensory Analysis of Yogurt
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EE | Encapsulation Efficiency |
| GAE | Gallic Acid Equivalents |
| MEE | Microbial Encapsulation Efficiency |
| PCEE | Phenolic Compounds Encapsulation Efficiency |
| RGS | Resistance of Gastrointestinal Simulation |
References
- Santeramo, F.G.; Carlucci, D.; De Devitiis, B.; Seccia, A.; Stasi, A.; Viscecchia, R.; Nardone, G. Emerging trends in European food, diets and food industry. Food Res. Int. 2018, 104, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Abud-Archila, M.; Mendoza, C. Jícama mínimamente procesada fortificada con probióticos y compuestos fenólicos de café verde microencapsulados. Biotecnia 2024, 26, e2350. [Google Scholar] [CrossRef]
- Rahmani, F.; Gandomi, H.; Noori, N.; Faraki, A.; Farzaneh, M. Microbial, physiochemical and functional properties of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium bifidum enriched by green tea aqueous extract. Food Sci. Nutr. 2021, 9, 5536–5545. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Favaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]
- De Prisco, A.; Van, H.J.; Flogiano, V.; Mauriello, G. Microencapsulated starter culture during yogurt manufacturing, effect on technological features. Food Bioprocess Technol. 2017, 10, 1767–1777. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D. Development of antioxidant rich fruit supplemented probiotic yogurts using free and microencapsulated Lactobacillus rhamnosus culture. J. Food Sci. Technol. 2016, 53, 667–675. [Google Scholar] [CrossRef]
- Ramírez-Pérez, J.I.; Álvarez-Gutiérrez, P.E.; Luján-Hidalgo, M.C.; Ovando-Chacón, S.L.; Soria-Guerra, R.E.; Ruiz-Cabrera, M.A.; Grajales-Lagunes, A.; Abud-Archila, M. Effect of linear and branched fructans on growth and probiotic characteristics of seven Lactobacillus spp. isolated from an autochthonous beverage from Chiapas, Mexico. Arch. Microbiol. 2022, 204, 364. [Google Scholar] [CrossRef]
- Bartoszek, M.; Polak, J. An electron paramagnetic resonance study of antioxidant properties of alcoholic beverages. Food Chem. 2012, 132, 2089–2093. [Google Scholar] [CrossRef]
- Belščak, A.; Komez, D.; Karlović, S.; Djaković, S.; Špolijarć, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulation of caffeine in alginate in alginate hidrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef]
- Li, Q.; Duan, M.; Hou, D.; Chen, X.; Shi, J.; Zhou, W. Fabrication and characterization of Ca (II)-alginate-based beads combined with different polysaccharides as vehicles for delivery, release and storage of tea polyphenols. Food Hydrocoll. 2021, 112, 106274. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food Bioprocess Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef]
- Heeger, A.; Konsińka, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee Cherry pulp and its utilisation for production of cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.F.; Luzia, D.M.M.; Jorge, N. Antioxidant compounds extraction from coffe husk: The influence of solvent type and ultrasound exposure time. Acta Sci. Technol. 2019, 41, 36451. [Google Scholar] [CrossRef]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract. Food Chem. 2021, 328, 129094. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Morales-Ruiz, M.C. Fortificación de Yogurt con Fitonanopartículas de ZnO y Lactiplantibacillus fabifermentans BAL-27-ITTG. Master’s Thesis, BAL-27-ITTG. Instituto Tecnológico Nacional de Tuxtla Gutiérrez, Tuxtla Gutiérrez, Mexico, 2022. [Google Scholar]
- Zainoldin, K.; Baba, A.H. The Effect of Hylocereus polyrhizus and Hylocereus undatus on physicochemical, proteolysis, and antioxidant activity in yogurt. World Acad. Sci. Eng. Technol. 2009, 60, 361–366. [Google Scholar]
- NOM-243-SSA1-2010; Productos y Servicios. Leche, Fórmula Láctea, Producto Lácteo Combinado y Derivados Lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba. Available online: https://dof.gob.mx/normasOficiales/4156/salud2a/salud2a.htm (accessed on 10 July 2023).
- Das, A.B.; Goud, V.V.; Das, C. Phenolic compounds as functional ingredients in beverages. In Value-Added Ingredients and Enrichments of Beverages; Academic Press: New York, USA, 2019. [Google Scholar]
- FAO. OMS Organización de las Naciones Unidad para la Alimentación y la Agricultura y Organización Mundial de la Salud. 2001. Available online: https://www.fao.org/3/a0512s/a0512s.pdf (accessed on 15 February 2025).
- Izadi, Z.; Nasirpour, A.; Garoosi, G.A.; Tamjidi, F. Rheological and physical properties of yogurt enriched with phytosterol during storage. J. Food Sci. Technol. 2015, 52, 5341–5346. [Google Scholar] [CrossRef]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Burton, E.; Arief, I.I.; Taufik, E. Formulasi yogurt probiotik karbonasi dan potensi sifat fungsionalnya. J. Ilmu Produksi Dan Teknol. Has. Peternak. 2014, 2, 213–218. [Google Scholar]
- Abbaszadeh, S.; Gandomi, H.; Misaghi, A.; Bokaei, S.; Noori, N. The effect of alginate and chitosan concentrations on some properties of chitosan-coated alginate beads and survivability of encapsulated Lactobacillus rhamnosus in simulated gastrointestinal conditions and during heat processing. J. Sci. Food Agric. 2014, 94, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and characteristics of alginate microparticles for food, pharmaceutical and cosmetic applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef] [PubMed]
- Gedam, S.; Jadhav, P.; Talele, S.; Jadhav, A. Effect of crosslinking agent on development of gastroretentive mucoadhesive microspheres of risedronate sodium. Int. J. Appl. Pharm. 2018, 10, 133–140. [Google Scholar] [CrossRef]
- Phùng, T.; Dinh, H.; Ureña, M.; Oliete, B.; Denimal, E.; DuPont, S.; Beney, L.; Karbowiak, T. Sodium Alginate as a promising encapsulating material for extremely oxygen sensitive probiotics. Food Hydrocoll. 2025, 160, 110857. [Google Scholar] [CrossRef]
- Machado, A.R.; Silva, P.M.P.; Vicente, A.A.; Souza-Soares, L.A.; Pinheiro, A.C.; Cerqueira, M.A. Alginate particles for encapsulation of phenolic extract from Spirulina sp. LEB-18: Physicochemical characterization and assessment of in vitro gastrointestinal behavior. Polymers 2022, 14, 4759. [Google Scholar] [CrossRef]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Lucini, L.; Rocchetti, G.; Dalmina, M.; Pearson, J.P.; Amboni, R.D.D.M.C. Encapsulation of Stevia rebaudiana Bertoni aqueous crude extracts by ionic gelation–Effects of alginate blends and gelling solutions on the polyphenolic profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef]
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring alginate beads with different biopolymers for the development of functional ingredients loaded with olive leaves phenolic extract. Food Hydrocoll. 2020, 108, 105849. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucic-Kojic, A. Physicochemical characterization and evaluation of gastrointestinal in vitro behavior of alginate-based microbeads with encapsulated Grape pomace extracts. Fharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Gonçalves, I.; Moreno, P.; Gonçalves, R.; Santos, S.; Pêgo, A.; Amaral, I. Chitosan. Comprehensive Biomaterials II, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Azam, M.; Saeed, M.; Pasha, I.; Shahid, M. A prebiotic-based biopolymeric encapsulation system for improved survival of Lactobacillus rhamnosus. Food Biosci. 2020, 37, 100679. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry byproducts and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Machado, M.; Galrinho, M.; Passos, C.; Espírito, L.; Simona, M.; Ranga, F.; Puga, H.; Palmeira, J.; Coimbra, M.; Oliveira, M.; et al. Prebiotic potential of a coffee silverskin extract obtained by ultrasound-assisted extraction on Lacticaseibacillus paracasei subsp. paracasei. J. Funct. Foods 2024, 120, 106378. [Google Scholar] [CrossRef]
- Chaikham, P. Stability of probiotics encapsulated with Thai herbal extracts in fruit juices and yogurt during refrigerated storage. Food Biosci. 2015, 12, 61–66. [Google Scholar] [CrossRef]
- Ahmed, H.; Rahaman, A.; Uddin, E.; Rafid, M.; Hosen, S.; Layek, K. Development and characterization of chitosan-based antimicrobial films: A sustainable alternative to plastic packaging. Cleaner Chem. Eng. 2025, 11, 100157. [Google Scholar] [CrossRef]
- Chan, E.S.; Lee, B.B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J. Colloid. Interface Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 2012, 64, 194–205. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Đorđević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarsky, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef]
- del Castillo, S.R.; Mestres Lagarriga, J. Productos Lácteos: Tecnología; Edicions UPC: Mexico City, Mexico, 2004; 159p. [Google Scholar]
- Feng, Y.; Niu, L.; Li, D.; Zeng, Z.; Sun, C.; Xiao, J. Effect of calcium alginate/collagen hydrolysates beads encapsulating high-content tea polyphenols on quality characteristics of set yogurt during cold storage. LWT-Food Sci. Technol. 2024, 191, 115608. [Google Scholar] [CrossRef]
- Pourjafar, H.; Noori, N.; Gandomi, H.; Basti, A.A.; Ansari, F. Viability of microencapsulated and nonmicroencapsulated Lactobacilli in a commercial beverage. Biotechnol. Rep. 2020, 25, e00432. [Google Scholar] [CrossRef]
- Budianto, E.; Saepudin, E.; Nasir, M. The encapsulation of Lactobacillus casei probiotic bacteria based on sodium alginate and chitosan. IOP Conf. Ser. Earth Environ. Sci. 2020, 483, 012043. [Google Scholar]
- Kailasapathy, K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yogurt. LWT-Food Sci. Technol. 2006, 39, 1221–1227. [Google Scholar] [CrossRef]
- Sert, D.; Mercan, E.; Dertli, E. Characterization of lactic acid bacteria from yogurtlike product fermented with pine cone and determination of their role on physicochemical, textural and microbiological properties of product. LWT Food Sci. Technol. 2017, 78, 70–76. [Google Scholar] [CrossRef]


| Treatment | Viability (Log10 CFU/g Bead) | MEE (%) | PC (mg GAE/g Bead) | PCEE (%) | RGS (%) |
|---|---|---|---|---|---|
| T1 | 8.83 c | 82.97 b | 0.48 a | 14.22 c | 84.38 ab |
| T2 | 9.09 abc | 82.86 b | 0.49 a | 17.02 bc | 83.82 b |
| T3 | 9.45 a | 84.70 a | 0.47 a | 19.75 ab | 85.34 a |
| T4 | 9.47 a | 84.84 a | 0.51 a | 20.99 a | 73.65 e |
| T5 | 8.91 bc | 81.00 c | 0.48 a | 20.86 a | 75.51 d |
| T6 | 9.00 abc | 81.79 c | 0.52 a | 19.12 ab | 84.08 ab |
| T7 | 9.29 abc | 83.47 b | 0.52 a | 19.06 ab | 77.94 c |
| T8 | 9.38 ab | 83.31 b | 0.52 a | 20.89 a | 76.11 d |
| Tukey | 0.532 | 1.01 | 0.07 | 3.03 | 1.50 |
| Treatment | Storage Time (Days) | Tukey | |
|---|---|---|---|
| 0 | 28 | ||
| T1 | 0.48 Aa | 0.43 Bb | 0.04 |
| T2 | 0.49 Aa | 0.45 Bb | 0.04 |
| T3 | 0.47 Aa | 0.41 Babc | 0.08 |
| T4 | 0.51 Aa | 0.52 Aa | 0.07 |
| T5 | 0.48 Aa | 0.47 Aabc | 0.13 |
| T6 | 0.52 Aa | 0.48 Babc | 0.02 |
| T7 | 0.52 Aa | 0.52 Aa | 0.04 |
| T8 | 0.52 Aa | 0.50 Aab | 0.02 |
| Tukey | 0.07 | 0.04 | |
| Treatment | RGS (%) |
|---|---|
| T1 | 89.82 a |
| T2 | 83.82 c |
| T3 | 84.22 c |
| T4 | 79.86 d |
| T5 | 73.25 f |
| T6 | 88.09 b |
| T7 | 77.23 e |
| T8 | 71.88 g |
| Tukey | 2.8104 |
| Time (Days) | ||||||
|---|---|---|---|---|---|---|
| Treatment | 0 | 7 | 14 | 21 | 28 | Tukey |
| T1 | 20.65 Ac | 23.40 Bc | 25.52 Cc | 25.29 Cc | 28.48 Dc | 1.42 |
| T2 | 21.03 Acd | 24.74 Bc | 24.74 Bc | 24.91 Bc | 25.09 Bb | 0.97 |
| T3 | 21.84 Ade | 24.1 Bc | 24.21 Bc | 25.41 Cc | 25.91 Cb | 1.01 |
| T4 | 29.70 Ag | 31.37 Ad | 34.57 Bf | 39.26 Cf | 39.93 Cf | 1.88 |
| T5 | 22.42 Ae | 24.45 Bc | 27.37 Cd | 29.91 Dd | 35.45 Ed | 1.50 |
| T6 | 17.60 Ab | 18.84 Ab | 21.33 Bb | 23.36 Cb | 25.56 Db | 1.88 |
| T7 | 23.73 Af | 27.69 Bd | 29.65 Ce | 30.46 Cd | 38.33 De | 1.90 |
| T8 | 23.06 Af | 25.65 Bcd | 28.52 Cde | 32.86 De | 37.54 Ee | 0.86 |
| Control | 13.63 Aa | 15.29 Ba | 17.71 Ca | 20.14 Da | 20.58 Da | 1.33 |
| Tukey | 1.00 | 2.77 | 1.95 | 1.04 | 1.02 | |
| Time (Days) | ||||||
|---|---|---|---|---|---|---|
| Treatment | 0 | 7 | 14 | 21 | 28 | Tukey |
| T1 | 4.34 Aa | 4.09 Bd | 4.00 BCc | 3.97 Cb | 3.97 Ccd | 0.08 |
| T2 | 4.32 Aab | 4.09 Bd | 4.03 Bc | 3.96 Cb | 3.93 Cd | 0.06 |
| T3 | 4.26 Aabc | 4.13 Bcd | 4.02 Cc | 3.97 CDb | 3.96 Dcd | 0.05 |
| T4 | 4.29 Aabc | 4.15 Bbc | 4.09 Bb | 4.00 Cab | 4.00 Cabc | 0.08 |
| T5 | 4.26 Aabc | 4.17 Bbc | 4.09 Cb | 4.01 Dab | 4.00 Dabc | 0.04 |
| T6 | 4.26 Aabc | 4.18 ABb | 4.09 BCb | 4.03 Cab | 3.98 Cbcd | 0.13 |
| T7 | 4.27 Aabc | 4.17 Bbc | 4.10 Cb | 4.04 Dab | 4.04 Dab | 0.05 |
| T8 | 4.27 Aabc | 4.15 Bbc | 4.10 Cb | 4.04 Dab | 4.00 Eabc | 0.03 |
| Control | 4.34 Aa | 4.27 Ba | 4.20 Ca | 4.11 Da | 4.05 Ea | 0.03 |
| Tukey | 0.08 | 0.04 | 0.05 | 0.12 | 0.06 | |
| Treatment | Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | Tukey | |
| 1 | 0.96 Abc | 0.98 Aab | 0.99 Aab | 1.04 Bab | 1.11 Cabc | 0.03 |
| 2 | 0.94 Aab | 0.96 Aa | 1.04 Bab | 1.09 BCab | 1.12 Cbc | 0.06 |
| 3 | 0.94 Aab | 1.06 Bc | 1.06 Bb | 1.14 Cb | 1.14 Cc | 0.02 |
| 4 | 0.94 Aab | 0.99 ABab | 1.01 ABab | 1.03 ABa | 1.08 Ba | 0.10 |
| 5 | 0.92 Aa | 0.95 Ba | 0.98 Ca | 1.05 Dab | 1.09 Eab | 0.01 |
| 6 | 0.96 Abc | 1.03 ABbc | 1.05 BCb | 1.12 Cab | 1.14 Cc | 0.08 |
| 7 | 0.95 Abc | 1.02 Bbc | 1.06 Cb | 1.10 Dab | 1.12 Eab | 0.02 |
| 8 | 0.98 Ac | 1.03 ABbc | 1.06 BCb | 1.11 Cab | 1.09 BCab | 0.07 |
| Control | 0.92 Aa | 0.96 ABa | 1.00 Bab | 1.05 Cab | 1.09 Cab | 0.04 |
| Tukey | 0.02 | 0.05 | 0.02 | 0.05 | 0.04 | |
| Treatment | L. fabifermentans (CFU/g Yogurt) | Starter Culture (CFU/g Yogurt) |
|---|---|---|
| T1 | ~104 | ~109 |
| T2 | ~103 | ~109 |
| T3 | ~104 | ~109 |
| T4 | ND | ~109 |
| T5 | ND | ~109 |
| T6 | ~103 | ~109 |
| T7 | ND | ~109 |
| T8 | ND | ~109 |
| Descriptor | Time (Days) | Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | Tukey | ||
| Color | 0 | 6.26 Aa | 5.25 cdA | 5.08 dA | 5.56 bcdA | 6.05 abA | 5.38 cdA | 5.66 bcdA | 6.25 abA | 5.80 abcA | 0.56 |
| 28 | 6.50 Aa | 5.46 cA | 5.57 cA | 5.89 abcA | 6.39 abA | 6.16 abcA | 5.7 bcA | 6.36 abA | 6.40 abA | 0.77 | |
| Odor | 0 | 6.05 aA | 5.36 bA | 5.41 bA | 5.71 abA | 5.48 abA | 5.18 bA | 5.78 abA | 5.80 abA | 5.50 abA | 0.63 |
| 28 | 6.14 Aa | 5.46 aA | 5.67 aA | 5.82 aA | 5.82 aA | 5.56 aA | 5.50 aA | 5.70 aA | 5.86 aA | 0.90 | |
| Flavor | 0 | 6.29 Aa | 4.73 dA | 5.21 cdA | 5.3 bcdA | 5.38 bcdA | 5.70 abcA | 5.55 bcA | 5.91 abA | 5.51 bcA | 0.69 |
| 28 | 6.28 aA | 5.35 abA | 5.42 abA | 5.67 abA | 6.0 abA | 5.60 abA | 5.16 abA | 5.66 abA | 6.16 aA | 0.98 | |
| Texture | 0 | 5.85 aA | 4.6 cA | 4.91 bcA | 5.15 abcA | 5.16 abcA | 4.56 cA | 4.98 bcA | 5.43 abA | 5.11 bcA | 0.84 |
| 28 | 6.17 aA | 5.07 bcA | 4.28 cA | 5.10 bcA | 5.75 abcA | 5.33 abA | 5.33 abcA | 5.76 abA | 5.86 abA | 0.95 | |
| Global Appearance | 0 | 6.25 aA | 5.01 bA | 5.2 cA | 5.40 bcA | 5.41 bcA | 5.50 bcA | 5.45 bcA | 5.95 abA | 5.46 bcA | 0.11 |
| 28 | 6.42 aA | 5.42 abcA | 5.17 cdA | 5.5 bcdA | 5.96 abcA | 5.73 abcdA | 5.10 dA | 5.76 abcdA | 6.06 abA | 0.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toalá-Gómez, A.V.; Mendoza-Avendaño, C.; Lujan-Hidalgo, M.C.; Ruiz-Cabrera, M.A.; Grajales-Lagunes, A.; Estudillo-Diaz, E.B.; Canseco, L.M.C.V.; Palacios-Pola, G.; Abud-Archila, M. Development of a Functional Yogurt Containing Probiotics and Phenolic Compounds of Coffee Encapsulated in Alginate Beads. Fermentation 2025, 11, 328. https://doi.org/10.3390/fermentation11060328
Toalá-Gómez AV, Mendoza-Avendaño C, Lujan-Hidalgo MC, Ruiz-Cabrera MA, Grajales-Lagunes A, Estudillo-Diaz EB, Canseco LMCV, Palacios-Pola G, Abud-Archila M. Development of a Functional Yogurt Containing Probiotics and Phenolic Compounds of Coffee Encapsulated in Alginate Beads. Fermentation. 2025; 11(6):328. https://doi.org/10.3390/fermentation11060328
Chicago/Turabian StyleToalá-Gómez, Aurora Viridiana, Claudia Mendoza-Avendaño, Maria Celina Lujan-Hidalgo, Miguel Angel Ruiz-Cabrera, Alicia Grajales-Lagunes, Enna Berenice Estudillo-Diaz, Lucia Maria Cristina Ventura Canseco, Gabriela Palacios-Pola, and Miguel Abud-Archila. 2025. "Development of a Functional Yogurt Containing Probiotics and Phenolic Compounds of Coffee Encapsulated in Alginate Beads" Fermentation 11, no. 6: 328. https://doi.org/10.3390/fermentation11060328
APA StyleToalá-Gómez, A. V., Mendoza-Avendaño, C., Lujan-Hidalgo, M. C., Ruiz-Cabrera, M. A., Grajales-Lagunes, A., Estudillo-Diaz, E. B., Canseco, L. M. C. V., Palacios-Pola, G., & Abud-Archila, M. (2025). Development of a Functional Yogurt Containing Probiotics and Phenolic Compounds of Coffee Encapsulated in Alginate Beads. Fermentation, 11(6), 328. https://doi.org/10.3390/fermentation11060328







