Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods
Abstract
1. Introduction
1.1. Importance of Traditional Fermented Foods
1.2. Challenges in Traditional Fermentation (Microbial Inconsistency, Variable Quality)
1.3. Need for Smart Technologies in Fermentation
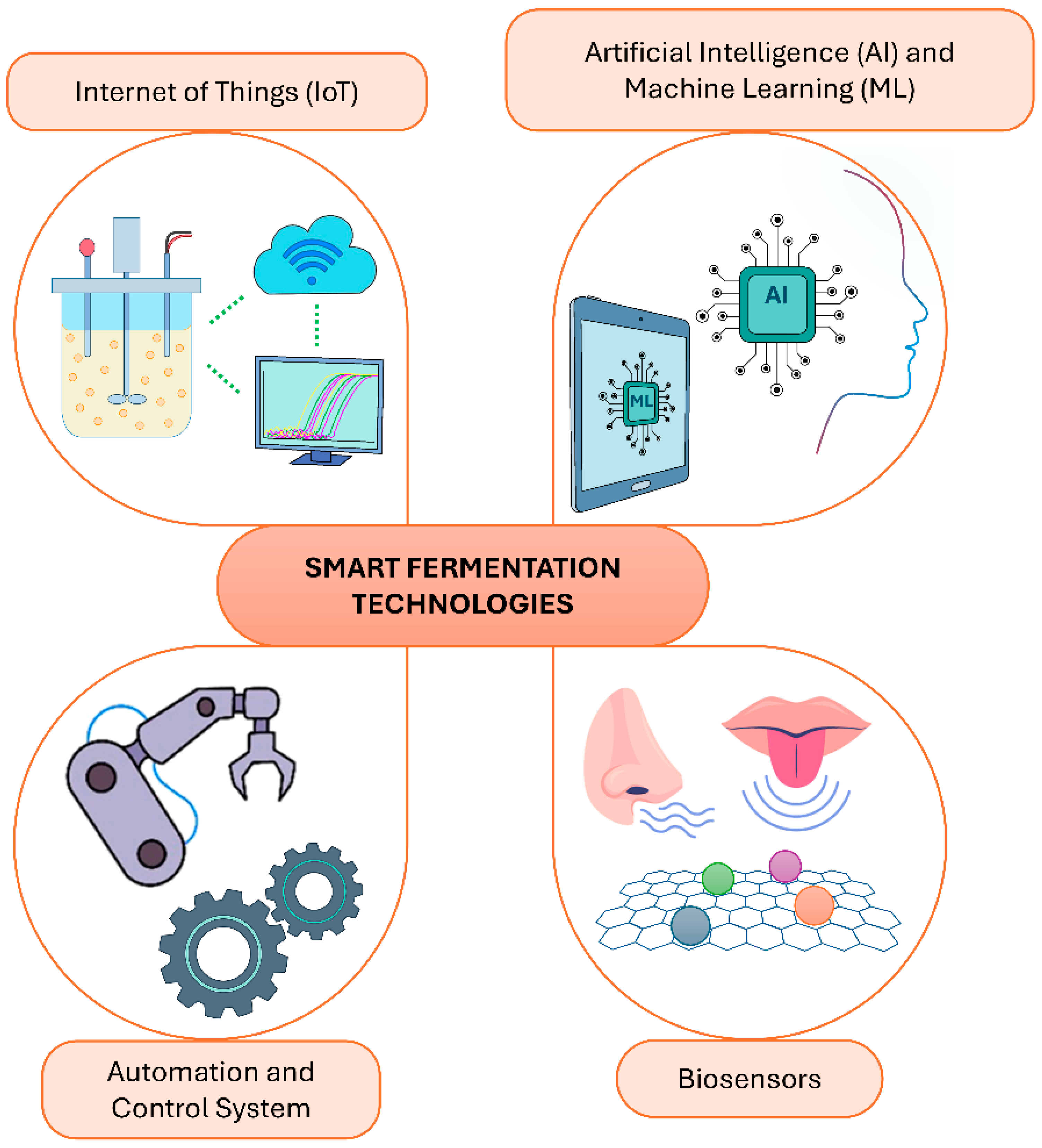
2. Overview of Smart Fermentation Technologies
2.1. Definition and Scope of Smart Technologies
2.2. Key Technologies in Smart Fermentation
2.2.1. Internet of Things (IoT): Real-Time Sensors, Cloud-Based Data
2.2.2. Biosensors and Electronic Noses/Tongues
2.2.3. Artificial Intelligence and Machine Learning
2.2.4. Automation and Control Systems (Proportional-Integral-Derivative (PID) Control, Neural Networks)
| Technologies | Applications | References |
|---|---|---|
| IoT (real-time sensors and cloud-based data) | Wine fermentation monitoring— Utilized real-time sensors to monitor temperature and sugar concentrations, relaying data to a cloud platform for analysis. This configuration facilitated real-time oversight and regulation, guaranteeing optimal fermentation conditions and product excellence. | [79,80] |
| Tea fermentation detector— An IoT-based methodology using CNNs and majority voting was presented to determine optimal tea fermentation stages. The method used a Raspberry Pi camera to take pictures of fermenting tea and ML algorithms to estimate its fermentation level. | [81,82] | |
| Beer fermentation control— Wireless sensor networks (WSNs) in beer fermentation provide real-time pH, density, and temperature monitoring. | [83,84] | |
| Sourdough fermentation—Gas sensor arrays that analyze exhaust gases and correlate them with pH and TTA levels. | [85] | |
| Biosensors, E-noses, and E-tongues | Detection of adulteration in fermented products— E-tongues to detect adulteration in whey protein powders and olive oil. | [86,87] |
| Monitoring fermentation process— Maintain authenticity, consistency and quality of food. | [29,88] | |
| AI an ML | Brewing industry— Energy consumption prediction and recipe optimization have been primarily handled by artificial neural networks (ANNs) and support vector machines (SVMs). | [89,90] |
| Biofuel production—Artificial neural networks (ANN) were used to forecast yields of bioethanol and biohydrogen to produce the optimum cellulosic biomass fermentation. | [91] | |
| Arabica coffee fermentation— ML improves the sensory attributes of coffee, augmenting the product’s value by creating varied sensory characteristics. | [92] | |
| Automation and control systems | Antibiotic production— Back-propagation neural network (BPNN) with adaptive genetic algorithm (AGA) enhanced nutrient delivery, resulting in elevated cell concentration and improved fermentation efficiency. | [93] |
| Ethanol production— The use of PID and fuzzy logic controllers for improved temperature and pH regulation has led to increased yields and enhanced process stability. | [76,94] | |
| Lactic acid fermentation— PID enhanced the management of process nonlinearities, resulting in greater product consistency. | [95] |
3. Microbial Process Control in Traditional Fermented Foods
3.1. Importance of Microbial Control in Fermentation
3.2. Microbial Fermentation Instability and Its Impact on Product Quality
3.3. Microbial Process Optimization Using Smart Technologies
4. Advances in Real-Time Microbial Monitoring
4.1. Sensor-Based Approaches for In-Process Microbial Detection
4.1.1. Optical Density (OD) Sensors
4.1.2. Inline or At-Line Flow Cytometry (FCM)
4.1.3. Fluorescence Sensors
4.1.4. Raman and Infrared (IR) Spectroscopy
4.1.5. Infrared Spectroscopy (NIR and FTIR)
4.1.6. Electrochemical Biosensors
4.1.7. E-Nose and E-Tongue
4.1.8. Microfluidic Impedance Cytometry
4.2. Rapid Molecular and Omics-Driven Monitoring Platforms
4.2.1. qPCR and Isothermal Amplification
4.2.2. CRISPR-Based Diagnostics
4.2.3. Next-Generation Sequencing (NGS) and Omics
4.3. Data Integration, Visualization & Digital Twins
5. Machine Learning in Fermentation
5.1. Principles of Predictive Modeling
5.2. Machine Learning Application in Traditional Fermented Foods
5.2.1. Artificial Neural Networks (ANNs)
5.2.2. Support Vector Machines (SVMs)
5.2.3. Decision Trees (DTs)
5.2.4. Random Forest (RF)
5.2.5. Extreme Gradient Boosting (XGB)
| Algorithm/Model | Traditional Fermented Food | Functionality | Accuracy | References |
|---|---|---|---|---|
| ANNs | Cream cheese | Prediction of cream cheese fermentation pH | R2 > 99% | [191] |
| Cocoa beans | Determination of the fermentation degree of cocoa beans | Misclassification rate = 12.8% | [192] | |
| Beer | Classification of beers into the top, bottom, and spontaneous fermentation | R2 = 97% | [195] | |
| Soy sauce | Classification of soy sauce by fermentation and geographic region | Fermentation (R2 = 100%) Geographic region (R2 = 100%) | [196] | |
| Determination of soy sauce concentration | RMSE = 5.396 | [193] | ||
| Bread | Determination of the electrical properties of bread doughs during fermentation | MSE = 0.007 | [194] | |
| SVMs | Black tea | Prediction of fermentation degree of black tea based on electrical properties | R2 = 77% | [200] |
| Yogurt | Prediction of consumer preferences based on sensory attributes | Preferred (RMSE = 3.56 Disliked (RMSE = 3.30) | [199] | |
| Wine | Early detection of abnormal wine fermentation | R2 = 90% | [198] | |
| DTs | Arabica coffee | Analysis of the relationship between the average concentrations of organic compounds and sensory evaluation scores | R2 = 92% | [92] |
| Cream cheese | Prediction of the ultrafiltration concentration factor to maximize yield | R2 = 91% | [202] | |
| RF | Kimchi | Prediction of titratable acidity (TA) and reducing sugar content (RSC) changes, and total lactic acid bacteria (TLAB) growth | Overall R2 > 90% | [203] |
| Cocoa beans | Determination of the fermentation degree of cocoa beans | Misclassification rate = 9.4% | [192] | |
| XGB | Kimchi | Prediction of titratable acidity (TA) and reducing sugar content (RSC) changes, and total lactic acid bacteria (TLAB) growth | Overall R2 > 90% | [203] |
| Kombucha | Prediction of the yield of SCOBY (Symbiotic consortium of bacteria and yeast) | R2 = 90% | [206] | |
| Mozzarella and cheddar cheese | Prediction of the level of proteolysis (breakdown of protein) | Mozzarella (R2 = 92%) Cheddar (R2 = 97%) | [208] |
6. Adaptive Control Systems in Fermentation Automation
6.1. Concept of Adaptive Control in Fermentation
6.1.1. Gain Scheduling Control (GSC)
6.1.2. Self-Tuning Control (STC)
6.1.3. Model Reference Adaptive Control (MRAC)
6.2. Benefits of Automation in Small-Scale Industries
| Benefit | Description | References |
|---|---|---|
| Consistent Product Quality | Real-time adjustments lead to uniform fermentation outcomes across batches. | [225,231] |
| Improved Food Safety and Traceability | Digital logs from constant 24 h monitoring support hazard tracking and compliance with food safety standards. | [232] |
| Enhanced Scalability | Automation allows growth without compromising quality or requiring proportional labor increases via efficient workflow. | [231] |
| Resource and Energy Efficiency | Reduces waste and optimizes utility use via responsive control of energy-intensive processes such as feature selection and feature extraction. | [232] |
| Reduced Labor Demand | Minimizes need for continuous supervision, ideal for low-labor rural or family-owned operations. | [232] |
| Resilience in Tropical Climates | Adapts to unpredictable environmental conditions to prevent microbial imbalance or spoilage. | [232] |
| Lower Learning Curve | User-friendly interfaces and configurations facilitate new entrepreneurs in managing fermentation as well as gaining useful data in an instant. | [231,232] |
| Competitive Differentiation | Supports branding around technology, quality assurance, and eco-consciousness. | [233] |
6.3. Industry Applications
7. Challenges in Smart Fermentation Adoption
7.1. High Costs and Technological Barriers
7.2. Standardization Issues
7.3. Accessibility for Small-Scale and Artisanal Producers
7.4. Limited Technical Expertise and Workforce Skills
7.5. Cybersecurity and Data Privacy Concerns
7.6. Environmental and Sustainability Considerations
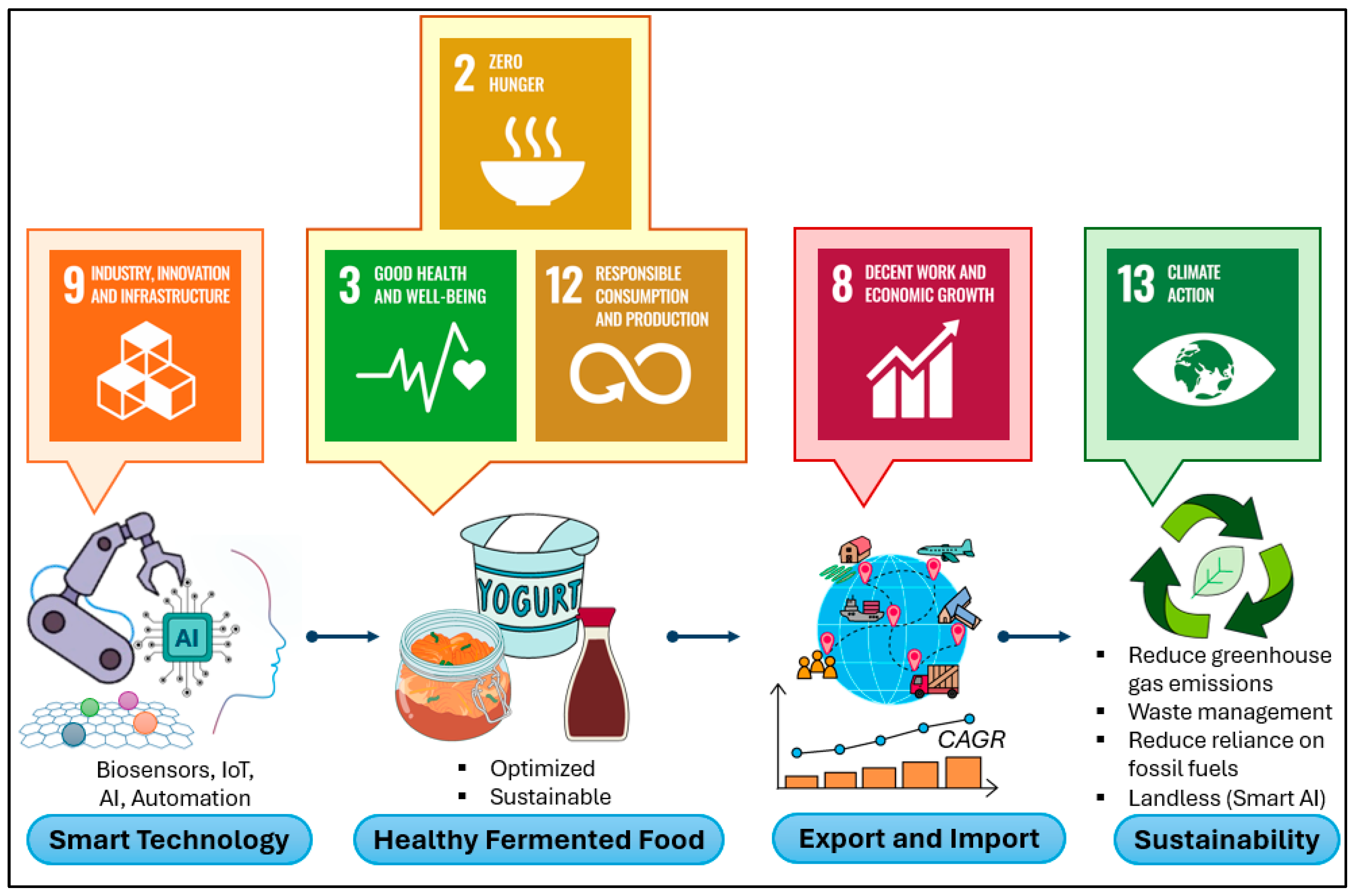
8. Future Directions and Roadmap for Smart Fermentation Technologies
8.1. Bridging the Gap Between Traditional Practices and Industry 4.0
8.2. Potential for Scaling Up and Global Adoption
8.3. Research Priorities and Technological Advancements
8.3.1. Developing Affordable, Durable Biosensors
8.3.2. Advancing AI for Artisanal Contexts
8.3.3. Promoting Open-Source Innovation
8.3.4. Ensuring Environmental Sustainability
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ANN/ANNs | Artificial Neural Network(s) |
| ATP | Adenosine Triphosphate |
| CP | Conducting Polymer |
| CNN | Convolutional Neural Network |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| DT | Decision Tree |
| E-nose | Electronic Nose |
| E-tongue | Electronic Tongue |
| FCM | Flow Cytometry |
| FTIR | Fourier-Transform Infrared |
| GSC | Gain Scheduling Control |
| HACCP | Hazard Analysis and Critical Control Points |
| IR | Infrared |
| IoT | Internet of Things |
| ITS | Internal Transcribed Spacer |
| LAB | Lactic Acid Bacteria |
| LAMP | Loop-mediated Isothermal Amplification |
| LED | Light Emitting Diode |
| MIR | Mid-Infrared |
| ML | Machine Learning |
| MOS | Metal Oxide Sensor |
| MRAC | Model Reference Adaptive Control |
| MSE | Mean Squared Error |
| NGS | Next-Generation Sequencing |
| NIR | Near-Infrared |
| OD | Optical Density |
| PID | Proportional-Integral-Derivative |
| PLC | Programmable Logic Controller |
| qPCR | Quantitative Polymerase Chain Reaction |
| R² | Coefficient of Determination |
| RF | Random Forest |
| RMSE | Root Mean Squared Error |
| RSC | Reducing Sugar Content |
| SCOBY | Symbiotic Culture of Bacteria and Yeast |
| SDG | Sustainable Development Goal |
| SME | Small and Medium-sized Enterprises |
| STC | Self-Tuning Control |
| SVM/SVMs | Support Vector Machine(s) |
| TTA | Total Titratable Acidity |
| TLAB | Total Lactic Acid Bacteria |
| TRL | Technology Readiness Level |
| UV | Ultraviolet |
| VIS | Visible Spectrum |
| XGB | Extreme Gradient Boosting |
References
- Ray, R.C.; Paramithiotis, S.; Thekkangil, A.; Nethravathy, V.; Rai, A.K.; Martin, J.G.P. Food Fermentation and Its Relevance in the Human History. In Trending Topics on Fermented Foods; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–57. [Google Scholar]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Gondaliya, S.M.; Sheth, J.; Shah, S. Exploring the culinary and health significance of fermented foods: A comprehensive review. World J. Adv. Res. Rev. 2024, 21, 2609–2616. [Google Scholar] [CrossRef]
- Okoye, J.; Oni, K. Promotion of indigenous food preservation and processing knowledge and the challenge of food security in Africa. J. Food Secur. 2017, 5, 75–87. [Google Scholar]
- Juodeikiene, G.; Bartkiene, E.; Viskelis, P.; Urbonaviciene, D.; Eidukonyte, D.; Bobinas, C. Fermentation processes using lactic acid bacteria producing bacteriocins for preservation and improving functional properties of food products. Adv. Appl. Biotechnol. 2012, 2012, 63–100. [Google Scholar]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional Applications of Lactic Acid Bacteria: Enhancing Safety, Quality, and Nutritional Value in Foods and Fermented Beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, E.; Lawrence, R. Microbial fermentation for reduction of antinutritional factors. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–260. [Google Scholar]
- Nagpal, R.; Kumar, A.; Kumar, M.; Behare, P.V.; Jain, S.; Yadav, H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012, 334, 1–15. [Google Scholar] [CrossRef]
- Bagchi, T. Traditional food & modern lifestyle: Impact of probiotics. Indian J. Med. Res. 2014, 140, 333–335. [Google Scholar]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tasting the terroir of wine yeast innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef]
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B.; Hall, R.L.; Heldman, D.R.; Karwe, M.V. Feeding the world today and tomorrow: The importance of food science and technology: An IFT scientific review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 572–599. [Google Scholar] [CrossRef]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef] [PubMed]
- Elazzazy, A.M.; Baeshen, M.N.; Alasmi, K.M.; Alqurashi, S.I.; Desouky, S.E.; Khattab, S.M. Where Biology Meets Engineering: Scaling up microbial nutraceuticals to bridge nutrition, therapeutics, and global impact. Microorganisms 2025, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.; Martins, A.; Correia, H. Raw materials: The importance of quality and safety. A review. Flavour Fragr. J. 2010, 25, 253–271. [Google Scholar] [CrossRef]
- Yan, J.; Guo, Z.; Xie, J. A critical analysis of the opportunities and challenges of phage application in seafood quality control. Foods 2024, 13, 3282. [Google Scholar] [CrossRef]
- Navarret-Bolaños, J.L. Improving traditional fermented beverages: How to evolve from spontaneous to directed fermentation. Eng. Life Sci. 2012, 12, 410–418. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the physicochemical composition and microbial diversity in apple juice fermentation process: A review. Molecules 2020, 25, 3698. [Google Scholar] [CrossRef]
- Leroy, F.; Geyzen, A.; Janssens, M.; De Vuyst, L.; Scholliers, P. Meat fermentation at the crossroads of innovation and tradition: A historical outlook. Trends Food Sci. Technol. 2013, 31, 130–137. [Google Scholar] [CrossRef]
- Akinsemolu, A.A.; Onyeaka, H.N. Microorganisms Associated with Food Spoilage and Foodborne Diseases. In Food Safety and Quality in the Global South; Springer: Berlin/Heidelberg, Germany, 2024; pp. 489–531. [Google Scholar]
- Medina-Pradas, E.; Pérez-Díaz, I.M.; Garrido-Fernández, A.; Arroyo-López, F.N. Review of vegetable fermentations with particular emphasis on processing modifications, microbial ecology, and spoilage. In The Microbiological Quality of Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 211–236. [Google Scholar]
- Rüßmann, M.; Lorenz, M.; Gerbert, P.; Waldner, M.; Justus, J.; Engel, P.; Harnisch, M. Industry 4.0: The future of productivity and growth in manufacturing industries. Boston Consult. Group 2015, 9, 54–89. [Google Scholar]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented food products in the era of globalization: Tradition meets biotechnology innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef]
- Maharjan, S.; Rayamajhee, B.; Chhetri, V.S.; Sherchan, S.P.; Panta, O.P.; Karki, T.B. Microbial quality of poultry meat in an ISO 22000: 2005 certified poultry processing plant of Kathmandu valley. Int. J. Food Contam. 2019, 6, 8. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global regulatory frameworks for fermented foods: A review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lavalle, L.; Carrasco, E.; Valero, A. Microbiological criteria: Principles for their establishment and application in food quality and safety. Ital. J. Food Saf. 2020, 9, 8543. [Google Scholar] [CrossRef] [PubMed]
- Boyte, M.-E.; Benkowski, A.; Pane, M.; Shehata, H.R. Probiotic and postbiotic analytical methods: A perspective of available enumeration techniques. Front. Microbiol. 2023, 14, 1304621. [Google Scholar] [CrossRef]
- Jackson, S.A.; Schoeni, J.L.; Vegge, C.; Pane, M.; Stahl, B.; Bradley, M.; Goldman, V.S.; Burguière, P.; Atwater, J.B.; Sanders, M.E. Improving end-user trust in the quality of commercial probiotic products. Front. Microbiol. 2019, 10, 739. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Vigneault, C. Electronic nose and electronic tongue in food production and processing. Stewart Postharvest Rev. 2006, 4, 1–6. [Google Scholar]
- Ma, S.; Luo, H.; Zhao, D.; Qiao, Z.; Zheng, J.; An, M.; Huang, D. Environmental factors and interactions among microorganisms drive microbial community succession during fermentation of Nongxiangxing daqu. Bioresour. Technol. 2022, 345, 126549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zheng, J.; Xie, J.; Zhao, D.; Qiao, Z.-W.; Huang, D.; Luo, H.-B. Effects of environmental factors on the microbial community changes during medium-high temperature Daqu manufacturing. Food Res. Int. 2022, 153, 110955. [Google Scholar] [CrossRef]
- Chong, S.; Ilham, Z.; Samsudin, N.; Soumaya, S.; Wan-Mohtar, W. Microbial consortia and up-to-date technologies in global soy sauce production: A review. Int. Food Res. J. 2023, 30, 1–24. [Google Scholar] [CrossRef]
- Nguyen, N.T.H.; Huang, M.B.; Liu, F.Y.; Huang, W.-L.; Tran, H.-T.; Hsu, T.-W.; Huang, C.-L.; Chiang, T.-Y. Deciphering microbial community dynamics along the fermentation course of soy sauce under different temperatures using metagenomic analysis. Biosci. Microbiota Food Health 2023, 42, 104–113. [Google Scholar] [CrossRef]
- Geilings, B. Using Artificial Intelligence to Positively Impact the Beer Brewing Process. Bachelor’s Thesis, Haaga-Helia University of Applied Sciences, Helsinki, Finland, 2021. [Google Scholar]
- Schlechter, T. Impact of AI on the Brewing Industry: A Comprehensive Summary. Brew. Sci. 2024, 77, 18–32. [Google Scholar]
- Choudhary, M.; Kaur, P. Integrating AI with Machine Learning (ML) for Real-Time Detection in Food Industry. In Artificial Intelligence in the Food Industry; CRC Press: Boca Raton, FL, USA, 2025; pp. 297–315. [Google Scholar]
- Zhang, Y.; Zhu, X.; Wang, N.; Liu, X.; Wang, L.; Ning, K. Synergy of Traditional Practices and Modern Technology: Advancing the Understanding and Applications of Microbial Resources and Processes in Fermented Foods. Trends Food Sci. Technol. 2025, 157, 104891. [Google Scholar] [CrossRef]
- Vinestock, T.; Short, M.; Ward, K.; Guo, M. Computer-aided chemical engineering research advances in precision fermentation. Curr. Opin. Food Sci. 2024, 58, 101196. [Google Scholar] [CrossRef]
- Adeleke, I.; Nwulu, N.; Adebo, O.A. Internet of Things (IoT) in the food fermentation process: A bibliometric review. J. Food Process Eng. 2023, 46, e14321. [Google Scholar] [CrossRef]
- Bogueva, D.; Danova, S. Comparing Precision Fermentation and Traditional Fermentations: Consumer Views. In Consumer Perceptions and Food; Springer: Berlin/Heidelberg, Germany, 2024; pp. 563–588. [Google Scholar]
- Izquierdo-Bueno, I.; Moraga, J.; Cantoral, J.M.; Carbú, M.; Garrido, C.; González-Rodríguez, V.E. Smart Viniculture: Applying Artificial Intelligence for Improved Winemaking and Risk Management. Appl. Sci. 2024, 14, 10277. [Google Scholar] [CrossRef]
- Ali, S.A.; Elsaid, S.A.; Ateya, A.A.; ElAffendi, M.; El-Latif, A.A.A. Enabling technologies for next-generation smart cities: A comprehensive review and research directions. Future Internet 2023, 15, 398. [Google Scholar] [CrossRef]
- Hassan, S.A.; Elakhdar, B.E.; Saied, W.M.; Hassan, D.G. Leveraging new technologies for building a comprehensive smart MIS: Integrating ERP, blockchain, IoT, context-awareness, and cloud computing. In Proceedings of the 2024 6th International Conference on Computing and Informatics (ICCI), Cairo, Egypt, 6–7 March 2024; pp. 459–465. [Google Scholar]
- Carrera-Rivera, A.; Larrinaga, F.; Lasa, G. Context-awareness for the design of Smart-product service systems: Literature review. Comput. Ind. 2022, 142, 103730. [Google Scholar] [CrossRef]
- Rashid, A.B.; Kausik, A.K. AI revolutionizing industries worldwide: A comprehensive overview of its diverse applications. Hybrid Adv. 2024, 7, 100277. [Google Scholar] [CrossRef]
- Bhujbal, S.A.; Bhosure, A.G.; Sonawane, G.S.; Patel, P.R.; Jadhav, V.R.; Dhivare, J. Study smart road with glowing lines. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 3238–3241. [Google Scholar] [CrossRef]
- Crofton, E.C.; Botinestean, C.; Fenelon, M.; Gallagher, E. Potential applications for virtual and augmented reality technologies in sensory science. Innov. Food Sci. Emerg. Technol. 2019, 56, 102178. [Google Scholar] [CrossRef]
- Rantamaa, V. The Aesthetic Significance of Augmented Reality Experiences. Bachelor’s Thesis, Aalto University, Espoo, Finland, 2023. [Google Scholar]
- Agarwal, P.; Swami, S.; Malhotra, S.K. Artificial intelligence adoption in the post COVID-19 new-normal and role of smart technologies in transforming business: A review. J. Sci. Technol. Policy Manag. 2024, 15, 506–529. [Google Scholar] [CrossRef]
- Viardot, E.; Brem, A.; Nylund, P.A. Post-pandemic implications for crisis innovation: A technological innovation view. Technol. Forecast. Soc. Change 2023, 194, 122680. [Google Scholar] [CrossRef]
- Borgosz, L.; Dikicioglu, D. Industrial internet of things: What does it mean for the bioprocess industries? Biochem. Eng. J. 2024, 201, 109122. [Google Scholar] [CrossRef]
- Baicu, L.M.; Andrei, M.; Ifrim, G.A.; Dimitrievici, L.T. Embedded IoT Design for Bioreactor Sensor Integration. Sensors 2024, 24, 6587. [Google Scholar] [CrossRef]
- Gargalo, C.L.; Lopez, P.C.; Hasanzadeh, A.; Udugama, I.A.; Gernaey, K.V. On-line monitoring of process parameters during fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–164. [Google Scholar]
- Hosseini, N.; Baghelani, M. Selective real-time non-contact multi-variable water-alcohol-sugar concentration analysis during fermentation process using microwave split-ring resonator based sensor. Sens. Actuators A Phys. 2021, 325, 112695. [Google Scholar] [CrossRef]
- Al-Jumaili, A.H.A.; Muniyandi, R.C.; Hasan, M.K.; Paw, J.K.S.; Singh, M.J. Big data analytics using cloud computing based frameworks for power management systems: Status, constraints, and future recommendations. Sensors 2023, 23, 2952. [Google Scholar] [CrossRef]
- Vošahlík, J.; Hart, J. Measurability of quality in fermentation process of rice wine by IoT in the field of industry 4.0. Agron. Res. 2021, 19, 1318–1324. [Google Scholar]
- Soumyalatha, S.G.H. Study of IoT: Understanding IoT architecture, applications, issues and challenges. In Proceedings of the 1st International Conference on Innovations in Computing & Net-working (ICICN16), CSE, RRCE, Bengaluru, Karnataka, 12–13 May 2016; p. 14. [Google Scholar]
- Tetyana, P.; Shumbula, P.M.; Njengele-Tetyana, Z. Biosensors: Design, development and applications. In Nanopores; IntechOpen: London, UK, 2021. [Google Scholar]
- Zhou, Z.; Tian, D.; Yang, Y.; Cui, H.; Li, Y.; Ren, S.; Han, T.; Gao, Z. Machine learning assisted biosensing technology: An emerging powerful tool for improving the intelligence of food safety detection. Curr. Res. Food Sci. 2024, 8, 100679. [Google Scholar] [CrossRef]
- Onyeaka, H.; Akinsemolu, A.; Miri, T.; Nnaji, N.D.; Emeka, C.; Tamasiga, P.; Pang, G.; Al-sharify, Z. Advancing food security: The role of machine learning in pathogen detection. Appl. Food Res. 2024, 4, 100532. [Google Scholar] [CrossRef]
- Kaur, G.; Bhari, R.; Kumar, K. Electronic noses and tongue-based sensor systems in food science. In Nanosensing and Bioanalytical Technologies in Food Quality Control; Springer: Berlin/Heidelberg, Germany, 2022; pp. 357–384. [Google Scholar]
- Poeta, E.; Liboà, A.; Mistrali, S.; Núñez-Carmona, E.; Sberveglieri, V. Nanotechnology and E-sensing for food chain quality and safety. Sensors 2023, 23, 8429. [Google Scholar] [CrossRef]
- Kühl, N.; Schemmer, M.; Goutier, M.; Satzger, G. Artificial intelligence and machine learning. Electron. Mark. 2022, 32, 2235–2244. [Google Scholar] [CrossRef]
- Raihan, A. A comprehensive review of artificial intelligence and machine learning applications in energy sector. J. Technol. Innov. Energy 2023, 2, 1–26. [Google Scholar] [CrossRef]
- Xia, J.; Long, D.; Chen, M.; Chen, A. Optimization of fermentation processes in intelligent biomanufacturing: On online monitoring, artificial intelligence, and digital twin technologies. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2025, 41, 1179–1196. [Google Scholar]
- Nian, R.; Liu, J.; Huang, B. A review on reinforcement learning: Introduction and applications in industrial process control. Comput. Chem. Eng. 2020, 139, 106886. [Google Scholar] [CrossRef]
- Li, C.; Zheng, P.; Yin, Y.; Wang, B.; Wang, L. Deep reinforcement learning in smart manufacturing: A review and prospects. CIRP J. Manuf. Sci. Technol. 2023, 40, 75–101. [Google Scholar] [CrossRef]
- Espinel-Ríos, S.; Walser, R.; Zhang, D. Reinforcement learning for robust dynamic metabolic control. arXiv 2025, arXiv:2504.00735. [Google Scholar]
- Cheng, Y.; Bi, X.; Xu, Y.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Machine learning for metabolic pathway optimization: A review. Comput. Struct. Biotechnol. J. 2023, 21, 2381–2393. [Google Scholar] [CrossRef]
- Jang, W.D.; Kim, G.B.; Kim, Y.; Lee, S.Y. Applications of artificial intelligence to enzyme and pathway design for metabolic engineering. Curr. Opin. Biotechnol. 2022, 73, 101–107. [Google Scholar] [CrossRef]
- Espinel-Ríos, S.; Avalos, J.L. Hybrid physics-informed metabolic cybergenetics: Process rates augmented with machine-learning surrogates informed by flux balance analysis. Ind. Eng. Chem. Res. 2024, 63, 6685–6700. [Google Scholar] [CrossRef]
- Wainaina, S.; Taherzadeh, M.J. Automation and artificial intelligence in filamentous fungi-based bioprocesses: A review. Bioresour. Technol. 2023, 369, 128421. [Google Scholar] [CrossRef]
- Hassan, M.M.; Yi, X.; Sayada, J.; Zareef, M.; Shoaib, M.; Chen, X.; Li, H.; Chen, Q. Progress of machine learning-based biosensors for the monitoring of food safety: A review. Biosens. Bioelectron. 2024, 267, 116782. [Google Scholar] [CrossRef]
- Mitra, S.; Murthy, G.S. Bioreactor control systems in the biopharmaceutical industry: A critical perspective. Syst. Microbiol. Biomanuf. 2022, 2, 91–112. [Google Scholar] [CrossRef]
- Noor, J.; Chaudhry, A.; Batool, S. Microfluidic technology, artificial intelligence, and biosensors as advanced technologies in cancer screening: A review article. Cureus 2023, 15, e39634. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, N.Y.; Bamaalabong, P.P.; Johnson, J.E.; Bonsu, J.K.; Addo, A. Modeling, simulations, and Simulink developments in the analysis of optimal control of temperature and pH in a batch ethanol fermentation process. J. Eng. Appl. Sci. 2024, 71, 195. [Google Scholar] [CrossRef]
- Mohindru, P. Review on PID, fuzzy and hybrid fuzzy PID controllers for controlling non-linear dynamic behaviour of chemical plants. Artif. Intell. Rev. 2024, 57, 97. [Google Scholar] [CrossRef]
- Patil, R.S.; Jadhav, S.P.; Patil, M.D. Review of intelligent and nature-inspired algorithms-based methods for tuning PID controllers in industrial applications. J. Robot. Control (JRC) 2024, 5, 336–358. [Google Scholar] [CrossRef]
- Sánchez-Gil, J.J.; Cañete-Carmona, E.; Brox, M.; Gersnoviez, A.; Molina-Espinosa, M.Á.; Gámez-Granados, J.C.; Moreno, J. An electronic barrel bung to wirelessly monitor the biological aging process of Fino Sherry wine. IEEE Access 2024, 12, 35337–35350. [Google Scholar] [CrossRef]
- Tomtsis, D.; Kontogiannis, S.; Kokkonis, G.; Zinas, N. IoT architecture for monitoring wine fermentation process of debina variety semi-sparkling wine. In Proceedings of the SouthEast European Design Automation, Computer Engineering, Computer Networks and Social Media Conference, Kastoria, Greece, 25–27 September 2016. [Google Scholar]
- Kimutai, G.; Ngenzi, A.; Rutabayiro Ngoga, S.; Ramkat, R.C.; Förster, A. An internet of things (IoT)-based optimum tea fermentation detection model using convolutional neural networks (CNNs) and majority voting techniques. J. Sens. Sens. Syst. 2021, 10, 153–162. [Google Scholar] [CrossRef]
- Mathe, S.E.; Kondaveeti, H.K.; Vappangi, S.; Vanambathina, S.D.; Kumaravelu, N.K. A comprehensive review on applications of Raspberry Pi. Comput. Sci. Rev. 2024, 52, 100636. [Google Scholar] [CrossRef]
- Violino, S.; Figorilli, S.; Costa, C.; Pallottino, F. Internet of beer: A review on smart technologies from mash to pint. Foods 2020, 9, 950. [Google Scholar] [CrossRef]
- Watson, N.; Escrig Escrig, J.; Ren, N.; Zaidi, S.A.R.; Hafeez, M. BrewNet: Intelligent Cloud-Connected Sensors for Economic Small-Scale Process Optimisation; University of Nottingham and University of Leeds: Nottingham, UK, 2018; Available online: https://connectedeverything.ac.uk/wp-content/uploads/2019/08/brewnet-project-.pdf (accessed on 29 April 2025).
- Anker, M.; Yousefi-Darani, A.; Zettel, V.; Paquet-Durand, O.; Hitzmann, B.; Krupitzer, C. Online Monitoring of Sourdough Fermentation Using a Gas Sensor Array with Multivariate Data Analysis. Sensors 2023, 23, 7681. [Google Scholar] [CrossRef]
- Tsopelas, F. Electroanalytical Approaches to Combatting Food Adulteration: Advances in Non-Enzymatic Techniques for Ensuring Quality and Authenticity. Molecules 2025, 30, 876. [Google Scholar] [CrossRef] [PubMed]
- Zaukuu, J.-L.Z. Application of Electronic Tongue and Near Infrared Spectroscopy to Detect Adulteration of Some Foods with High Economic Value. Ph.D. Dissertation, Magyar Agrár-és Élettudományi Egyetem, Gödöllő, Hungary, 2021. [Google Scholar]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R. Electronic Sensing Technologies in Food Quality Assessment: A Comprehensive Literature Review. Appl. Sci. 2025, 15, 1530. [Google Scholar] [CrossRef]
- Addanki, M.; Patra, P.; Kandra, P. Recent advances and applications of artificial intelligence and related technologies in the food industry. Appl. Food Res. 2022, 2, 100126. [Google Scholar] [CrossRef]
- Nettesheim, P.; Burggräf, P.; Steinberg, F. Applications of machine learning in the brewing process: A systematic review. Discov. Artif. Intell. 2024, 4, 80. [Google Scholar] [CrossRef]
- Naveed, M.H.; Khan, M.N.A.; Mukarram, M.; Naqvi, S.R.; Abdullah, A.; Haq, Z.U.; Ullah, H.; Al Mohamadi, H. Cellulosic biomass fermentation for biofuel production: Review of artificial intelligence approaches. Renew. Sustain. Energy Rev. 2024, 189, 113906. [Google Scholar] [CrossRef]
- Rocha, R.A.; Cruz, M.A.d.; Silva, L.C.; Costa, G.X.; Amaral, L.R.; Bertarini, P.L.; Gomes, M.S.; Santos, L.D. Evaluation of arabica coffee fermentation using machine learning. Foods 2024, 13, 454. [Google Scholar] [CrossRef]
- Li, Z.; Atique, F.; Shahzad, M.; Rehman, K.U. Intelligent Control Strategy Based on Back-Propagation Neural Network with Adaptive Genetic Algorithm for Lincomycin Fermentation Process. Ind. Biotechnol. 2022, 18, 98–105. [Google Scholar] [CrossRef]
- Cruz, M.L.; de Resende, M.M.; Ribeiro, E.J. Improvement of ethanol production in fed-batch fermentation using a mixture of sugarcane juice and molasse under very high-gravity conditions. Bioprocess Biosyst. Eng. 2021, 44, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ye, J.; Su, L.; Liu, Z. Growth and metabolism control of lactic acid fermentation based on Internet of Things. J. Phys. Conf. Ser. 2021, 1757, 012158. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Admassie, M. A review on food fermentation and the biotechnology of lactic acid bacteria. World J. Food Sci. Technol. 2018, 2, 19–24. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Verardo, V.; Gómez-Caravaca, A.M.; Tabanelli, G. Bioactive components in fermented foods and food by-products. Foods 2020, 9, 153. [Google Scholar] [CrossRef]
- Yee, C.S.; Ilham, Z.; Cheng, A.; Abd Rahim, M.H.; Hajar-Azhari, S.; Yuswan, M.H.; Zaini, N.A.M.; Reale, A.; Di Renzo, T.; Wan, W.A.A.Q.I. Optimisation of fermentation conditions for the production of gamma-aminobutyric acid (GABA)-rich soy sauce. Heliyon 2024, 10, e33147. [Google Scholar]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Nout, M.R.; Aidoo, K.E. Asian fungal fermented food. In Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 29–58. [Google Scholar]
- Sieuwerts, S.; De Bok, F.A.; Hugenholtz, J.; van Hylckama Vlieg, J.E. Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Appl. Environ. Microbiol. 2008, 74, 4997–5007. [Google Scholar] [CrossRef]
- Hamad, S.H. Factors affecting the growth of microorganisms in food. In Progress in Food Preservation; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 405–427. [Google Scholar]
- Horlacher, N.; Oey, I.; Agyei, D. Learning from tradition: Health-promoting potential of traditional lactic acid fermentation to drive innovation in fermented plant-based dairy alternatives. Fermentation 2023, 9, 452. [Google Scholar] [CrossRef]
- Ajayeoba, T.A.; Ijabadeniyi, O.A. Transforming Food for the Future: Precision Fermentation as a Key to Sustainability, Nutrition, and Health. Nutr. Health 2025, 31, 1–23. [Google Scholar] [CrossRef]
- Navarro-Díaz, M.; Aparicio-Trejo, V.; Valdez-Vazquez, I.; Carrillo-Reyes, J.; Avitia, M.; Escalante, A.E. Levels of microbial diversity affect the stability and function of dark fermentation bioreactors. Front. Ind. Microbiol. 2024, 2, 1386726. [Google Scholar] [CrossRef]
- Nikoloudaki, O.; Aheto, F.; Di Cagno, R.; Gobbetti, M. Synthetic microbial communities: A gateway to understanding resistance, resilience, and functionality in spontaneously fermented food microbiomes. Food Res. Int. 2024, 192, 114780. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Huang, X.; Li, R.; Zhang, Y.; Chen, X.-X.; Han, B.-Z. Deciphering the core microbes and their interactions in spontaneous baijiu fermentation: A comprehensive review. Food Res. Int. 2024, 188, 114497. [Google Scholar] [CrossRef]
- Duperray, M.; Delvenne, M.; François, J.-M.; Delvigne, F.; Capp, J.-P. Long-term phenotypic and genomic instability of an industrial ethanol-producing and C5-utilizing Saccharomyces cerevisiae strain. bioRxiv 2023. [Google Scholar] [CrossRef]
- Beschkov, V.N.; Angelov, I.K. Volatile Fatty Acid Production vs. Methane and Hydrogen in Anaerobic Digestion. Fermentation 2025, 11, 172. [Google Scholar] [CrossRef]
- Yao, S.; Hao, L.; Zhou, R.; Jin, Y.; Huang, J.; Wu, C. Multispecies biofilms in fermentation: Biofilm formation, microbial interactions, and communication. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3346–3375. [Google Scholar] [CrossRef]
- dos Santos, V.M.; Tan, Y.; Zhu, Y.; Wijffels, R.; Zhang, H.; Scott, W., Jr.; Xu, Y. Controlling metabolic stability of food microbiome for stable indigenous liquor fermentation. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Thomashoff, U.L. Survival of Oxidative Stress-Adapted Bifidobacterium spp. in Yoghurt. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2024. [Google Scholar]
- Naveed, M.; Memon, Z.-n.; Abdullah, M.; Makhdoom, S.I.; Azeem, A.; Mehmood, S.; Salahuddin, M.; Rajpoot, Z.; Majeed, M. Use Cases and Future Aspects of Intelligent Techniques in Microbial Data Analysis. In Microbial Data Intelligence and Computational Techniques for Sustainable Computing; Springer: Berlin/Heidelberg, Germany, 2024; pp. 259–280. [Google Scholar]
- Arain, S.; John, G.T.; Krause, C.; Gerlach, J.; Wolfbeis, O.S.; Klimant, I. Characterization of microtiterplates with integrated optical sensors for oxygen and pH, and their applications to enzyme activity screening, respirometry, and toxicological assays. Sens. Actuators B Chem. 2006, 113, 639–648. [Google Scholar] [CrossRef]
- Lafirenza, M. Development of In-Situ Optical Sensor-Based Monitoring Methodologies for Additive Manufacturing Processes. Ph.D. Thesis, Politecnico di Bari, Bari, Italy, 2025. [Google Scholar]
- Nguyen, D.; Gadhamshetty, V.; Nitayavardhana, S.; Khanal, S.K. Automatic process control in anaerobic digestion technology: A critical review. Bioresour. Technol. 2015, 193, 513–522. [Google Scholar] [CrossRef]
- Rodriguez, M.; Dhameliya, N.; Patel, B. AI-Driven Programmable Logic Controllers: Enhancing Automation in Industrial Processes. Robot. Xplore USA Tech Dig. 2024, 1, 1–14. [Google Scholar]
- Seifan, M.; Samani, A.K.; Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO 3). Appl. Microbiol. Biotechnol. 2017, 101, 3131–3142. [Google Scholar] [CrossRef]
- Bolmanis, E.; Dubencovs, K.; Suleiko, A.; Vanags, J. Model predictive control—A stand out among competitors for fed-batch fermentation improvement. Fermentation 2023, 9, 206. [Google Scholar] [CrossRef]
- Ferrocino, I.; Rantsiou, K.; McClure, R.; Kostic, T.; de Souza, R.S.C.; Lange, L.; FitzGerald, J.; Kriaa, A.; Cotter, P.; Maguin, E. The need for an integrated multi-OMICs approach in microbiome science in the food system. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1082–1103. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Kamilari, E. Contribution of the Microbiome as a Tool for Estimating Wine’s Fermentation Output and Authentication. In Advances in Grape and Wine Biotechnology; IntechOpen: London, UK, 2019; p. 97. [Google Scholar]
- Venkataramanan, K.P.; Min, L.; Hou, S.; Jones, S.W.; Ralston, M.T.; Lee, K.H.; Papoutsakis, E.T. Complex and extensive post-transcriptional regulation revealed by integrative proteomic and transcriptomic analysis of metabolite stress response in Clostridium acetobutylicum. Biotechnol. Biofuels 2015, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, K.; Chen, M.; Fan, J.; Han, S.; Hou, J.; Chi, L.; Liu, Y.; Wei, T. Profiling the composition and metabolic activities of microbial community in fermented grain for the Chinese strong-flavor Baijiu production by using the metatranscriptome, high-throughput 16S rRNA and ITS gene sequencings. Food Res. Int. 2020, 138, 109765. [Google Scholar] [CrossRef]
- Yu, H.; Liu, S.; Qin, H.; Zhou, Z.; Zhao, H.; Zhang, S.; Mao, J. Artificial intelligence-based approaches for traditional fermented alcoholic beverages’ development: Review and prospect. Crit. Rev. Food Sci. Nutr. 2024, 64, 2879–2889. [Google Scholar] [CrossRef]
- Zhu, X.; Rehman, K.U.; Wang, B.; Shahzad, M. Modern soft-sensing modeling methods for fermentation processes. Sensors 2020, 20, 1771. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Soni, A.; Brightwell, G.; Reis, M.M.; Wang, Z.; Wang, J.; Wu, Q.; Ding, Y. The potential new microbial hazard monitoring tool in food safety: Integration of metabolomics and artificial intelligence. Trends Food Sci. Technol. 2024, 149, 104555. [Google Scholar] [CrossRef]
- Sharma, V.; Mottafegh, A.; Joo, J.-U.; Kang, J.-H.; Wang, L.; Kim, D.-P. Toward microfluidic continuous-flow and intelligent downstream processing of biopharmaceuticals. Lab A Chip 2024, 24, 2861–2882. [Google Scholar] [CrossRef]
- Knychala, M.M.; Boing, L.A.; Ienczak, J.L.; Trichez, D.; Stambuk, B.U. Precision fermentation as an alternative to animal protein, a review. Fermentation 2024, 10, 315. [Google Scholar] [CrossRef]
- Goodman, D. Alternative Proteins: Bio-Mimicry, Structuring the New Protein Industry, ‘Promissory Narratives’, and ‘Substitutionism 4.0’. In Transforming Agriculture and Foodways; Bristol University Press: Bristol, UK, 2023; pp. 36–52. [Google Scholar]
- Malkawi, H.I.; Kapiel, T.Y.S. Microbial biotechnology: A key tool for addressing climate change and food insecurity. Eur. J. Biol. Biotechnol. 2024, 5, 1–15. [Google Scholar] [CrossRef]
- Bockisch, A.; Kielhorn, E.; Neubauer, P.; Junne, S. Process analytical technologies to monitor the liquid phase of anaerobic cultures. Process Biochem. 2019, 76, 1–10. [Google Scholar] [CrossRef]
- Chakraborty, J.; Das, S. Application of spectroscopic techniques for monitoring microbial diversity and bioremediation. Appl. Spectrosc. Rev. 2017, 52, 1–38. [Google Scholar] [CrossRef]
- Ha, N.S.; de Raad, M.; Golini, A.; Petzold, C.J.; Northen, T.R. Faster, better, and cheaper: Harnessing microfluidics and mass spectrometry for biotechnology. RSC Chem. Biol. 2021, 2, 1331–1351. [Google Scholar] [CrossRef]
- Chandra, S.; Chapman, J.; Power, A.; Roberts, J.; Cozzolino, D. The application of state-of-the-art analytic tools (biosensors and spectroscopy) in beverage and food fermentation process monitoring. Fermentation 2017, 3, 50. [Google Scholar] [CrossRef]
- López-Gálvez, J.; Schiessl, K.; Besmer, M.D.; Bruckmann, C.; Harms, H.; Müller, S. Development of an automated online flow cytometry method to quantify cell density and fingerprint bacterial communities. Cells 2023, 12, 1559. [Google Scholar] [CrossRef]
- Hristovski, K.D.; Burge, S.R.; Boscovic, D.; Burge, R.G.; Babanovska-Milenkovska, F. Real-time monitoring of kefir-facilitated milk fermentation using microbial potentiometric sensors. J. Environ. Chem. Eng. 2022, 10, 107491. [Google Scholar] [CrossRef]
- Assawajaruwan, S.; Kuon, F.; Funke, M.; Hitzmann, B. Feedback control based on NADH fluorescence intensity for Saccharomyces cerevisiae cultivations. Bioresour. Bioprocess. 2018, 5, 24. [Google Scholar] [CrossRef]
- He, H.; Sun, D.-W.; Pu, H.; Chen, L.; Lin, L. Applications of Raman spectroscopic techniques for quality and safety evaluation of milk: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2019, 59, 770–793. [Google Scholar] [CrossRef]
- Arango, O.; Trujillo, A.J.; Castillo, M. Inline control of yoghurt fermentation process using a near infrared light backscatter sensor. J. Food Eng. 2020, 277, 109885. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. On-line monitoring of food fermentation processes using electronic noses and electronic tongues: A review. Anal. Chim. Acta 2013, 804, 29–36. [Google Scholar] [CrossRef]
- Wen, L.; Yang, L.; Chen, C.; Li, J.; Fu, J.; Liu, G.; Kan, Q.; Ho, C.-T.; Huang, Q.; Lan, Y. Applications of multi-omics techniques to unravel the fermentation process and the flavor formation mechanism in fermented foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 8367–8383. [Google Scholar] [CrossRef] [PubMed]
- Ebert, F.V.; Reitz, C.; Cruz-Bournazou, M.N.; Neubauer, P. Characterization of a noninvasive on-line turbidity sensor in shake flasks for biomass measurements. Biochem. Eng. J. 2018, 132, 20–28. [Google Scholar] [CrossRef]
- Muncan, J.; Tei, K.; Tsenkova, R. Real-time monitoring of yogurt fermentation process by aquaphotomics near-infrared spectroscopy. Sensors 2020, 21, 177. [Google Scholar] [CrossRef]
- Reardon, K.F. Practical monitoring technologies for cells and substrates in biomanufacturing. Curr. Opin. Biotechnol. 2021, 71, 225–230. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Sun, G. Flow cytometry and sorting in Arabidopsis. Methods Mol. Biol. 2021, 2200, 255–294. [Google Scholar] [CrossRef] [PubMed]
- Blundell, M.P.; Sanderson, S.L.; Long, T.A. Flow cytometry as an important tool in proteomic profiling. In Proteomic Profiling: Methods and Protocols; Humana: New York, NY, USA, 2021; pp. 213–227. [Google Scholar]
- Hutter, K.J. Flow cytometry—A new tool for direct control of fermentation processes. J. Inst. Brew. 2002, 108, 48–51. [Google Scholar] [CrossRef]
- Torello Pianale, L.; Rugbjerg, P.; Olsson, L. Real-time monitoring of the yeast intracellular state during bioprocesses with a toolbox of biosensors. Front. Microbiol. 2022, 12, 802169. [Google Scholar] [CrossRef]
- Čakić Semenčić, M.; Biedrzycka, A.; Kiczor, A.; Beluhan, S.; Šupljika, F. Spectrofluorimetric Analysis of Riboflavin Content during Kombucha Fermentation. BioTech 2024, 13, 20. [Google Scholar] [CrossRef]
- Mishra, A.; Aghaee, M.; Tamer, I.M.; Budman, H. Spectroscopic Advances in Real Time Monitoring of Pharmaceutical Bioprocesses: A Review of Vibrational and Fluorescence Techniques. Spectrosc. J. 2025, 3, 12. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Stefkov, G.; Cvetkovikj Karanfilova, I.; Stoilkovska Gjorgievska, V.; Trajkovska, A.; Geskovski, N.; Karapandzova, M.; Kulevanova, S. Analytical techniques for phytocannabinoid profiling of cannabis and cannabis-based products—A comprehensive review. Molecules 2022, 27, 975. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Olsen, P.M.; Byrtusová, D.; Tafintseva, V.; Shapaval, V.; Horn, S.J.; Kohler, A.; Szotkowski, M.; Marova, I.; Zimmermann, B. Raman spectroscopy online monitoring of biomass production, intracellular metabolites and carbon substrates during submerged fermentation of oleaginous and carotenogenic microorganisms. Microb. Cell Factories 2023, 22, 261. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman spectroscopy for food quality assurance and safety monitoring: A review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Rodriguez, L.; Zhang, Z.; Wang, D. Recent advances of Raman spectroscopy for the analysis of bacteria. Anal. Sci. Adv. 2023, 4, 81–95. [Google Scholar] [CrossRef]
- Seesaard, T.; Wongchoosuk, C. Recent progress in electronic noses for fermented foods and beverages applications. Fermentation 2022, 8, 302. [Google Scholar] [CrossRef]
- Hosseini, S.-N.; Das, P.S.; Lazarjan, V.K.; Gagnon-Turcotte, G.; Bouzid, K.; Gosselin, B. Recent advances in CMOS electrochemical biosensor design for microbial monitoring: Review and design methodology. IEEE Trans. Biomed. Circuits Syst. 2023, 17, 202–228. [Google Scholar] [CrossRef]
- Subjakova, V.; Oravczova, V.; Tatarko, M.; Hianik, T. Advances in electrochemical aptasensors and immunosensors for detection of bacterial pathogens in food. Electrochim. Acta 2021, 389, 138724. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef]
- Lee, W.-H.; Oh, I.-N.; Choi, S.; Park, J.-T. Classification of geographical origin of kimchi by volatile compounds analysis using an electronic nose. Food Sci. Biotechnol. 2021, 30, 1313–1319. [Google Scholar] [CrossRef]
- Kumar, A.; Castro, M.; Feller, J.-F. Review on sensor array-based analytical technologies for quality control of food and beverages. Sensors 2023, 23, 4017. [Google Scholar] [CrossRef]
- Podrażka, M.; Bączyńska, E.; Kundys, M.; Jeleń, P.S.; Witkowska Nery, E. Electronic tongue—A tool for all tastes? Biosensors 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, D.; Lee, K.W.; Chang, J.Y. Kimchi Lactic Acid Bacteria Starter Culture: Impact on Fermented Malt Beverage Volatile Profile, Sensory Analysis, and Physicochemical Traits. J. Microbiol. Biotechnol. 2024, 34, 1653. [Google Scholar] [CrossRef]
- Cheung, K.C.; Di Berardino, M.; Schade-Kampmann, G.; Hebeisen, M.; Pierzchalski, A.; Bocsi, J.; Mittag, A.; Tárnok, A. Microfluidic impedance-based flow cytometry. Cytom. Part A 2010, 77, 648–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Huang, C.-H.; Pai, P.-C.; Seo, J.; Lei, K.F. A review on microfluidics-based impedance biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Lu, X.; Liu, Q. Isothermal nucleic acid amplification based microfluidic “lab-on-a-chip” for the detection of pathogenic bacteria and viruses in agri-foods. Trends Food Sci. Technol. 2024, 148, 104482. [Google Scholar] [CrossRef]
- Rafiq, S.M.; Majumder, R.; Joshi, D.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Sidiqi, U.S. Lab-on-a-chip Device for Food Quality Control and Safety. Food Control 2024, 164, 110596. [Google Scholar] [CrossRef]
- Son, H. Harnessing CRISPR/Cas Systems for DNA and RNA Detection: Principles, Techniques, and Challenges. Biosensors 2024, 14, 460. [Google Scholar] [CrossRef]
- Li, X.; Zhong, J.; Li, H.; Qiao, Y.; Mao, X.; Fan, H.; Zhong, Y.; Imani, S.; Zheng, S.; Li, J. Advances in the application of CRISPR-Cas technology in rapid detection of pathogen nucleic acid. Front. Mol. Biosci. 2023, 10, 1260883. [Google Scholar] [CrossRef]
- Kurniawan, Y.N.; Shinohara, Y.; Takesue, N.; Sakai, H.; Magarifuchi, T.; Suzuki, K. Development of a rapid and accurate nanopore-based sequencing platform for on-field identification of beer-spoilage bacteria in the breweries. J. Am. Soc. Brew. Chem. 2021, 79, 240–248. [Google Scholar] [CrossRef]
- Kim, N.; Park, K.-r.; Park, I.-S.; Cho, Y.-J.; Bae, Y.M. Application of a taste evaluation system to the monitoring of Kimchi fermentation. Biosens. Bioelectron. 2005, 20, 2283–2291. [Google Scholar] [CrossRef]
- Mu’azzam, K.; da Silva, F.V.S.; Murtagh, J.; Gallagher, M.S. A roadmap for model-based bioprocess development. Biotechnol. Adv. 2024, 73, 108378. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Koo, I. Real-time remote patient monitoring: A review of biosensors integrated with multi-hop IoT systems via cloud connectivity. Appl. Sci. 2024, 14, 1876. [Google Scholar] [CrossRef]
- Zhao, S.; Jiao, T.; Adade, S.Y.-S.S.; Wang, Z.; Ouyang, Q.; Chen, Q. Digital twin for predicting and controlling food fermentation: A case study of kombucha fermentation. J. Food Eng. 2025, 393, 112467. [Google Scholar] [CrossRef]
- Helmy, M.; Elhalis, H.; Rashid, M.M.; Selvarajoo, K. Can digital twin efforts shape microorganism-based alternative food? Curr. Opin. Biotechnol. 2024, 87, 103115. [Google Scholar] [CrossRef]
- Udugama, I.A.; Öner, M.; Lopez, P.C.; Beenfeldt, C.; Bayer, C.; Huusom, J.K.; Gernaey, K.V.; Sin, G. Towards digitalization in bio-manufacturing operations: A survey on application of big data and digital twin concepts in Denmark. Front. Chem. Eng. 2021, 3, 727152. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, A.; Gupta, P.; Chowdary, V. Machine learning applications for precision agriculture: A comprehensive review. IEEe Access 2020, 9, 4843–4873. [Google Scholar] [CrossRef]
- Khaleghi, M.K.; Savizi, I.S.P.; Lewis, N.E.; Shojaosadati, S.A. Synergisms of machine learning and constraint-based modeling of metabolism for analysis and optimization of fermentation parameters. Biotechnol. J. 2021, 16, 2100212. [Google Scholar] [CrossRef]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of conventional statistical methods with machine learning in medicine: Diagnosis, drug development, and treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine learning in agriculture: A review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef]
- Niazian, M.; Niedbała, G. Machine learning for plant breeding and biotechnology. Agriculture 2020, 10, 436. [Google Scholar] [CrossRef]
- Oliveira, A.L. Biotechnology, big data and artificial intelligence. Biotechnol. J. 2019, 14, 1800613. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.H.; Sablani, S.S.; Nayak, R.; Gu, Y. Machine learning-based modeling in food processing applications: State of the art. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1409–1438. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Niyigaba, T.; Küçükgöz, K.; Kołożyn-Krajewska, D.; Królikowski, T.; Trząskowska, M. Advances in Fermentation Technology: A Focus on Health and Safety. Appl. Sci. 2025, 15, 3001. [Google Scholar] [CrossRef]
- Ahamed, K.I.; Akthar, S. A study on neural network architectures. Comput. Eng. Intell. Syst 2016, 7, 1–7. [Google Scholar]
- Nagata, Y.; Chu, K.H. Optimization of a fermentation medium using neural networks and genetic algorithms. Biotechnol. Lett. 2003, 25, 1837–1842. [Google Scholar] [CrossRef]
- Li, B.; Lin, Y.; Yu, W.; Wilson, D.I.; Young, B.R. Application of mechanistic modelling and machine learning for cream cheese fermentation pH prediction. J. Chem. Technol. Biotechnol. 2021, 96, 125–133. [Google Scholar] [CrossRef]
- Tan, J.; Balasubramanian, B.; Sukha, D.; Ramkissoon, S.; Umaharan, P. Sensing fermentation degree of cocoa (Theobroma cacao L.) beans by machine learning classification models based electronic nose system. J. Food Process Eng. 2019, 42, e13175. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Z.; Cui, F.; Pei, S.; Wei, L.; Weng, Z.; Li, L. Innovative rapid liquid concentration measurement based on thermal lens effect and machine learning. Opt. Express 2024, 32, 17837–17852. [Google Scholar] [CrossRef]
- Massah, J.; Nomanfar, P.; Soufi, M.D.; Vakilian, K.A. Electrical properties measurement: A nondestructive method to determine the quality of bread doughs during fermentation. J. Cereal Sci. 2022, 107, 103530. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Low-cost methods to assess beer quality using artificial intelligence involving robotics, an electronic nose, and machine learning. Fermentation 2020, 6, 104. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Xu, N.; Hu, Y.; Wang, C.; He, J.; Cao, Y.; Chen, S.; Li, D. Soy sauce classification by geographic region and fermentation based on artificial neural network and genetic algorithm. J. Agric. Food Chem. 2014, 62, 12294–12298. [Google Scholar] [CrossRef]
- Loddo, A.; Di Ruberto, C.; Armano, G.; Manconi, A. Detecting coagulation time in cheese making by means of computer vision and machine learning techniques. Comput. Ind. 2025, 164, 104173. [Google Scholar] [CrossRef]
- Urtubia, A.; León, R.; Vargas, M. Identification of chemical markers to detect abnormal wine fermentation using support vector machines. Comput. Chem. Eng. 2021, 145, 107158. [Google Scholar] [CrossRef]
- Bi, K.; Qiu, T.; Huang, Y. A deep learning method for yogurt preferences prediction using sensory attributes. Processes 2020, 8, 518. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, F.; Ye, Y.; Chen, L.; Liu, J.; Gui, A.; Zhang, J.; Dong, C. Application of machine learning algorithms in quality assurance of fermentation process of black tea-based on electrical properties. J. Food Eng. 2019, 263, 165–172. [Google Scholar] [CrossRef]
- Mienye, I.D.; Jere, N. A survey of decision trees: Concepts, algorithms, and applications. IEEE Access 2024, 12, 86716–86727. [Google Scholar] [CrossRef]
- Parrenin, L.; Dupuis, A.; Danjou, C.; Agard, B. Machine Learning Tool for Yield Maximization in Cream Cheese Production. In Proceedings of the International Conference on Innovative Intelligent Industrial Production and Logistics, Porto, Portugal, 22–23 November 2024; pp. 97–114. [Google Scholar]
- Park, S.Y.; Kang, M.; Yun, S.-M.; Eun, J.-B.; Shin, B.-S.; Chun, H.H. Changes and machine learning-based prediction in quality characteristics of sliced Korean cabbage (Brassica rapa L. pekinensis) kimchi: Combined effect of nano-foamed structure film packaging and subcooled storage. Lwt 2022, 171, 114122. [Google Scholar] [CrossRef]
- Alfian, G.; Syafrudin, M.; Farooq, U.; Ma’arif, M.R.; Syaekhoni, M.A.; Fitriyani, N.L.; Lee, J.; Rhee, J. Improving efficiency of RFID-based traceability system for perishable food by utilizing IoT sensors and machine learning model. Food Control 2020, 110, 107016. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Y.; Zhao, J.; Fei, F.; Gao, M.; Wang, Q. Explainable machine learning-driven predictive performance and process parameter optimization for caproic acid production. Bioresour. Technol. 2024, 410, 131311. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshini, T.; Nageshwari, K.; Vimaladhasan, S.; Prakash, S.P.; Balasubramanian, P. Machine learning prediction of SCOBY cellulose yield from Kombucha tea fermentation. Bioresour. Technol. Rep. 2022, 18, 101027. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Zhao, C.; Peng, Y.; Wang, H. An ensemble feature selection method for high-dimensional data based on sort aggregation. Syst. Sci. Control Eng. 2019, 7, 32–39. [Google Scholar] [CrossRef]
- Golzarijalal, M.; Ong, L.; Neoh, C.R.; Harvie, D.J.; Gras, S.L. Machine learning for the prediction of proteolysis in Mozzarella and Cheddar cheese. Food Bioprod. Process. 2024, 144, 132–144. [Google Scholar] [CrossRef]
- Hassoun, A.; Jagtap, S.; Garcia-Garcia, G.; Trollman, H.; Pateiro, M.; Lorenzo, J.M.; Trif, M.; Rusu, A.V.; Aadil, R.M.; Šimat, V. Food quality 4.0: From traditional approaches to digitalized automated analysis. J. Food Eng. 2023, 337, 111216. [Google Scholar] [CrossRef]
- Henson, M.A. Biochemical reactor modeling and control. IEEE Control Syst. Mag. 2006, 26, 54–62. [Google Scholar]
- Yadav, H.; Singh, S.; Sinha, R. Fermentation Technology for Microbial Products and Their Process Optimization. In Industrial Microbiology and Biotechnology: A New Horizon of the Microbial World; Springer: Berlin/Heidelberg, Germany, 2024; pp. 35–64. [Google Scholar]
- Stavrov, D.; Nadzinski, G.; Deskovski, S.; Stankovski, M. Quadratic model-based dynamically updated PID control of CSTR system with varying parameters. Algorithms 2021, 14, 31. [Google Scholar] [CrossRef]
- Petre, E.; Selişteanu, D.; Roman, M. Advanced nonlinear control strategies for a fermentation bioreactor used for ethanol production. Bioresour. Technol. 2021, 328, 124836. [Google Scholar] [CrossRef]
- Ritonja, J.; Goršek, A.; Pečar, D. Model reference adaptive control for milk fermentation in batch bioreactors. Appl. Sci. 2020, 10, 9118. [Google Scholar] [CrossRef]
- Canatan, M.; Alkhulaifi, N.; Watson, N.; Boz, Z. Artificial Intelligence in Food Manufacturing: A Review of Current Work and Future Opportunities. Food Eng. Rev. 2025, 1–31. [Google Scholar] [CrossRef]
- Lisci, S. Development of Monitoring and Control Systems for Biotechnological Processes. Ph.D. Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2023. [Google Scholar]
- Hals, J.; Falnes, J.; Moan, T. A comparison of selected strategies for adaptive control of wave energy converters. J. Offshore Mech. Arct. Eng. 2011, 133, 031101. [Google Scholar] [CrossRef]
- Charfeddine, S.; Jerbi, H. A survey on non-linear gain scheduling design control for continuous and discrete time systems. Int. J. Model. Identif. Control 2013, 19, 203–216. [Google Scholar] [CrossRef]
- de Medeiros, M.L.B.; de Oliveira Junior, A.M.; Fonseca, R.R. Gain scheduling control applied to oil and gas separator level loop. Res. Soc. Dev. 2021, 10, e55010414397. [Google Scholar] [CrossRef]
- Burnham, K.J.; Larkowski, T. Self-Tuning and Adaptive Control; WUT: Wroclaw, Poland, 2011. [Google Scholar]
- Sovik, G.; Urkin, T.; Masandilov, E.E.; Peretz, M.M. Optimal Self-Tuning Control for Data-Centers’ 48V-12V ZCS-STC. In Proceedings of the 2020 IEEE Applied Power Electronics Conference and Exposition (APEC), New Orleans, LO, USA, 15–19 March 2020; pp. 455–462. [Google Scholar]
- Barkana, I. Simple adaptive control–a stable direct model reference adaptive control methodology–brief survey. Int. J. Adapt. Control Signal Process. 2014, 28, 567–603. [Google Scholar] [CrossRef]
- Joshi, G.; Chowdhary, G. Deep model reference adaptive control. In Proceedings of the 2019 IEEE 58th Conference on Decision and Control (CDC), Nice, France, 11–13 December 2019; pp. 4601–4608. [Google Scholar]
- Landau, Y.D. Adaptive control: The model reference approach. IEEE Trans. Syst. Man Cybern. 1984, SMC-14, 169–170. [Google Scholar] [CrossRef]
- Petre, E.; Selişteanu, D. Adaptive control of a fermentation bioprocess for lactic acid production. Math. Probl. Eng. 2012, 2012, 936034. [Google Scholar] [CrossRef]
- George, A.S. Leveraging industry 4.0 for efficiency gains in food production. Partn. Univers. Int. Res. J. 2024, 3, 86–108. [Google Scholar]
- Okuyelu, O.; Adaji, O. AI-driven real-time quality monitoring and process optimization for enhanced manufacturing performance. J. Adv. Math. Comput. Sci 2024, 39, 81–89. [Google Scholar] [CrossRef]
- ifm electronic gmbh. Perfecting Wine Fermentation Through Real-Time Monitoring. Available online: https://www.ifm.com/my/en/shared/successstories/casestudies/perfecting-wine-fermentation-through-real-time-monitoring (accessed on 29 April 2025).
- Jaywant, S.A.; Singh, H.; Arif, K.M. Sensors and instruments for brix measurement: A review. Sensors 2022, 22, 2290. [Google Scholar] [CrossRef]
- Valyasevi, R.; Rolle, R.S. An overview of small-scale food fermentation technologies in developing countries with special reference to Thailand: Scope for their improvement. Int. J. Food Microbiol. 2002, 75, 231–239. [Google Scholar] [CrossRef]
- Chandrasiri, G.; Wijenayake, A.; Arachchige, U. Development of automated systems for the implementation of food processing. J. Res. Technol. Eng 2022, 3, 8–18. [Google Scholar]
- Kuppusamy, S.; Meivelu, M.; Praburaman, L.; Alam, M.M.; Al-Sehemi, A.G. Integrating AI in food contaminant analysis: Enhancing quality and environmental protection. J. Hazard. Mater. Adv. 2024, 16, 100509. [Google Scholar] [CrossRef]
- Mor, R.S.; Kumar, D.; Singh, A.; Neethu, K. Robotics and automation for agri-food 4.0: Innovation and challenges. In Agri-Food 4.0: Innovations, Challenges and Strategies; Emerald Publishing Limited: Leeds, UK, 2022; pp. 189–199. [Google Scholar]
- Viejo, C.G.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Kaunkid, S.; Aurasopon, A.; Khamsen, W.; Takeang, C.; Piladaeng, N.; Lloret, J. Resistive Measurement Method for MQ Sensors Based on ADCs of Microcontrollers. IEEE Access 2024, 12, 144364–144376. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Torrico, D.D.; Godbole, A.; Dunshea, F.R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef]
- Chui, M.; Harryson, M.; Valley, S.; Manyika, J.; Roberts, R. Notes from the AI Frontier: Applying AI for Social Good—Discussion Paper; McKinsey Global Institute: San Frncisco, CA, USA, 2018; Available online: https://www.mckinsey.com/~/media/mckinsey/featured%20insights/artificial%20intelligence/applying%20artificial%20intelligence%20for%20social%20good/mgi-applying-ai-for-social-good-discussion-paper-dec-2018.pdf (accessed on 29 April 2025).
- Kimball, R. The Evolving Role of the Enterprise Data Warehouse in the Era of Big Data Analytics; Kimball Group: Seattle, WA, USA, 2011; Available online: https://www.kimballgroup.com/wp-content/uploads/2011/04/Evolving-Role-of-EDW-in-the-Era-of-Big-Data-Analytics.pdf (accessed on 29 April 2025).
- Lang, V. Digitalization and digital transformation. In Digital Fluency: Understanding the Basics of Artificial Intelligence, Blockchain Technology, Quantum Computing, and Their Applications for Digital Transformation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–50. [Google Scholar]
- Sanogo, K.; Touré, I.; Arinloye, D.-D.A.; Dossou-Yovo, E.R.; Bayala, J. Factors affecting the adoption of climate-smart agriculture technologies in rice farming systems in Mali, West Africa. Smart Agric. Technol. 2023, 5, 100283. [Google Scholar] [CrossRef]
- Yépez-Ponce, D.F.; Salcedo, J.V.; Rosero-Montalvo, P.D.; Sanchis, J. Mobile robotics in smart farming: Current trends and applications. Front. Artif. Intell. 2023, 6, 1213330. [Google Scholar] [CrossRef]
- Rikala, P.; Braun, G.; Järvinen, M.; Stahre, J.; Hämäläinen, R. Understanding and measuring skill gaps in Industry 4.0—A review. Technol. Forecast. Soc. Change 2024, 201, 123206. [Google Scholar] [CrossRef]
- Knapp, J.N.R. Cyberbiosecurity Importance in Relation to Small Fermentation Businesses and How to Integrate it into Known Hazard Prevention Tools. Ph.D. Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2024. [Google Scholar]
- Goel, A.; Masurkar, S.; Pathade, G.R. An Overview of Digital Transformation and Environmental Sustainability: Threats, Opportunities, and Solutions. Sustainability 2024, 16, 11079. [Google Scholar] [CrossRef]
- Henderson, P.; Hu, J.; Romoff, J.; Brunskill, E.; Jurafsky, D.; Pineau, J. Towards the systematic reporting of the energy and carbon footprints of machine learning. J. Mach. Learn. Res. 2020, 21, 1–43. [Google Scholar]
- Jain, M.; Kumar, D.; Chaudhary, J.; Kumar, S.; Sharma, S.; Verma, A.S. Review on E-waste management and its impact on the environment and society. Waste Manag. Bull. 2023, 1, 34–44. [Google Scholar] [CrossRef]
- Perkins, D.N.; Drisse, M.-N.B.; Nxele, T.; Sly, P.D. E-waste: A global hazard. Ann. Glob. Health 2014, 80, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Guo, J.; Xie, J.; Zhou, X.; Liu, A.; Gu, X.; Mourtzis, D.; Qi, Q.; Liu, Q.; Shen, W. Review of manufacturing system design in the interplay of Industry 4.0 and Industry 5.0 (Part I): Design thinking and modeling methods. J. Manuf. Syst. 2024, 76, 158–187. [Google Scholar] [CrossRef]
- Sharma, K.K.; Verma, P.K.; Garg, P. IoT-Enabled Energy Management Systems For Sustainable Energy Storage: Design, Optimization, And Future Directions. Front. Health Inform. 2024, 13, 2234–2255. [Google Scholar]
- Zhang, J. Design Guidelines of A Low Power Communication Protocol for Zero Energy Devices. Master’s Thesis, KTH, School of Electrical Engineering and Computer Science (EECS), Stockholm, Sweden, 2023. [Google Scholar]
- Musa, S.F.P.D.; Basir, K.H. Smart farming: Towards a sustainable agri-food system. Br. Food J. 2021, 123, 3085–3099. [Google Scholar] [CrossRef]
- Jia, W.; Huang, X. Digital literacy and vocational education: Essential skills for the modern workforce. Int. J. Acad. Res. Bus. Soc. Sci. 2023, 13, 2195–2202. [Google Scholar] [CrossRef]
- Obasi, S.; Aa, T.V.; Obasi, C.; Jokthan, G.; Adjei, E.; Keyagha, E. Harnessing Artificial Intelligence For Sustainable Agriculture: A Comprehensive Review of African Applications in Spatial Analysis And Precision Agriculture. Big Data Agric. 2024, 6, 1–13. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, K.; Grover, S. Real-time data analysis with smart sensors. In Application of Artificial Intelligence in Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2024; pp. 127–153. [Google Scholar]
| Technology | Principle | Example Application | References |
|---|---|---|---|
| Optical Density Sensors | Light scattering (VIS/NIR turbidity) | Turbidity tracking in wine fermentation | [137] |
| Flow Cytometry (Online) | Laser scatter and fluorescence (single-cell) | Fingerprinting of different LAB during fermentation | [138] |
| Microfluidic Impedance Cytometry | Impedance pulses per cell (microchannel) | Production of metabolites during kefir fermentation | [139] |
| Fluorescence Spectroscopy | NADH/tryptophan or dye fluorescence | Yeast metabolic activity in tempeh | [140] |
| Raman Spectroscopy | Inelastic light scattering (molecular “fingerprints”) | Milk composition and microorganisms monitoring | [141] |
| Infrared (NIR/MIR/FTIR) Spectroscopy | Vibrational absorbance of biomolecules | Lactose conversion and acid production in yogurt | [142] |
| Electrochemical Biosensors | Potentiometric/amperometric signals (redox, enzymes) | Redox potential monitoring in kefir fermentation | [139] |
| E-nose and E-tongue (Gas Sensor Array) | The E-nose uses MOS/CP sensor arrays to sniff VOC patterns, while the E-tongue uses potentiometric or voltammetric sensors to “taste” dissolved compounds | Geographical origin discrimination of kimchi volatiles and sourness | [143] |
| Sequencing and Omics (Molecular Approach) | Nanopores, CRISPR, NGS | On-site microbial community profiling in tempeh | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yee, C.S.; Zahia-Azizan, N.A.; Abd Rahim, M.H.; Mohd Zaini, N.A.; Raja-Razali, R.B.; Ushidee-Radzi, M.A.; Ilham, Z.; Wan-Mohtar, W.A.A.Q.I. Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods. Fermentation 2025, 11, 323. https://doi.org/10.3390/fermentation11060323
Yee CS, Zahia-Azizan NA, Abd Rahim MH, Mohd Zaini NA, Raja-Razali RB, Ushidee-Radzi MA, Ilham Z, Wan-Mohtar WAAQI. Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods. Fermentation. 2025; 11(6):323. https://doi.org/10.3390/fermentation11060323
Chicago/Turabian StyleYee, Chong Shin, Nur Asyiqin Zahia-Azizan, Muhamad Hafiz Abd Rahim, Nurul Aqilah Mohd Zaini, Raja Balqis Raja-Razali, Muhammad Ameer Ushidee-Radzi, Zul Ilham, and Wan Abd Al Qadr Imad Wan-Mohtar. 2025. "Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods" Fermentation 11, no. 6: 323. https://doi.org/10.3390/fermentation11060323
APA StyleYee, C. S., Zahia-Azizan, N. A., Abd Rahim, M. H., Mohd Zaini, N. A., Raja-Razali, R. B., Ushidee-Radzi, M. A., Ilham, Z., & Wan-Mohtar, W. A. A. Q. I. (2025). Smart Fermentation Technologies: Microbial Process Control in Traditional Fermented Foods. Fermentation, 11(6), 323. https://doi.org/10.3390/fermentation11060323









