Characterizations and In Vitro Gut Microbiome Modulatory Effects of Gluco-Oligosaccharides Synthesized by the Acceptor Reactions of Glucansucrase 53
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Production of Gluco-Oligosaccharides with the Acceptor Reaction of GS53
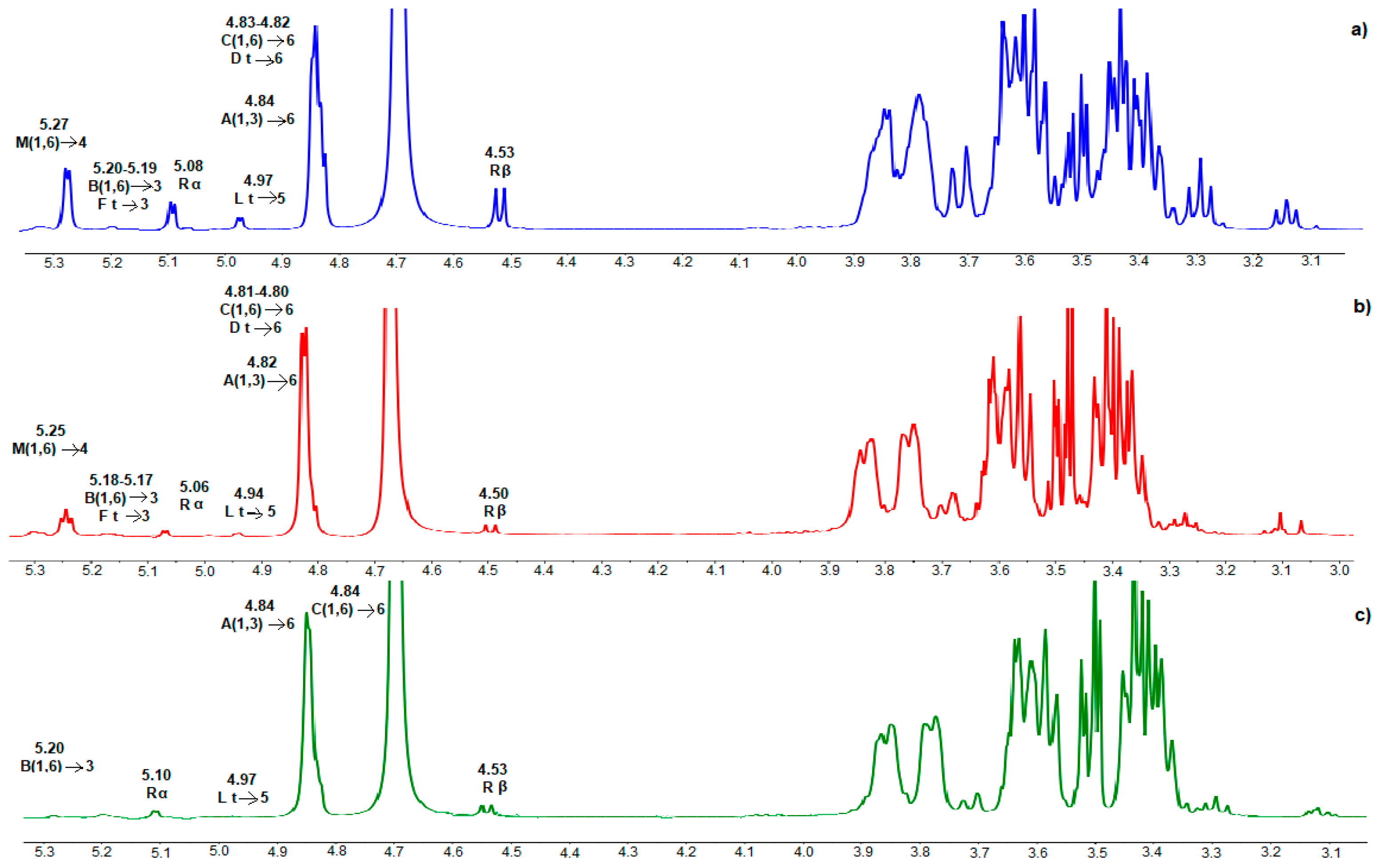
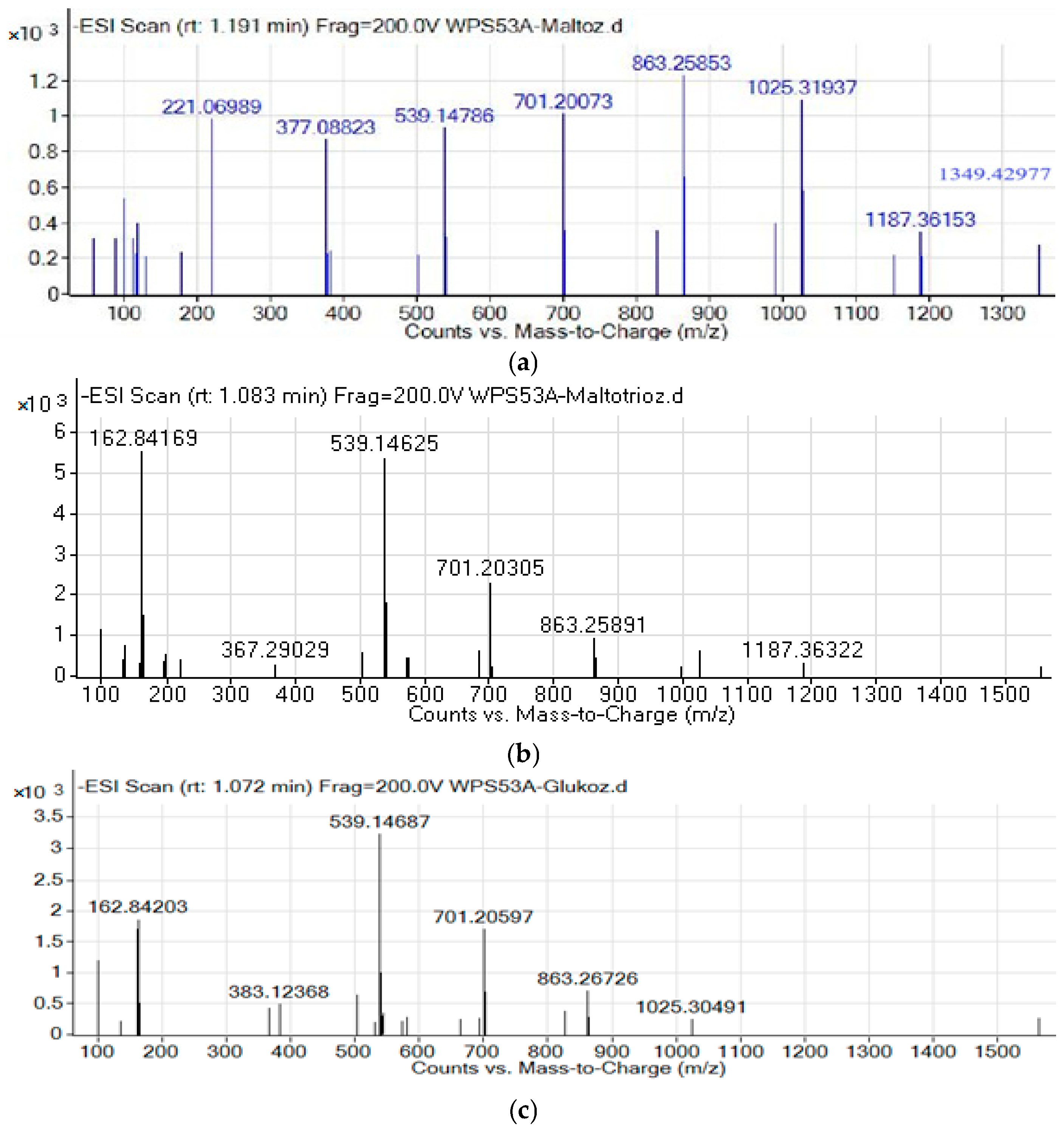
2.3. Structural Characterization of Gluco-Oligosaccharides by NMR and LC-MS/QTOF
2.4. In Vitro Fecal Fermentation of Gluco-Oligosaccharides
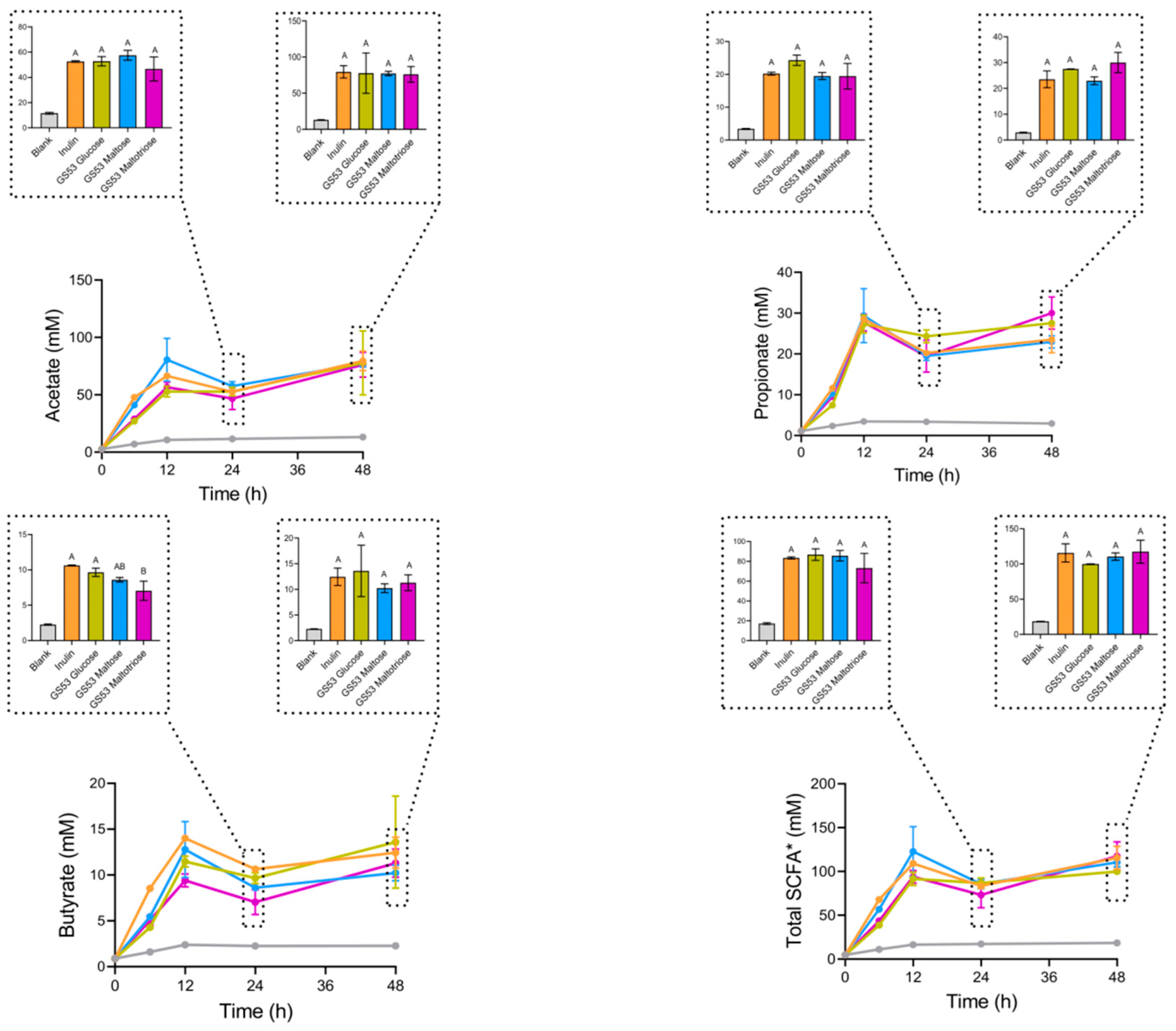
2.5. Determination of SCFA Levels During Fecal Fermentation
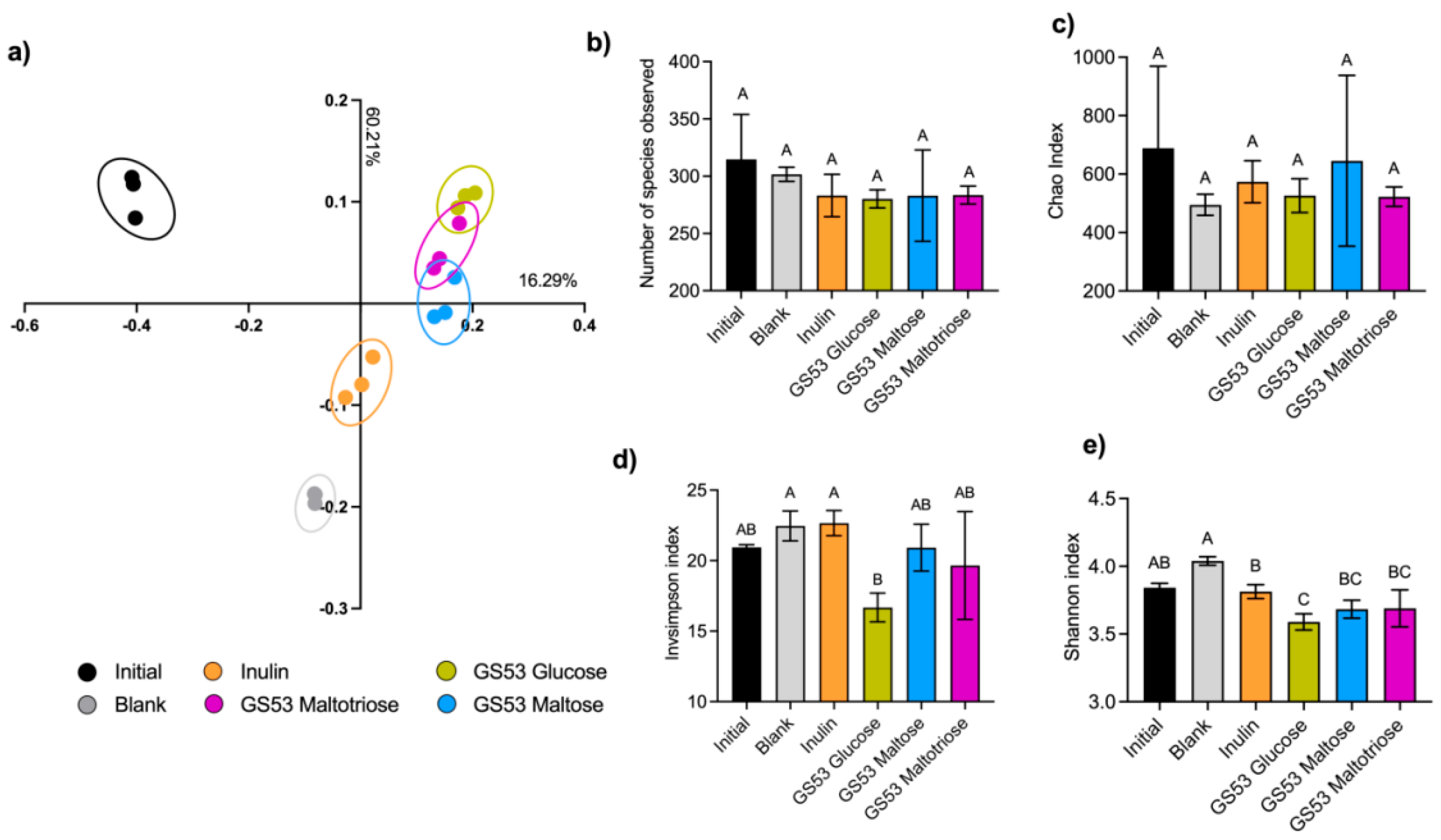
2.6. Microbiota Composition Analysis
2.6.1. Extraction of DNA from Fecal Material
2.6.2. 16S rRNA Sequencing, Sequence Processing and Community Analysis
2.7. Bioinformatics and Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Gluco-Oligosaccharides Produced by GS53 Acceptor Reactions
3.2. GS53 Gluco-Oligosaccharides Induced Short-Chain Fatty Acid (SCFA) Production Comparable to That of Inulin
3.3. GS53 Oligosaccharides Influenced the Structure (β-Diversity) and α-Diversity of Microbial Communities
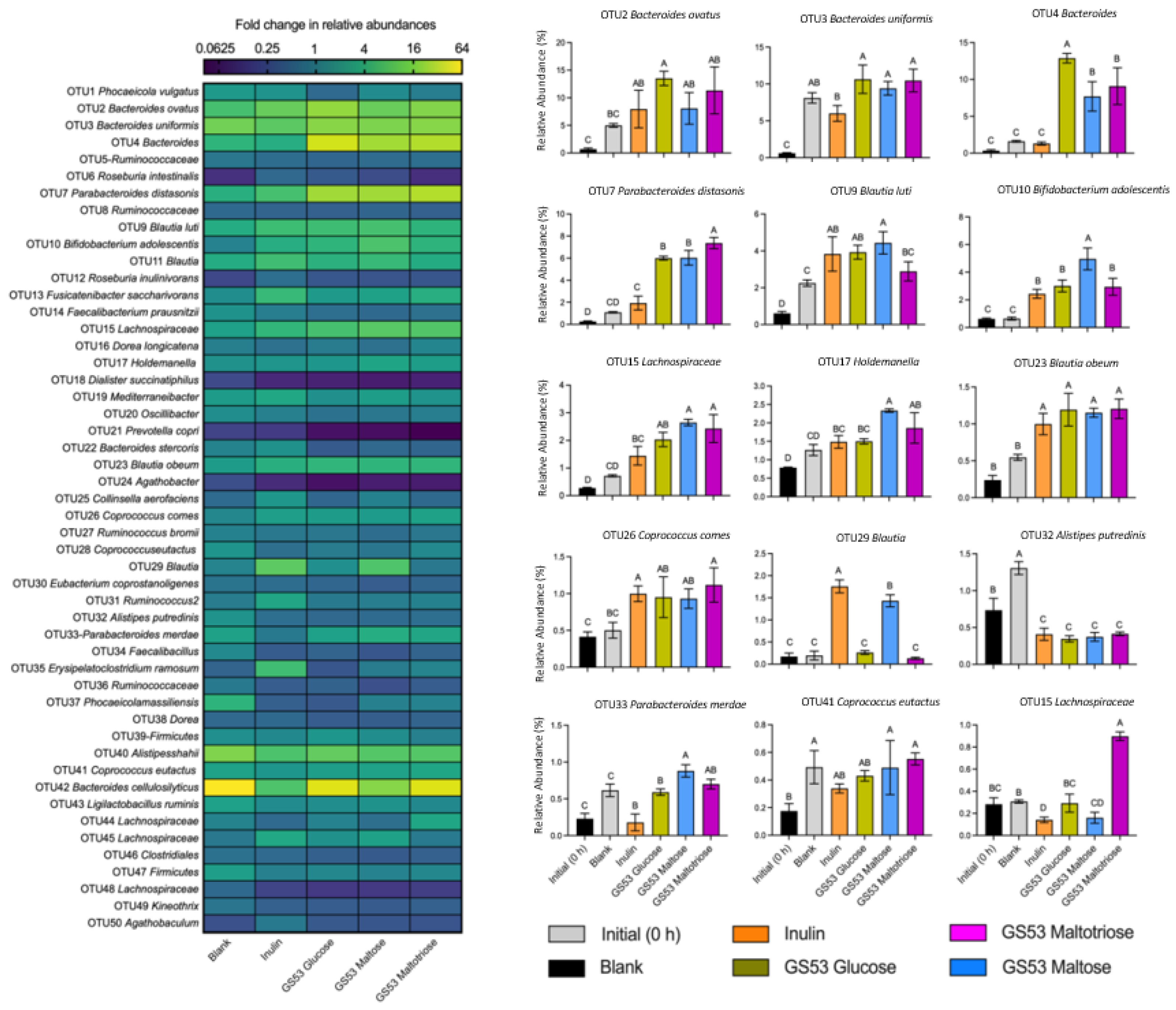
3.4. GS53 Gluco-Oligosaccharides Promoted Butyrate-Producing Bacteria and OTUs Within Bifidobacterium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GS | Glucansucrase |
| SCFA | Short-chain fatty acid |
| OTU | Operational taxonomic unit |
References
- Rolim, P.M. Development of prebiotic food products and health benefits. Food Sci. Technol. 2015, 35, 3–10. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a tool for the prevention and treatment of obesity and diabetes: Classification and ability to modulate the gut microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shi, B. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Li, Q.; Zhang, C.; Zhang, L.; Qu, H.; Wang, W.; Zhou, Y.; Huang, Y.; Xiao, L.; et al. Effect of complex prebiotics on the intestinal colonization ability of Limosilactobacillus fermentum DALI02. Fermentation 2022, 9, 25. [Google Scholar] [CrossRef]
- Manning, T.S.; Gibson, G.R. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Mano, M.C.R.; Neri-Numa, I.A.; Da Silva, J.B.; Paulino, B.N.; Pessoa, M.G.; Pastore, G.M. Oligosaccharide biotechnology: An approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018, 102, 17–37. [Google Scholar] [CrossRef]
- Chavan, A.R.; Singh, A.K.; Gupta, R.K.; Nakhate, S.P.; Poddar, B.J.; Gujar, V.V.; Purohit, H.J.; Khardenavis, A.A. Recent trends in the biotechnology of functional non-digestible oligosaccharides with prebiotic potential. Biotechnol. Genet. Eng. Rev. 2023, 39, 465–510. [Google Scholar] [CrossRef]
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; van Leeuwen, S.S.; Kralj, S.; Dijkstra, B.W.; Dijkhuizen, L. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 2013, 163, 250–272. [Google Scholar] [CrossRef]
- Miao, M.; Ma, Y.; Jiang, B.; Cui, S.W.; Jin, Z.; Zhang, T. Characterisations of Lactobacillus reuteri SK24. 003 glucansucrase: Implications for α-gluco-poly-and oligosaccharides biosynthesis. Food Chem. 2017, 222, 105–112. [Google Scholar] [CrossRef]
- İspirli, H.; Kaya, Y.; Dertli, E. Bifidogenic effect and in vitro immunomodulatory roles of melibiose-derived oligosaccharides produced by the acceptor reaction of glucansucrase E81. Process Biochem. 2020, 91, 126–131. [Google Scholar] [CrossRef]
- Côté, G.L.; Holt, S.M.; Miller-Fosmore, C. Prebiotic Oligosaccharides via Alternansucrase Acceptor Reactions; ACS Publications: Washington, DC, USA; Columbus, OH, USA, 2003; pp. 76–89. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Tuncil, Y.E.; Thakkar, R.D.; Arioglu-Tuncil, S.; Hamaker, B.R.; Lindemann, S.R. Subtle variations in dietary-fiber fine structure differentially influence the composition and metabolic function of gut microbiota. mSphere 2020, 5, e00180-20. [Google Scholar] [CrossRef] [PubMed]
- İspirli, H.; Dertli, E. Production of lactose derivative hetero-oligosaccharides from whey by glucansucrase E81 and determination of prebiotic functions. LWT 2021, 137, 110471. [Google Scholar] [CrossRef]
- İspirli, H.; Bowman, M.J.; Skory, C.D.; Dertli, E. Synthesis and characterization of Bifidogenic raffinose-derived oligosaccharides via acceptor reactions of glucansucrase E81. LWT 2021, 147, 111525. [Google Scholar] [CrossRef]
- Kabli, M.; İspirli, H.; Balubaid, M.; Taylan, O.; Yılmaz, M.T.; Dertli, E. Optimization of lactose derivative hetero-oligosaccharides production using whey as the acceptor molecule by an active glucansucrase. Biocatal. Biotransform. 2022, 40, 9–16. [Google Scholar] [CrossRef]
- Hu, X.; Song, L.; Yang, Y.; Jin, Z.; Miao, M. Synthesis of potential prebiotic α-glucooligosaccharides using microbial glucansucrase and their in vitro fecal fermentation. Food Funct. 2020, 11, 1672–1683. [Google Scholar] [CrossRef]
- Gu, J.; Jiao, Z.; Wang, T.; Zhang, B.; Zhao, H. Glucans with Different Degrees of Polymerization from Leuconostoc mesenteroides CICC6055: Analysis of Physicochemical Properties and Intestinal Prebiotic Function. Int. J. Mol. Sci. 2023, 25, 258. [Google Scholar] [CrossRef]
- Ernst, L.; Offermann, H.; Werner, A.; Wefers, D. Comprehensive structural characterization of water-soluble and water-insoluble homoexopolysaccharides from seven lactic acid bacteria. Carbohydr. Polym. 2024, 323, 121417. [Google Scholar] [CrossRef]
- İspirli, H.; Yüzer, M.O.; Skory, C.; Colquhoun, I.J.; Sağdıç, O.; Dertli, E. Characterization of a glucansucrase from Lactobacillus reuteri E81 and production of malto-oligosaccharides. Biocatal. Biotransform. 2019, 37, 421–430. [Google Scholar] [CrossRef]
- Hu, Y.; Winter, V.; Chen, X.Y.; Gänzle, M.G. Effect of acceptor carbohydrates on oligosaccharide and polysaccharide synthesis by dextransucrase DsrM from Weissella cibaria. Food Res. Int. 2017, 99, 603–611. [Google Scholar] [CrossRef] [PubMed]
- İspirli, H.; Colquhoun, I.J.; Şahin, E.; Sagdic, O.; Dertli, E. Preparation of gentiobiose-derived oligosaccharides by glucansucrase E81 and determination of prebiotic and immune-modulatory functions. Carbohydr. Res. 2019, 486, 107837. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.D.; Tuncil, Y.E.; Hamaker, B.R.; Lindemann, S.R. Maize bran particle size governs the community composition and metabolic output of human gut microbiota in in vitro fermentations. Front. Microbiol. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Meyer, A.; Johnson, P.J.; Ericsson, A.C. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PLoS ONE 2015, 10, e0143334. [Google Scholar] [CrossRef]
- Wagner MacKenzie, B.; Waite, D.; Taylor, M.W. Evaluating variation in human gut microbiota profiles due to DNA extraction method and inter-subject differences. Front. Microbiol. 2015, 6, 130. [Google Scholar] [CrossRef]
- Lindemann, S.R.; Moran, J.J.; Stegen, J.C.; Renslow, R.S.; Hutchison, J.R.; Cole, J.K.; Dohnalkova, A.C.; Tremblay, J.; Singh, K.; Malfatti, S.A.; et al. The epsomitic phototrophic microbial mat of Hot Lake, Washington: Community structural responses to seasonal cycling. Front. Microbiol. 2013, 4, 323. [Google Scholar] [CrossRef]
- Tuncil, Y.E.; Thakkar, R.D.; Arioglu-Tuncil, S.; Hamaker, B.R.; Lindemann, S.R. Fecal microbiota responses to bran particles are specific to cereal type and in vitro digestion methods that mimic upper gastrointestinal tract passage. Food Chem. 2018, 66, 12580–12593. [Google Scholar] [CrossRef]
- Tuncil, Y.E.; Thakkar, R.D.; Marcia, A.D.R.; Hamaker, B.R.; Lindemann, S.R. Divergent short-chain fatty acid production and succession of colonic microbiota arise in fermentation of variously-sized wheat bran fractions. Sci. Rep. 2018, 8, 16655. [Google Scholar] [CrossRef]
- Dobruchowska, J.M.; Meng, X.; Leemhuis, H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P. Gluco-oligomers initially formed by the reuteransucrase enzyme of Lactobacillus reuteri 121 incubated with sucrose and malto-oligosaccharides. Glycobiology 2013, 23, 1084–1096. [Google Scholar] [CrossRef]
- Bivolarski, V.; Vasileva, T.; Gabriel, V.; Iliev, I. Synthesis of glucooligosaccharides with prebiotic potential by glucansucrase URE 13–300 acceptor reactions with maltose, raffinose and lactose. Eng. Life Sci. 2018, 18, 904–913. [Google Scholar] [CrossRef]
- Meng, X.; Pijning, T.; Tietema, M.; Dobruchowska, J.M.; Yin, H.; Gerwig, G.J.; Kralj, S.; Dijkhuizen, L. Characterization of the glucansucrase GTF180 W1065 mutant enzymes producing polysaccharides and oligosaccharides with altered linkage composition. Food Chem. 2017, 217, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Miao, M.; Jiang, B.; Xu, T.; Cui, S.W.; Zhang, T. Leuconostoc citreum SK24. 002 glucansucrase: Biochemical characterisation and de novo synthesis of α-glucan. Int. J. Biol. Macromol. 2016, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Cherbut, C. Inulin and oligofructose in the dietary fibre concept. Br. J. Nutr. 2002, 87 (Suppl. S2), S159–S162. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Yang, J.; Saito, M.; Hartanto, T.; Nakayama, Y.; Ichinohe, T.; Fukuda, S. Prebiotic inulin ameliorates SARS-CoV-2 infection in hamsters by modulating the gut microbiome. npj Sci. Food 2024, 8, 18. [Google Scholar] [CrossRef]
- Leser, T.; Baker, A. Bifidobacterium adolescentis—A beneficial microbe. Benef. Microbes 2023, 14, 525–551. [Google Scholar] [CrossRef]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Immunol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Z.; Wang, Q.; Wu, C.; Sun, Y.; Wang, Z.; Xu, X.; Xue, W.; Cao, Z.; Zhang, M.; et al. Bacteroides methylmalonyl-CoA mutase produces propionate that promotes intestinal goblet cell differentiation and homeostasis. Cell Host Microbe 2024, 32, 63–78.e7. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Romero Marcia, A.D.; Yao, T.; Chen, M.H.; Oles, R.E.; Lindemann, S.R. Fine carbohydrate structure of dietary resistant glucans governs the structure and function of human gut microbiota. Nutrients 2021, 13, 2924. [Google Scholar] [CrossRef]
- Qu, Z.; Liu, H.; Yang, J.; Zheng, L.; Huang, J.; Wang, Z.; Xie, C.; Zuo, W.; Xia, X.; Sun, L.; et al. Selective utilization of medicinal polysaccharides by human gut Bacteroides and Parabacteroides species. Nat. Commun. 2025, 16, 638. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Ma, X.; Zhao, J.-C.; Wang, X.-Q.; Gao, C.-Q. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes 2023, 15, 2190300. [Google Scholar] [CrossRef]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef]
- Sun, Y.; Nie, Q.; Zhang, S.; He, H.; Zuo, S.; Chen, C.; Yang, J.; Chen, H.; Hu, J.; Li, S.; et al. Parabacteroides distasonis ameliorates insulin resistance via activation of intestinal GPR109a. Nat. Commun. 2023, 14, 7740. [Google Scholar] [CrossRef]
- Duan, J.; Li, Q.; Cheng, Y.; Zhu, W.; Liu, H.; Li, F. Therapeutic potential of Parabacteroides distasonis in gastrointestinal and hepatic disease. MedComm 2024, 5, e70017. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The role of next-generation probiotics in obesity and obesity-associated disorders: Current knowledge and future perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayaman, R.Y.; Alkay, Z.; Ispirli, H.; Arioglu-Tuncil, S.; Dere, S.; Can, H.; Alvarez Gonzales, M.A.; Sagdic, O.; Lindemann, S.R.; Tuncil, Y.E.; et al. Characterizations and In Vitro Gut Microbiome Modulatory Effects of Gluco-Oligosaccharides Synthesized by the Acceptor Reactions of Glucansucrase 53. Fermentation 2025, 11, 324. https://doi.org/10.3390/fermentation11060324
Bayaman RY, Alkay Z, Ispirli H, Arioglu-Tuncil S, Dere S, Can H, Alvarez Gonzales MA, Sagdic O, Lindemann SR, Tuncil YE, et al. Characterizations and In Vitro Gut Microbiome Modulatory Effects of Gluco-Oligosaccharides Synthesized by the Acceptor Reactions of Glucansucrase 53. Fermentation. 2025; 11(6):324. https://doi.org/10.3390/fermentation11060324
Chicago/Turabian StyleBayaman, Rabia Yusra, Zuhal Alkay, Humeyra Ispirli, Seda Arioglu-Tuncil, Sevda Dere, Hasan Can, Miguel Angel Alvarez Gonzales, Osman Sagdic, Stephen R. Lindemann, Yunus Emre Tuncil, and et al. 2025. "Characterizations and In Vitro Gut Microbiome Modulatory Effects of Gluco-Oligosaccharides Synthesized by the Acceptor Reactions of Glucansucrase 53" Fermentation 11, no. 6: 324. https://doi.org/10.3390/fermentation11060324
APA StyleBayaman, R. Y., Alkay, Z., Ispirli, H., Arioglu-Tuncil, S., Dere, S., Can, H., Alvarez Gonzales, M. A., Sagdic, O., Lindemann, S. R., Tuncil, Y. E., & Dertli, E. (2025). Characterizations and In Vitro Gut Microbiome Modulatory Effects of Gluco-Oligosaccharides Synthesized by the Acceptor Reactions of Glucansucrase 53. Fermentation, 11(6), 324. https://doi.org/10.3390/fermentation11060324






