Abstract
Probiotics play a significant role in human nutrition. Heat stress is one of the most important factors leading to the inactivation and loss of viability of probiotics during their production. Therefore, there is a need to develop probiotics that can withstand higher temperatures during processing and storage. Thermotolerant bacteria are a group of microorganisms that survive at elevated temperatures due to the presence of active proteins in bacterial cells. In this review, the molecular mechanisms underlying the maintenance of the structural integrity of beneficial bacterial cells during heat stress are discussed. In addition, the possibilities of stimulating the resistance and adaptation of bacterial cells to high temperature are indicated. Finally, the possibilities of application of such solutions in the food industry are discussed. On the basis of a thorough literature review, it is concluded that it is still worth continuing the research on the mechanisms of resistance to heat stress in beneficial bacteria, because the molecular mechanisms of this process are not fully understood. It is believed that such knowledge could accelerate the development of the probiotic market in the future.
1. Introduction
The term “beneficial bacteria” in relation to human health could be defined as microorganisms that serve several health benefits to the host through the gut–microbiota interactions. The specific anatomical, cellular, and molecular characteristics cause the human gut to be a key habitat for beneficial bacteria growth. During our lives, we come into contact with a large and diverse population of different environmental microorganisms, especially derived from fermented food, soil, and water, that inhabit the human gut. Therefore, the gut microbiota comprises bacteria that have been evolutionarily selected for their ability to survive and proliferate in the human digestive tract [1]. Evidence suggests that gut bacteria play a crucial role in maintaining human health. It has been proven that beneficial gut bacteria could train the immune system, prevent the growth of pathogens, regulate gut cell development, maintain epithelial integrity, and shape neuronal cell development. The mechanisms of action of these bacteria are connected with the production of specific metabolites like organic acids, short-chain fatty acids, bacteriocins, and many others [2].
The Human Microbiome Project (HMP) has provided significant evidence of a link between microbiota and human health [3]. In recent years, attempts have been made to manipulate the human gut microbiota positively. The tools in these processes are specific microorganisms isolated from the digestive tract of healthy people, which, when administered as supplements or in the form of functional foods, can affect the microbiome. These bacteria with beneficial properties are called probiotics. Probiotics are defined as ’live microorganisms which, when administered in adequate amounts, confer health benefits on the host’ [4]. Bacteria included in the former Lactobacillus and Bifidobacterium genera and yeasts like Saccharomyces boulardii are the most often utilized probiotic species. All of the mentioned lactobacilli and bifidobacteria belong to the informal group of lactic acid bacteria (LAB), producers of lactic acid, that hold significant importance in diverse fields, including the food industry as adjunct starter cultures, industrial biotechnology, and medicine. However, there are also other bacteria that have been identified as probiotics through their specific characteristics, for example, Enterococcus, Streptococcus, Pediococcus, Leuconostoc, Bacillus, and E. coli, as well as next-generation probiotics like Akkermansia, Faecalibacterium, Roseburia, and others [5].
There are several challenges to the use of probiotics in food. First of all, bacteria must be alive and present in food in adequate amounts, meaning at least 109 CFU/g. However, the adequate dose is strain-specific and depends on the bacterial strain properties, target group, method, and form of administration [4]. Moreover, the bacteria must be resistant to preservation methods, mostly freeze-drying or spray-drying, and storage conditions to be suitable for use in food technology. All these processes involve several stress factors, including temperature fluctuations, osmotic and pH shocks, exposure to oxidants and ultraviolet radiation, substrate deprivation, mechanical damage, and many others, which can affect the survival of probiotic bacteria. To survive these adversities, probiotics use a wide range of stress-response strategies, for example, metabolic rearrangement, expression of specialized biomolecules (e.g., chaperones and antioxidants), synthesis of exopolysaccharides, and complex repair and regulatory systems [6,7]. Most of the above-mentioned features can develop in subsequent generations through adaptation. Analysis of recent findings in this field leads to the conclusion that bacteria can enhance stress resistance mechanisms when exposed to minor stress factors [8].
Therefore, the aim of this work was to review the current state of knowledge in the field of thermotolerant bacteria, heat-adaptation mechanisms, and technical approaches to increase the stress resistance of beneficial bacteria. The possibilities of applying these solutions in food technology are also discussed.
2. The Mechanism Underlying Heat Resistance in Beneficial Bacteria
All free-living microbes are often exposed to environmental stress conditions, one of which is temperature changes. The heat-shock response is one of the well-known physiological characteristics of the cell. Living bacterial cells respond to a sudden increase in temperature by changes in gene expression, resulting in the secretion of proteins called heat-shock proteins (HSPs). Most of the HSP genes are highly conserved during evolution, indicating that the HSP function has been conserved among diverse organisms. The HSPs assist in folding, assembling, transporting, and degrading proteins. The two most common classes of HSPs are molecular chaperones and ATP-dependent proteases. Whereas the chaperones prevent misfolding and aggregation of partially denatured proteins, proteases are involved in the degradation of denatured proteins [7,8].
The most commonly tested model microorganism is Escherichia coli. One of the E. coli strains, Nissle 1917, is also considered a probiotic. The first extensive pulse-labeling experiments that revealed the characteristic profile of E. coli HSPs started in the early 1970s. It was found that the heat-shock response to temperature upshift from 30 to 42 °C consists of the rapid, up to 15-fold, induction of the synthesis of more than 20 HSPs, followed by an adaptation period where the rate of HSP synthesis decreases to reach a new steady-state level [9]. Further research has shown that the E. coli heat-shock response is positively controlled at the transcriptional level by the product of the rpoH gene, the heat-shock promoter-specific sigma factor σ32 of RNA polymerase. When the environmental temperature increases, a rapid increase in σ32 levels occurs, which indicates HSP production. The DnaK, DnaJ, and GrpE heat-shock proteins act as negative modulators by mediating the repression of rpoH mRNA translation during the shut-off phase of the heat-shock response, as well as an efficient degradation and repression of σ32 activity. This mode of thermotolerance regulation allows E. coli cells to respond rapidly to sudden stress [10].
On the other hand, B. subtilis is the model organism for the Gram-positive bacteria. Bacilli are spore-forming bacteria, usually tolerant to environmental stress factors like heat, acid, bile, or salt. Because of their ability to smoothly pass through the human gastrointestinal tract, some of the Bacillus coagulans and Bacillus licheniformis strains are considered probiotics [11]. It was found that the heat-shock response of B. subtilis is composed of at least five classes of heat-shock-inducible genes: class I genes, encoding the chaperones DnaK, GroES, and GroEL, are controlled by the HrcA repressor; class II genes encode general stress proteins, and their expression is dependent on the σB sigma factor; class III heat-shock genes are regulated by CtsR (Class three stress gene Repressor), which recognizes a specific tandem direct repeat; class IV genes are not controlled by HrcA, σB, or CtsR; and class V genes are regulated by the two-component system [7,12]. It appears that chaperones play a crucial role in the temperature resistance of bacteria. The thermotolerance feature of Bacillus species is also related to lipid functions. The fatty acid composition changes when the temperature increases. It was found that temperature adaptation in heat stress is regulated by changing the composition of iso/anteiso fatty acids in bacterial cells [13]. Moreover, during heat stress, the fluidity of the bacterial membrane increases, aiming to maintain the proton permeability of the cytoplasmic membrane at a constant level [14]. The additional mechanism that helps withstand the high temperature stress is hopanoids, which are inserted into the bacterial membrane. This structure possibly plays a role in counteracting the increased fluidity of the cell membrane [15].
Heat stress is also commonly present in many LAB environments. High temperatures (50–60 °C) are used for the pasteurization of raw materials or during the fermentation processes. Physiologically, high temperature can increase the membrane fluidity and, consequently, affect cell activity. To prevent the degradation of bacterial cells, specific and diverse adaptation mechanisms are applied; for instance, the synthesis of specialized biomolecules that help bacterial cells to adapt and withstand adverse conditions. The secreted proteins include HSPs, phosphoenolpyruvate-protein phosphotransferase, chaperones, chaperonins, and cofactors that are regulated by CtsR- and/or HrcA-encoding genes and conserved binding sites in the upstream regions of several clp genes. Under heat stress conditions, the expression of chaperones and proteases is rapidly increased. They can refold the denatured protein to the correct configuration or degrade it when damage is irreversible. Proteases serve as a last line of defense, restoring homeostasis and promoting the recycling of denatured protein amino acids. Moreover, proteins that have been damaged are folded to the correct configuration by chaperones, like the well-known DnaK (heat-shock protein). They are crucial for the heat stress response, facilitating the correct packaging and ultimate transport of nascent polypeptides [8]. Interestingly, several LAB HSPs, known as lipochaperones, can attach and stabilize cell membranes. However, these heat-shock response mechanisms are diverse and rather poorly characterized in LAB [7,16]. In addition to the above-mentioned mechanisms, probiotic bacteria that grow under heat stress produce specific saturated and straight-chain fatty acids, which help reduce the membrane fluidity. It is well known that heat stress increases cell membrane flexibility, affects cell activity, and may cause irreversible damage or death of the cell [16]. Moreover, exopolysaccharide production [17] and the presence of DNA-binding proteins, biomolecules akin to DNA in cells [18], can serve as heat protection factors.
To sum up, taking into account the recent studies regarding beneficial bacteria, several response mechanisms to thermal stress can be identified: chaperone complexes, such as GroEL and GroES production; induction of small heat-shock proteins (sHSP); degradation of misfolded and aggregated proteins; synthesis/upregulation of HSPs with protease activity; and increasing S-layer production [19,20,21,22]. It is also worth underlining that heat resistance stress response is strain-dependent and could be influenced by genetic variations, as well as environmental factors, such as culture media, NaCl concentration, aw, pH, and other environmental factors [7,16].
3. Methods for Adapting Lactic Acid Bacterial Cells to High Temperatures
Methods of inducing thermotolerance in LAB can be generally divided into three categories: exposure to environmental stress prior to heat challenge, adaptive laboratory evolution, and genetic modifications. All of these have been used successfully to improve LAB tolerance to heat, as shown in Table 1.
3.1. Environmental Stress
Exposure to environmental stress before subjecting LAB to high temperatures, also known as stress preadaptation, was shown to yield improved thermoadaptation. For example, Yang et al. [23] have demonstrated that exposing Tetragenococcus halophilus CGMCC 3792 to heat at 45 °C for 1.5 h preceding a heat challenge at 60 °C for 2.5 h increased its thermotolerance 18-fold. It was found that LAB can exhibit cross-protection—exposition to one type of stress may induce tolerance towards other types of stress. This was demonstrated by Shin et al. [24], where exposure to an acid stress (pH 5.0 for 30 min) improved Enterococcus faecium HL7 heat survival rate 17-fold. Another example was provided by Scheyhing et al. [25] in subjecting Lactobacillus sanfranciscensis DSM 20451T to sublethal high pressure of 80 MPa before exposure to 50 °C for 30 min. Such preadaptation improved the resistance to heat 3.6-fold. Also, the media supplementation might be used in conjunction with environmental stress preadaptation to improve bacterial thermotolerance. Yang et al. [26] employed heat preadaptation at 45 °C for 90 min together with 10% skim milk, 30% skim milk, 10% sucrose, and 5% trehalose media additions to improve Tetragenococcus halophilus CGMCC 3792 survival rates during spray drying. The addition of 10% skim milk proved to be the most effective in increasing survival rates, bettering them 3.71-fold. Milk media supplementation was also used by Suo et al. [27] to increase the thermotolerance of Lactobacillus casei Zhang, Lactobacillus plantarum P8, Lactobacillus rhamnosus GG, and Lactobacillus casei BL23. Bacteria grown with total reconstituted skim milk solid contents of 5.0, 10.0, 20.0, 25.0, 30.0, 35.0, and 40.0 wt% were exposed to a heat challenge of 65 °C or 70 °C for 10 min. The addition of 10.0–20.0 wt% skim milk solids proved most effective in increasing the heat tolerance. By supplementing the growth media of both Lactococcus lactis ssp. cremoris and Lactobacillus acidophilus NCFM with 10 mM CaCl2, Wang et al. [28] were able to improve spray-drying survival rates from 49.9% to 64.9% and 35.5% to 43.3%, respectively.

Table 1.
Methods applied to induce improved thermotolerance in lactic acid bacteria: selected examples.
Table 1.
Methods applied to induce improved thermotolerance in lactic acid bacteria: selected examples.
| Method | Organism | Adaptation Conditions | Results | Reference |
|---|---|---|---|---|
| Environmental stress | Lactobacillus acidophilus NCFM | Initial selection for thermotolerance: incubation at 65 °C for 10, 15, 20, 25, 30, 35, and 40 min. | Heat challenge: 65 °C for 10, 15, 20, 25, 30, 35, and 40 min. At least a 1-log higher percent survival in comparison to wild-type L. acidophilus; survival ratio up to 1000-fold higher than wild type was achieved. | [29] |
| Enterococcus faecium SFM1; SFM2 | Pasteurization—usually heated at 62.5 °C for 30 min or at 72–75 °C for 15 s(sic). | Heat challenge: 50 °C for 120 min. Heat-preadapted (pasteurized) E. faecium SFM1 and E. faecium SFM2 survived incubation at 50 °C for 2 h at rates of 28.20 ± 0.04% and 82.58 ± 0.01%, respectively. | [30] | |
| Tetragenococcus halophilus CGMCC 3792 | 45 °C for 90 min in 10% skim milk, 30% skim milk, 10% sucrose, and 5% trehalose. | Heat challenge: 120 °C for an unspecified amount of time (spray drying). Survival increased in 10% skim milk, 30% skim milk, 10% sucrose, and 5% trehalose 3.71-, 1.96-, 1.69-, and 2.32-fold, respectively. Spray drying conditions (as specified in the paper): inlet/outlet temperature = 120/75 °C, volumetric airflow = 40 m3/h, feed rates = 7.5 mL/min, and atomizing pressure < −50 mbar. | [26] | |
| Lactococcus lactis ssp. cremoris; Lactobacillus acidophilus NCFM; Lactobacillus rhamnosus GG. | Cultured at following temperatures: L. lactis, 33 °C; L. acidophilus, 42 °C; L. rhamnosus, 42 °C. | Heat challenge: 60 °C for 2, 4, 6, 8, 10, 12, and 14 min (L. cremoris); 60 °C for 6 min (L. rhamnosus); 60 °C for 4 min (L. acidophilus). Both L. cremoris and L. rhamnosus cultures showed increased survival rates compared to non-pretreated cultures. Preadapted L. acidophilus cultures showed lower survival rates than non-pretreated cultures. When L. acidophilus culture incubation time at preadaptation temperature (42 °C) was doubled (12 to 24 h), survival rates increased (from 60.1% to 63.7%), but viability decreased (from 8.2 to 6.8 log CFU/mL). | [31] | |
| Lactobacillus casei Zhang; Lactobacillus plantarum P8; Lactobacillus rhamnosus GG; Lactobacillus casei BL23. | Reconstituted milk powder; total solids contents of 5.0, 10.0, 20.0, 25.0, 30.0, 35.0, and 40.0 wt%; stationary incubation at 37 °C for 36 h. | Heat challenge: 65 °C or 70 °C for 10 min. Reconstituted skim milk with 20–30 wt% solid content proved most efficient in increasing survival rates of heat-challenged LAB. | [27] | |
| Environmental stress | Lactobacillus plantarum KLDS 1.0628 | 45 °C for 1 h, 15 °C for 1 h; 1 mmol/L H2O2 for 1 h at 37 °C; pH 4.0 for 1 h at 37 °C; 0.2% bile salt for 1 h at 37 °C; and 2% NaCl for 1 h at 37 °C. | Heat challenge: 60 °C for 1 h. Heat preadaptation proved most effective—heat tolerance of preadapted bacteria increased 31.38-fold in comparison to non-preadapted cells. | [32] |
| Tetragenococcus halophilus CGMCC 3792 | 45 °C for 1.5 h | Heat challenge: 60 °C for 2.5 h. Thermotolerance increased 18-fold. | [23] | |
| Lactococcus lactis ssp. cremoris; Lactobacillus acidophilus NCFM | Media supplementation with CaCl2. | Heat challenge: 97 +/− 5 °C for an unspecified amount of time (spray-drying). Both L. lactis and L. acidophilus grown with 10 mM CaCl2 showed enhanced thermotolerance with increased spray-drying survival rates from 49.9% to 64.9% and 35.5% to 43.3%, respectively. Spray-drying conditions (as specified in the paper): […] inlet and out temperatures were controlled at 97 +/− 5 and 58 +/− 2 °C. Mass flow rate was around 0.85 +/− 0.08 g/min. | [28] | |
| Enterococcus faecium HL7 | 15 min at 52 °C; exposure to sublethal levels of acid (pH 5.0 for 30 min). | Heat challenge: 60 °C for 40 min. Heat-adapted E. faecium survival rate was 103–105-fold higher than that of non-pretreated culture. Acid-adapted E. faecium survival rate was 17-fold higher than that of non-pretreated culture; cell viability equaled 92.73% and 5.19%, respectively. | [24] | |
| Lactobacillus rhamnosus hsryfm 1301 | Pretreatment under 50 °C(sic); 0.5 mM H2O2 for 1 h. | Heat challenge: 54 °C for 60 min. 0.5 mM H2O2-pretreated L. rhamnosus survival rates increased from approximately 5.5 Lg(CFU/mL) to approx. 7.8 Lg(CFU/mL). Heat-pretreated L. rhamnosus survival rates increased from approximately 5.5 Lg(CFU/mL) to approx. 7.5 Lg(CFU/mL). | [33] | |
| Lactobacillus kefiranofaciens M1 | 37 °C for 1 h, 20 °C for 1 h, pH 5.0 for 1 h at 30 °C, and 0.05% bile salts for 1 h at 30 °C. | Heat challenge: 52 °C for 2 h. Heat challenge survival rates were the highest in heat-adapted cells (0.21%), followed by bile salt- adapted cells (0.18%) then acid-adapted cells (0.07%). | [34] | |
| Environmental stress | Lactiplantibacillus plantarum Lp 790; Lp 813; Lp 998 | 45 °C for 30 min. | Heat challenge: 52 °C for 15 min. Thermal adaptation (30 min) clearly improved cell resistance against thermal shock. | [35] |
| Lactobacillus sanfranciscensis DSM 20451T | 1.9% NaCl at 30 °C; pH 3.7 at 30 °C; 80 MPa at 30 °C; 43 °C and 12.5 °C. | Heat challenge: 50 °C for 30 min. Application of sublethal high pressure (80 MPa) pretreatment resulted in increased heat resistance (3.6-fold). | [25] | |
| Adaptative Laboratory Evolution | L. lactis ssp. lactis bv. diacetylactis SD96. | ALE performed for 400 generations: 67 generations at 39 °C; 83 at 40 °C; and 250 generations at 41 °C. | Strains RD01 (150th generation isolate) and RD07 (400th generation isolate) acidified milk faster than parent strain at 30 °C and 37 °C; RD01 acidified milk 1.7-fold slower than RD07 at 40 °C; only RD07 strain could acidify milk at 41 °C. | [36] |
| Lacticaseibacillus casei N; Lactobacillus helveticus NRRL B-4526 | 500 generations cultured at 45 °C. | Biomass increased two-fold when grown at 45 °C in both studied strains. Continued ALE by subculturing at 47 °C for 300 generations, 50 °C for 20 generations, and 55 °C for 10 generations resulted in decreased biomass; not studied further. | [37] | |
| L. acidophilus EG004 | 60 °C for 1 min, then cultivation at 37 °C for 24 h; process repeated twice; identical procedure repeated with rising temperature in increments of 3 °C. Repeated in ranges from 60 °C to 72 °C. | Newly developed L. acidophilus EG008 showed improved thermal resistance (in comparison to L. acidophilus EG004), understood as survival rates in temperatures from 65 °C to 75 °C. | [38] | |
| Lactobacillus delbrueckii ssp. bulgaricus ET45 | Cultivation temperature was raised from 37 °C to 40 °C in increments of 1 °C and then from 40 °C to 45 °C in increments of 0.2 °C. ALE consisted of 68 passages. | Newly developed L. bulgaricus strain was able to perform simultaneous saccharification and fermentation at 45 °C. | [39] | |
| Adaptative Laboratory Evolution | Enterococcus faecium BIOPOP-3 | Screening for thermotolerant bacteria by thermal adaptation starting at 60 °C, rising in increments of 3 °C, then ALE: heat challenge at 75 °C, then cultivation at 37 °C for 24 h, repeated for 25 days. | Newly developed Enterococcus faecium BIOPOP-3 ALE strain showed 75.85% survival rate compared to ~5% survival rate of wild type and heat-preadapted strain. | [40] |
| Genetic modification | Streptococcus thermophilus sp. | Transformation of shsp gene-containing plasmid into S. thermophilus cells deprived of or lacking shsp gene. | Heat challenge: 60 °C for 60 min. Presence of shsp encoding plasmid increased resistance to heat challenge and allowed for growth at 52 °C in different S. thermophilus strains. The shsp gene codes for small heat-shock protein and is present on S. thermophilus plasmid pSt04. | [41] |
| Lactobacillus plantarum WCFS1 | Transient overproduction of hsp 18.5, hsp 18.55, and hsp 19.3 genes. | Heat challenge: 40 °C and 37 °C for 14 h. Unmodified strain viability decreased after heat challenge (~3 log CFU/mL at 37 °C and ~4 log CFU/mL at 40 °C after 14 h); recombinant strain viability was almost unchanged (≤0.2 log CFU/mL and ≤0.5 log CFU/mL, respectively). The hsp 18.5, hsp 18.55, and hsp 19.3 genes code for heat-shock proteins (HSPs) HSP 18.5, HSP 18.55, and HSP 19.3, respectively. | [42] | |
| Lactobacillus salivarius Ren | Homologous overexpression of oppA gene. | Heat challenge: 55 °C for 1 h. Recombinant L. salivarius Ren displayed higher resistance than unmodified strain: A 4 log CFU/mL viability increase was observed (6 log CFU/mL compared to 2 log CFU/mL). The oppA gene codes for a transport protein involved in oligopeptide uptake. | [43] | |
| Lactococcus lactis ssp. cremoris NZ9000 | The uvrA gene overexpression. | Heat challenge: 46 °C for 6 h. UvrA-overexpressing strain survival rate increased 1.22-fold in comparison to non-overexpressing strain. Gene uvrA codes for a protein involved in DNA repair, replication, and recombination. | [44] |
In the studies focusing on methods classified as environmental stress, heat challenge temperatures ranged from 37 °C to 70 °C, with the exception of spray-drying, where the applied temperatures reached as high as 120 °C. Both median and average heat challenge temperatures (excluding spray-drying) amounted to ~60 °C; meanwhile, the median duration equaled 20 min, while the average duration was ~40.5 min.
3.2. Adaptive Laboratory Evolution
Adaptive Laboratory Evolution (ALE) is a method that makes possible the induction of guided changes in populations of microorganisms, in a way mimicking natural evolutionary processes. Its mechanism relies on the accumulation of adaptive changes acquired under prolonged culturing of subsequent microbial generations under specific environmental pressures [45]. Bommasamudram et al. [37] have shown that culturing both Lacticaseibacillus casei N and Lactobacillus helveticus NRRL B-4526 at 45 °C for 500 generations resulted in a two-fold increase in biomass accumulation in comparison to non-evolved strains. Dorau et al. [36] subjected L. lactis ssp. lactis bv. diacetylactis SD96, an industrial LAB strain widely used in cheesemaking, to ALE. The experiments resulted in two evolved strains, RD01 and RD07; this pair acidified milk at a faster rate and at higher temperatures than their shared parent strain. Moreover, L. acidophilus EG004 subjected to ALE in a study carried out by Jeon et al. [38] showed greater heat tolerance than its parent strain when subjected to heat challenge at temperatures from 65 °C to 75 °C. ALE conducted during this study differed somewhat from previously described ones in two aspects. Before being subjected to ALE, bacteria were screened for thermotolerance, and after every 24 h, the bacterial populations were exposed to a successively more intense heat challenge, with temperature rising every two iterations by 3 °C. Another noteworthy way of conducting ALE was carried out by Min et al. [40]. Before subjecting Enterococcus faecium BIOPOP-3 (a strain derived from a fermented dairy product) to ALE, bacteria were preadapted by thermal adaptation starting at 60 °C, rising in increments of 3 °C. After this screening, ALE was performed in a similar way as described by Jeon et al. [38]: heat challenge at 75 °C, then cultivation at 37 °C for 24 h repeated for 25 days. This process resulted in the development of a new strain, Enterococcus faecium BIOPOP-3 ALE, that showed survival rates approx. 70 percentage points greater than wild type (75.85% compared to ~5%, respectively). On the other hand, as described by Vishnu Prasad et al. [39], Lactobacillus delbrueckii ssp. bulgaricus ET45 subjected to ALE of 68 passages, with slowly rising temperatures from 37 °C to 40 °C in increments of 1 °C, and then from 40 °C to 45 °C in increments of 0.2 °C, showed improved thermotolerance in comparison to its parent strain. This was shown in the ability of the evolved strain to grow and perform simultaneous saccharification and fermentation in 45 °C—conditions deemed prohibitive to wild-type Lactobacillus delbrueckii ssp. bulgaricus ET45.
To sum up, the number of generations that ALE consists of varies between studies from under 20, as in ALE implemented by Jeon et al. [38], up to 500, as described by Bommasamudram et al. [37]. ALE temperatures ranged from 39 °C, as used by Dorau et al. [36], to 75 °C, as described by Min et al. [40].
3.3. Genetic Manipulation
Although genetic manipulation of LAB shows promising results regarding heat tolerance [46], the use of genetically modified microorganisms (GMM) in commercially available probiotics is currently restricted in many parts of the world [47]. Despite these limitations, studies aimed at inducing genetic changes in LAB resulting in improved thermotolerance were carried out, although research regarding this specific subject seems to be less developed. As was shown by El Demerdash, Heller, and Geis [41], species of Streptococcus thermophilus lacking the shsp gene that codes for a small heat-shock protein, when transformed with a newly constructed pAGS4E plasmid containing the said gene, demonstrated increased resistance to heat challenge at 60 °C for 60 min and showed growth at 52 °C. Overexpression of genes coding for different heat-shock proteins as a means for improving LAB heat tolerance was studied by Fiocco et al. [42], who analyzed hsp 18.5, hsp 18.55, and hsp 19.3 genes coding the following heat-shock proteins (HSPs): HSP 18.5, HSP 18.55, and HSP 19.3, respectively. Their overexpression in Lactobacillus plantarum WCFS1, achieved via the use of the pGIZ906 vector, resulted in improved thermotolerance. When exposed to heat challenge either at 40 °C or 37 °C for 14 h, recombinant strain viability decreased only slightly (≤0.2 log CFU/mL and ≤0.5 log CFU/mL, respectively) in comparison to the unmodified strain (~3 log CFU/mL at 37 °C and ~4 log CFU/mL decrease at 40 °C after 14 h).
While both previously described studies focused on different heat-shock-protein-coding genes, Wang et al. [43] showed that homologous overexpression of the oppA gene, coding for a transport protein involved in the uptake of oligopeptides, can improve LAB thermotolerance. Overexpression of the aforementioned gene was accomplished by transforming Lactobacillus salivarius Ren with the pNZ8148 plasmid. When subjected to a heat challenge of 55 °C for 1 h, the recombinant strain showed improved thermotolerance, resulting in a 4 log CFU/mL increase in comparison to an unmodified strain. Another study in which bacteria were transformed with the use of pNZ8148 plasmid was carried out by Moghaddam et al. [44] in Lactococcus lactis ssp. cremoris NZ9000. LABs in this study were modified to overexpress the uvrA gene. When exposed to 46 °C for 6 h, the recombinant strain showed a 1.22-fold greater survival rate than the non-overexpressing strain.
In studies focused on methods classified as genetic manipulation, heat challenge temperatures ranged from 37 °C to 60 °C. The median heat challenge temperature was 46 °C, and the average heat challenge temperature came to ~47.6 °C. Heat challenge duration times varied from 1 h to 14 h, with a median of 4 h and an average of 5.5 h.
4. Application of Thermotolerant Beneficial Microorganisms in Food Technology and Industry
Beneficial microorganisms that thrive at relatively high temperatures (typically between 45 and 60 °C) are called thermophilic probiotics [46]. On the other hand, thermotolerant bacteria can tolerate higher temperatures but do not necessarily thrive at them. They are usually mesophilic (optimal growth in the range of 20 °C to 45 °C) but can survive at higher temperatures without optimal growth. These properties are often acquired and result from adaptation to the current environmental conditions. In both cases, the bacteria are able to survive at high temperatures [7,8]. Their resistance to heat makes them particularly valuable in food technology, especially in processes involving heat treatment, which, until recently, was a major limitation of using probiotics in the food industry. Probiotic foods produced using thermophilic or thermotolerant microorganisms are divided into fermented and non-fermented products.
Fermented foods mainly use beneficial starter cultures of LAB, including Lactobacillus spp. (e.g., L. plantarum, L. acidophilus, L. delbrueckii ssp. bulgaricus) and some Streptococcus thermophilus species. Also, among the group of LAB, the species that demonstrate technological usefulness can be distinguished and are most often used as starter cultures for food fermentation. Moreover, LABs have the US Food and Drug Administration (FDA) GRAS or the European Union Food Safety Authority (EFSA) QPS status, which means that they are considered microorganisms safe for humans and animals and can be used in food and feed technology processes [48]. On the other hand, probiotics used in non-fermented products are produced mainly with the addition of thermoresistant probiotic spores of Bacillus bacteria. Although some Bacillus spp. pose a significant health risk to humans by producing harmful metabolites and toxins, as well as transferring antibiotic resistance genes, some specific strains of B. coagulans and B. licheniformis are being reported as safe and present a wide range of probiotic properties [49,50].
Microorganisms with probiotic properties can play an important role in improving the nutritional and functional value of bakery products. Their addition to bread can not only support the health of the digestive system but also affect the sensory properties and durability of products. However, using probiotics in baking poses technological challenges for producers, including ensuring their survival during the baking and storage processes. A few LAB cultures can be used in the baking industry, but unlike Bacillus spp., their participation is mainly limited to the fermentation of sourdough for bread. It was shown that the application of thermophilic L. acidophilus, L. bulgaricus, or L. rossiae sourdough increased bread porosity, elasticity, crumbliness, and moisture content but did not influence crumb hardness. Moreover, the fungal spoilage on the bread crust surface was suppressed using sourdough prepared with thermophilic LAB; hence, the LAB strain used for sourdough preparation influenced the shelf life of the bread [51]. A good example of a beneficial strain of bacteria used in food technology is B. coagulans, which is resistant to high-temperature processing, making it suitable for baking. Almada-Erix et al. [52] presented the usefulness of the B. coagulans GBI-30 6086 strain in the bread manufacturing process, particularly the baking conducted at very high temperatures. Besides, white and whole wheat bread carrying GBI-30 6086 was stable during storage for ten days. Additionally, B. coagulans MTCC 5856 was found to be stable during baking and storage at frozen conditions of banana muffins (92% viability) and waffles (86% viability) for up to 12 months [53].
Thermophilic beneficial bacteria also play a key role in the dairy industry, especially in the production of fermented products such as yogurts, kefirs, and cheeses. Thermophilic probiotic bacteria such as Lactobacillus delbrueckii ssp. bulgaricus or Streptococcus thermophilus are characterized by the ability to grow at elevated temperatures (40–45 °C), which allows for quick and effective fermentation of milk. Their presence not only improves the sensory and textural properties of dairy products but also contributes to health benefits for consumers, such as supporting the intestinal microbiota, strengthening immunity, and improving lactose digestion [48,51]. One of the most popular genera is L. acidophilus, a thermophilic lactic acid bacterium used worldwide for the elaboration of probiotic fermented milk products, mainly yogurts [48]. L. acidophilus is subdivided into many strain types, including L. acidophilus LA-1, LA-5, NCFM, ATCC4356 and DDS-1. Different strains also confer differing probiotic properties and technological functions [48,54]. L. acidophilus exhibits important technological properties, i.e., thermostability and retention of activity at a wide pH range, along with strong inhibitory actions against food spoilage and pathogenic bacteria, making them an important class of bio preservatives. L. acidophilus can be added as an adjunct in many plant- and animal-origin food fermentations, contributing to unique flavor and texture. It also preserves the products by producing organic acids and bacteriocins. The other thermoresistant bacterium is Lactobacillus helveticus, a homofermentative and thermophilic bacterium with an optimum growth temperature of 42 to 45 °C. The selected species are characterized as having the ability to grow at a relatively high temperature (~55 °C) and being proteolytic. It is a crucial food-associated species traditionally used to manufacture Swiss-type cheeses and long-ripened Italian cheeses such as Emmental, Gruyere, and Provolone [55]. The strain DPC4571 has emerged as a promising flavor adjunct culture for Cheddar cheese, given that it is consistently associated with improved flavor [48,55]. Swiss-type cheese includes, among other microorganisms, a thermophilic LAB, especially L. helveticus, L. delbrueckii ssp. lactis, and Streptococcus thermophilus, which produce aroma compounds from three amino acids, leucine, phenylalanine, and methionine, during a ripening process [56]. In summary, there are definitely more studies on the resistance of technological LAB strains to high temperatures, but not exceeding 55 °C. On the other hand, an example of the use of beneficial bacteria outside the LAB group is B. coagulans-70, which showed a positive impact on the appearance and texture of yogurt products. This microorganism increased the levels of 2-heptanone, 2-nonanone, pentanol, and 2-hydroxy-3-pentanone, making it suitable as a strain for the development of functional dairy products [48,57].
The growing interest in plant-based diets creates new opportunities for using beneficial bacteria in producing plant products. Particular attention is paid to the use of thermophilic strains, including mainly species of Bacillus. Thermophilic bacteria can effectively ferment plant substrates, thanks to their ability to grow at elevated temperatures and the intensive production of beneficial metabolites. Using these microorganisms allows not only to improve the sensory properties of products, such as taste, aroma, and consistency, but also to enrich them with health-promoting properties, supporting intestinal microbiota and immunity. However, adapting thermophilic probiotics to plant environments requires appropriate adjustment of the fermentation technology and the composition of raw materials. Majeed et al. [53] found that thermoresistant spores of B. coagulans MTCC 5856 showed over 95% viability in chocolate fudge frosting, hot fudge toppings, peanut butter, strawberry preserve, and vegetable oil at room temperature for up to 12 months. Additionally, it was found to be stable in apple juice for up to 6 months under refrigerated conditions and in concentrated glucose syrup at 4 and 25 °C for up to 24 months. Date pastes as carriers of spores of the probiotic B. coagulans BC4 are an example of using this bacteria in dried fruit bar or paste technologies with low water activity (aw ≈ 0.37–0.87) and moisture content (8–15%) [58,59]. It was also shown that Bacillus spores are heat-resistant during brewing beverages. Therefore, probiotic coffee or tea beverages are another food application solution where probiotic spores or cells are applied. Coffee is developed by using Bacillus spores to produce non-fermented formulations (B. coagulans MTCC 5856 spores). After brewing coffee at 90 °C for 2 min, there was 87% viability, and the bacteria retained 66% viability even after maintaining the temperature at 77 °C for 4 h. Also, B. licheniformis is widely used both in the processing of raw materials, the biosynthesis of edible compounds, fermentation, and the biological treatment of waste from food manufacturing [60,61,62]. It can also be used to produce high yields of beneficial food additives, such as aromas and phenols, in the fermentation process of white wine, or compounds such as acetoin, which is widely used as an edible flavoring. Another example of food applications of B. licheniformis is Daqu (a saccharifying and fermenting starter in the production of Chinese Baijiu). It becomes a dominant bacterium in the Daqu fermentation [60,63]. Moreover, the ability of the probiotic spore-forming bacteria to survive the cooking process and the storage stage demonstrates their capacity for usage, especially in functional cooked food products [49,64]. For example, probiotic strains of B. coagulans ATCC 31284 and Bacillus subtilis var. Natto ATCC 15245 (as spores) were positively evaluated during the processing and refrigerated storage of cooked sausage [64]. Furthermore, B. licheniformis is widely used in the meat food waste technology for keratinase production by the biodegradation of feathers and fish bones [60].
There are only a few examples in the literature of thermotolerant bacteria where different laboratory methods of adaptation to heat stress at high temperatures were used. Most of the below-discussed strains are listed in Table 1. For example, the thermotolerant Lactococcus lactis ssp. cremoris RD07 strain was used to develop a new cheese starter culture. It was found that the tested cheese starter culture was less sensitive to cooking when cultured in milk and autolyzed well after entering the stationary phase upon facing sugar starvation, resulting in a more rapid release of free amino acids. It was proven to perform better than the commercial starter and can reduce the ripening time of a mature cheese from 25 weeks to only 13 weeks [65]. In the study by Dorau et al. [36], the same strain, RD07, blended with the commercial starter culture revealed better acidification and flavor formation than the pure starter culture during cheese production. Also, Zhang et al. [66] indicated that mild heat stress treatment could be a promising strategy to improve the shelf life of probiotic fermented milk. The authors used heat stress (46 °C, 1 h) as a tool to regulate Lactobacillus rhamnosus hsryfm 1301 metabolism during probiotic fermented milk technology. It was shown that mild heat stress treatment could limit the post-acidification effect, while the viable count of bacterial strain remained at a high level.
Thermotolerant LAB can also be used in meat technology. For example, four thermotolerant strains of Lactobacillus plantarum, Lactobacillus curvatus, Pediococcus pentosaceus, and Pediococcus acidilacti were inoculated in sausage batters before cooking in order to determine their effect on color, texture, acceptance, and overall microbiological quality. The thermotolerant capacity was positively identified after the strains survived thermal treatment (70 °C during 60 min). The added thermotolerant LAB strains did not affect texture and sensory characteristics but improved the microbiological quality of sausage samples [67].
Regarding plant-origin food examples, Bommasamudram et al. [68] investigated the usefulness of thermotolerant Lactobacillus helveticus H-45 and Lacticaseibacillus casei N (N-45) strains for synbiotic preparation with galactooligosaccharides. The developed synbiotic spray-dried powder was added to five fruit-flavored drinks (pineapple, orange, grape, mango, and lemon ginger). It was found that the varied amounts of added synbiotic powder did not significantly alter the physicochemical properties of the fruit drinks and may find application in developing functional foods. In another study, the addition of 1010 CFU/mL thermotolerant probiotic E. faecalis R22B during the fermentation of bekasam and rusip (traditional foods of Indonesia) was suggested to acquire the minimum concentration of probiotic bacteria after heat treatment at 70 °C [69].
Also, the biotechnological application of thermotolerant lactic acid bacteria was investigated. For example, D-lactic acid production from rice-straw biomass was increased by using a thermo-tolerant Lactobacillus delbrueckii subsp. bulgaricus ET45 [39]. Other thermotolerant LAB strains (L. paraplantarum St1 and St3, L. fermentum N4, and P. pentosaceus R4) isolated from the fermented juice of tropical crops were used to improve the quality of tropical crop silage. Four selected strains were shown to improve the fermentation quality and nutritive values (protein concentration) of Stylo silage in comparison to the control [70].
With a growing interest in functional foods, the use of thermophilic and thermotolerant probiotics in the food industry is becoming an increasingly important element of both production technology and marketing strategies. In both cases, there are many benefits of using probiotics in food technology that are resistant to higher temperatures (Figure 1).
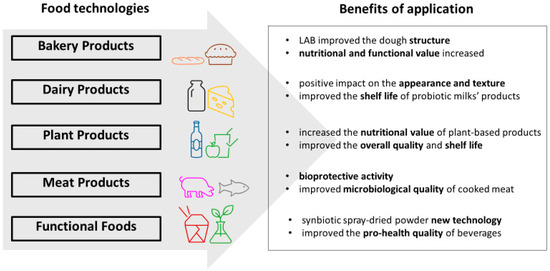
Figure 1.
Applications of thermophilic and thermotolerant probiotics in food technology and benefits.
Thermoresistant-selected beneficial bacteria and their pro-health metabolites have been used as probiotics or postbiotics in functional foods technology. Their beneficial effects are highly related to generating extracellular enzymes, bacteriocins or bacteriocin-like substances, L-lactic acid, amino acids, vitamins, exopolysaccharides (EPS), and GABA [21]. Due to thermal stability, thermophilic and thermotolerant species can be used in the dairy, bakery, meat, and fermentation food industries, especially where the functional food products undergo industrial processing involving high temperatures. During heat treatment, most bacterial cells are inactivated; however, thermotolerant bacteria could survive. Very few studies have been devoted to the thermotolerance of probiotics, especially lactic acid bacteria, during food technology processes. Thermotolerance of probiotic microorganisms used in such processing is important to make it effective during consumption and administration in an appropriate pro-health amount.
5. Conclusions
Probiotics must remain alive in food to produce health-promoting effects in the human body. However, during the industrial application of LAB, bacteria are subjected to various environmental stresses, among which heat stress is the most common. Bacterial cell damage can be reduced by adapting LAB in physiological and metabolic aspects, which was widely discussed in the manuscript. Stress adaptation methods, including laboratory adaptive evolution, environmental stress, or genetic modification, can influence the synthesis of stress proteins, changes in the fatty acid composition of the cell membrane, and other minor mechanisms. These techniques can be applied to improve the probiotic strains’ features. Although the results are promising, it can be concluded that the specific mechanism of heat resistance and adaptation is often strain-dependent.
So far, thermophilic and thermotolerant probiotic strains have been used in bakery, dairy, plant, meat, and functional food technology; however, this refers more to the use of spore-forming Bacillus rather than lactobacilli. Thanks to the high heat stability, the shelf life and health benefits of the products can be enhanced. Moreover, such probiotics can be applied to products that need mild heat treatment, like instant food. However, some challenges can also be identified. First of all, the regulatory approval for using such genetically modified strains of bacteria is not clear. Although stress adaptation is a natural evolutionary mechanism, genetic modification may be unacceptable. Secondly, the impact on sensory attributes of food should be confirmed before use. Also, the viability of probiotic bacterial cells should be checked during storage. Perhaps in the future, the solution will be the use of postbiotics, which are gaining popularity.
In summary, it is still worth continuing research on the mechanisms of resistance to heat stress in beneficial bacteria because the molecular mechanisms of this process are not fully understood. It is believed that such knowledge could accelerate the development of the probiotic market in the future.
Author Contributions
Conceptualization, D.Z.; methodology, M.K. and D.Z.; software, M.K., K.N.-S., and D.Z.; validation, D.Z.; formal analysis, D.Z.; writing—original draft preparation, M.K., K.N.-S., and D.Z.; writing—review and editing, D.Z.; visualization, M.K. and K.N.-S.; supervision, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, H.; Wei, C.X.; Min, L.; Zhu, L.Y. Good or Bad: Gut Bacteria in Human Health and Diseases. Biotechnol. Biotechnol. Equip. 2018, 32, 1075–1080. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- NIH Human Microbiome Project—About the Human Microbiome. Available online: https://www.hmpdacc.org/hmp/overview/ (accessed on 20 May 2025).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Sionek, B.; Szydłowska, A.; Zielińska, D.; Neffe-Skocińska, K.; Kołożyn-Krajewska, D. Beneficial Bacteria Isolated from Food in Relation to the next Generation of Probiotics. Microorganisms 2023, 11, 1714. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wu, C. Cross Protection of Lactic Acid Bacteria during Environmental Stresses: Stress Responses and Underlying Mechanisms. LWT 2021, 144, 111203. [Google Scholar] [CrossRef]
- Varmanen, P.; Savijoki, K. Responses of Lactic Acid Bacteria to Heat Stress. In Stress Responses of Lactic Acid Bacteria; Springer: Boston, MA, USA, 2011; pp. 55–66. [Google Scholar]
- Papadimitriou, K.; Alegria, A.; Bron, P.A.; Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Res. 2016, 80, 837–890. [Google Scholar] [CrossRef]
- Yamamori, T.; Ito, K.; Yura, T.; Suzuki, T.; Iino, T. Ribonucleic Acid Polymerase Mutant of Escherichia coli Defective in Flagella Formation. J. Bacteriol. 1977, 132, 254–261. [Google Scholar] [CrossRef]
- Arsène, F.; Tomoyasu, T.; Bukau, B. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 2000, 55, 3–9. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, T.; Yan, F. Probiotic Role and Application of Thermophilic Bacillus as Novel Food Materials. Trends Food Sci. Technol. 2023, 138, 1–15. [Google Scholar] [CrossRef]
- Darmon, E.; Noone, D.; Masson, A.; Bron, S.; Kuipers, O.P.; Devine, K.M.; Dijl, J.M.V. A Novel Class of Heat and Secretion Stress-Responsive Genes Is Controlled by the Autoregulated CssRS Two-Component System of Bacillus Subtilis. J. Bacteriol. 2002, 184, 5661–5671. [Google Scholar] [CrossRef]
- Mansilla, M.C.; Cybulski, L.E.; Albanesi, D.; Mendoza, D. Control of Membrane Lipid Fluidity by Molecular Thermosen-Sors. J. Bacteriol. 2004, 186, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Konings, W.N.; Albers, S.V.; Koning, S.; Driessen, A.J. The Cell Membrane Plays a Crucial Role in Survival of Bacteria and Archaea in Extreme Environments. Antonie Van. Leeuwenhoek 2002, 81, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Siristova, L.; Melzoch, K.; Sigler, K. Hopanoids in Bacteria and Cyanobacteria-Their Role in Cellular Biochemistry and Physiology, Analysis and Occurrence. Mini-Rev. Org. Chem. 2010, 7, 300–313. [Google Scholar] [CrossRef]
- Kathiriya, M.R.; Vekariya, Y.V.; Hati, S. Understanding the Probiotic Bacterial Responses Against Various Stresses in Food Ma-Trix and Gastrointestinal Tract: A Review. Probiotics Antimicro Prot. 2023, 15, 1032–1048. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Razafindralambo, H.; Blecker, C.; N’Yapo, C.; Thonart, P.; Delvigne, F. Stochastic Exposure to Sub-Lethal High Tem-Perature Enhances Exopolysaccharides (EPS) Excretion and Improves Bifidobacterium Bifidum Cell Survival to Freeze–Drying. Bio-Chem. Eng. J. 2014, 88, 85–94. [Google Scholar]
- Angelis, M.; Cagno, R.; Huet, C.; Crecchio, C.; Fox, P.F.; Gobbetti, M. Heat Shock Response in Lactobacillus Plantarum. Ap-Plied Environ. Microbiol. 2004, 70, 1336–1346. [Google Scholar] [CrossRef]
- Russo, P.; Luz Mohedano, M.; Capozzi, V.; Palencia, P.F.; López, P.; Spano, G.; Fiocco, D. Comparative Proteomic Analysis of Lactobacillus Plantarum WCFS1 and Δ ctsR Mutant Strains under Physiological and Heat Stress Conditions. Int. J. Mol. Sci. 2012, 13, 10680–10696. [Google Scholar] [CrossRef]
- Khaskheli, G.B.; Zuo, F.; Yu, R.; Chen, S. Overexpression of Small Heat Shock Protein Enhances Heat- and Salt-Stress Tolerance of Bifidobacterium Longum NCC2705. Curr. Microbiol. 2015, 71, 8–15. [Google Scholar] [CrossRef]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ismail, M.B.; Bakhrouf, A. Change in Cell Surface Properties of Lactobacillus Casei under Heat Shock Treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar] [CrossRef]
- Pandi, S.; Basheer, S. Adaptation of Lactobacillus sp. and Saccharomyces sp. to Heat Stress. Int. J. Microbiol. Allied Sci. 2016, 283, 7–16. [Google Scholar]
- Yang, H.; Yao, S.; Zhang, M.; Wu, C. Heat Adaptation Induced Cross Protection Against Ethanol Stress in Tetragenococcus halophilus: Physiological Characteristics and Proteomic Analysis. Front. Microbiol. 2021, 12, 686672. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Kang, C.-H.; Kim, W.; So, J.-S. Heat Adaptation Improved Cell Viability of Probiotic Enterococcus Faecium HL7 upon Various Environmental Stresses. Probiotics Antimicrob. Proteins 2019, 11, 618–626. [Google Scholar] [CrossRef]
- Scheyhing, C.H.; Hörmann, S.; Ehrmann, M.A.; Vogel, R.F. Barotolerance Is Inducible by Preincubation under Hydrostatic Pressure, Cold-, Osmotic- and Acid-Stress Conditions in Lactobacillus Sanfranciscensis DSM 20451T. Lett. Appl. Microbiol. 2004, 39, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, P.; Hao, L.; Che, Y.; Dong, S.; Wang, Z.; Wu, C. Enhancing Viability of Dried Lactic Acid Bacteria Prepared by Freeze Drying and Spray Drying via Heat Preadaptation. Food Microbiol. 2023, 112, 104239. [Google Scholar] [CrossRef]
- Suo, X.; Huang, S.; Wang, J.; Fu, N.; Jeantet, R.; Chen, X.D. Effect of Culturing Lactic Acid Bacteria with Varying Skim Milk Concentration on Bacteria Survival during Heat Treatment. J. Food Eng. 2021, 294, 110396. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, F.; Lu, W.; Suo, X.; Bellenger, E.; Fu, N.; Jeantet, R.; Chen, X.D. Enhanced Thermal Stability of Lactic Acid Bacteria during Spray Drying by Intracellular Accumulation of Calcium. J. Food Eng. 2020, 279, 109975. [Google Scholar] [CrossRef]
- Kulkarni, S.; Haq, S.F.; Samant, S.; Sukumaran, S. Adaptation of Lactobacillus Acidophilus to Thermal Stress Yields a Thermotolerant Variant Which Also Exhibits Improved Survival at pH 2. Probiotics Antimicrob. Proteins 2018, 10, 717–727. [Google Scholar] [CrossRef]
- Zhao, J.; Gong, J.; Liang, W.; Zhang, S. Microbial Diversity Analysis and Isolation of Thermoresistant Lactic Acid Bacteria in Pasteurized Milk. Sci. Rep. 2024, 14, 29705. [Google Scholar] [CrossRef]
- Hao, F.; Fu, N.; Ndiaye, H.; Woo, M.W.; Jeantet, R.; Chen, X.D. Thermotolerance, Survival, and Stability of Lactic Acid Bacteria After Spray Drying as Affected by the Increase of Growth Temperature. Food Bioprocess Technol. 2021, 14, 120–132. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Liu, F.; Hou, J.; Shao, H.; Yu, W. Stress Adaptation and Cross-Protection of Lactobacillus Plantarum KLDS 1.0628. CyTA—J. Food 2021, 19, 72–80. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Yang, D.; Chen, X.; Huang, Y.; Gu, R. Stress Influenced the Aerotolerance of Lactobacillus Rhamnosus Hsryfm 1301. Biotechnol. Lett. 2018, 40, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Tang, H.-Y.; Chiang, M.-L. Effects of Heat, Cold, Acid and Bile Salt Adaptations on the Stress Tolerance and Protein Expression of Kefir-Isolated Probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Ferrando, V.; Quiberoni, A.; Reinhemer, J.; Suárez, V. Resistance of Functional Lactobacillus plantarum Strains against Food Stress Conditions. Food Microbiol. 2015, 48, 63–71. [Google Scholar] [CrossRef]
- Dorau, R.; Chen, J.; Liu, J.; Ruhdal Jensen, P.; Solem, C. Adaptive Laboratory Evolution as a Means To Generate Lactococcus Lactis Strains with Improved Thermotolerance and Ability To Autolyze. Appl. Environ. Microbiol. 2021, 87, e01035-21. [Google Scholar] [CrossRef]
- Bommasamudram, J.; Kumar, P.; Kapur, S.; Sharma, D.; Devappa, S. Development of Thermotolerant Lactobacilli Cultures with Improved Probiotic Properties Using Adaptive Laboratory Evolution Method. Probiotics Antimicrob. Proteins 2023, 15, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, H.; Choi, Y.; Cho, S.; Seo, M.; Kim, H. Complete Genome Sequence of the Newly Developed Lactobacillus Acidophilus Strain With Improved Thermal Adaptability. Front. Microbiol. 2021, 12, 697351. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Prasad, J.; Sahoo, T.K.; Naveen, S.; Jayaraman, G. Evolutionary Engineering of Lactobacillus Bulgaricus Reduces Enzyme Usage and Enhances Conversion of Lignocellulosics to D-Lactic Acid by Simultaneous Saccharification and Fermentation. Biotechnol. Biofuels 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Yoo, D.; Lee, Y.; Seo, M.; Kim, H. Complete Genomic Analysis of Enterococcus Faecium Heat-Resistant Strain Developed by Two-Step Adaptation Laboratory Evolution Method. Front. Bioeng. Biotechnol. 2020, 8, 828. [Google Scholar] [CrossRef]
- El Demerdash, H.A.M.; Heller, K.J.; Geis, A. Application of the Shsp Gene, Encoding a Small Heat Shock Protein, as a Food-Grade Selection Marker for Lactic Acid Bacteria. Appl. Environ. Microbiol. 2003, 69, 4408–4412. [Google Scholar] [CrossRef]
- Fiocco, D.; Capozzi, V.; Goffin, P.; Hols, P.; Spano, G. Improved Adaptation to Heat, Cold, and Solvent Tolerance in Lactobacillus Plantarum. Appl. Microbiol. Biotechnol. 2007, 77, 909–915. [Google Scholar] [CrossRef]
- Wang, G.; Li, D.; Ma, X.; An, H.; Zhai, Z.; Ren, F.; Hao, Y. Functional Role of oppA Encoding an Oligopeptide-Binding Protein from Lactobacillus Salivarius Ren in Bile Tolerance. J. Ind. Microbiol. Biotechnol. 2015, 42, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, T.K.; Zhang, J.; Du, G. UvrA Expression of Lactococcus Lactis NZ9000 Improve Multiple Stresses Tolerance and Fermentation of Lactic Acid against Salt Stress. J. Food Sci. Technol. 2017, 54, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Dragosits, M.; Mattanovich, D. Adaptive Laboratory Evolution—Principles and Applications for Biotechnology. Microb. Cell Factories 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Derunets, A.S.; Selimzyanova, A.I.; Rykov, S.V.; Kuznetsov, A.E.; Berezina, O.V. Strategies to Enhance Stress Tolerance in Lactic Acid Bacteria across Diverse Stress Conditions. World J. Microbiol. Biotechnol. 2024, 40, 126. [Google Scholar] [CrossRef]
- Johansen, E. Future Access and Improvement of Industrial Lactic Acid Bacteria Cultures. Microb. Cell Factories 2017, 16, 230. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus Acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, S.; Li, M.; Lee, J.H.; Zhu, Y.; Liang, D.; Zhi, H.; Ding, Q.; Zhao, G.; Ma, Y.; et al. Bibliometric Analysis of Probiotic Bacillus in Food Science: Evolution of Research Trends and Systematic Evaluation. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.; Heo, S.; Lee, J.H.; Jeong, D.W. Technology and Safety Evaluation of Bacillus coagulans Exhibiting Antimi-Crobial Activity for Starter Development. LWT 2021, 137, 110464. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J.; Stankevicius, M.; Maruska, A. Thermophilic Lactic Acid Bacteria Affect the Characteristics of Sourdough and Whole-Grain Wheat Bread. Food Biosci. 2020, 38, 100791. [Google Scholar] [CrossRef]
- Almada-Érix, C.N.; Almada, C.N.; Pedrosa, G.T.S.; Biachi, J.P.; Bonatto, M.S.; Schmiele, M.; Nabeshima, E.H.; Clerici, M.T.P.; Magnani, M.; Sant’Ana, A.S. Bread as Probiotic Carriers: Resistance of Bacillus coagulans GBI-30 6086 Spores through Processing Steps. Food Res. Int. 2022, 155, 111040. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Arumugam, S.; Beede, K.; Ali, F. Evaluation of Probiotic Bacillus coagulans MTCC 5856 Viability after Tea and Coffee Brewing and Its Growth in GIT Hostile Environment. Food Res. Int. 2019, 121, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Plummer, S.; Marchesi, J.; Mahenthiralingam, E. The Life History of Lactobacillus Acidophilus as a Probiotic: A Tale of Revisionary Taxonomy, Misidentification and Commercial Success. FEMS Microbiol. Lett. 2013, 349, 77–87. [Google Scholar] [CrossRef]
- Slattery, L.; O’Callaghan, J.; Fitzgerald, G.F.; Beresford, T.; Ross, R.P. Invited Review: Lactobacillus helveticus—A Thermophilic Dairy Starter Related to Gut Bacteria. J. Dairy Sci. 2021, 93, 4435–4454. [Google Scholar] [CrossRef]
- Helinck, S.; Le Bars, D.; Moreau, D.; Yvon, M. Ability of Thermophilic Lactic Acid Bacteria to Produce Aroma Compounds from Amino Acids. Appl. Environ. Microbiol. 2004, 70, 3855–3861. [Google Scholar] [CrossRef]
- Ma, S.; Cao, J.; Liliu, R.; Li, N.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Effects of Bacillus coagulans as an Adjunct Starter Culture on Yogurt Quality and Storage. J. Dairy. Sci. 2021, 104, 7466–7479. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Pjaca, A.S.; Andersen, C.J.; Knøchel, S.; Nielsen, D.S. Dried Date Paste as Carrier of the Proposed Probiotic Bacillus coagulans BC4 and Viability Assessment during Storage and Simulated Gastric Passage. LWT 2019, 99, 197–201. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Pereira, C.; López-Corrales, M.; Córdoba, M.G. Evaluation of Different Drying Systems as an Alternative to Sun Drying for Figs (Ficus carica L.). Innov. Food Sci. Emerg. Technol. 2016, 36, 156–165. [Google Scholar] [CrossRef]
- He, H.; Yu, Q.; Ding, Z.; Zhang, L.; Shi, G.; Li, Y. Biotechnological and Food Synthetic Biology Potential of Platform Strain: Bacillus Licheniformis. Synth. Syst. Biotechnol. 2023, 8, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ohair, J.; Jin, Q.; Yu, D.; Wu, J.; Huang, H. Non-sterile fermentation of food waste using thermophilic and alkaliphilic Bacillus licheniformis YNP5-TSU for 2,3-butanediol production. Waste Manag. 2021, 120, 248–256. [Google Scholar] [CrossRef]
- Ghosh, A.; Sutradhar, S.; Baishya, D. Delineating Thermophilic Xylanase from Bacillus Licheniformis DM5 towards Its Po-Tential Application in Xylooligosaccharides Production. World J. Microbiol. Biotechnol. 2019, 35, 34. [Google Scholar] [CrossRef]
- Yan, Z.; Zheng, X.W.; Han, B.Z.; Yan, Y.Z.; Zhang, X.; Chen, J.Y. 1H NMR-Based Metabolomics Approach for under-Standing the Fermentation Behaviour of Bacillus Licheniformis. J. Inst. Brew. 2015, 121, 425–431. [Google Scholar] [CrossRef]
- Voigt, B.; Schroeter, R.; Schweder, T.; Jürgen, B.; Albrecht, D.; van Dijl, J.M.; Maurer, K.H.; Hecker, M. A Proteomic View of Cell Physiology of the Industrial Workhorse Bacillus Licheniformis. J. Biotechnol. 2014, 191, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Dorau, R.; Tømmerholt, L.; Gu, L.; Tadesse, B.T.; Zhao, G.; Solem, C. Simple & Better–Accelerated Cheese Ripening Using a Mesophilic Starter Based on a Single Strain with Superior Autolytic Properties. Int. J. Food Microbiol. 2023, 407, 110398. [Google Scholar]
- Zhang, C.; Yang, L.; Gu, R.; Ding, Z.; Guan, C.; Lu, M.; Gu, R. Mild Heat Stress Limited the Post-Acidification Caused by Lactobacillus Rhamnosus Hsryfm 1301 in Fermented Milk. Biotechnol. Lett. 2019, 41, 633–639. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.D.L.; Totosaus, A.; Guerrero, I. Evaluation of Thermotolerant Capacity of Lactic Acid Bacteria Isolated from Com-Mercial Sausages and the Effects of Their Addition on the Quality of Cooked Sausages. Ciênc. Tecnol. Aliment. Camp. 2008, 28, 132–138. [Google Scholar] [CrossRef]
- Bommasamudram, J.; Muthu, A.; Devappa, S. Effect of Prebiotics on Thermally Acclimatized Lactobacilli Cultures and Their Application as Synbiotics in RTD Fruit Drinks. 3 Biotech 2023, 13, 311. [Google Scholar] [CrossRef]
- Lestari, S.D.; Rinto, R.; Wahyuni, I.S.; Ridhowati, S.; Wulandari, W. Heat Resistance of Probiotic Candidate Enterococcus Faecalis R22B in Different Matrices. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2021, 16, 19–27. [Google Scholar] [CrossRef]
- Pitiwittayakul, N.; Bureenok, S.; Schonewille, J.T. Selective Thermotolerant Lactic Acid Bacteria Isolated from Fermented Juice of Epiphytic Lactic Acid Bacteria and Their Effects on Fermentation Quality of Stylo Silages. Front. Microbiol. 2021, 12, 673946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).