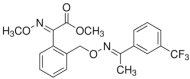
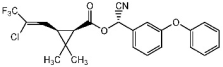
1. Introduction
Viticulture is a globally significant agricultural sector, with annual wine production in 2023 surpassing 235 million hectolitres [
1]. The use of pesticides is crucial for safeguarding grapevines against fungal diseases, insect pests, and weeds, which helps maintain both crop yield and quality. However, the presence of pesticide residues in wine has raised significant safety concerns due to potential health risks and stringent regulatory controls [
2,
3,
4].
Good Agricultural Practices (GAP) aim to minimise pesticide residue levels by following recommended safety intervals and dosages. Assessing pesticide dissipation under GAP conditions offers a thorough understanding of how residues behave during winemaking and their potential implications for wine safety. In this sense, chronic exposure to certain pesticide compounds has been linked to endocrine disruption, neurotoxicity, and carcinogenicity. This has prompted international food safety authorities, including the European Food Safety Authority (EFSA) and the United States Environmental Protection Agency, to establish Maximum Residue Limits (MRLs) for wine and related products [
5,
6].
The dissipation of pesticide residues throughout vinification is influenced by multiple physicochemical and biochemical mechanisms, including hydrolysis, oxidation, microbial metabolism, adsorption, and volatilisation [
7,
8]. The extent of residue reduction depends on the pesticide’s solubility, chemical stability, and interaction with wine matrix components. For instance, hydrophobic pesticides tend to adsorb onto grape solids, including skins, seeds, and yeast lees, which are removed during pressing, racking, and clarification, while hydrophilic compounds persist in the liquid phase and are more likely to be retained in the final product [
9].
Several commonly used pesticides in viticulture, including boscalid, penconazole, tebufenozide, kresoxim-methyl, trifloxystrobin, chlorpyrifos or lambda-cyhalothrin, have been studied for their dissipation behaviour in wine [
7,
10]. The behaviour of these pesticides in wine production varies based on their water solubility, volatility, and affinity for solid or liquid phases within the wine matrix. For instance, boscalid, tebufenozide, and kresoxim-methyl are moderately to highly hydrophobic (partition coefficient log
p > 3), which encourages their adsorption onto grape solids and aids in their removal during the clarification and racking stages. Trifloxystrobin is also hydrophobic but tends to persist during fermentation. This persistence is likely due to its high organic carbon/water partition coefficient (K
oc) and resistance to microbial degradation. In contrast, chlorpyrifos and lambda-cyhalothrin have very low water solubility and are primarily removed through adsorption. However, some residues are still detectable in the later stages of winemaking. Penconazole displays intermediate properties and shows moderate dissipation throughout the vinification process. In this sense, factors such as pH, ethanol content, temperature, and interactions with yeast and fining agents can affect the degradation kinetics and distribution of each compound during winemaking [
11,
12,
13].
Among vinification processes, clarification and racking are crucial for reducing pesticide residues. These stages involve removing solid particles where pesticides may be adsorbed, which significantly lowers residue levels. Fermentation also aids in pesticide dissipation, mainly through enzymatic degradation, hydrolysis, and adsorption onto yeast biomass. However, its effectiveness can vary based on factors such as fermentation temperature, ethanol concentration, and the choice of yeast strain [
14,
15,
16]. Some studies suggest that ethanol improves the breakdown of certain pesticide residues, while other studies emphasise the importance of yeast cell walls in adsorbing and immobilising these compounds. The log
p value of each pesticide influences its affinity for organic or aqueous phases, which in turn affects its behaviour during fermentation [
17].
In addition to fermentation, techniques for clarification and stabilisation further reduce pesticide residues. Fining agents, such as bentonite, gelatine, and activated carbon, can help to precipitate suspended pesticide residues, while filtration techniques, including microfiltration and ultrafiltration, can improve removal efficiency [
1,
18]. The degree of pesticide elimination differs between red and white wines. Red wines generally show a greater reduction in pesticide levels, primarily due to longer maceration periods that improve the adsorption of pesticides onto grape solids. Furthermore, the polyphenols found in red wines interact with pesticides, affecting their stability and the speed at which they degrade [
12,
19].
Processing factor (F) is a useful metric for assessing how vinification affects pesticide dissipation. An F value less than 1 indicates a net reduction in pesticide concentration due to the winemaking processes, while an F value greater than 1 suggests an increase in concentration. This increase often results from volume reduction or phase partitioning [
20,
21].
From a regulatory standpoint, ensuring consumer safety necessitates rigorous monitoring of pesticide residues in wine. Advances in analytical chemistry have greatly enhanced detection capabilities, with LC-MS/MS and GC-MS being commonly used to assess residue dissipation during vinification [
22,
23,
24,
25,
26,
27]. In this sense, to evaluate the potential health risks from residual pesticides, regulatory agencies determine the Acceptable Daily Intake (ADI). This defines the maximum amount of a pesticide that can be consumed daily over a lifetime without causing adverse effects [
28]. The Estimated Daily Intake (EDI) is utilised to assess whether wine consumption surpasses established safety limits.
The assessment of health risks of each pesticide is calculated by dividing the EDI of each residue by their respective ADI. Some studies indicate that, even in worst-case exposure scenarios, the EDI/ADI ratio remains within acceptable safety thresholds. This reinforces the idea that pesticide residues in wine present minimal toxicological risks, provided that regulatory limits are adhered to [
3,
12,
13]. However, despite natural reductions during vinification, some pesticides still persist at trace levels, requiring additional mitigation strategies in order to ensure consumer safety necessities and to provide additional data on the behaviour of each pesticide regulated.
This study aims to investigate the dissipation of pesticide residues (boscalid, penconazole, tebufenozide, kresoxim-methyl, trifloxystrobin, chlorpyrifos, and lambda-cyhalothrin) across different winemaking stages, providing insights into the mechanistic pathways influencing residue reduction and their implications for food safety and regulatory compliance, performing an evaluation of the potential health risk of each pesticide studied.
2. Materials and Methods
2.1. Materials and Reagents
The pesticides selected for this study are boscalid, and kresoxim-methyl (Collis: boscalid 20% + kresoxim-methyl 10%
w/
v), penconazole (Topas 100 EC: penconazole 10%
w/
v), trifloxystrobin (Flint: 50% trifloxystrobin
w/
v), tebufenozide (Mimic: tebufenozide 24%
w/
v), chlorpyrifos (Dursban: chlorpyrifos 48%
w/
v), and lambda-cyhalothrin (Karate Zeon: lambda-cyhalothrin 10%
w/
v), which are commonly used in viticulture in Spain and frequently detected in grape treatments [
29]. In addition, these pesticides were chosen based on their prevalence in grape protection programs, persistence, and regulatory relevance [
7].
To better understand the behaviour of each pesticide during the vinification process, their key physicochemical properties are summarised in
Table 1. This includes water solubility, the octanol–water partition coefficient (log P), molecular weight, and K
oc.
2.2. Field Trials
The experimental vineyard was located in the Region of Murcia (Spain). This area is characterised by an arid climate and an extensive viticultural tradition. The study was carried out on Vitis vinifera L. Monastrell grapevines, a variety widely cultivated in south-eastern Spain. The vineyard was divided into eight experimental plots (10 × 10 m each), with seven treated plots and one untreated control plot. The control plot was located at a sufficient distance to avoid contamination and to ensure comparable soil and climatic conditions.
Applications were made according to each pesticide’s pre-harvest interval (PHI) at 7, 10, 14, 15 and 21 days before harvest [
29]. Each pesticide formulation was applied according to the manufacturer’s recommendations and independently to ensure that its respective PHI was met on the day of sampling. Treatments were applied in a range of relative humidities between 70% and 80% and an ambient temperature ranging between 24 and 28 °C using a Maruyama MS073D backpack sprayer (Auburn, WA, USA) equipped with a 2 mm nozzle. No rainfall was recorded on the days when the treatment was applied.
Each pesticide treatment was applied in seven separate plots and pesticide residues were analysed individually per treatment. The pesticide residue values reported in this study represent the mean concentration of all samples analysed from the treated plots.
Grape samples were collected at the specific PHI of each pesticide to ensure that residue levels reflected real commercial conditions. For each treatment, 15 kg of grapes were randomly harvested from the treated plots and packed in opaque polyethylene bags to prevent degradation by light. Samples were kept at room temperature during transport to the laboratory to ensure minimal physical damage.
On arrival, the grapes were homogenised with dry ice under controlled conditions to obtain a representative analytical sample. All samples were immediately frozen at −20 °C until analysis. To ensure data reliability, pesticide residues were quantified within two weeks of freezing, with all extractions and analyses performed on the same day to minimise degradation.
2.3. Processing Factors
The vinification process was carried out at a controlled laboratory scale, simulating the industrial conditions used in commercial wineries.
Figure 1 illustrates the flowchart of the vinification process, showing the main stages of vinification and the monitoring points for the reduction or concentration of pesticide residues at each stage, from harvest to final filtration.
The process began with the grape harvest (M1), where Monastrell (Vitis vinifera) grapes were selected and harvested under controlled conditions. Pesticide residue concentrations were quantified in the harvested grapes to establish baseline levels.
Following harvest, the grapes were crushed (M2), and the resulting must was analysed for pesticide residues. The first processing factor (F1) was determined by comparing the residue concentrations in the crushed must (M2) to those in the harvested grapes (M1). This stage is crucial as it begins the mechanical breakdown of the grape structure, which influences pesticide dispersion.
The next step was maceration (M3), where the must remained in contact with the skins to extract phenolic compounds and colour. Pesticide residues were measured again, and the second processing factor (F2) was calculated by comparing the residue levels in the must after maceration (M3) with those in the crushed must (M2). This stage improves extraction and the effect of maceration on pesticide residue levels was studied by following changes in residue concentrations.
After maceration, the must was fermented (M4) in stainless steel tanks at 24–28 °C for 10–14 days. Commercial Saccharomyces cerevisiae yeast strains were used to convert sugars to ethanol, and during fermentation microbial metabolism and enzymatic hydrolysis contributed to the degradation of pesticide residues. Samples were taken at various intervals during fermentation and the third processing factor (F3) was calculated by comparing the pesticide levels in the fermented wine (M4) with those in the must before fermentation (M3). This step is critical in assessing how fermentation affects pesticide degradation.
After fermentation, the wine was separated from the solid residues [racking (M5)]. At this point, the wine was decanted, and the remaining solids (pomace) were pressed to extract additional liquid. The fourth processing factor (F4) was calculated by comparing the pesticide residue concentrations in the wine after racking (M5) with those before racking (M4), highlighting the role of solid particle removal in pesticide dissipation.
After racking, the wine underwent clarification (M6) where clarifying agents, such as bentonite and gelatine, were added to promote precipitation of suspended solids and further reduce pesticide residue concentrations. The fifth processing factor (F5) was calculated by comparing the residue concentrations in the clarified wine (M6) with those in the wine before clarification (M5).
After clarification, the wine was filtered (M7) through a 0.45 µm membrane to remove any remaining particles. The sixth processing factor (F6) was calculated by comparing the pesticide concentrations in the filtered wine (M7) with those in the clarified wine (M6). Filtration is an important step in ensuring that residual pesticide molecules are removed from the wine before bottling.
After filtration, the wine underwent cold stabilisation (M8), where it was chilled at 0–4 °C to prevent tartaric acid precipitation for seven days. Although the main purpose of this step is to improve the stability of the wine, it may also affect the degradation of pesticide residues. The seventh processing factor (F7) was calculated by comparing the pesticide concentrations in the cold-stabilised wine (M8) with those in the filtered wine (M7).
After cold stabilisation, the wine was sent to clarification to obtain clarified (M9) and filtered (M12) wine. The wine from M8 was also sent for clarification to obtain unclarified wine (M10), where the lees were also obtained (M11). Residue concentrations were measured at these stages and the eighth, ninth and tenth processing factors (F8, F9 and F10, respectively) were calculated to assess the total residue reduction from harvest (M1) to final bottling (M9 and M10). The global processing factor (Fglobal) was also calculated (M12/M1). These stages contribute to any final adjustments in pesticide residue levels through physical interactions with the wine matrix.
2.4. Extraction and Analysis of Pesticide Residues
The extraction of pesticide residues was carried out using the QuEChERS multi-residue method [
31,
32]. Briefly, a 10 mL sample of wine was placed into a 50 mL polypropylene centrifuge tube, followed by the addition of 10 mL of acetonitrile. The tube was sealed and manually shaken vigorously for one minute while placed in an ice bath. A buffered salt mixture (4 g of anhydrous magnesium sulphate, 0.5 g of disodium citrate sesquihydrate, and 1 g of trisodium citrate dihydrate) was added. The tube was hand-shaken vigorously for another minute and centrifuged at 3500 rpm for 5 min. The resulting extract was acidified (pH ≈ 4.3) with 5% formic acid and directly injected into the chromatographic system. In the analytical sequence, duplicate samples were included at the beginning and end as quality control measures to ensure sample stability, and they met the established acceptance and rejection criteria.
Pesticide residues were analysed using an Agilent Infinity Liquid Chromatograph, model 1260, coupled to a triple quadrupole mass spectrometer (Agilent Triple Quad LC/MS 6410B) operating in dynamic MRM scan mode (Agilent Technologies, Palo Alto, CA, USA). Chromatographic separation was performed on an Agilent Poroshell 120 EC-C18 column (3 mm × 100 mm, 2.7 µm) thermostated at 40 °C, with a flow rate of 0.6 mL/min. The injection volume consisted of 5 µL of sample plus 95 µL of mobile phase. The mobile phase consisted of acetonitrile with 0.1% of formic acid (phase A) and water with 0.1% of formic acid and 2 mM ammonium formate (phase B). The elution gradient started at 20% phase A, increasing linearly to 100% A in 10 min, holding for 6 min, before returning to the initial composition within 1 min. The total analysis time was 14 min, with an additional 5 min for stabilisation [
31,
32].
Quantification and identification of all pesticides analysed were performed using multiple reaction monitoring with an electrospray ionisation source in positive mode. The nebuliser gas was synthetic air at 40 psi, with a flow rate of 9 L/min. Ionisation voltage was set at 5500 V, and solvent evaporation was carried out with synthetic air at 350 °C.
2.5. Validation of the Analytical Method
The analytical method was validated following the guidelines outlined in the European Union document SANTE/11312/2021 v.2 on analytical quality control and validation procedures for pesticide residue analysis in food and feed [
33]. The validation included an assessment of potential interferences in the quantification of the target compounds, evaluation of detector response linearity in matrix extracts, calculation of Relative Standard Deviation (RSD) for response factors, back-calculated concentration and correlation coefficients (R
2), as well as recovery at two concentration levels of the limit of quantification (LOQ) (LOQ and 10LOQ) under repeatability and reproducibility conditions. Additionally, a series of quality control checks were implemented in each analysis sequence to ensure method robustness.
Each analytical sequence included the following: calibration curve, solvent blank, matrix extract, duplicate test samples, fortified samples at the lowest concentration level, and control standards. Detector response linearity was determined in triplicate using blank matrix extracts (must and wine) fortified with the selected pesticides at five concentrations (1, 10, 50, 100 and 500 µg/kg). To identify each pesticide, the limit of detection (LOD) was defined as the lowest concentration yielding a signal-to-noise (S/N) ratio greater than 3, while the limit of quantification (LOQ) was the lowest concentration with an S/N ratio greater than 10 and acceptable accuracy and precision.
Repeatability and reproducibility of the method were evaluated using six consecutively analysed fortified samples at LOQ and 10LOQ levels for all pesticides. The acceptance criterion was that the RSD should be ≤20%. Reproducibility was assessed by processing samples on six different days under identical conditions. Accuracy was determined by recovery experiments, where fortified samples at LOQ and 10LOQ levels were analysed, and results were compared with known standards analysed within the same sequence. The acceptance criterion for accuracy required mean recovery rates between 70% and 120%, with an RSD ≤ 20%.
2.6. Dietary Risk Assessment
The dietary risk assessment was conducted to evaluate the potential health risks associated with the consumption of wine containing pesticide residues. The Estimated Daily Intake (EDI) of pesticide residues was calculated following the methodology proposed by EFSA [
3,
23] and considering national wine consumption patterns in Spain. The Risk Quotient (RQ) was determined as the ratio between the estimated intake of each pesticide and its respective Acceptable Daily Intake (ADI), as defined by international regulatory agencies such as EFSA and FAO/WHO [
28]. The equations used for risk assessment were Equations (1) and (2) as follows:
where C represents the estimated national consumption of wine, R is the concentration of pesticide residues in wine (mg/L), and kg bw represents the mean adult body weight in Spain, considered as 60 kg for standard calculations [
28]. ADI indicates the maximum amount of a pesticide that can be ingested daily over a lifetime without appreciable health risk [
3].
RQ ≤ 1 indicates an acceptable risk to human health. Whereas RQ > 1 indicates that the risk of a pesticide to humans is unacceptable, and higher RQ values indicate higher risks [
34,
35].
2.7. Statistical Analysis
Descriptive statistical parameters, including mean, standard deviation and coefficient of variation, were calculated for all pesticide residues measured at different vinification stages. Data processing and statistical analyses were performed using IBM SPSS Statistics (version 24.0).
To evaluate the correlation of the variables used in the pesticides studied, as well as the relationship with the ADI, the software was Rstudio IDE Desktop 2023.06.01 and R 4.4.2 was used [
36]. Different R libraries were used depending on the analysis performed: for example, data import into R was performed using the Readxl library, and graph analysis was performed using ggplot2, rstatix, patchwork, dplyr and tidyverse.
3. Results and Discussion
3.1. Validation
The analytical method for pesticide residue detection was validated following the SANTE guidelines [
33]. Both LOD and LOQ were determined for each pesticide, together with recovery rates and repeatability. The method showed an excellent performance with LOD values in the range of 0.3 µg/kg to 0.8 µg/kg and LOQ values in the range of 1.0 µg/kg to 2.1 µg/kg. Recovery rates for all pesticides ranged from 89% to 108%, and the repeatability was between 1.5% and 6.8%, meeting with the criteria set by the SANTE guidelines [
33]. Further details of the validation parameters are shown in
Table 2.
3.2. Pesticide Residues Behaviour
Pesticide residues were quantified at each stage of the winemaking process, from the harvested grapes (M1) to the final wine products (M12), paying particular attention to those with the highest and lowest dissipation rates.
Table 3 shows the residue concentrations of each pesticide studied (boscalid, penconazole, tebufenozide, kresoxim-methyl, trifloxystrobin, chlorpyrifos and lambda-cyhalothrin) at each vinification stage (M1 to M12).
Penconazole and kresoxim-methyl were not detected at any vinification stage. Trifloxystrobin was not detected at the beginning of the vinification process; however, it was detected in M6 (17.45 µg/L) and M7 (22.79 µg/L) vinification stages, likely due to the fact that these are the stages of pressing and concentration, and that the coefficient of variation and the recovery have an uncertainty of about 40%. Chlorpyrifos and lambda-cyalothrin were detected at the beginning of the vinification stage; however, they were eliminated throughout all vinification stages, showing a 100% elimination rate.
Boscalid showed the greatest reduction during the vinification process, with a concentration below the LOQ at the last stage of vinification. The initial concentration in the harvested grapes (M1) was 43.68 µg/kg, which decreased to <10 µg/kg by the final filtration stage (M12), showing a reduction of 100%. In contrast, tebufenozide showed the smaller reduction. Its initial concentration in the harvested grapes (M1) was 182.76 µg/kg and, although it decreased during vinification, the final concentration at M12 was 21.71 µg/kg, reflecting the lower dissipation (88.1%). In this sense, it can be said that the stages that showed the higher elimination rates were M5, M6 and M11.
Similar results have been found in other studies on wine and other foods, both from our research group and from others. These results depend on the greater or lesser elimination of the physicochemical characteristics of the pesticide studied [
12,
13,
16,
37].
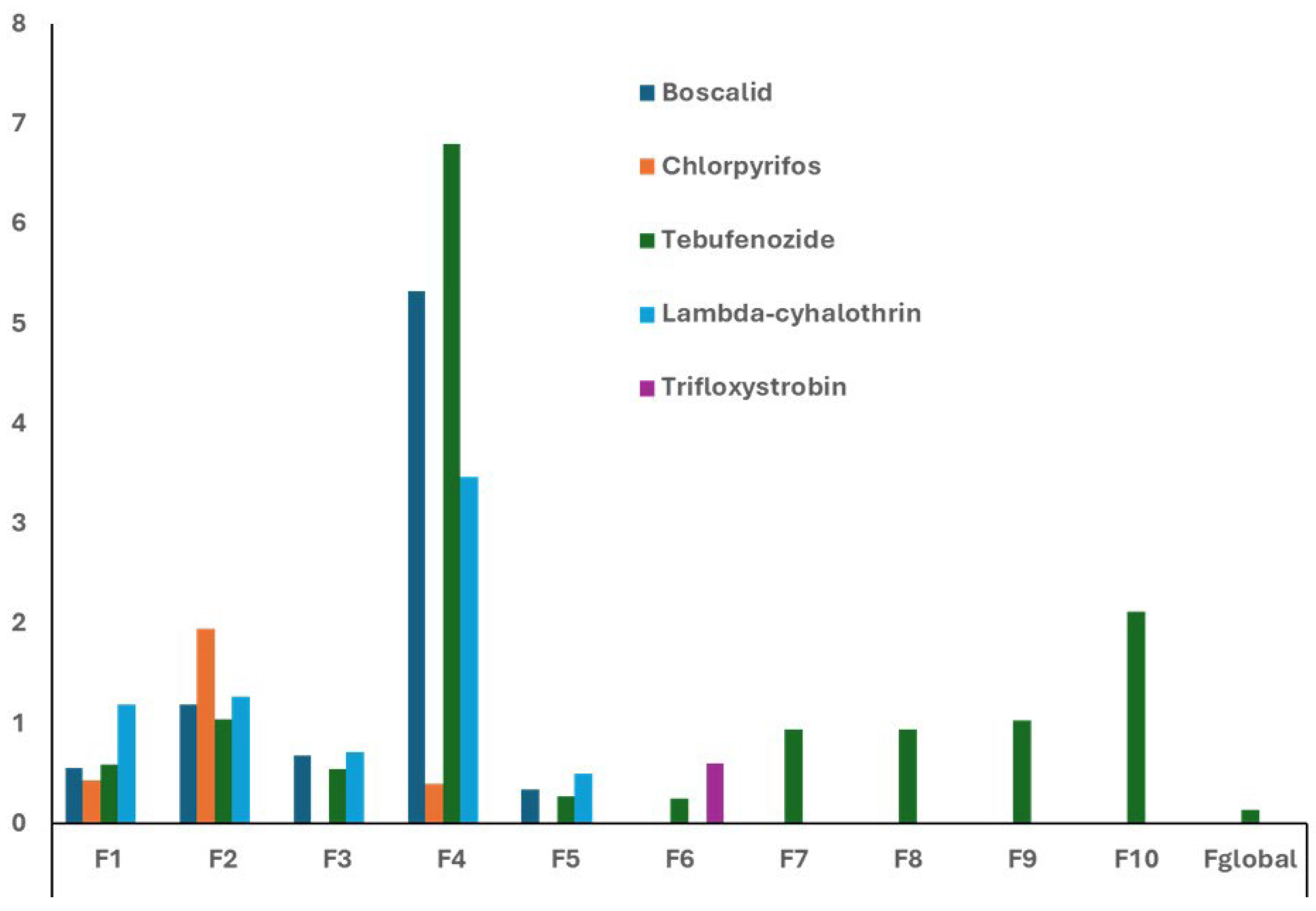
Figure 2 shows the corresponding processing factors (F1–F10 and F
global) which help to quantify removal efficiency at each stage of vinification, providing a comprehensive overview of how each step contributes to pesticide removal. As can be seen in
Figure 2, reductions in pesticide residues were observed during stages involving the removal of solids, such as crushing, maceration and racking, particularly F4, F5 and F
global. These stages are particularly effective because they remove byproducts such as pomace, lees and marc, which absorb significant amounts of pesticide residues.
These results indicate that there is no toxicological risk in the final wine product, as nearly all the pesticide residues found in the grapes were removed during the alcoholic fermentation and clarification processes. However, the wine byproducts—such as pomace, lees, and marc—that can be used in other industries, may present a risk due to the accumulation of pesticides in these materials.
Microbial activity during fermentation also contributed to the dissipation of pesticide residues. This finding is consistent with the work of Lozowicka et al. [
38], which emphasised the role of microbial metabolism in pesticide breakdown during the fermentation process. Furthermore, the EFSA report highlighted filtration as a highly effective method for removing pesticide residues during the final stages of winemaking [
3].
It can be concluded that the vinification process effectively reduced pesticide residues at all stages, with key steps such as crushing, maceration, fermentation and filtration playing a crucial role in the removal of pesticide residues from the wine.
3.3. Risk Assessment
Risk evaluation assessment for human health aims to estimate the nature and likelihood of adverse effects from pesticide exposure through wine consumption. The chronic risk relating to ingestion of the pesticides studied (boscalid, penconazole, tebufenozide, kresoxim-methyl, chlorpyrifos and lambda-cyhalothrin) was assessed by comparing pesticide residue levels in the wine with the EDI (calculated based on the average daily wine consumption, according to national dietary guidelines [
33]), and the ADI (sourced from the EU Pesticides Database [
39] and EFSA [
3], with the lowest ADI values considered in cases of joint toxicological actions) for each pesticide.
Table 4 shows EDI, ADI, and RQ values calculated for wine under GAP conditions. These values reflect the potential health risk from wine consumption based on average exposure.
As can be observed, on the one hand, the mean RQ values were all below 5% of the ADI, except for chlorpyrifos in wine, which reached 7.5% of the ADI showing a RQ of 0.0070. However, in all instances, the RQ values remained well below the safety threshold of 1, indicating a very low potential risk for human health. For other fruits using the same pesticides, similar results have been reported elsewhere [
37].
On the other hand, wine byproducts, such as pomace, lees, and marc, may present a higher risk due to pesticide accumulation, suggesting that they could pose a greater risk if used in applications like animal feed.
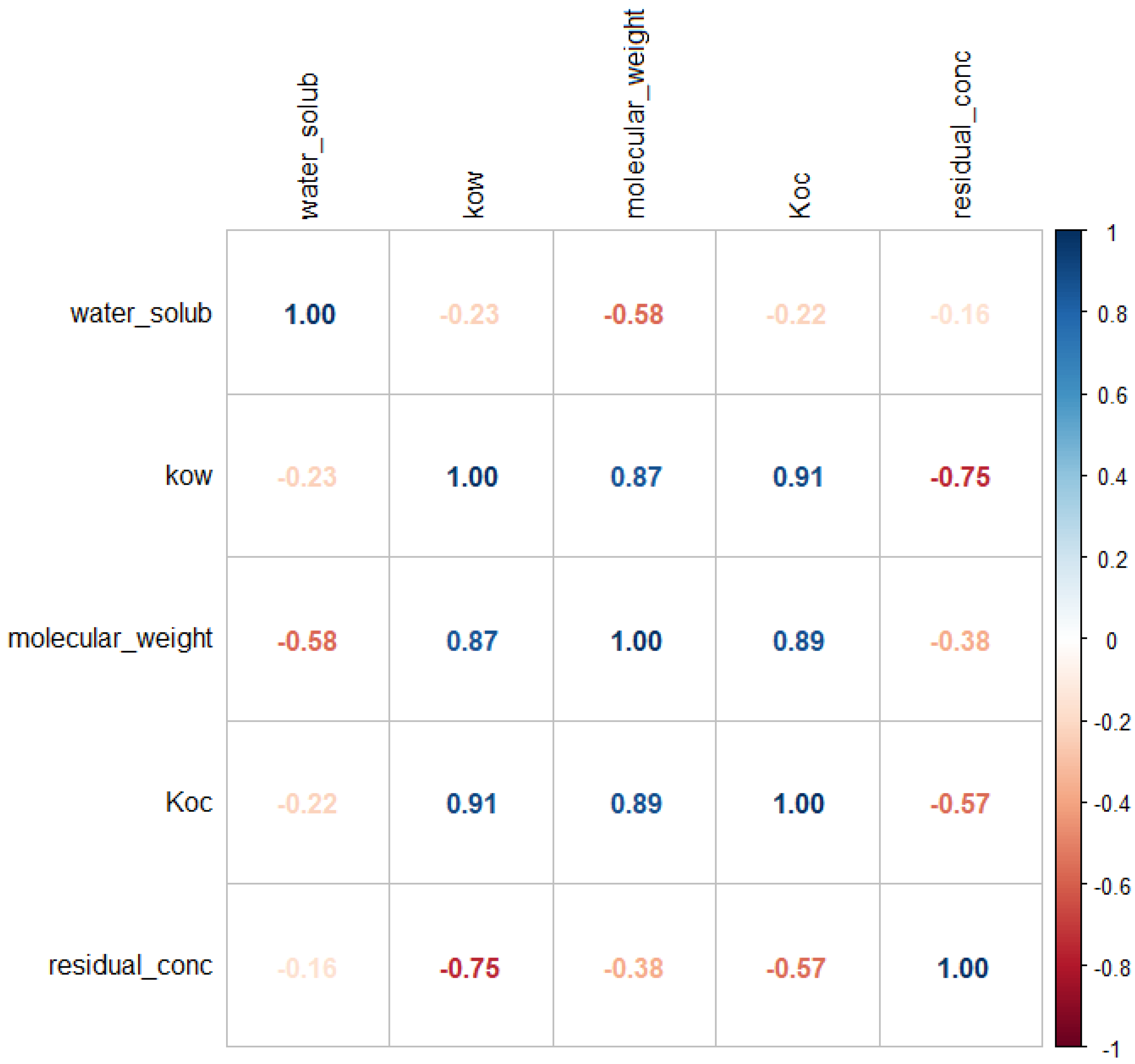
Further, a correlation analysis of the variables used for the pesticides studied (water solubility, k
ow, molecular weight, k
oc, residual concentration), and the correlation matrix is shown in
Figure 3.
As expected, there is a significant correlation of residual concentration with k
ow and k
oc, and to a lesser extent with molecular weight and insignificantly with water solubility, as reported in previous studies [
40,
41].
Applying a multiple regression model using all variables (including those from the health risk analysis), the following model was obtained with a significant
p-value of 0.00306 (<0.05) and an adjusted R
2 value of 1:
As can be observed, this model is totally independent of the molecular weight variable and the variable that most influenced the model is the residual concentration, according to Equation (1), and as reported elsewhere [
42].
4. Conclusions
This study confirms that the winemaking process plays a significant role in the degradation of pesticide residues, mainly through physicochemical transformations, microbial metabolism and adsorption to solid matrices. The effectiveness of each winemaking step varies according to the chemical nature of the pesticide, with fermentation, clarification, racking and filtration contributing to residue reduction to different extents.
The results show that fermentation contributes to pesticide degradation via microbial enzymatic activity and adsorption to yeast biomass. However, the efficiency of this process depends on the hydrophobicity of the pesticide, as hydrophilic compounds tend to persist in the aqueous phase. Clarification and racking further reduce pesticide residues by removing suspended solids that retain pesticides, with the efficiency of fining agents, such as bentonite and gelatine, varying depending on the molecular structure of the pesticides involved. Filtration completes the removal process, particularly for pesticides with a high adsorption affinity for colloidal organic matter, ensuring that residual contaminants are minimised.
The Ftotal calculations confirm that vinification generally results in net pesticide dissipation, with most residues having F values below 1, indicating that reduction mechanisms outweigh potential concentration effects.
Risk assessment-based EDI and ADI confirms that pesticide concentrations in the final wine remain well below the safety limits set by regulatory authorities. These results reinforce the role of winemaking as a natural mitigation process that enhances consumer safety.
Despite the reductions observed, certain pesticide residues show limited degradation, particularly those with high water solubility and chemical stability, highlighting the need for additional mitigation strategies. Optimisation of microbial metabolism and yeast strain selection could further improve pesticide degradation during fermentation. In addition, evaluation of the interaction of fining agents with pesticide residues could improve clarification efficiency, while further development of filtration techniques, such as nanofiltration or adsorption-based methods, could provide more effective residue removal. Furthermore, extending studies to assess the effect of maceration time, fermentation temperature and storage conditions on pesticide stability would provide a more comprehensive understanding of dissipation patterns in different oenological scenarios.
These findings provide a scientific basis for improving winemaking processes to minimise pesticide residues and ensure compliance with increasingly stringent food safety regulations, while maintaining wine quality. The integration of optimised processing techniques will be crucial in reducing chemical contaminants in wine production and increasing consumer confidence in the safety of the final product.