Abstract
Erythromycin fermentation residue (EFR) is difficult to dispose of due to its high content of macrolide erythromycin. An alternative economic method was proposed in this study for erythromycin elimination in EFR through volatile fatty acid (VFA) production via an anaerobic digestion process. Different parameters were applied to evaluate the effects on energy recovery of VFA together with erythromycin elimination from EFR through batch assays under mesophilic conditions. Results demonstrated that anaerobic digestion technology for VFA production can significantly enhance erythromycin elimination in EFR. The highest removal efficiency of 86.7–87.5% was obtained at conditions of controlled pH at 11.0, with erythromycin decreasing from an initial 100.2 to 12.6–14.0 mg/L. Additionally, controlled pH during the digestion process was reported to positively improve VFA yield to a maximum of 1.04 g-COD/g-VS than the adjustment of initial pH (0.46 g-COD/g-VS). Metabolic analysis alongside high-throughput sequence analysis further demonstrated the high hydrolysis and acidogenesis activities of EFR during the VFA accumulation process. Dominate enzymes EC:3.2.1.40, EC:6.2.1.3, EC:4.1.2.14, EC:2.7.2.1, and EC:1.1.1.27 well balanced the whole process from organic to VFA at pH controlled 11.0. The current study provided a new feasible choice for the economical treatment of antibiotic fermentation residues due to the tolerable antibiotic removal efficiency and satisfactory VFA yield.
1. Introduction
Erythromycin is a macrolide antibiotic which is widely used in disease privation of human beings and livestock [1]. However, erythromycin can only be obtained by fermentation due to its complex structure, which inevitably produces a large number of fermentation residues. Statistical reports showed that the yield ratio of antibiotics to their fermentation residue is around 1:8–1:12 [2], equivalent to around 2.2 million tons of antibiotic fermentation residues produced annually in China [3]. Erythromycin fermentation residue (EFR) contains a relatively high content of erythromycin of around 300–600 g/kg [4], leading to the spread of residual antibiotic and resistance genes if improperly handled. From this point, EFR as well as other antibiotic fermentation residues, are currently listed as “National Hazardous Waste List (2025 edition)” [5] in China. Furthermore, erythromycin is an antibiotic from the macrolide group with a complex matric structure, which has a central lactone ring with 12 to 16 atoms [4]. A few technologies can be applied for erythromycin elimination in EFR, for instance, spray drying flowered by incineration [6,7], hydrothermal pretreatment with ultra-high temperature and high pressure for a long time (>160 °C, >60 min) [3,4,8]. However, the above methods are too expensive for pharmaceutical companies as well as leading to organic waste. Other economic approaches, such as heat-activated persulfate oxidation combined with thermal treatment [9] or thermal pretreatment (<100 °C) [10], were reported to have poor dissipation of such a high content of erythromycin in raw EFR. Thereafter, it is urgent to find an economical and efficient method to dispose of EFR and degrade erythromycin.
Reports have shown that an anaerobic digestion process can help to degrade erythromycin [4,11,12]. Around 94.4% of degradation efficiency was reached in 10 days under mesophilic conditions with an initial erythromycin concentration of 40 mg/L [12]. Another research work showed that more than 80% of erythromycin was removed during an anaerobic digestion process dealing with EFR with an initial erythromycin concentration of 100–500 mg/L at 37 °C [4]. In addition, EFR has a high content of protein, carbohydrate, and lipid, with a ratio of volatile solids (VSs) to total solids (TSs) higher than 80% [13]. Anaerobic digestion was assumed to be a promising technology for EFR treatment to recover energy, together with erythromycin reduction. However, opposite conclusions have been reported in a large number of studies that erythromycin can inhibit the anaerobic digestion process, especially methanogenesis, which might be the reason why few reports have been carried out directly with EFR as raw material. Specifically, Cetecioglu et al. [14] reported that a drastic negative impact of around 68% on methane generation occurred at a 100 mg/L dose of erythromycin, and methanogenesis was almost completely inhibited at an erythromycin concentration of 500 mg/L. This was consistent in previous research dealing with macrolide antibiotics, such as clarithromycin [15,16], roxithromycin [17], tylosin [2], etc., that these antibiotics have inhibitory effects on methane generation. Therefore, methane production from EFR might not be a good choice. How to degrade erythromycin economically through anaerobic digestion and recover bioenergy is the biggest challenge at present.
Recently, volatile fatty acid (VFA) generation via anaerobic digestion has become a research hotspot, as it is well known that methanogens are more sensitive to environmental changes than hydrolysis and acidogenesis bacteria. Concretely, parameters that inhibit methane yield might stimulate the accumulation of VFA [18]. Additionally, VFA components, including acetic acid, propionic acid, (iso)butyric acid, (iso)valeric acid, and caproic acid, have been recognized with numerous industrial applications supported by the food and pharmaceuticals industries, whether individually or as mixtures [19]. For instance, acetic acid has been widely used as a preservative, acidity regulator in food industries, and terephthalic acid for chemical industries, etc. [19]. According to Expert Market Research 2020 [20], the global market for acetic acid will reach up to 24.51 tons and 12 billion dollars by 2025. Generally, VFA, as an essential intermediate product of anaerobic digestion, was expected with various applications and a higher economy value than biomethane [21,22]. Furthermore, VFA accumulation instead of methane yield might help to eliminate erythromycin due to its characteristic of extreme acid sensitivity [23]. Thereby, anaerobic digestion for VFA production was suggested to be an alternative promising approach for EFR treatment and erythromycin elimination. However, to the authors’ knowledge, no research has been conducted to evaluate the possibility of VFA generation directly from EFR without any pretreatment for erythromycin elimination. Further research was expected to explore the upper limits of the erythromycin elimination via the anaerobic VFA production process.
In this study, different factors of initial pH and heat-shock pretreatment were first arranged to evaluate the possibility of anaerobic fermentation from EFR. Secondly, batch assays were carried out based on the above results to achieve higher VFA concentration to enhance erythromycin degradation with parameters of pH control, higher EFR added, and different inoculum-to-substrate ratio (ISR). Decreasing erythromycin was supplied to evaluate the effect of erythromycin dissipation followed by VFA production. Finally, mass balance and biodegradability were calculated to analyze material flow and microbial activities.
2. Materials and Methods
2.1. Substrate and Inoculum
The EFR was obtained from Yili Chuanning Biotechnology Co., Ltd. (Yining, Xinjiang, China), with TS and VS contents of around 134.52 and 119.28 g/L, respectively. To ensure consistency, the raw feeding material was diluted with tap water to a VS of around 10%, mixed well, and stored at 4 °C until usage. The inoculum used in the current research was collected from a continuously stirred tank reactor treating swine manure at 37 °C and contained 50.92 g/L of TS and 43.36 g/L of VS. The inoculum was incubated for one week at 35 °C in an incubator and was thermally shocked at 80 °C for 15 min to inhibit the activity of methanogens before use. The characteristics of the substrate and inoculum are presented in Table 1.

Table 1.
Characteristics of erythromycin fermentation residue and inoculum.
2.2. Experimental Setup and Design
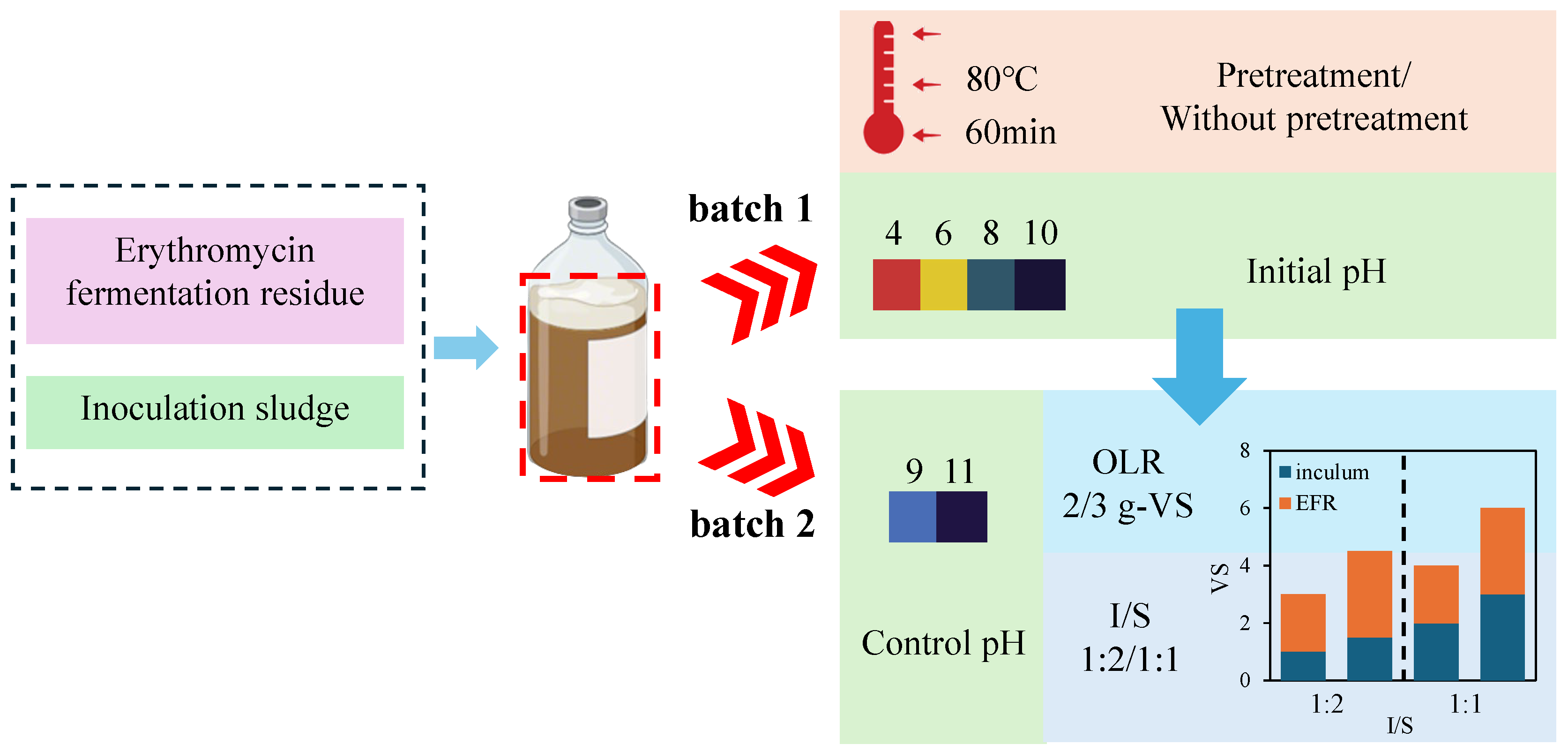
Batch assays (Figure 1) were carried out to evaluate the effects of initial pH and heat shock pretreatment on VFA yield from EFR via anaerobic digestion process. Around 0.33 g-VS of inoculum and 1 g-VS of diluted EFR or heat-shocked (HS, 80 °C for 60 min) EFR (ISR 1:3) were added into 120 mL serum bottles. Deionized water was added to the serum bottles to a total working volume of 100 mL for each. HCl or NaOH solution of 3 mol/L was used to adjust the initial pH to a certain value of 4.0, 6.0, or 10.0. Control conditions were checked with initial pH unchanged with a pH value of around 8.0. Well-mixed digestate was sampled from each bioreactor to analyze the initial characteristics on day zero. The bottles were sealed with butyl rubber stoppers and aluminum seals, and the headspace in each serum bottle was immediately flushed with N2 for 5 min to ensure strict anaerobic conditions. All experimental setups were carried out in triplicates. Finally, all bioreactors were incubated in a water bath shaker at 37 °C and 100 rpm. Analyzing the biogas volume and their composition (H2, CH4, CO2) with gas chromatography (GC) as mentioned in Section 2.3 and sampling 1 mL of well-mixed digestate every 2–3 days during the digestion period for pH and VFA analysis. Total chemical oxygen demand (TCOD), soluble chemical oxygen demand (SCOD), and NH4+-N were analyzed before and after digestion. The investigation period was 18 days as VFA concentrations began to decline in most experimental groups.
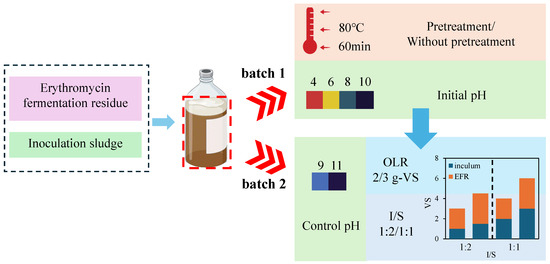
Figure 1.
Graphical diagram of the experimental design.
In order to enhance the removal efficiency of erythromycin as well as VFA conversion, another batch assays were carried out to evaluate the effects of pH control (9.0 and 11.0), organic loading rates (OLR, 2 and 3 g-VS/reactor) of EFR and ISR (1:1 and 1:2). The pH was adjusted every two days to ensure pH maintained at certain level during digestion processes with 3 mol/L NaOH solution. EFR applied in this batch of assays was untreated via heat shock. Residual erythromycin and chemical characteristics were analyzed in the same way as Section 2.3. The investigation period was set as 30 days, according to the changes in VFA concentration.
2.3. Analytical Methods and Material Balance
TS, VS, ammonium nitrogen (NH4+-N), crude protein, and carbohydrate were measured, referring to previous research (Yin et al., 2019 [24]). The pH values were measured with a standard pH meter (FE28, Mettler Toledo, Greifensee, Switzerland). The concentration of TCOD and SCOD were analyzed using solutions (LH-3BA) made in Lianhua Technology (Shanghai, China) and a spectrophotometer (UV2600, Shimadzu, Kyoto, Japan). VFA concentrations and biogas composition were determined by HPLC (Ultimate 3000, Thermo Fisher, Waltham, MA, USA) and GC (GC 2014, Shimadzu, Japan), respectively. Erythromycin was first extracted from digestate (1 mL) with 5 mL of methyl alcohol, vortexed for 2 min, and diluted with ultrapure water, followed by 0.22 μm filters. The concentrations of erythromycin were measured with ultra-performance liquid chromatography (UPLC, Xevo TQD, Waters, Milford, MA, USA) with a C18 column (2.1 × 100 mm, 1.7 μm). The volumetric methane values were converted to standard conditions at a pressure of 1.01 bar and a temperature of 0 °C.
Mass balance was calculated based on COD, where VFA including acetic acid, propionic acid, butyric acid, valeric acid, and caproic acid can be converted to COD with coefficients of 1.07, 1.51, 1.81, 2.04, and 2.21 g-COD/g-VFA, and methane to COD of 0.35 L-CH4/g-COD.
The biodegradation activity was calculated to evaluate the metabolic activity of hydrolysis, acidogenesis, and acetogenesis based on the conversion rates of COD and the addition of inoculum, expressed as follows [24]:
where VSS (volatile suspend solid) was the content of additional inoculum in each reactor, g/L; COD was the concentration of the digested solution, g.
2.4. High-Throughput Microbial Structure
Digestion conditions with the highest erythromycin removal efficiency and relatively high VFA yield (pH control 11.0&OLR 3.0&ISR 1:1) and EFR digestate as control, were chosen for microbial structure analysis, named EFR 11.0 and EFR, respectively. Sludge samples of around 5 mL were stored at −80 °C obtained from the last day of fermentation. DNA extraction was performed with the cetyltrimethylammonium bromide-sodium dodecyl sulfate (CTAB/SDS) method and purified with 1% agarose gels. Primers of 338F and 806R were applied for polymerase chain reaction (PCR) amplifications for bacteria and archaea, respectively. After purification, PCR products were applied to construct libraries at Major Bio and sequenced on the Illumina MiSeq platform (Illumina, San Diego, CA, USA).
2.5. Statistical Analysis
To evaluate the influence of the above factors, statistical significance was calculated using Duncan’s test. The means of the significantly different main effects were compared at p < 0.05. The statistical analysis was performed using SPSS 2023.
3. Results and Discussion
EFR, as the by-product of erythromycin production, is rich in organic matter but a threat to the environment. Its moisture content of over 90% due to its high viscosity made traditional treatment methods for EFR, such as incineration and hydrothermal (>160 °C) pretreatment, too expensive to utilize. Anaerobic digestion is supposed to be an alternative economic approach for energy conservation and erythromycin elimination. However, the existence of high concentrations of hazardous compounds would inhibit the sensitive methanogens to make it unattractive for biogas industries. The hypothesis of this work was to turn this challenge into an opportunity and explore the possibility of using EFR as feedstock for anaerobic digestion for the production and accumulation of VFA instead of methane. Acidic (<6.0) or alkaline (>9.0) pH environment was conducive to both the degradation of erythromycin and the accumulation of VFA. Therefore, in the current work, batch assays with different initial pH followed by pH control during the digestion period were carried out to obtain optimal conditions for VFA generation from EFR. Erythromycin concentration followed by microorganism structure were further analyzed to evaluate the anaerobic digestion effectiveness of erythromycin removal combined with VFA production.
3.1. VFA Production Performance and Erythromycin Elimination Under Different Initial pH with or Without Heat-Shock Pretreatment
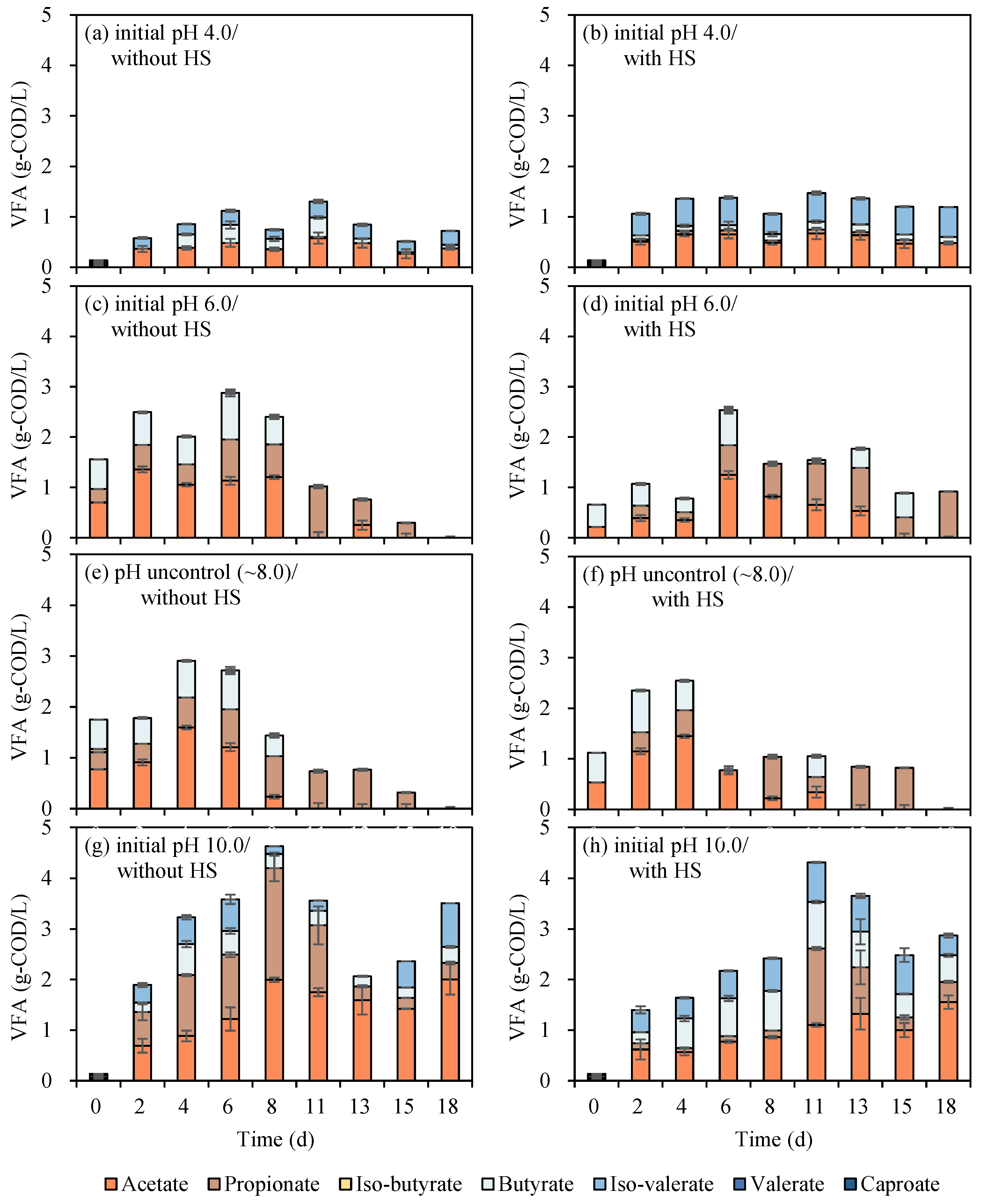
It was critical to inhibit methane generation in order to improve VFA accumulation. Several approaches have been applied to inhibit methanogens, such as thermal shock pretreatment of inoculum [25,26] and an initial pH lower than 5.0 [22]. Erythromycin remaining in EFR might help to inhibit the methanogenesis process at the same time [10,11]. From another point, thermal shock pretreatment was considered to be a promising approach that can both enhance the hydrolysis process and decrease erythromycin. Hence, in the first batch cultivations, the effects of initial pH (4.0, 6.0, 8.0, and 10.0) and HS pretreatment (80 °C, 60 min) and the interaction between parameters toward biogas production and VFA yield were investigated through a batch experiment (Figure 2).
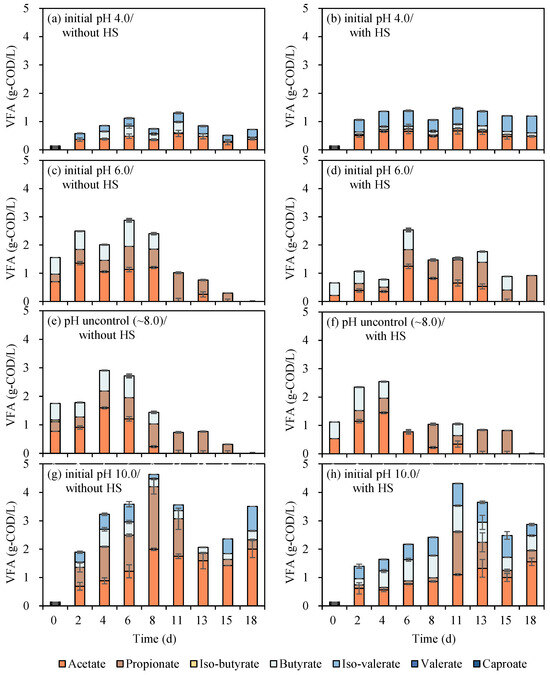
Figure 2.
VFAs production from EFR under different initial pH of 4.0 (a,b), 6.0 (c,d), 8.0 (e,f), and 10.0 (g,h) with or without heat-shock pretreatment (p < 0.05).
In general, the VFA concentration was increased with the increase in initial pH, regardless of whether the substrate was HS or not (Figure 2). Relatively, the higher VFA concentrations were obtained with an initial pH of 10.0, and the highest VFA concentration was 4.63 g-COD/L generated on the 8th day without HS pretreatment (Figure 2g). It was shown that the VFA concentration sharply increased from the 2nd to the 8th day and fluctuated during the following digestion period. However, there was no biogas produced combined with VFA decrease; this might be due to the conversion of aldehydes, lactic acid, and other types of intermediates. Followed by 4.31 g-COD/L occurred in the cultivation of initial pH 10.0 with HS pretreatment (Figure 2h). It has been reported that the hydrolysis and acidogenesis bacteria in anaerobic digestion of EFR mainly consist of genus Bacteroides spp., Escherichia spp., and Parabacteroides spp. [10], with the optimal pH ranging from 5.5 to 7.0, this may lead to the accumulation of VFA. However, the inhibition of both methanogenesis and hydrolysis/acidogenesis at initial pH 4.0 might result in both lower methane and VFA production (Figure 2). These reports were consistent with the current results that pick of VFA concentrations under initial pH 4.0 were the lowest compared to other initial pH conditions ranging from 1.30 to 1.47 g/L (Figure 2a,b). In the view of HS pretreatment, no obvious effect was reflected in the current research shown in Figure 1. It might be because of the high content of protein and carbohydrate in EFR, which was easily degraded with no need to be pretreated to enhance hydrolysis [3]. In other words, the higher initial content of erythromycin in EFR without heat shock has no significant effect on VFA generation.
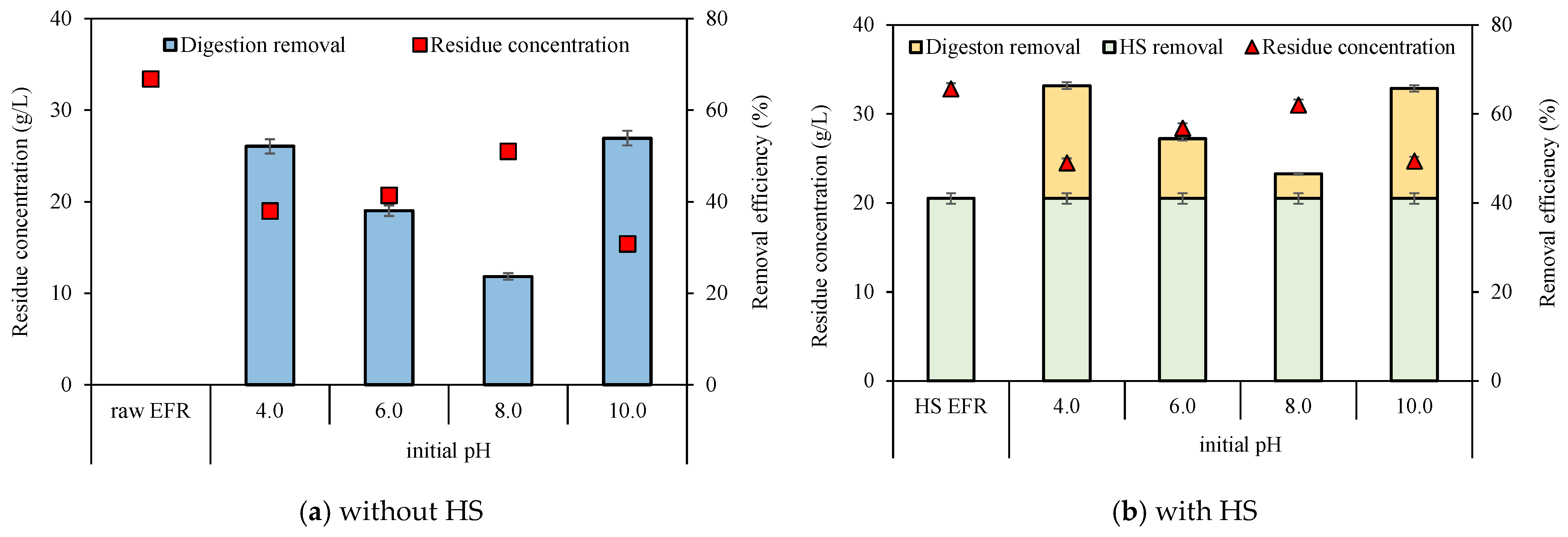
Retained erythromycin concentrations before and after digestion were analyzed to investigate the elimination performance in combination with the VFA production process. The initial content of erythromycin in raw EFR was 401 ± 5 mg/L and declined to 237 ± 5 mg/L after heat-shock pretreatment at 80 °C, 60 min (Table 1), with a removal efficiency of around 41%. This removal rate was extremely higher than 5% (80 °C, 60 min), 18% (120 °C, 60 min), 27% (90 °C, 120 min) [3,9] and slightly lower than 60% (90 °C, 60 min) with an initial concentration of 185 mg/L reported in previous researches [10]. The residue erythromycin concentration and removal efficiency of EFR with or without HS under different initial pH were calculated (Figure 3). The residual erythromycin concentrations were analyzed in each serum bottle. Due to the different VS concentrations between HS EFR and raw EFR, after adding EFR with the same VS to the serum bottle, the initial concentrations of erythromycin without and with HS were 33.4 and 32.8 mg/L, respectively. Generally, HS pretreatment can slightly enhance erythromycin elimination, with removal efficiency ranging from 47 to 66%, which was 12–23% higher than direct digestion of raw EFR under similar initial erythromycin concentration. From the point of initial pH, previous research reported that erythromycin was extremely acid sensitive [23]. Additional results in current research showed that extreme acid and alkali conditions helped to inactivate and dispose of erythromycin, and pH unadjusted of around 8.0 obtained the lowest removal efficiency no matter with or without HS. In short, for the degradation of erythromycin, the initial pH is 4.0 ≈ 10.0 > 6.0 > 8.0.
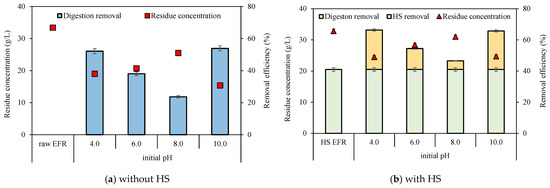
Figure 3.
Residue erythromycin concentrations and their removal efficiencies under different initial pH without (a) and with (b) heat-shock pretreatment (p < 0.05).
The effect of anaerobic digestion on erythromycin elimination was further investigated (Figure 3b). For raw EFR directly digested, elimination can be degraded from 33.4 mg/L to 15.4–20.7 mg/L, with removal efficiency varying from 24 to 54% (Figure 3a). Whereas, for HS EFR, the total removal efficiency of erythromycin reached up to 47% to 66% after 18 d digestion, of which 41% was contributed by thermal shock pretreatment (Figure 3b). It meant that after HS pretreat, the anaerobic digestion process contributed to 12–38% of erythromycin dissipation under different initial pH from a raw concentration of 32.8 mg/L. Such low contribution from anaerobic fermentation compared to raw EFR could be attributed to two aspects: (1) the lower initial concentration due to the dilution effect of inoculum (32.8 mg/L of day zero vs. 237 mg/L of HS EFR) and (2) more soluble organics were released via HS hydrolysis, enabling anaerobic microorganisms to utilize the organic matter in shorter time before erythromycin inhibition [10,27].
3.2. Effect of pH Control, ISR, and OLR on Biogas and VFA Yield
According to the above results, pH has the strongest influence on VFA accumulation. Research dealing with citrus waste and food waste have concluded that pH control during the digestion process could improve VFA yield by around 4–8-fold under acidic conditions [28]. At the same time, another semi-continuous research dealing with livestock manure reported that an uncontrolled pH of around 8.2 was preferred to achieve a higher VFA yield than that control at 6.0 [26]. Briefly, pH control alongside digestion was supposed to positively enhance VFA concentration and yield than adjustment of initial pH. From the above results, the pH values for initial pH 10.0 ranged from 9.0 to 10.0 during digestion. Therefore, pH control at 9.0 and 11.0 was designed in the following experiment. In addition, it was well known that VFA concentration would increase with the increase of substrate loading [25,29]. Furthermore, ISR influenced the VFA yield due to the addition of microorganisms [22]. Thus, in order to improve VFA concentration from anaerobic digestion of EFR, two levels of pH control, ISR as well as OLR, were chosen to determine the effects of these parameters.
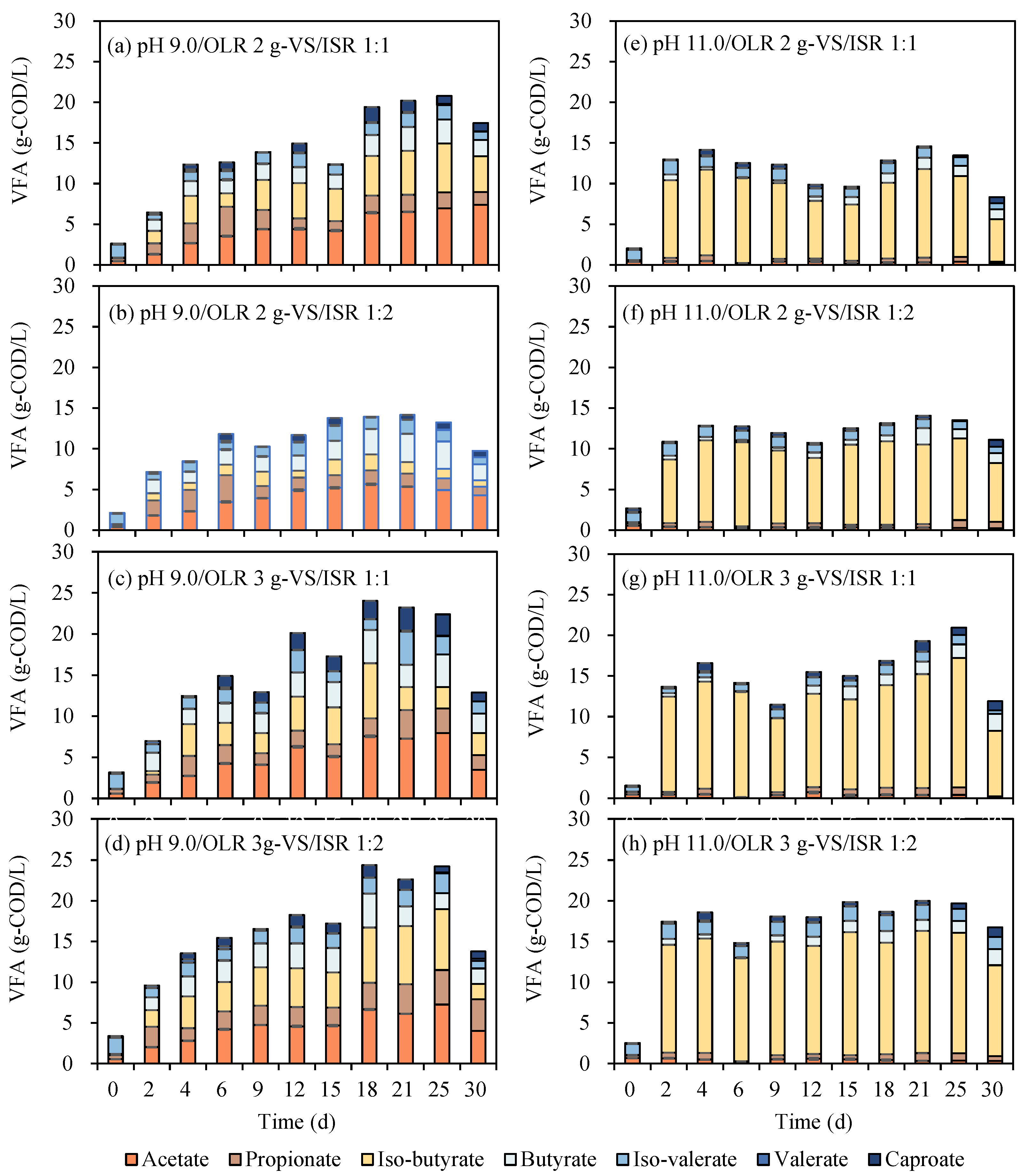
For factors of controlled pH (Figure 4a–d vs. Figure 4e–h), trends for VFA concentrations with pH control at 9.0 were improved step by step during the first 15 days, remained stable for around one week and dropped sharply after 30 days’ digestion (Figure 4a–d). VFA caught up and was kept around certain concentrations for pH control at 11.0. VFA dropped from the 25th to 30th days might be due to the consumption of biogas. Additionally, it was shown that the selections of VFA for pH control at 9.0 ranging from 14.2 to 24.2 g-COD/L were relatively higher than pH 11.0 (14.1–20.9 g-COD/L). Correspondingly, the highest net VFA yield (minus initial VFA of day 0) ranged from 0.70 to 1.04 g-COD/g-VS for different digestion conditions under controlled pH 9.0 (Table 2). A great distinction was presented on the maximum, medium, and mean net VFA yields between different pH values. Controlled pH 9.0 was a preferred condition for VFA conversion for EFR. This result was consistent with previous research dealing with kitchen waste through a continuous reactor has demonstrated that pH controlled close to neutral levels led to better VFA production [28]. A similar result carried out from another batch reactor showed that pH control at 9.0 obtained a higher VFA concentration than pH 11.0 [30]. Positively, even though it was a lower concentration of VFA, the components under pH controlled at 11.0 were more homogeneous, of which a content of up to 81–83% (5.7–8.8 g/L) iso-butyrate was produced (Table 2). This result was similar to previous research that the utilization of butyric acid would be inhibited when erythromycin existed (200 mg/L) [31]. Iso-butyric acid, an important raw material for the synthesis of flavors, was reported with a higher market price of around 2500 $/t [21]. In the current work, a controlled pH of 11.0 was suggested to be an economic method for the generation of iso-butyrate with a lower cost for purification.
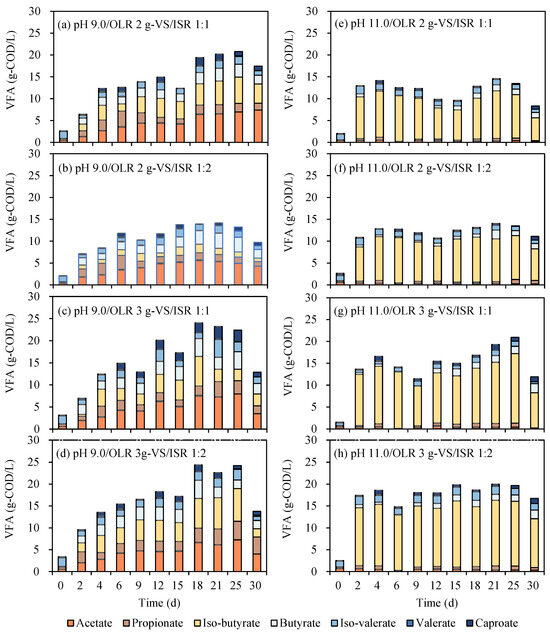
Figure 4.
VFAs production from EFR under different controlled pH, OLR and ISR.

Table 2.
Summary of VFA yield and performance of the second batch.
The VFA concentrations increased with OLR changed from 2 to 3 g-VS/reactor whether pH 9.0 or 11.0. The highest VFA produced from EFR in this study was 24.2 g-COD/L presented on the 25th day under OLR of 3 g-VS per reactor at a controlled pH of 9.0 (Table 2). However, the highest net VFA yield would decline with the increase in OLR under different conditions, except for the condition of pH control 9.0 and ISR 1:2, of which the selections of net VFA yield were promoted from 0.70 to 0.81 g-COD/g-VS. Scilicet, OLR 3 g-VS in strum bottles was not the limitation for VFA generation from EFR. Research has illustrated that higher OLR is possible with the VFA accumulation process than for biogas production [22,32]. Moreover, 3 g-VS/(L·d) was supposed as the limit OLR for biogas industries [33]. At the same time, OLR up to 6–8 g-VS/(L·d) was evaluated as suitable OLRs for VFA generation for food waste and animal manure [25,34]. Optimal OLR and continuous experiments were expected to be further carried out to achieve higher VFA concentrations and conversion efficiencies.
With the additional increase in inoculum (ISR 1:2 to 1:1), a bit higher or significantly higher VFA generation was obtained under different conditions. ISR 1:1 was examined as a better condition than ISR 1:2 for VFA production from EFR both on concentrations and yields (Table 2). Even though EFR has similar characteristics, for example, high organic content, excellent biodegradability and low C/N ratio with food waste and animal manure. Contrary results were carried out from EFR on the effects of ISR. Previous research illustrated that ISR 1:3 was suggested as the optimal condition for VFA production from food waste when ISR changes between 1:1 and 1:3 [35]. A similar conclusion was obtained from anaerobic digestion of animal manure that VFA would decline with the increase in inoculum under mesophilic digestion [25]. This opposite effect of ISR on the digestion of EFR may be due to (1) a higher content of microorganisms needed for the initial digestion of EFR against the unfavorable fermentation environment (mycelium and erythromycin) [6] or (2) a certain amount of anaerobic microorganisms carried in food waste, especially in animal manure themselves, which was more favorable for the enrichment of microbial flora. Therefore, a greater content of inoculum was supposed to be inoculated for the anaerobic digestion of EFR.
In short, the highest VFA concentration from EFR in the current literature was 24.2 g-COD/L. This concentration was inferior to results from food waste (over 30 g/L) [30,34]. However, competitive VFA yield obtained from EFR was higher than nearly all kinds of organic wastes, such as food waste (0.54–0.80 g-COD/g-VS) [34,35], chicken manure (0.64–0.91 g-COD/g-VS) [26], cow slurry (0.41 g-VFA/g-VS) [25], and slaughterhouse wastewater (0.61 g-COD/g-COD) [36]. Thereby, it can be inferred that EFR was a promising alternative substrate for the production of VFA. Further experiments were suggested to illustrate the continuous anaerobic acidogenic performance of EFR, and higher VFA concentrations can be expected.
3.3. Enhanced Erythromycin Elimination During VFA-Accumulating Processes
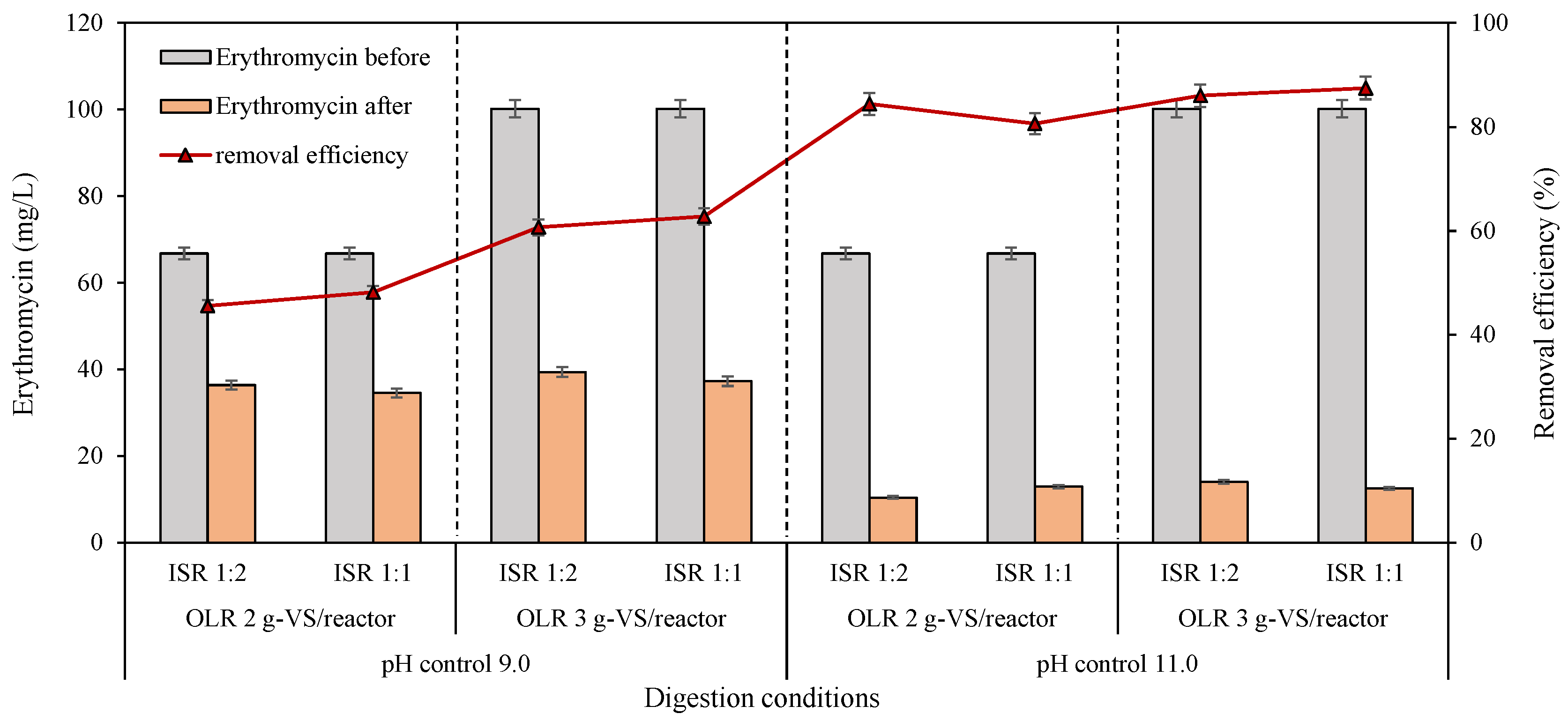
The initial erythromycin concentrations were 66.8 and 100.2 mg/L for OLR 2 and 3 g-VS/reactor, respectively, for VFA generation reactors in the second batch assays (Figure 5). Obviously, the controlled pH significantly influenced the erythromycin elimination performance during the VFA generation process in anaerobic digesters. Extraordinarily higher erythromycin removal efficiencies of over 80% were carried out at pH 11.0 than at pH 9.0. Concretely, after 30 days of fermentation, the removal efficiencies of the antibiotic ranged from 46% to 63% at controlled pH 9.0 and stabilized at an average of 85% ± 3% at pH control 11.0 (Figure 5). The same conclusion was achieved in 1994 that increased pH from 7.5 to 11.0 sharply improved the decomposition of erythromycin both at 20 °C and 40 °C, of which the first order rates were 6.01 × 10−3 and 6.84 × 10−2 for pH 9.0 and 11.0, respectively [37]. A similar inference was verified by Li and Zhang [38] dealing with municipal wastewater. Both the highest removal efficiency of erythromycin (88%) and the lowest residual erythromycin concentration (12.6 mg/L) appeared at pH control 11.0 in the current research during anaerobic acid generation processes (Figure 5). About a 47% removal rate was reported in an anaerobic digestion process from initially 38 to 20 mg/L [10]. This extremely high removal efficiency in this work is equivalent to the effect of 140–160 °C with hydrothermal pretreatment [3,39]. Importantly, this high removal efficiency and low concentration of residual erythromycin have never been obtained through the bio-degradation process in previous research dealing with EFR. The current work first identified the possibility and feasibility of erythromycin elimination through anaerobic digestion from an initial concentration of above 100 mg/L.
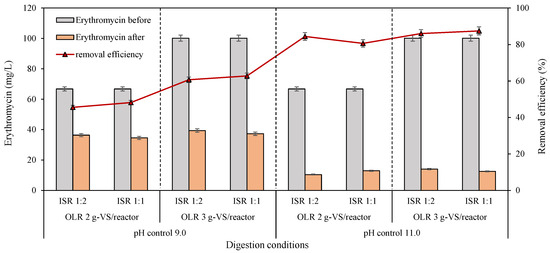
Figure 5.
Erythromycin declination and removal efficiency during VFA production processes under different controlled pH, OLR, and ISR.
From the point of OLR, its increase has led to the enhancement of erythromycin elimination. In other words, removal efficiency has been enhanced with the increased initial erythromycin concentration (Figure 5). This trend is consistent with the previous literature on deteriorating tylosin in an up-flow anaerobic stage reactor in that the removal rates increased from 70% to 99% when OLR increased from 1.86 to 3.73 kg-COD/(m3·d) [40]. However, the residual erythromycin in digestate approximately ranged from 34.6 to 39.4 mg/L under pH control at 9.0 and 10.4 to 14.0 mg/L under pH control at 11.0. The enhanced performance for erythromycin degradation with increasing OLR might be due to the relatively low removal efficiencies at pH 9.0. Ren and his colleagues [41] have demonstrated that in pure solutions, 100 mg/L of erythromycin could be degraded within 36 h by a microbial degradation technique, but only 58.5% was removed in EFR. It was well known that biological degradation was ultimately the role of enzymes, for example, erythromycin esterase enzymes, including EreA and EreB [42,43]. Several troubles can affect the enzyme activity and inhibit erythromycin degradation; for example, (a) the binding site for erythromycin was competitively occupied, or (b) the enzyme activity was lost over time [44]. Speculatively, erythromycin of around 35 and 10 mg/L in the current experiment might be the limiting concentrations for anaerobic microorganisms with pH 9.0 and 11.0, respectively.
Interestingly, no significant difference was observed with the changes in ISR from 1:2 to 1:1 (Figure 4), which meant that the erythromycin dissipation process would not be improved with increased anaerobic microorganisms in the inoculum. Two possible interpretations for this phenomenon were as follows: (I) the number of microorganisms and enzymes from the inoculum was enough for erythromycin elimination at ISR of 1:2 [14], or (II) the accumulated high content of organic acids, pH, or the anaerobic environment itself helped to remove erythromycin, while the contribution of microorganisms was negligible [12]. Further experiments were needed to distinguish the specific contributions of the two factors for erythromycin removal and verify mechanisms.
3.4. Metabolic Activities and Material Balance During VFA Production Processes
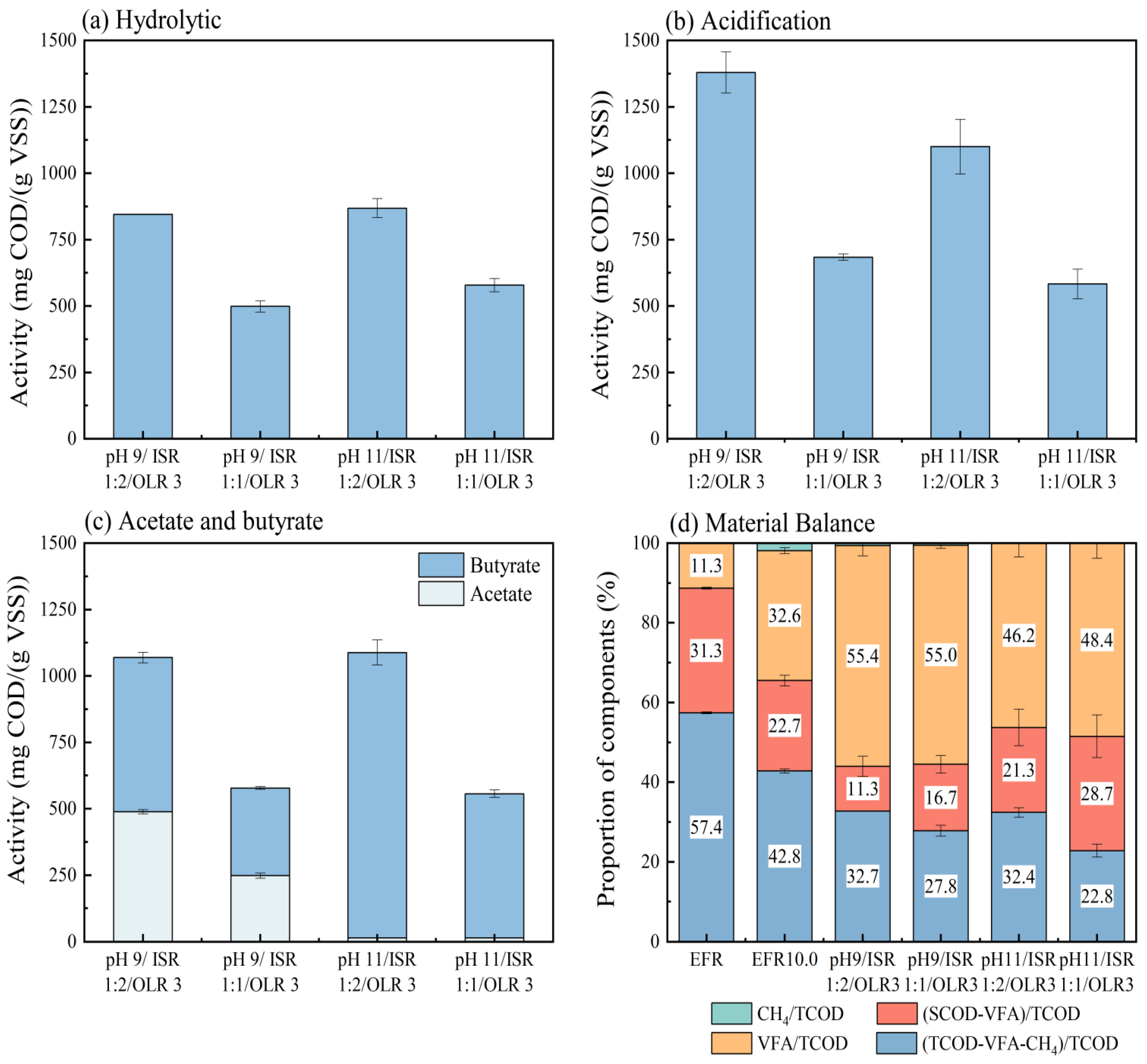
In order to evaluate what happened during the VFA accumulation process, the metabolic activities of hydrolysis, acidogenesis, and acetogenesis (Table 3) and material balance based on COD conversion were calculated for different conditions with higher VFA concentrations from the second assays (Figure 6). The percentages of remaining COD unconverted were 21.4% to 41.7% from raw EFR of 57.4% (Figure 6), which meant that around 15.7% to 36.0% of COD was hydrolyzed during anaerobic digestions. Correspondingly, the solubilization performance for VFA production under different conditions ranged from 253 to 499 mg-COD/(g-VSS·d) (Table 3). The highest hydrolysis activity was 499 ± 16 mg-COD/(g-VSS·d) appeared at pH control 11.0, OLR 2 and ISR 1:2, followed by 436 (pH 11.0, OLR 3, ISR 1:2) and 433 mg-COD/(g-VSS·d) (pH 9.0, OLR 3, ISR 1:1). The hydrolysis activities of EFR under mesophilic conditions could be considered comparable with an enhanced activity of the hyper-thermophilic condition dealing with chicken manure at a hydraulic retention time of 2 days (556 mg-COD/(g-VSS·d)) [24] and extremely higher than other kinds of organic wastes under mesophilic and thermophilic conditions (230–260 mg-COD/(g-VSS·d)) [33]. It can be inferred that raw EFR without any pretreatment can be well hydrolyzed via anaerobic digestion. However, a totally opposite performance was carried out from hydrolysis conversion rates based on COD (Figure 6d). The four groups with the lowest hydrolysis activities (ISR 1:1) had the highest hydrolysis conversion efficiencies. Specifically, the addition of seed sludge positively helped to enhance the conversion of organic matters from TCOD to SCOD, but the hydrolysis activity decreased with the increase in ISR ratio from 1:2 to 1:1 under other conditions except for conditions of pH control 9.0 and OLR 2 g-VS/reactor. There was still a large amount of organic matter unconverted in reactors, enhanced hydrolysis conversion rates with higher ISR, indicating that the hydrolysis was the rate-limiting step for anaerobic digestion of EFR due to the existence of non-degradable organic matters such as mycelium [1,10]. Similar trends were observed for OLR; its increase helped to improve the hydrolysis activity. At the same time, the controlled pH (9.0 and 11.0) did not show significant effects of hydrolytic activity (Table 3).

Table 3.
Metabolic activities of hydrolysis, acidogenesis, and iso-butyrate production under different digestion conditions of controlled pH, OLR, and ISR.
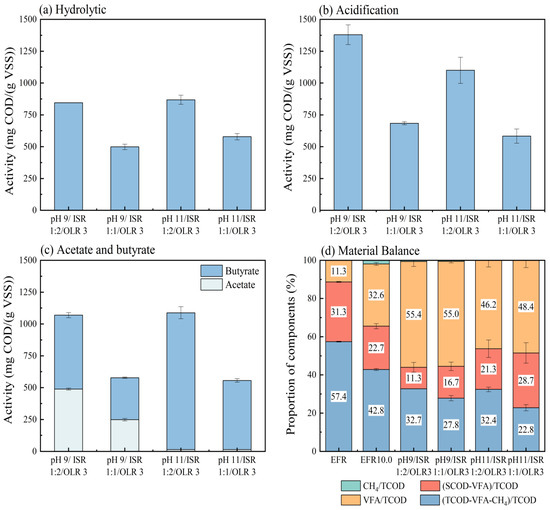
Figure 6.
Metabolic activities of hydrolysis (a), acidogenesis (b), and major acids’ conversion (c) and mass balance based on COD (d) under different conditions of controlled pH, OLR, and ISR.
The acidogenesis process showed higher metabolic activities (292–690 mg-COD/(g-VSS·d)) in this study for the acid digestion of EFR than hydrolytic activities because of the relatively high content of initial SCOD (42.6%, Figure 5) that does not need to be converted from solid content and can be utilized to VFA [24]. Consistent with hydrolysis, the acidogenesis activities sharply declined as the ISR changed from 1:2 to 1:1 (Table 3). The highest acidogenesis activity was 690 mg-COD/(g-VSS·d) appeared at controlled pH 9.0, ISR 1:2, and OLR 3 g-VS/reactor, followed by 603 and 596 mg-COD/(g-VSS·d) both at ISR 1:2, OLR 2 g-VS/reactor and pH 9.0 and 11.0, respectively (Table 3). The acidogenesis activities of EFR under mesophilic conditions during acid digestion performance were much higher than many other organic wastes, such as chicken manure (220–240 mg-COD/(g-VSS·d)) [33], food waste (192–228 mg-COD/(g-VSS·d)) [22,34], and sewage sludge (367 mg-COD/(g-VSS·d)) [22]. For conditions of ISR 1:1, the relatively higher activity (471 mg-COD/(g-VSS·d), pH control 9.0, and OLR 2 g-VS/reactor) led to the highest percentage of VFA to TCOD of 71.3% based on COD, equivalent to 60% of VFA converted from COD. This result was consistent with the VFA yield of 1.04 g-COD/g-VS achieved through the same digester (Table 3). It is worth noting that ISR 1:2 gives higher metabolic activity, while ISR 1:1 gives higher conversion. This seemingly contradictory result precisely indicates that an increase in the amount of seed sludge can promote the overall activity, but per unit of sludge (microorganisms), an adequate substrate is more beneficial [24,33]. Except for pH control 9.0 and ISR 1:2, the acidogenesis activities decreased with the increase in OLR from 2 to 3 g-VS/reactor under different conditions. In addition, controlled pH 9.0 seemed to be more suitable for acid-producing microbials with higher acidogenesis activities than pH control 11.0 (Table 3). The same trends appeared in COD conversion efficiencies that higher content of SCOD unconverted to VFA (21.3–28.7%, Figure 6b) in cultivations of controlled pH 11.0 compared to conditions of pH control 9.0 (6.5–16.7%, Figure 6a).
The metabolic activities for acetic acid and iso-butyric acid production were calculated as these two acids were the major components (Table 3). It was shown that the acetogenesis activities at pH 11.0 were very low in agreement with the low acetate concentrations (Figure 4a–d). Oppositely, the highest activity for iso-butyric acid production was 558 mg-COD/(g-VSS·d) under conditions of pH control 11.0, OLR 2 g-VS/reactor, and ISR 1:2, close to the corresponding acidogenesis activity.
3.5. Bacterial Structures and Predicted Functional Enzyme Abundances
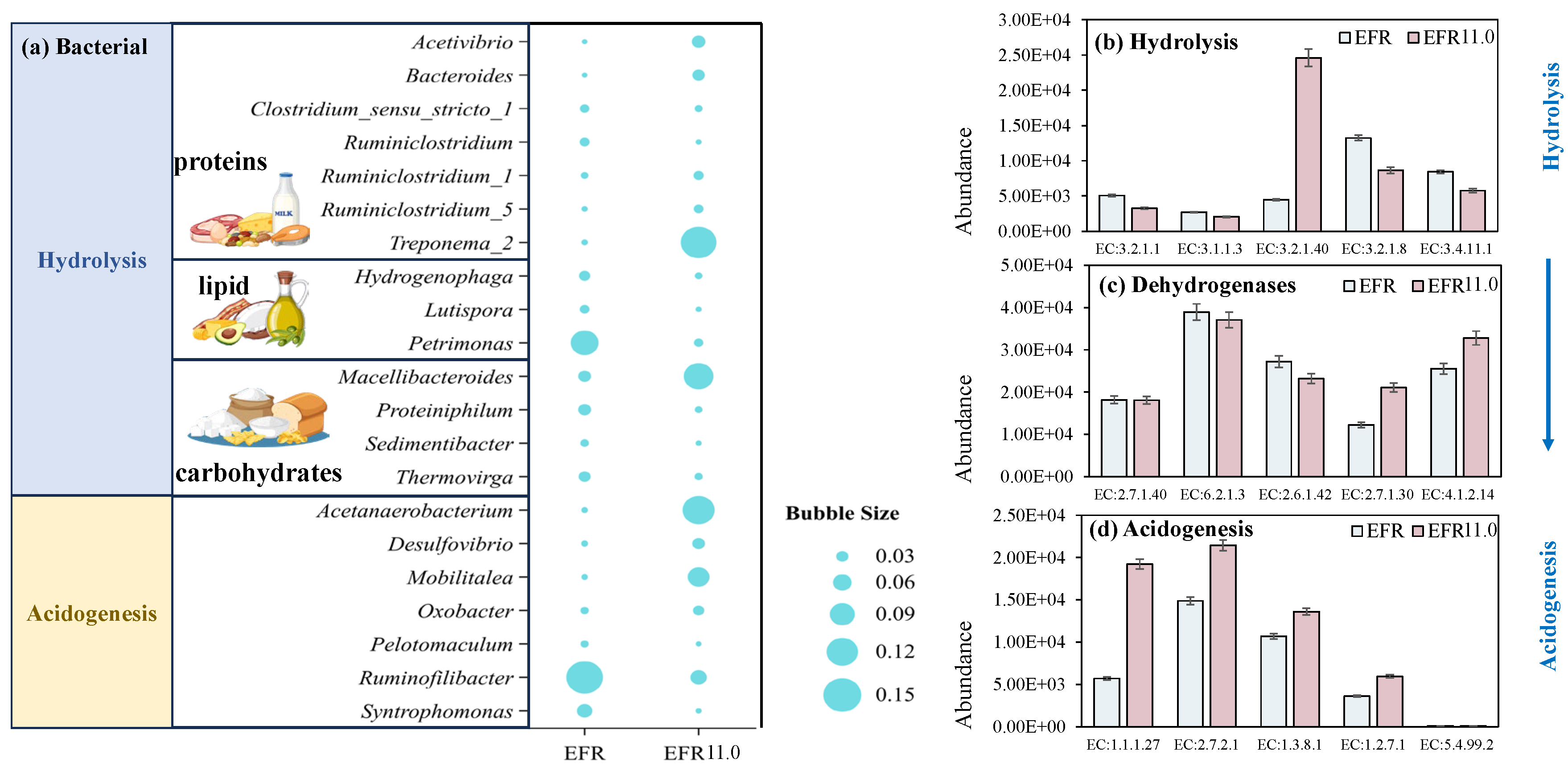
Bacterial structures were operated via 16S rRNA sequence for EFR (without pH adjustment as control) and EFR 11.0 (pH controlled at 11.0 with the highest erythromycin removal efficiency) to evaluate the changes under pH control conditions. The relative abundance of bacteria at the genus level of EFR and EFR 11.0 was presented in Figure 7a. Results showed that Petrimonas (10.3% ± 1.1%) and Ruminofilibacter (14.4% ± 0.8%) were the most dominant genera for the digestion of EFR, which were catalyzed with the function of lipid hydrolysis and acidogenesis to produce acid, respectively [44]. However, bacterial function as protein and carbohydrate hydrolysis were inhibited with relatively low abundance, leading to a low organic matter conversion rate in EFR, which may be the fundamental reason for the low VFA yield. Positively, potential protein hydrolysis bacteria Treponema_2 [45], carbohydrate hydrolysis bacteria Macellibacteroides [10], and acidogenesis bacteria Acetanaerobacterium [10,38] were well balanced in the anaerobic conversion process of EFR at pH controlled at 11.0 with relative abundances of 13.9%, 11.2%, and 12.3%, respectively.
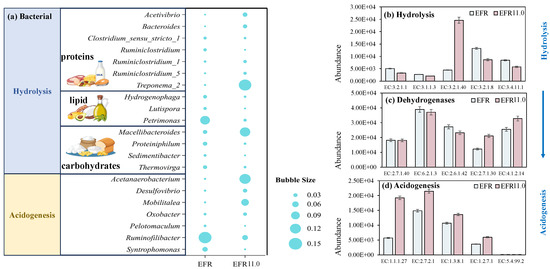
Figure 7.
Bacterial structure at genus level (a) and the relative abundance of functional enzyme via PICRUSt2 for the process of hydrolysis (b), dehydrogenases (c), and acidogenesis (d) for the anaerobic digestion of EFR and EFR 11.0 with the highest erythromycin removal efficiency.
Based on the 16S rRNA gene sequencing profile, PICRUSt2 was utilized in this study to predict functional enzyme abundances, enabling the identification of key enzymes involved in carbohydrate, protein, and lipid degradation across both EFR and EFR 11.0. Figure 7b illustrates the enzymatic activity profiles and substrate degradation patterns of distinct reactors during the anaerobic digestion process. Results revealed that during the hydrolysis process, significantly higher abundances of enzymes were exhibited for the digestion of EFR compared to the condition of EFR 11.0. Among the enzymes associated with substrate degradation, the activity hierarchy was ranked as follows: glycoside hydrolase (EC 3.2.1.8) > peptidase (EC 3.4.11.1) > triacylglycerol lipase (EC 3.1.1.3). Nevertheless, particle-associated carboxymethylcellulase (EC 3.2.1.40) and xylanase (EC 3.2.1.8) [4,46] were present in much higher abundance in EFR 11.0 helped to harmonize the hydrolysis process.
During the acidogenic phase, enzymatic activity predictions indicated the involvement of both the Embden–Meyerhof–Parnas (EMP) and Entner–Doudoroff (ED) pathways. The highly abundant expression of 2-keto-3-deoxy-6-phosphogluconate aldolase (EC 4.1.2.14) suggested a preference for the ED pathway [47]. Furthermore, the EFR 11.0 group exhibited elevated abundances of key enzymes compared to the EFR digestion. Notably, the high abundances of acetate kinase (EC 2.7.2.1) and butyryl-CoA dehydrogenase (EC 1.3.8.1) implied a metabolic trend toward acetate and butyrate production, consistent with the observed predominance of these acids in the study. Acetate kinase (EC 2.7.2.1), a hallmark enzyme of acetogenic bacteria, catalyzed the conversion of acetyl phosphate to acetate while generating ATP. Its high abundance indicated active acetate production within the system, likely driven by abundant substrates (e.g., glucose, lactate) and the dominance of acetogenic communities [48,49]. Conversely, butyryl-CoA dehydrogenase (EC 1.3.8.1) participates in the β-oxidation of fatty acid or the reverse butyrate synthesis pathway, directing the metabolic flux of butyryl-CoA. In the butyrate synthesis pathway, butyryl-CoA is hydrolyzed to butyrate, and its elevated abundance suggests a substrate enriched in long-chain fatty acids or proteins, promoting butyrogenic activity [50,51]. The results demonstrated that substrate degradation during the hydrolysis phase via elevated activities of glycoside hydrolases, peptidases, and lipases, prioritizing carbohydrate breakdown, dominated for anaerobic digestion of EFR. In contrast, for pH controlled at 11.0, the ED pathway was coupled with a high expression of acetate kinase (EC 2.7.2.1) and butyryl-CoA dehydrogenase (EC 1.3.8.1) during the acidogenic phase, driving the production of acetate and butyrate.
4. Conclusions
Anaerobic digestion was thought of as a potentially promising technology for EFR disposal to eliminate erythromycin and recover energy. Enhanced erythromycin removal efficiencies of 45.5% to 87.5% were achieved dealing with EFR in this study under mesophilic conditions, as well as VFA yield ranging from 0.67 to 1.04 g-COD/g-VS. This study demonstrated that anaerobic digestion for the VFA production process was an alternative economic technology for the disposal of EFR without thermal pretreatment to eliminate erythromycin. This innovative idea was assumed to be applied to the treatment of different kinds of antibiotic fermentation residues; higher VFA yield can be obtained through further research. However, for continuous fermentation, the continuous accumulation of residual antibiotics and antibiotic-resistance genes may lead to different results, which needs more research.
Author Contributions
Conceptualization, M.J.T. and D.Y.; data curation, X.X., L.Z., C.L. and X.Q.; formal analysis, X.X. and C.X.; funding acquisition, J.R. and D.Y.; investigation, H.M.; methodology, H.M.; project administration, J.R. and D.Y.; software, X.X. and L.Z.; supervision, J.R., M.J.T. and D.Y.; validation, R.T.; visualization, D.N.; writing—original draft, X.X.; writing—review and editing, M.J.T., Y.C. and D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Changzhou Science and Technology Bureau, CJ20235062, CQ20230112, Natural Science Foundation of Jiangsu Higher Education Institutions of China, 23KJA610001, 20KJB230004 and Postgraduate Research & Practice Innovation Program of Jiangsu Province, SJCX_1640, SJCX24_1719.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to express their gratitude to Li-ping Dong and Li-li Han from Yili Chuanning Biotechnology Co., Ltd., in Xinjiang Province, for their help in offering raw materials and the analysis of erythromycin. Special thanks are also given to the Analysis and Testing Center of Changzhou University for their support in chemical analysis and Major Bio for microbial analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EFR | Erythromycin fermentation residue |
| VS | Volatile solids |
| TS | Total solids |
| VFA | Volatile fatty acids |
| GC | Gas chromatography |
| UPLC | Ultra-performance liquid chromatography |
| CTAB/SDS | Cetyltrimethylammonium bromide-sodium dodecyl sulfate |
| PCR | Polymerase chain reaction |
| ISR | Inoculum-to-substrate ratio |
| TCOD | Total chemical oxygen demand |
| SCOD | Suspended chemical oxygen demand |
| HS | Heat shocked |
| OLR | Organic loading rates |
| VSS | Volatile suspend solids |
References
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Felsa, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, M.; Ma, X.X.; Xie, D.F.; Wang, Q.H. Effect of co-digestion of tylosin fermentation dreg and food waste on anaerobic digestion performance. Bioresour. Technol. 2021, 325, 124693. [Google Scholar] [CrossRef]
- Cai, C.; Hua, Y.; Li, H.; Li, L.; Dai, L.; Liu, H.; Dai, X. Hydrothermal treatment of erythromycin fermentation residue: Harmless performance and bioresource properties. Resour. Conserv. Recycl. 2020, 161, 104952. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, S.; Wang, M. Effect of hydrothermal pretreatment on anaerobic digestion of erythromycin fermentation dregs: Biogas production, antibiotic resistance gene evolution, and microbial community dynamics. Front. Environ. Sci. 2022, 10, 905494. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. National Hazardous Waste List. Available online: https://www.mee.gov.cn/gzk/gz/202411/t20241129_1097688.shtml (accessed on 1 January 2025).
- Zhang, Y.; Wang, G.; Liu, H.; Dai, X. Application of spray-dried erythromycin fermentation residue as a soil amendment: Antibiotic resistance genes, nitrogen cycling, and microbial community structure. Environ. Sci. Pollut. Res. 2023, 30, 20547–20557. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Wu, H.; Shi, L.H.; Wang, X.M.; Shen, Y.P.; Tian, S.L.; Hou, L.A. Sustainable on farm strategy for the disposal of antibiotic fermentation residue: Co-benefits for resource recovery and resistance mitigation. J. Hazard. Mater. 2023, 446, 130705. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Ma, D.; Zhang, Z.; Xu, G. Anaerobic digestion of antibiotic residue in combination with hydrothermal pretreatment for biogas. Bioresour. Technol. 2015, 192, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Xin, Y.; Shen, Y.; Wang, J.; Cai, C.; Wang, M. Erythromycin degradation and ERY-resistant gene inactivation in erythromycin mycelial dreg by heat-activated persulfate oxidation. Chem. Eng. J. 2019, 358, 1446–1453. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, T.; He, S.; Wang, J. Volatile fatty acids recovery and antibiotic degradation from erythromycin fermentation residues by combined thermal pretreatment and anaerobic fermentation: Insights into microbial communities and metabolic pathways. Bioresour. Technol. 2023, 387, 129691. [Google Scholar] [CrossRef]
- Jiang, M.; Song, S.; Fu, H.; Cai, C.; Liu, H. Research of feasibility for anaerobic digestion of erythromycin mycelial dreg and degradation rules of erythromycin. Environ. Prot. Sci. 2017, 43, 62–67. [Google Scholar]
- Zhang, H.; Yin, M.; Li, S.; Zhang, S.; Han, G. The removal of erythromycin and its effects on anaerobic fermentation. Int. J. Environ. Res. Public Health 2022, 19, 7256–7276. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, Z.; Peng, J.; Mo, J.; Li, Q.; Guo, J.; Yang, F. Transcriptomic analysis of Raphidocelis subcapitata exposed to erythromycin: The role of DNA replication in hormesis and growth inhibition. J. Hazard. Mater. 2021, 402, 123512. [Google Scholar] [CrossRef] [PubMed]
- Cetecioglu, Z.; Ince, B.; Ince, O.; Orhon, D. Acute effect of erythromycin on metabolic transformations of volatile fatty acid mixture under anaerobic conditions. Chemosphere 2015, 124, 129–135. [Google Scholar] [CrossRef]
- Wu, Q.; Zou, D.; Zheng, X.; Liu, F.; Li, L.; Xiao, Z. Effects of antibiotics on anaerobic digestion of sewage sludge: Performance of anaerobic digestion and structure of the microbial community. Sci. Total Environ. 2022, 845, 157384. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Sun, J.; Chen, Z.; Xu, Q.; Wei, W.; Wang, D.; Ni, B.J. The impact and fate of clarithromycin in anaerobic digestion of waste activated sludge for biogas production. Environ. Res. 2021, 195, 110792. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, X.; Zhou, Y.; Yang, X.; Lam, S.S.; Wang, D. Influence of roxithromycin as antibiotic residue on volatile fatty acids recovery in anaerobic fermentation of waste activated sludge. J. Hazard. Mater. 2020, 394, 122570. [Google Scholar] [CrossRef]
- Xu, H.; Wang, T.; Zhou, Y.; Zhao, M.; Shi, W.; Huang, Z.; Ruan, W. Insights into the phenol disinfectant on the methane performance from wastewater by mesophilic anaerobic digestion: Single and two stages analysis. Process. Saf. Environ. Prot. 2023, 170, 19–27. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the world of volatile fatty acids production and application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Expert Market Research. Acetic Acid Market Report and Forecast 2020–2025. 2020. Available online: https://www.expertmarketresearch.com/reports/acetic-acid-market (accessed on 1 January 2020).
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-popiel, P. Volatile fatty acids production during mixed culture fermentation—The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa Awasthi, M.K.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Tang, M.; Gu, Y.; Wei, D.; Tian, Z.; Tian, Y.; Yang, M.; Zhang, Y.U. Enhanced hydrolysis of fermentative antibiotics in production wastewater: Hydrolysis potential prediction and engineering application. Chem. Eng. J. 2020, 391, 123626. [Google Scholar] [CrossRef]
- Yin, D.M.; Qiao, W.; Negri, C.; Adani, F.; Fan, R.; Dong, R.J. Enhancing hyper-thermophilic hydrolysis pre-treatment of chicken manure for biogas production by in-situ gas phase ammonia stripping. Bioresour. Technol. 2019, 287, 121470. [Google Scholar] [CrossRef] [PubMed]
- Jomnonkhaow, U.; Uwineza, C.; Mahboubi, A.; Wainaina, S.; Reungsang, A.; Taherzadeh, M.J. Membrane bioreactor-assisted volatile fatty acids production and in situ recovery from cow manure. Bioresour. Technol. 2021, 321, 124456. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; Wever, H.D.; Qiao, W.; Taherzadeh, M.J. Volatile fatty acids (VFA) production and recovery from chicken manure using a high-solid anaerobic membrane bioreactor (AnMBR). Membranes 2022, 12, 1133–1149. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Alexander, J.; Schwartz, T.; Fatta- Kassinos, D. Investigation of the potential of a Membrane BioReactor followed by solar Fenton oxidation to remove antibiotic-related microcontaminants. Chem. Eng. J. 2017, 310, 491–502. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.L.; Zhang, S.C.; Shi, H.Z.; Cai, W.M. The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environ. Technol. 2005, 26, 329–340. [Google Scholar] [CrossRef]
- Luo, J.Y.; Fang, S.Y.; Huang, W.X.; Wang, F.; Zhang, L.; Fang, F.; Cao, J.S.; Wu, Y.; Wang, D.B. New insights into different surfactants’ impacts on sludge fermentation: Focusing on the particular metabolic processes and microbial genetic traits. Front. Environ. Sci. Eng. 2022, 16, 106. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Mohan, S.V. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Amin, M.; Bina, B.; Movahedian, H.; Morgenroth, E.; Raskin, L.; Torkian, A. Influence of the antibiotic erythromycin on anaerobic treatment of a pharmaceutical wastewater. J. Res. Med. Sci. 2004, 9, 52–58. [Google Scholar] [CrossRef]
- Tampio, E.A.; Blasco, L.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile fatty acids (VFAs) and methane from food waste and cow slurry: Comparison of biogas and VFA fermentation processes. GCB Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Hu, W.; Mahdy, A.; Dong, T.; Sun, Y.; Qiao, W.; Dong, R. The metabolic performance and microbial communities of anaerobic digestion of chicken manure under stressed ammonia condition: A case study of a 10-year successful biogas plant. Renew. Energy 2021, 167, 644–651. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Horvath, I.S.; Taherzadeh, M.J. Anaerobic digestion of food waste to volatile fatty acids and hydrogen at high organic loading rates in immersed membrane bioreactors. Renew. Energy 2020, 152, 1140–1148. [Google Scholar] [CrossRef]
- Lukitawesa Patinvoh, R.J.; Millati, R.; Sarvari-Horvath, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguirre, J.; Aymerich, E.; Gonzalez-Mtnez De Goni, J.; Esteban-Gutierrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- Paesen, J.; Khan, K.; Roets, E.; Hoogmartens, J. Study of the stability of erythromycin in neutral and alkaline solutions by liquid chromatography on poly(styrene-divinylbenzene). Int. J. Pharm. 1995, 113, 215–222. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. pH significantly affects removal of trace antibiotics in chlorination of municipal wastewater. Water Res. 2012, 46, 3703–3713. [Google Scholar] [CrossRef]
- Song, S.; Jiang, M.; Yao, J.; Liu, H.; Dai, X.H. Anaerobic digestion of spectinomycin mycelial residues pretreated by thermal hydrolysis: Removal of spectinomycin and enhancement of biogas production. Environ. Sci. Pollut. Res. 2020, 27, 39297–39307. [Google Scholar] [CrossRef]
- Chelliapan, S.; Wilby, T.; Sallis, P.J.; Yuzir, A. Tolerance of the antibiotic tylosin on treatment performance of an up-flow anaerobic stage reactor (UASR). Water Sci. Technol. 2011, 63, 1599–1606. [Google Scholar] [CrossRef]
- Ren, J.J.; Ni, S.S.; Shen, Y.P.; Niu, D.Z.; Sun, R.M.; Wang, C.G.; Deng, L.J.; Zhang, Q.P.; Tang, Y.; Jiang, X.M.; et al. Charecterization of the erythromycin degradation pathway and related enzyme in Rhodococcus gordoniae rjjtx-2. J. Clean. Prod. 2022, 379, 134758. [Google Scholar] [CrossRef]
- De Cazes, M.; Belleville, M.P.; Petit, E.; Salomo, M.; Bayer, S.; Czaja, R.; De Gunzburg, J.; Sanchez-Marcano, J. Erythromycin degradation by esterase (EreB) in enzymatic membrane reactors. Biochem. Eng. J. 2016, 114, 70–78. [Google Scholar] [CrossRef]
- Kim, H.E.; Park, K.R. Purification and characterization of an esterase from Acinetobacter lwoffii I6C-1. Curr. Microbiol. 2002, 44, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Zitomer, D.; Maki, J.; Venkiteshwaran, K.; Bocher, B. Relating anaerobic digestion microbial community and process function. Microbiol. Insights 2016, 8, 37–46. [Google Scholar]
- Klaus, H.; Ingo, B.; Kirsten, H.; Göbel, U.B. Proteolytic activity among various oral treponema species and cloning of a prtp-like gene of treponema socranskii subsp. socranskii. FEMS Microbiol. Lett. 2001, 201, 169–176. [Google Scholar]
- Huhtanen, P.; Khalili, H. The effect of sucrose supplements on particle-associated carboxymethylcellulase (EC 3.2.1.4) and xylanase (EC 3.2.1.8) activities in cattle given grass-silage-based diet. Br. J. Nutr. 1992, 67, 245–255. [Google Scholar] [CrossRef]
- Luo, L.; Pradhan, N. Anaerobic mono-digestion and co-digestion of food waste and mixed sewage sludge: A comparative analysis of metabolic patterns and taxonomic profiles. Chem. Eng. J. 2024, 489, 151397. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Q.; Ping, Q.; Zhang, S.; Li, D.; Wang, L.; Li, Y. Effect of Maillard reaction products on anaerobic digestion of waste activated sludge: Mechanisms from molecular docking and metagenomics. Chem. Eng. J. 2025, 507, 160494. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Niu, X.; Su, X.; He, X.; Hu, Y.; Zhao, D.; Chen, D.; Lin, Y.; Li, K. Ferrihydrite enhance performance in anaerobic digestion of pig manure: Methane production, Feammox and metabolic pathway. J. Water Process. Eng. 2025, 72, 107621. [Google Scholar] [CrossRef]
- Cao, Q.; Meng, X.; Jia, F.; Li, J.; Liu, X.; Li, D. The metabolic redundancy relieving VFAs shocks in anaerobic digestion system exposed sequentially to increasing acetic acid loading. Chem. Eng. J. 2025, 505, 159791. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Huang, H.; Duan, X.; Dong, L. Improved anaerobic digestion under ammonia stress by regulating microbiome and enzyme to enhance VFAs bioconversion: The new role of glutathione. Chem. Eng. J. 2022, 433, 134562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).