Ensilage and Secondary Fermentation of Maize Stalk and Their Effect on Methane Production and Microbial Community Dynamics in Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Harvest and Ensilage of Maize Stalks
2.3. Secondary Fermentation of Maize Stalks
2.4. Anaerobic Digestion of Maize Stalks
2.5. Kinetic Model Fitting of Organic Dry Matter (ODM) Loss and Methane Yield Loss During Secondary Fermentation
2.6. Chemical Analysis
2.7. Microbial Analysis of Maize Stalks
2.7.1. Hydrolytic Enzyme Activity Determination
2.7.2. Microbial Enumeration
2.8. Microbial Analysis of Anaerobic Digestion (AD) Samples
2.8.1. Quantitative Real-Time Polymerase Chain Reaction of Bacteria in Anaerobic Digestion (AD)
2.8.2. High-Throughput Sequencing Analysis
2.8.3. Hydrolytic Enzyme Activity Determination
2.9. Data Analysis
3. Results and Discussion
3.1. Ensilage and Secondary Fermentation of Maize Stalk
3.1.1. Characteristic Changes in Maize Stalk During Ensiling
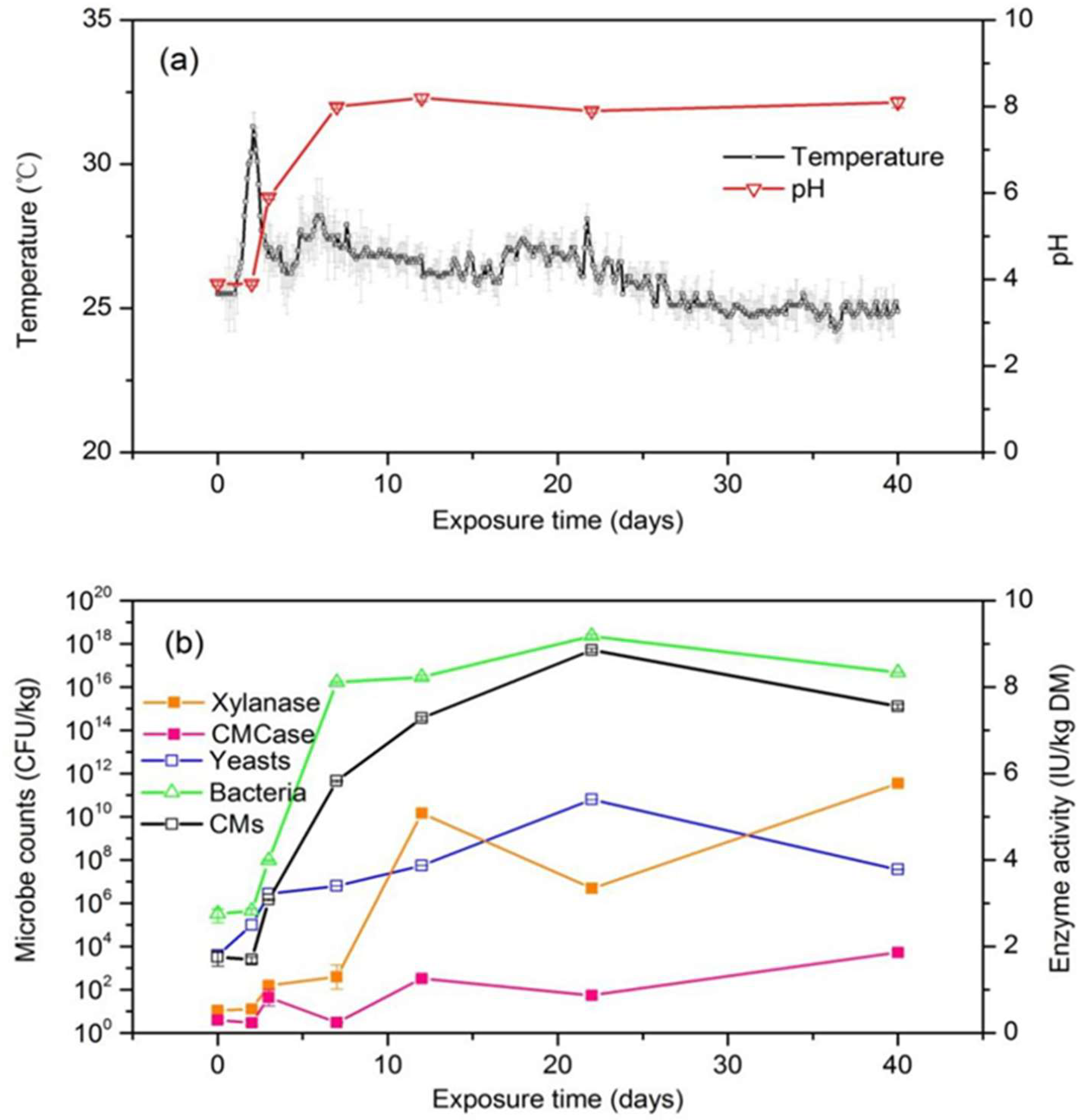
3.1.2. Characteristic Changes, Microbial Dynamics, and Organic Dry Matter (ODM) Loss of Silage During Air Exposure
3.2. Effect of Ensiling and Air Exposure on Methane Production
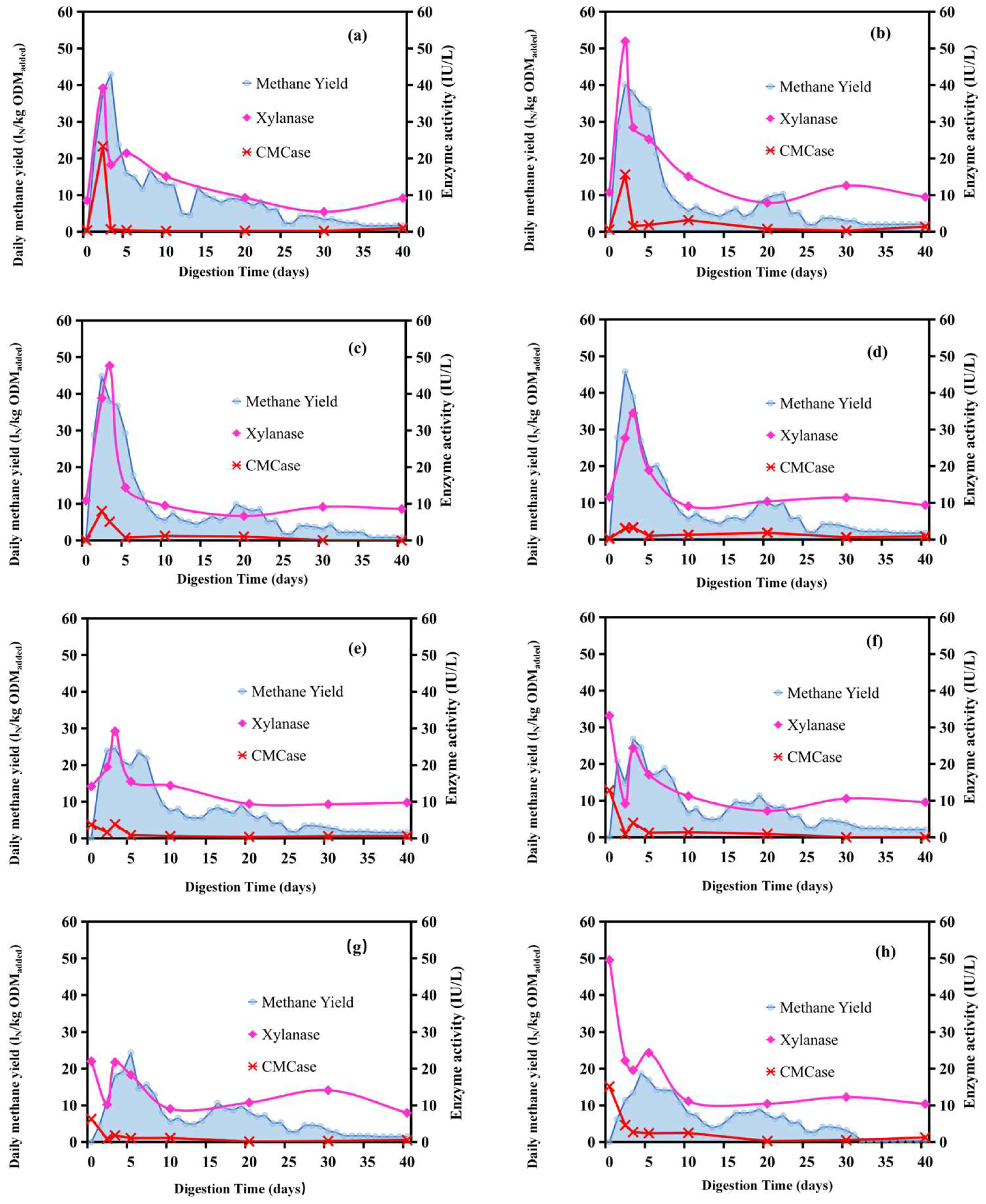
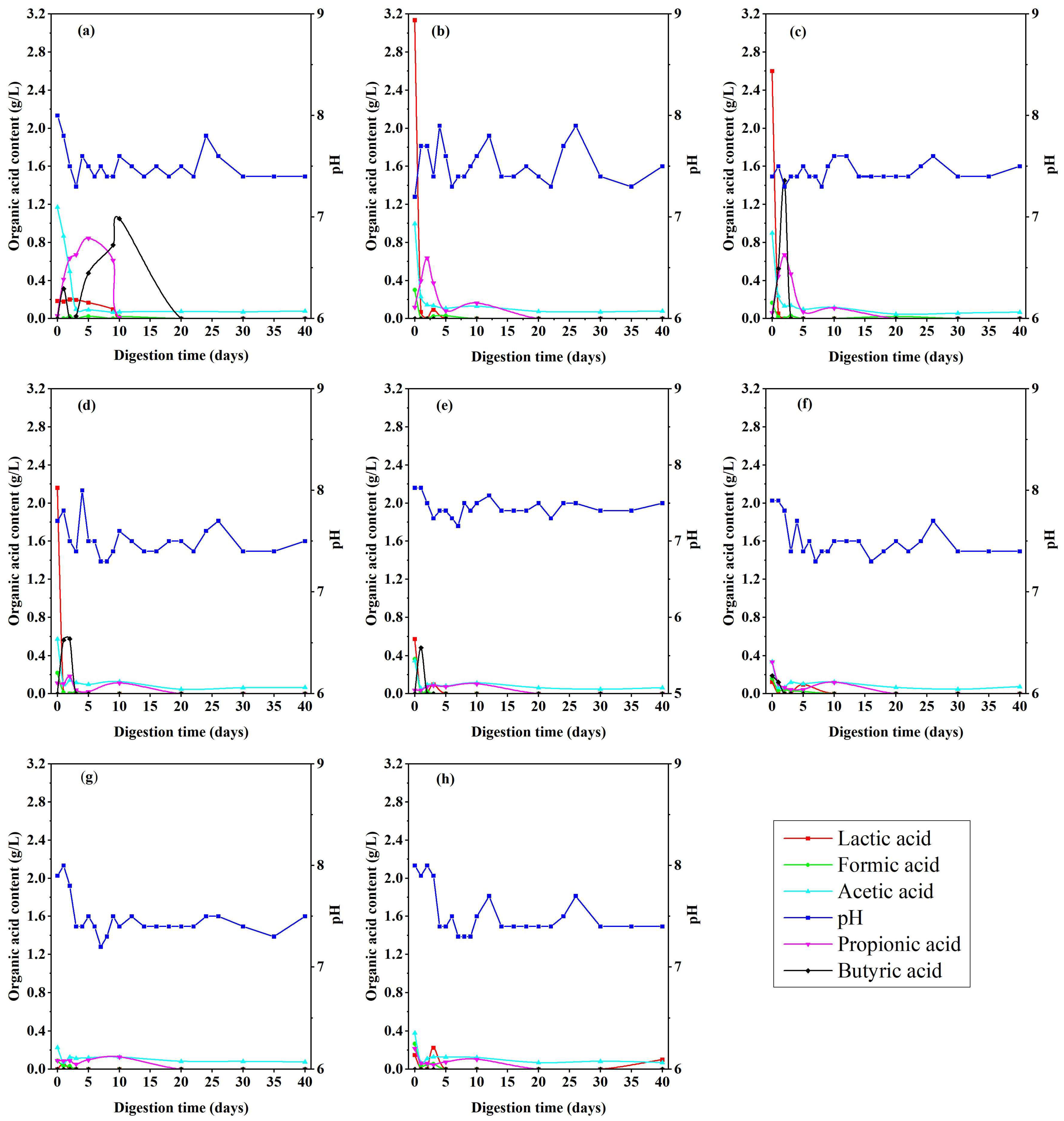
3.2.1. Fermentation Dynamics in Anaerobic Digestion (AD) of Maize Stalks
3.2.2. Methane Yield Potential of Maize Stalks During Ensiling and Air Exposure
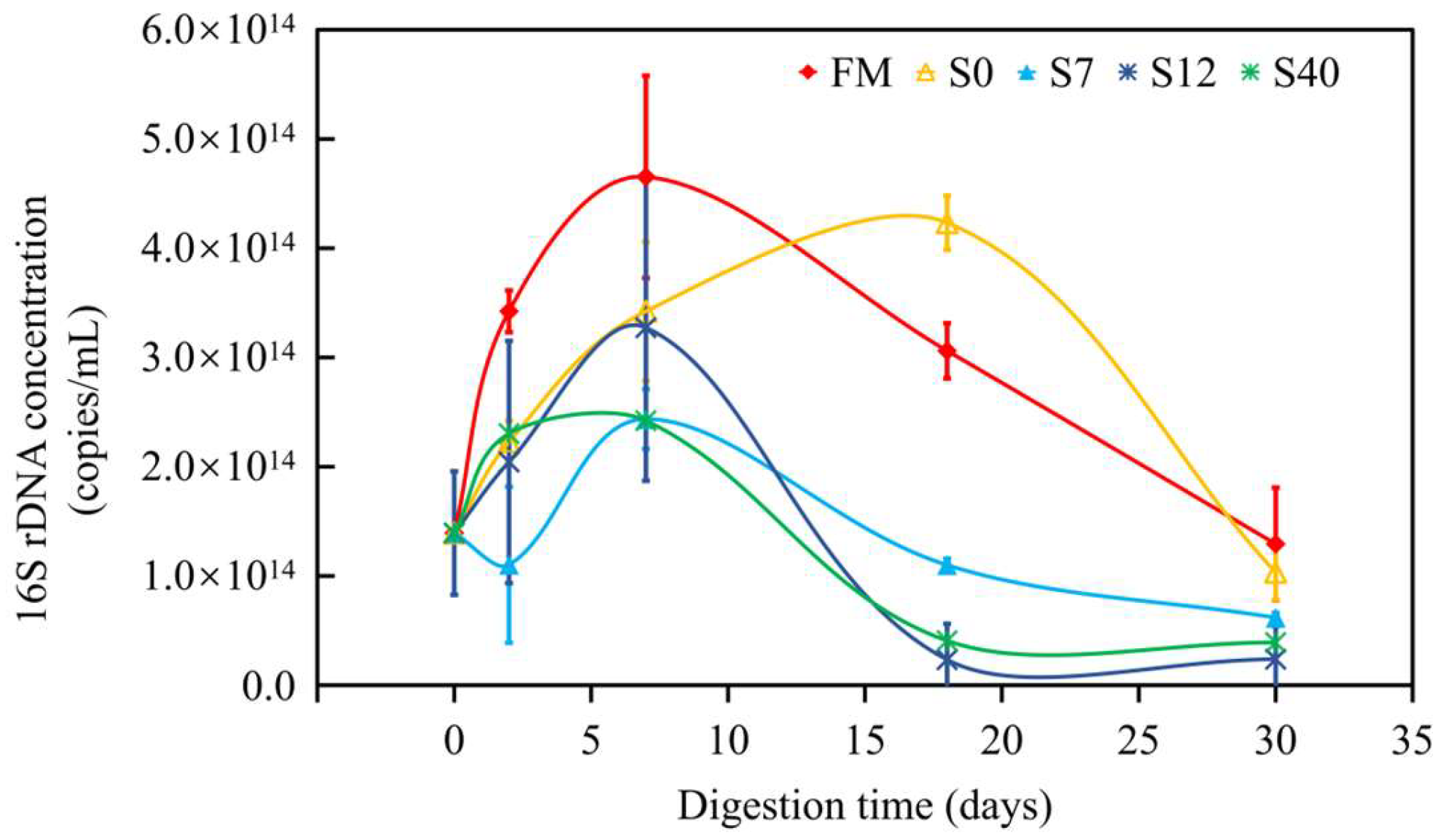
3.2.3. Quantitative Polymerase Chain Reaction of Bacteria in Anaerobic Digestion (AD) of Maize Stalks
3.2.4. Metagenomic Analysis of Bacterial Community in Anaerobic Digestion (AD) of Maize Stalks Following Ensilage and Air Exposure
3.2.5. Methane Yield Loss of Maize Stalks During Ensiling and Air Exposure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AT | ambient temperature |
| AD | anaerobic digestion |
| ADF | acid detergent fiber |
| ADL | acid detergent lignin |
| CMC | carboxymethyl cellulose |
| CMCase | carboxymethyl cellulase |
| CMs | cellulolytic microbes |
| CP | crude protein |
| DM | dry matter |
| FM | fresh maize stalk |
| MY | methane yield |
| NDF | neutral detergent fiber |
| ODM | organic dry matter |
| OTUs | operational taxonomic units |
| sCOD | soluble chemical oxygen demand |
| SF | secondary fermentation |
| SMY | specific methane yield |
| VFAs | volatile fatty acids |
| WSCs | water-soluble carbohydrates |
References
- National Bureau of Statistics of China. China Statistical Yearbook 2024; China Statistics Press: Beijing, China, 2025. [Google Scholar]
- Cao, G.; Zhang, X.; Wang, Y.; Zheng, F. Estimation of emissions from field burning of crop straw in China. Chin. Sci. Bull. 2008, 53, 784–790. [Google Scholar] [CrossRef]
- Menardo, S.; Balsari, P.; Tabacco, E.; Borreani, G. Effect of conservation time and the addition of lactic acid bacteria on the biogas and methane production of corn stalk silage. Bioenerg. Res. 2015, 8, 1810–1823. [Google Scholar] [CrossRef]
- Sun, H.; Cui, X.; Stinner, W.; Mustafa Shah, G.; Cheng, H.; Shan, S.; Guo, J.; Dong, R. Synergetic effect of combined ensiling of freshly harvested and excessively wilted maize stover for efficient biogas production. Bioresour. Technol. 2019, 285, 121338. [Google Scholar] [CrossRef]
- Sun, H.; Cui, X.; Li, R.; Guo, J.; Dong, R. Ensiling process for efficient biogas production from lignocellulosic substrates: Methods, mechanisms, and measures. Bioresour. Technol. 2021, 342, 125928. [Google Scholar] [CrossRef]
- Herrmann, C.; Heiermann, M.; Idler, C. Effects of ensiling, silage additives and storage period on methane formation of biogas crops. Bioresour. Technol. 2011, 102, 5153–5161. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Zhao, X.; Zheng, Z.; Cai, Y.; Hu, Y.; Cui, Z.; Wang, X. The macro- and micro-prospects of the energy potential of the anaerobic digestion of corn straw under different storage conditions. Bioresour. Technol. Rep. 2019, 7, 100189. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Gao, L.; Yu, J.; Yuan, X.; Zhu, W.; Wang, X.; Cui, Z. Aerobic deterioration of corn stover silage and its effect on methane production and microbial community dynamics in anaerobic digestion. Bioresour. Technol. 2018, 250, 828–837. [Google Scholar] [CrossRef]
- Wu, S.; Huang, P.; Zhang, C.; Zhou, W.; Chen, X.; Zhang, Q. Novel strategy to understand the aerobic deterioration of corn silage and the influence of Neolamarckia cadamba essential oil by multi-omics analysis. Chem. Eng. J. 2024, 482, 148715. [Google Scholar]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2012, 68, 1–19. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Improving aerobic stability and biogas production of maize silage using silage additives. Bioresour. Technol. 2015, 197, 393–403. [Google Scholar] [CrossRef]
- Pakarinen, A.; Maijala, P.; Jaakkola, S.; Stoddard, F.; Kymäläinen, M.; Viikari, L. Evaluation of preservation methods for improving biogas production and enzymatic conversion yields of annual crops. Biotechnol. Biofuels 2011, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.; Rodriguez, L.O.; Fenech, C.; Anika, O.C. Ensiling for anaerobic digestion: A review of key considerations to maximise methane yields. Renew. Sustain. Energy Rev. 2020, 134, 110401. [Google Scholar] [CrossRef]
- Yan, J.; Sun, Y.; Kang, Y.; Meng, X.; Zhang, H.; Cai, Y.; Zhu, W.; Yuan, X.; Cui, Z. An innovative strategy to enhance the ensiling quality and methane production of excessively wilted wheat straw: Using acetic acid or hetero-fermentative lactic acid bacterial community as additives. Waste Manag. 2022, 149, 11. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Lu, D.; Dong, B.; Li, L.; Wei, H.; Sun, Y.; Liu, L.; Zheng, Y. Bioaugmented ensiling pretreatment to improve bioconversion of sweet sorghum: Effect of bio-additives on enzymatic saccharification and biomethane potential. Chem. Eng. J. 2024, 500, 156794. [Google Scholar] [CrossRef]
- Sun, H.; Liao, C.; Lu, G.; Zheng, Y.; Cheng, Q.; Xie, Y.; Wang, C.; Chen, C.; Li, P. Role of Lactiplantibacillus paraplantarum during anaerobic storage of ear-removed corn on biogas production. Bioresour. Technol. 2022, 364, 128061. [Google Scholar] [CrossRef]
- Vervaeren, H.; Hostyn, K.; Ghekiere, H.; Willems, B. Biological ensilage additives as pretreatment for maize to increase the biogas production. Renew. Energy 2010, 35, 2089–2093. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, J.; Liu, J.; Yang, H.; Gao, L.; Yuan, X.; Cui, Z.; Wang, X. Material and microbial changes during corn stalk silage and their effects on methane fermentation. Bioresour. Technol. 2016, 222, 89–99. [Google Scholar] [CrossRef]
- Ploechl, M.; Zacharias, H.; Herrmann, C.; Heiermann, M.; Prochnow, A. Influence of silage additives on methane yield and economic performance of selected feedstock. Agric. Eng. Int. CIGR. Ejournal 2009, 11, 1123. [Google Scholar]
- McEniry, J.; Allen, E.; Murphy, J.D.; O’Kiely, P. Grass for biogas production: The impact of silage fermentation characteristics on methane yield in two contrasting biomethane potential test systems. Renew. Energ. 2014, 63, 524–530. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhao, X.; Yan, P.; Yang, R.; Yan, J.; Yuan, X.; Cui, Z. Improving aerobic stability and methane production of maize stover silage with lactic acid bacteria inoculants: Focus on pentose-fermentation. Ind. Crop. Prod. 2023, 201, 116861. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, F.M.; Rombouts, F.M.; Riet, K.V. Modeling of the bacterial growth curve. Appl. Environ. Microb. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.G.; Murray, R.S. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 2001, 56, 405–411. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef]
- Castillo, M.; Martin-Orue, S.M.; Manzanilla, E.G.; Badiola, I.; Martin, M.; Gasa, J. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 2006, 114, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Lu, Y.; Wang, J.; Wen, B.; Cao, Y.; Wang, X.; Cui, Z. Dynamic changes in the composite microbial system MC1 during and following its rapid degradation of lignocellulose. Appl. Biochem. Biotech. 2014, 172, 951–962. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energ. 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, X.; Wang, S.; Li, J.; Shao, T. Separating the effects of chemical and microbial factors on fermentation quality and bacterial community of Napier grass silage by using gamma-ray irradiation and epiphytic microbiota transplantation. Anim. Feed. Sci. Tech. 2021, 280, 115082. [Google Scholar] [CrossRef]
- Tabacco, E.; Piano, S.; Cavallarin, L.; Bernardes, T.F.; Borreani, G. Clostridia spore formation during aerobic deterioration of maize and sorghum silages as influenced by Lactobacillus buchneri and Lactobacillus plantarum inoculants. J. Appl. Microbiol. 2009, 107, 1632–1641. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, G.; Zhang, J.; Chen, Y.; Yu, M.; Lu, L.; Li, H.; Zhu, Y.; Yuan, Y.; Huang, A.; et al. Response of denitrifying genes coding for nitrite (nirK or nirS) and nitrous oxide (nosZ) reductases to different physico-chemical parameters during agricultural waste composting. Appl. Microbiol. Biot. 2015, 99, 4059–4070. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energ. Combust. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresource Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman., P.J.; Jones., D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Chojnacka, A.; Szczęsny., P.; Błaszczyk., M.K.; Zielenkiewicz., U.; Detman., A.; Salamon., A.; Sikora., A. Noteworthy facts about a methane producing microbial community processing acidic effluent from sugar beet molasses fermentation. PLoS ONE 2015, 10, e0128008. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, Y.; Liu, B.; Zhao, Y.; Wu, J.; Yuan, X.; Zhu, W.; Cui, Z. Accelerated acidification by inoculation with a microbial consortia in a complex open environment. Bioresour. Technol. 2016, 216, 294–301. [Google Scholar] [CrossRef]
- St-Pierre, B.; Wright, A.G. Comparative metagenomic analysis of bacterial populations in three full-scale mesophilic anaerobic manure digesters. Appl. Microbiol. Biot. 2014, 98, 2709–2717. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Li, Q.; Liu, J.; Liang, C.; Wang, C.; Yang, B.; Wu, K.; Yin, F.; Zhang, W. Anaerobic digestion for degrading saponins from Panax notoginseng root and applying biogas slurry to promote degradation of autotoxic saponins in continuous cropping soil. Environ. Technol. Innov. 2023, 31, 103203. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef] [PubMed]
- Dinh, N.T.; Hatta, K.; Kwon, S.H.; Pollon, A.P.; Nakasaki, K. Changes in the microbial community during the acclimation stages of the methane fermentation for the treatment of glycerol. Biomass Bioenerg. 2014, 68, 240–249. [Google Scholar] [CrossRef]
- Baek, G.; Jaai, K.; Kyungjin, C.; Hyokwan, B.; Changsoo, L. The biostimulation of anaerobic digestion with (semi)conductive ferric oxides, their potential for enhanced biomethanation. Appl. Microbiol. Biot. 2015, 99, 10355–10366. [Google Scholar] [CrossRef]
- Stolze, Y.; Zakrzewski, M.; Maus, I.; Eikmeyer, F.; Jaenicke, S.; Rottmann, N.; Siebner, C.; Pühler, A.; Schlüter, A. Comparative metagenomics of biogas-producing microbial communities from production-scale biogas plants operating under wet or dry fermentation conditions. Biotechnol. Biofuels 2015, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, H.; Schluepmann, H.; Kristalijn, J.; Sulaiman, Z.; Marshallet, D.J. Molecular analysis of bacterial diversity in mudflats along the salinity gradient of an acidified tropical Bornean estuary (South East Asia). Aquat. Bot. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikeda-Ohtsubo, W.; Strassert Jürgen, F.H.; Tim, K.; Aram, M.; Ivan, G.; Alice, C.M.; Green, T.S.; Phil, H.; Renate, R.; Andreas, B. ʻCandidatus Adiutrix intracellularisʼ, an endosymbiont of termite gut flagellates, is the first representative of a deep-branching clade of Deltaproteobacteria and a putative homoacetogen. Environ. Microbiol. 2016, 18, 2548–2564. [Google Scholar] [CrossRef]
- Gaspari, M.; Treu, L.; Centurion, V.B.; Kotsopoulos, T.A.; Campanaro, S.; Kougias, P.G. Impacts of long chain fatty acids injection on biogas reactors performance stability and microbial community structure and function. J. Clean. Prod. 2023, 418, 138048. [Google Scholar] [CrossRef]
- Gompertz, B. XXIV. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. In a letter to Francis Baily, Esq. F.R.S. &C. Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar]
- Li, J.; Zicari, S.M.; Cui, Z.; Zhang, R. Processing anaerobic sludge for extended storage as anaerobic digester inoculum. Bioresour. Technol. 2014, 166, 201–210. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Liu, J.; Yang, F.; Zhu, W.; Yuan, X.; Hu, Y.; Cui, Z.; Wang, X. Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresour. Technol. 2017, 241, 349–359. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. The Gompertz model and its applications in microbial growth and bioproduction kinetics: Past, present and future. Biotechnol. Adv. 2024, 72, 108335. [Google Scholar] [CrossRef]

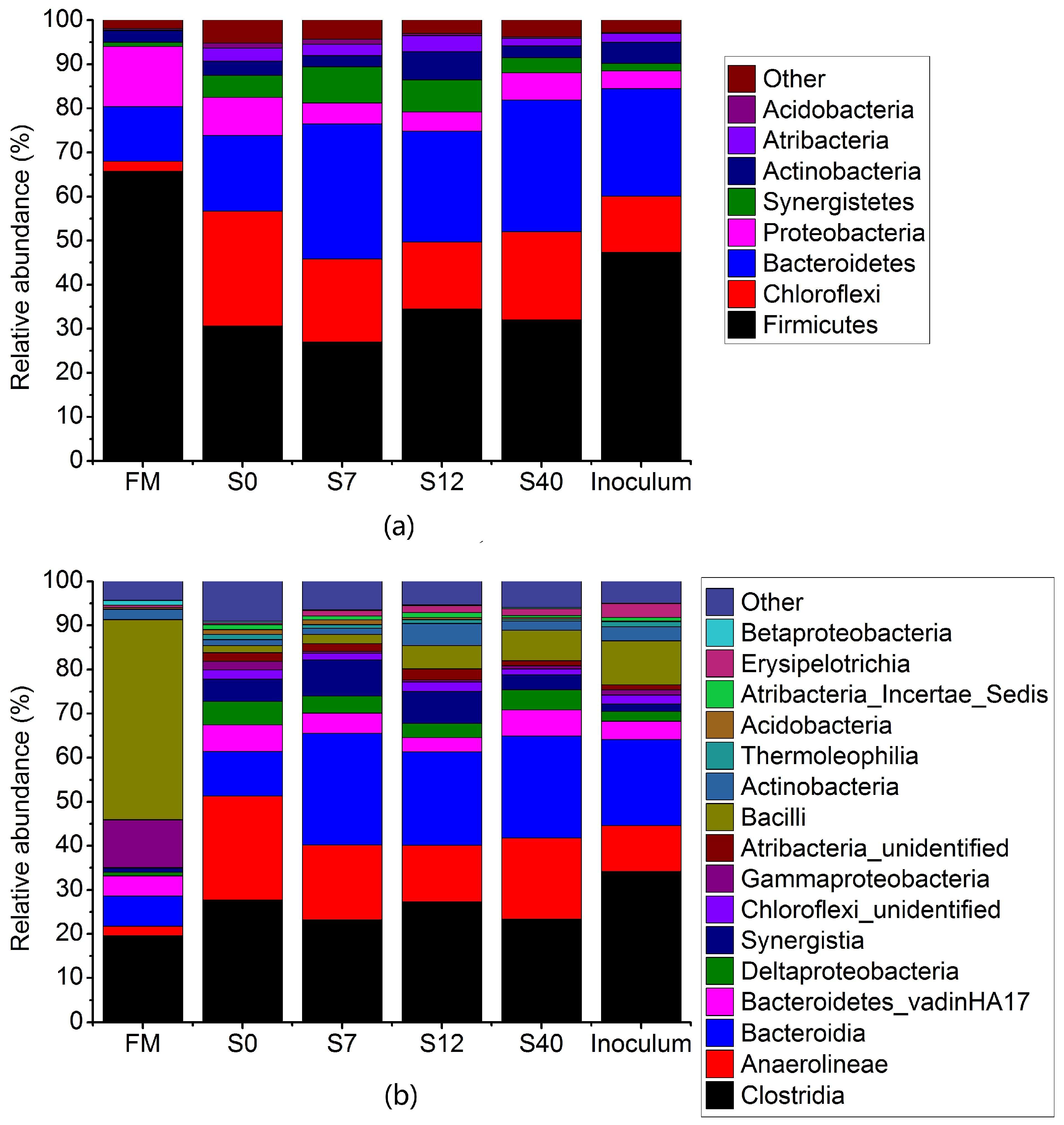
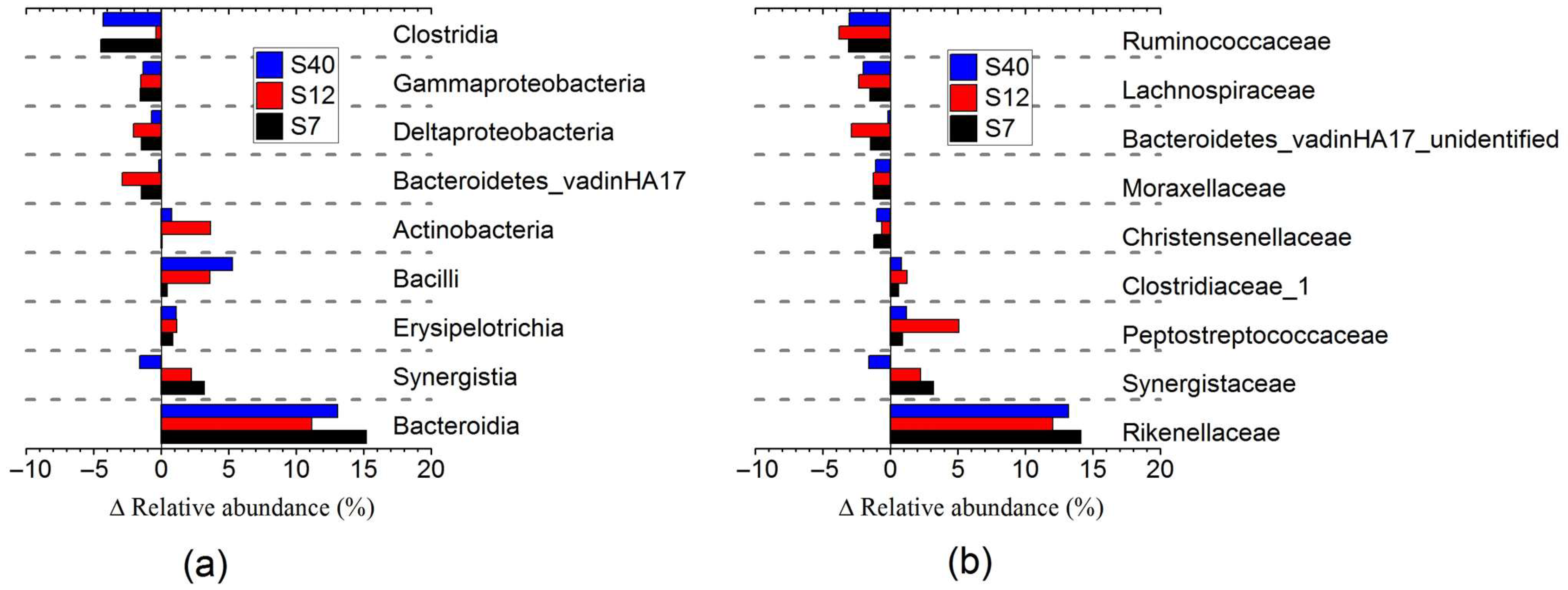
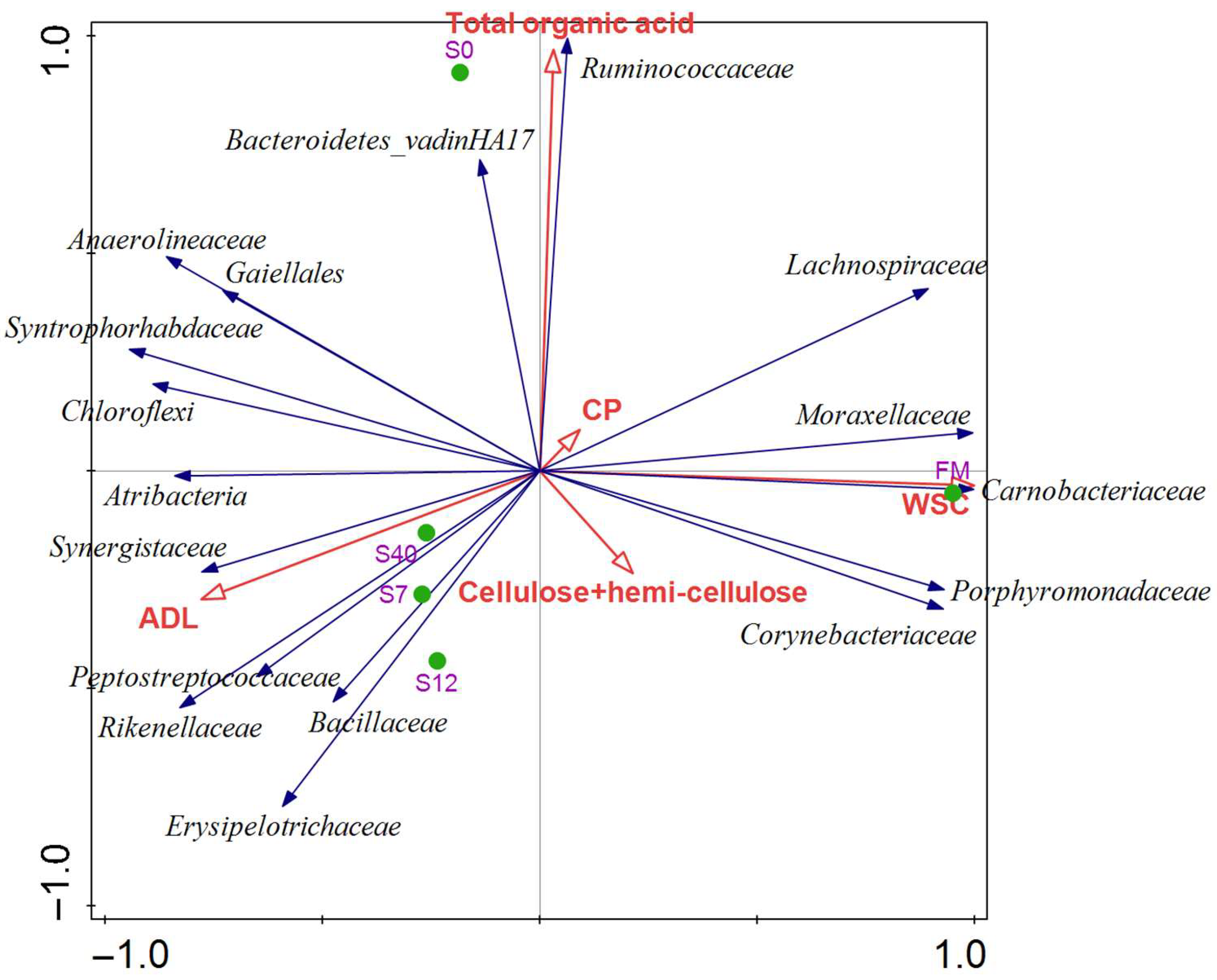
| Items | FM | S0 | S2 | S3 | S7 | S12 | S22 | S40 |
|---|---|---|---|---|---|---|---|---|
| DM (g/kg) | 301.0 ± 1.1 | 305.1 ± 10.3 | 299.4 ± 12.3 | 286.4 ± 0.8 | 266.3 ± 13.2 | 249.7 ± 12.1 | 251.2 ± 2.8 | 457.0 ± 4.8 |
| ODM (g/kg) | 276.6 ± 1.5 | 279.3 ± 11.4 | 274.0 ± 11.8 | 260.2 ± 1.0 | 240.2 ± 12.1 | 222.8 ± 11.0 | 220.9 ± 3.2 | 400.2 ± 5.7 |
| Ash (g/kg) | 24.7 ± 0.8 | 25.7 ± 0.1 | 25.4 ± 0.1 | 26.2 ± 0.1 | 26.1 ± 0.4 | 26.9 ± 0.2 | 30.3 ± 0.2 | 56.8 ± 0.3 |
| NDF (%DM) | 65.45 ± 0.65 | 64.59 ± 0.91 | 65.52 ± 2.10 | 70.22 ± 0.92 | 76.04 ± 1.04 | 73.27 ± 1.29 | 74.61 ± 1.64 | 62.33 ± 2.45 |
| ADL (%DM) | 3.69 ± 0.78 | 6.19 ± 0.56 | 5.78 ± 0.80 | 5.00 ± 0.80 | 7.73 ± 0.97 | 7.51 ± 0.58 | 5.65 ± 1.65 | 5.12 ± 1.37 |
| CP (%DM) | 5.35 ± 0.02 | 5.45 ± 0.02 | 5.62 ± 0.06 | 5.82 ± 0.21 | 5.87 ± 0.20 | 5.28 ± 1.31 | 5.27 ± 1.14 | 4.39 ± 0.05 |
| WSC (%DM) | 16.00 ± 0.21 | 1.30 ± 0.10 | 1.19 ± 0.11 | 1.50 ± 0.15 | 1.11 ± 0.10 | 0.78 ± 0.13 | 1.12 ± 0.05 | 1.08 ± 0.03 |
| sCOD (%DM) | 36.04 ± 1.43 | 27.53 ± 0.39 | 26.13 ± 2.66 | 18.26 ± 8.19 | 11.08 ± 0.31 | 8.10 ± 0.42 | 12.39 ± 0.34 | 15.16 ± 0.26 |
| NH3-N (‰DM) | 0.30 ± 0.03 | 0.94 ± 0.01 | 0.38 ± 0.00 | 0.24 ± 0.00 | 0.35 ± 0.00 | 0.39 ± 0.01 | 0.11 ± 0.00 | 0.05 ± 0.01 |
| Nitrate-N (‰DM) | 0.41 ± 0.02 | 0.11 ± 0.00 | 0.11 ± 0.00 | 0.20 ± 0.00 | 0.21 ± 0.00 | 0.24 ± 0.00 | 0.22 ± 0.00 | 0.20 ± 0.00 |
| ODMR (%) | NA | 97.06 | 94.18 | 87.84 | 81.15 | 72.98 | 65.24 | 62.95 |
| Items | FM | S0 | S2 | S3 | S7 | S12 | S22 | S40 |
|---|---|---|---|---|---|---|---|---|
| Formic acid (%DM) | 0.05 ± 0.01 | --- | --- | --- | --- | 0.11 ± 0.00 | 0.14 ± 0.01 | 0.13 ± 0.00 |
| Lactic acid (%DM) | --- | 11.91 ± 0.11 | 10.84 ± 0.02 | 3.94 ± 0.01 | 0.80 ± 0.12 | 0.59 ± 0.02 | 0.93 ± 0.01 | 0.00 ± 0.00 |
| Acetic acid (%DM) | 1.68 ± 0.01 | 3.24 ± 0.02 | 1.94 ± 0.00 | 0.50 ± 0.00 | 0.57 ± 0.00 | 1.02 ± 0.00 | 0.85 ± 0.01 | 1.04 ± 0.01 |
| Propionic acid (%DM) | 0.53 ± 0.01 | 0.15 ± 0.00 | 0.07 ± 0.00 | 0.32 ± 0.01 | 0.33 ± 0.00 | 0.40 ± 0.00 | --- | 0.07 ± 0.01 |
| Ethanol (%DM) | 0.02 | --- | --- | --- | --- | --- | --- | --- |
| Maize Stalk | Specific Methane Yield (lN/kg ODMadded) | Methane Yield (lN/kg DMorigin) | Methane Yield Recovery (%) |
|---|---|---|---|
| FM | 370.18 a ± 2.09 | NA | NA |
| S0 | 359.59 b ± 3.11 | 349.01 ± 3.02 | 94.28 |
| S2 | 362.99 ab ± 3.78 | 341.86 ± 3.56 | 92.35 |
| S3 | 355.23 b ± 2.82 | 312.05 ± 2.48 | 84.30 |
| S7 | 307.87 d ± 3.34 | 249.85 ± 2.71 | 67.49 |
| S12 | 325.67 c ± 6.24 | 237.68 ± 4.55 | 64.21 |
| S22 | 274.36 e ± 5.01 | 179.00 ± 3.27 | 48.35 |
| S40 | 245.07 f ± 3.74 | 154.27 ± 2.35 | 41.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yan, P.; Qin, Z.; Zhao, X.; Yuan, X.; Cui, Z.; Wu, J. Ensilage and Secondary Fermentation of Maize Stalk and Their Effect on Methane Production and Microbial Community Dynamics in Anaerobic Digestion. Fermentation 2025, 11, 309. https://doi.org/10.3390/fermentation11060309
Zhang H, Yan P, Qin Z, Zhao X, Yuan X, Cui Z, Wu J. Ensilage and Secondary Fermentation of Maize Stalk and Their Effect on Methane Production and Microbial Community Dynamics in Anaerobic Digestion. Fermentation. 2025; 11(6):309. https://doi.org/10.3390/fermentation11060309
Chicago/Turabian StyleZhang, Huan, Puxiang Yan, Ziyao Qin, Xiaoling Zhao, Xufeng Yuan, Zongjun Cui, and Jingwei Wu. 2025. "Ensilage and Secondary Fermentation of Maize Stalk and Their Effect on Methane Production and Microbial Community Dynamics in Anaerobic Digestion" Fermentation 11, no. 6: 309. https://doi.org/10.3390/fermentation11060309
APA StyleZhang, H., Yan, P., Qin, Z., Zhao, X., Yuan, X., Cui, Z., & Wu, J. (2025). Ensilage and Secondary Fermentation of Maize Stalk and Their Effect on Methane Production and Microbial Community Dynamics in Anaerobic Digestion. Fermentation, 11(6), 309. https://doi.org/10.3390/fermentation11060309





