Abstract
This study developed a solid-state fermentation system based on Trichoderma harzianum, which significantly enhanced the nutritional value of distiller’s grain (DG) feed through a multi-stage synergistic treatment process. During the cellulase production phase, rice husk was used as an auxiliary material, and specific degradation of DGs was effectively enhanced. Through optimization using response surface methodology, the optimal enzyme production conditions were determined. The filter paper enzyme activity reached a peak of 1.45 U/gds (enzyme activity per gram of dry substrate) when the moisture content was 53%, the fermentation time was 3 days, and the Tween-80 dosage was 0.015 mL/g (dry weight basis). Under these conditions, the crude enzyme solution was used to hydrolyze DGs. Compared to original DGs, the content of reducing sugars increased by 10.75%. In the stage of protein production, segmented hydrolysis fermentation (SHF) and simultaneous saccharification fermentation (SSF) processes were employed using yeast. The results showed that SSF pathway showed better performance, and the true protein content reached 15.16% after 11 days, an increase of 41.5% compared to the control. Finally, through secondary fermentation regulated by Lactobacillus fermentum, the flavor of the feed was significantly improved. This study innovatively integrated bio-enzymatic hydrolysis and multi-strain synergistic fermentation technologies, providing a novel strategy for the efficient and sustainable production of protein feed based on DGs.
1. Introduction
Distillers’ grains (DGs) are by-products generated during the production of baijiu (Chinese liquor), with an annual output reaching as high as 50 million tons [1]. The moisture content of fresh DGs typically ranges from 60% to 70%, and their pH value is low. If not properly handled, they are highly susceptible to mold growth. This not only leads to severe environmental pollution but also results in the waste of resources. Therefore, the value-added utilization of DGs has become an urgent issue that needs to be addressed.
Currently, researchers are more inclined to develop DGs for use in fertilization and feed utilization to promote their value [2]. However, during the fertilization process of DGs, nitrogen losses can reach up to 40%, which not only significantly reduces the nitrogen concentration in the fertilizer product, affecting fertilizer efficiency, but also increases odor production, thereby triggering a series of environmental issues [3]. Compared to composting, the utilization of DGs as feed appears to be more advantageous. Dennis et al. reported that due to the high crude protein content of DGs (25–35%), the early market primarily used DGs to produce animal protein feed as a substitute for soybean meal [4]. In China, the demand for meat is increasing with the continuous improvement of people’s living standards. However, this growing demand has also revealed some contradictions and problems, the most significant of which is the shortage of protein feed supply. Against this backdrop, high-quality protein feed undoubtedly has a broad development prospect [5]. A previous study reported that the protein content of DGs can be significantly increased through enzymatic hydrolysis and fermentation. However, the high cost of commercial enzymes has greatly increased the cost of enzymatic hydrolysis of DGs. At present, the industry mostly uses inexpensive waste biomass to cultivate fungi to produce cellulase. Liu et al. constructed a functionally complementary microbial community (Aspergillus niger, Penicillium, and Trichoderma reesei) for enzyme production. The crude enzyme showed good results, and the true protein content increased by 53.49% after enzymatic hydrolysis and fermentation [6]. Kong et al. produced protein feed from DGs through a three-stage fermentation process involving aerobic fermentation by Aspergillus niger, microaerobic fermentation by yeast, and anaerobic fermentation by lactic acid bacteria, increasing the true protein content from 10.8% to 16.4% [7]. Numerous studies have shown that Aspergillus niger has strong growth and colonization capability and performs excellently in degrading DGs. However, Aspergillus niger may produce mycotoxins during the feed utilization process, leading to a series of health problems for livestock after application [8]. In comparison, Trichoderma harzianum is a typical probiotic that can be used in animal feed to improve gut health and enhance animal immunity. In addition, Trichoderma harzianum plays a significant role in the production of cellulase, which can effectively degrade lignocellulose. Cellulase is a complex enzyme system composed of endoglucanase, exoglucanase, and β-glucosidase [9]. Compared to cellulase produced by Aspergillus niger, cellulase produced by Trichoderma harzianum has a more complete enzyme system, which makes it more efficient and synergistic in the process of cellulose degradation [10].
This study established a solid-state fermentation system based on Trichoderma harzianum to enhance the protein content of DGs through enzymolysis and fermentation. In the first stage, the culture medium for enzyme production by Trichoderma harzianum was screened, followed by optimization of the enzyme production conditions using response surface methodology. In the second stage, the DGs were subjected to enzymatic hydrolysis using the crude enzyme to increase the content of reducing sugar in the substrate. In the third stage, yeast and lactic acid fermentation were employed to enhance the protein content and flavor of the DGs. Finally, the digestibility and flavor of the DG-based protein feed was evaluated. This study provides an innovative strategy for the efficient conversion of DGs and paves a new way for the development of related fields.
2. Materials and Methods
2.1. Raw Materials and Microorganisms
The strong-aroma type DGs were provided by Luzhou Laojiao Co., Ltd., (Luzhou, China). The dry matter (DM) content of the fresh DGs was 35%. Fresh DGs were dried to constant weight at 105 °C in an electrically heated, constant-temperature blast drying oven (DHG-9140A, Shanghai Zhenghong Industrial Co., Ltd., Shanghai, China). Corn stover (CS) and wheat bran (WB) were purchased from a farm located in Zhengzhou, Henan. The rice husks (RH) were separated from the DGs of Luzhou Laojiao by sieving the dried DGs through a 10-mesh screen. All solid materials were ground into powder using a high-speed multifunctional grinder (RS-FS1401, Hefei Royalstar Home Appliance Co., Ltd., Hefei, China), and then the powder was passed through a 40-mesh sieve for subsequent use. The specific composition is shown in Table 1.

Table 1.
The composition of the main materials.
Trichoderma harzianum (KY644130) and Aspergillus niger (OP580494) were used for producing cellulase. Candida utilis (OP580494), Rhodotorula marincola (CICC1235), and Saccharomyces cerevisiae (CICC32281) were used for protein production. Lactobacillus fermentum (CCIC20176) was used for anaerobic fermentation period [6]. The mold was inoculated on Potato Dextrose Agar (PDA) medium and cultured at 30 °C for 3–5 d. Spores were harvested using 0.05% Tween-80, and the spore suspension concentration was adjusted to 1 × 107 spores/mL for inoculation [11]. The yeast was inoculated on Yeast Extract Peptone Dextrose (YPD) medium and cultured at 30 °C for 20–24 h, ensuring that the OD value of the yeast suspension was consistent for each inoculation [12].
2.2. Medium Screening
2.2.1. Spore Production Medium Screening
This study optimized the growth conditions of Trichoderma harzianum by screening different formulations of media. The design of the culture medium was based on PDA and the PSA (PSA is Potato Sucrose Agar, a medium primarily used for culturing molds). The detailed formulations of the media are shown in Table 2.

Table 2.
Formulation of growth medium for Trichoderma harzianum.
2.2.2. Enzyme-Producing Medium Screening
To optimize the enzyme production medium, this study selected WB medium and a mixture of WB and CS (CS:WB = 4:1) as the basic enzyme-producing substrates. To make cellulase more suitable for DGs with high RH content, 0%, 20%, 40%, 60%, 80%, and 100% (w/w) RH was used as the component of the two enzyme-producing substrates. The formulation of the enzyme production nutrient solution was as follows: (NH4)2SO4 2 g/L, MgSO4 5 g/L, KH2PO4 8 g/L [11]. Solid-state fermentation of enzyme production was carried out in 250 mL Erlenmeyer flasks. The medium consisted of 10 g of substrate combined with 10 mL of nutrient solution, and each flask was inoculated with 1 × 107 spores of Trichoderma harzianum. The flasks were placed in an incubator at 30 °C for fermentation for 4 d.
2.3. Experimental Design
2.3.1. Design of Enzyme-Producing Experiments
In this study, response surface optimization was designed based on Box–Behnken to investigate the effects of moisture content, time, and Tween-80 addition on cellulase activity, as well as the interaction effects among these three factors [13]. The enzyme-producing fermentation system contained 10 g of substrate. The moisture content levels were set at 40%, 50%, and 60%. The fermentation time levels were 3 d, 4 d, and 5 d. The Tween-80 addition levels were 0.05 mL, 0.10 mL, and 0.15 mL. The specific details of the enzyme production medium and fermentation system can be found at Section 2.2.2.
2.3.2. Design of Enzymatic Hydrolysis Optimization Experiments
In this study, surfactants including Tween-20, Tween-80, PEG4000, and PEG6000 were used to assist enzymatic hydrolysis. The specific method was as follows: 5% of the surfactant was added to the crude enzyme solution based on the dry weight of the substrate [14]. After pre-incubating the crude enzyme solution at 4 °C for 12 h, the crude enzyme solution was mixed with the substrate for enzymatic hydrolysis [15]. Each experimental group was conducted with three biological replicates.
2.3.3. Experimental Design to Produce Feed Protein from DGs Using Enzymatic Pretreatment
Segmented saccharification and fermentation (SSF): Firstly, the enzymatically hydrolyzed DGs were sterilized at 121 °C for 20 min. Subsequently, 2% urea (based on the dry weight of the substrate) was added to the system as the fermentation medium [6]. Candida utilis, Rhodotorula marincola, and Saccharomyces cerevisiae were inoculated into the fermentation medium at different ratios. Fermentation was carried out at 30 °C for 15 d, with samples taken every 2 d to investigate the effects of yeast inoculation ratios and fermentation time on protein content.
Separate hydrolysis and fermentation (SHF): After the enzyme-producing fermentation was completed, the crude enzyme solution was aseptically extracted using sodium acetate [16]. The DGs was sterilized at 121 °C for 20 min. Subsequently, 2% urea (based on the dry weight of the substrate) was added to the system, and Candida utilis, Rhodotorula marincola, and Saccharomyces cerevisiae were inoculated. The moisture content was adjusted to 60% using the crude enzyme solution. Fermentation was carried out at 30 °C for 15 d, with samples taken every 2 d to investigate the effects of yeast inoculation ratios and fermentation time on protein content.
2.3.4. Design of In Vitro Simulated Rumen Fluid Digestion Experiment
To ensure representativeness, rumen fluid was collected from beef cattle (fed a diet of soybean meal and straw) at a slaughterhouse in Zhengzhou, Henan Province, China. The rumen contents were collected from freshly slaughtered beef cattle at a local slaughterhouse, and the rumen fluid was obtained by squeezing through cheesecloth. The rumen fluid was rapidly collected and preserved in a collection bottle. The collected rumen fluid was stirred and mixed evenly, then filtered through four layers of cheesecloth to remove large particles and residues [17]. Carbon dioxide was continuously bubbled through the filtered rumen fluid. After filtration, the rumen fluid was mixed with Menke medium in a ratio of 1:2 to prepare the artificial rumen culture fluid [18]. Fresh rumen fluid was aseptically processed into artificial rumen fluid and immediately used in the experiment to preserve microbial viability. Note that during transfer, continuous shaking was necessary to ensure the homogeneity of the rumen fluid in the fermentation vessel.
The artificial rumen culture fluid was dispensed into 500 mL blue-capped bottles, with 200 mL in each bottle. About 1.5 g DG protein feed was added to each bottle, which was then sealed with an isobutyl rubber stopper and secured with an aluminum cap. The bottles were placed in a constant-temperature shaker at 39 °C, with the shaking frequency adjusting to 150 rpm. The experiment was designed with four sampling points: 4 h, 14 h, 24 h, and 48 h. A blank control (without substrate) was set up for each sampling point for comparison. After each sampling point, the samples were quickly removed from the water bath shaker and immediately placed in ice water to terminate fermentation. The rumen fluid was then filtered through four layers of cheesecloth and rinsed three times with water. The filtered solids were dried to constant weight at 105 °C to determine the DM degradation rate. The remaining fermentation liquid was mixed thoroughly and stored in a freezer at −80 °C.
2.3.5. Experimental Design for Lactic Acid Fermentation of Protein Feed
The protein feed product obtained from fermentation was dried at 105 °C. It was ground using a high-speed crusher and then sieved through a 40-mesh sieve. The pH of the substrate was adjusted to 5.6 using 1 M HCl, and the sample was sterilized at 121 °C for 20 min. A 250 mL conical flask was used as the fermentation reactor, with a loading amount of 10 g. Vacuum packaging bags and a vacuum sealer were employed for anaerobic fermentation by vacuum extraction, with sterile water replacing the microbial inoculum as the control group. Lactobacillus fermentum was selected as the fermentation strain. The moisture content was controlled at 50%, with an inoculation rate of 2% (dry weight) and glucose added at 2% (dry weight). The fermentation temperature was maintained at 37 °C for 15 d [19]. Sampling was conducted at 0, 3, 7, 11, and 15 d. After the experiment, the contents of lactic acid and volatile fatty acids were measured, and the feed flavor was assessed using an electronic nose and electronic tongue (see Section 2.4.5).
2.4. Methods of Measurement and Data Processing
2.4.1. Filter Paper Enzyme Activity Assay Method
In this experiment, filter paper enzyme (FPase) activity was used as an indicator to evaluate the cellulase activity. Prior to the enzyme activity assay, crude enzyme solution was prepared using a 0.1 M acetic acid–sodium acetate buffer at pH 4.8. The Whatman No. 1 filter paper strip (1 × 6 cm, ~50 mg) was placed in the crude enzyme solution. It was then incubated in a water bath shaker at 50 °C and 150 rpm for 1 h. After the reaction, 3 mL of 3,5-dinitrosalicylic acid (DNS) reagent was added, and the mixture was boiled for 10 min [20]. The volume was then adjusted to 25 mL with deionized water. Finally, the absorbance was measured at 540 nm using a spectrophotometer to calculate the cellulase activity [6].
2.4.2. Method for Determination of Lignocellulose Content
The determination of lignocellulose content was referred to the standard method reported by the National Renewable Energy Laboratory (NREL) of the United States [21]. Specifically, 0.3 g of the sample was hydrolyzed with 72 wt% H2SO4 at 30 °C for 1 h. Subsequently, the sample was further treated with 4 wt% H2SO4 at 121 °C for 1 h. The concentrations of glucose, xylose, and arabinose in the hydrolyzed products were determined using high-performance liquid chromatography (HPLC). The hydrolyzed products were filtered through a crucible to retain the precipitate, which was then dried at 105 °C and weighed. The precipitate was placed in a muffle furnace, heated to 575 °C, and maintained for 3 h. After cooling to 105 °C, it was removed and cooled in a desiccator and then weighed. Finally, the contents of cellulose, hemicellulose, and lignin were calculated using the formulas provided by the NREL method.
2.4.3. Methods for Determination of Sugar and Protein Content
The total reducing sugar content was determined using the DNS (3,5-dinitrosalicylic acid) method. The concentrations of glucose, xylose, and arabinose were measured by high-performance liquid chromatography (HPLC). For the determination of true protein, inorganic nitrogen was first removed from the sample using the copper sulfate precipitation method, and then the total nitrogen content was determined by the Kjeldahl method (Official Methods of Analysis of AOAC International). The results were multiplied by a factor of 6.25 to obtain the crude protein content [22]. The specific calculation formula is shown as Equation (1).
In the above formula, V refers to the volume (in mL) of the 0.1 M standard hydrochloric acid solution consumed during the titration of the sample, V0 refers to the volume (in mL) of the standard hydrochloric acid solution consumed during the titration of the blank, C refers to the amount concentration (in mol/L) of the standard hydrochloric acid solution, and m refers to the mass (in g) of the sample.
2.4.4. Methods for Determination of Ammonia Nitrogen, Lactic Acid, and Volatile Fatty Acids (VFA)
The determination of ammonia nitrogen (NH3-N) content was carried out using a modified alkaline phenol–hypochlorite method. The reaction principle was based on the Berthelot reaction, where ammonia reacts with alkaline hypochlorite and phenol in the presence of the catalyst, nitroprusside, to form blue indophenol [23]. Lactic acid and VFAs (acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric (including 2-methylbutyric) acid, and caproic acid) were determined using HPLC [24].
2.4.5. Detection Methods Using Electronic Nose and Tongue
The electronic tongue (SA402B) from Insent Japan used artificial lipid membrane sensors with broad selectivity to simulate the taste sensation mechanism of living organisms. It could evaluate five basic tastes (sour, sweet, bitter, salty, umami) and astringency by detecting changes in membrane potential resulting from electrostatic or hydrophobic interactions between various taste substances and the artificial lipid membrane (Table 3). The electronic nose (PEN3) from AireSense could detect volatile flavor compounds (Table 4). For sample preparation, the test sample was diluted 1:10 with pure water. The sample was homogenized and centrifuged, and then it was filtered before analysis using the electronic nose and electronic tongue [25].

Table 3.
Matching information between sensor and taste value.

Table 4.
Germany PEN3 electronic nose sensor sensitive substances.
2.5. Statistical Analysis
In this study, Excel 2020 and OriginPro 2024 were used for the calculation and analysis of experimental data. Design Expert 11 was employed to design the response surface experiments and process the data. The statistical significance of intergroup differences was determined by one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Screening of Culture Medium for Trichoderma harzianum
3.1.1. Screening of Growth Medium for Trichoderma harzianum
Figure 1 illustrates the growth characteristics and sporulation capacity of Trichoderma harzianum on different media formulations. As shown in Figure 1a, when cultured on the basic PDA medium, the strain only formed white mycelium without spores. In contrast, a characteristic green spore formation was observed in the modified media with different glucose concentrations (PDA0–PDA20) and sucrose-containing PSA10–PSA20. After the spore suspension was extracted with a 0.05% Tween-80 solution, quantitative analysis using a hemocytometer and fluorescence inverted microscope (Figure 1b) revealed that the sporulation yields on PDA20 and PSA15 media were 2.33 × 108 and 2.15 × 108 spores/mL, respectively, which were significantly higher than those in other treatments. Notably, despite the similar spore yields, the colonies on PDA20 exhibited clearer growth rings than PSA15. Meanwhile, mycelial distribution on PSA15 was more diffuse than PDA20. Based on both sporulation efficiency and colony morphology, PDA20 was ultimately selected as the standard medium for the sporulation of Trichoderma harzianum in this study.
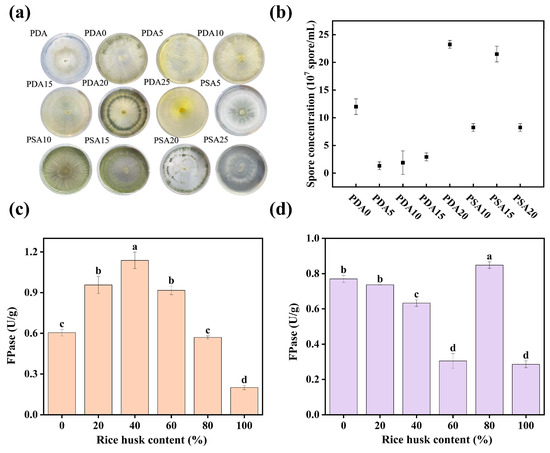
Figure 1.
Growth of Trichoderma harzianum under different conditions and FPase activity of enzyme-producing fermentation on different substrates. (a): Growth of Trichoderma harzianum under different conditions; (b): spore concentration in suspensions under different conditions; (c): effect of RH addition on FPase activity in WB-CS mixed medium; (d): effect of RH addition on FPase activity in pure WB medium. Different letters indicate significant differences among groups (p < 0.05).
3.1.2. Screening of Enzyme-Producing Medium for Trichoderma harzianum
Figure 1c shows the effect of adding RH at different proportions (0%, 20%, 40%, 60%, 80%, and 100%) in WB-CS mixed medium on FPase activity. FPase first increased and then decreased with the increasing addition of RH, reaching the highest value of 1.14 U/gds at an RH addition of 40%. Figure 1d shows the effect of adding RH at different proportions in the pure WB medium on FPase activity. The highest FPase of 0.79 U/gds was achieved when the RH content was 80%. In summary, the enzyme-producing fermentation was carried out using WB-CS mixed medium with a 40% addition of RH.
3.2. Response Surface Optimization of FPase Activity
Based on response surface optimization (Table 5), the FPase activity gradually decreased as the fermentation time increased from 3 to 5 d. The moisture content ranged from 40% to 60%, with the FPase activity first increasing and then decreasing, reaching a maximum value of around 50% moisture content. As the concentration of Tween-80 increased, the FPase activity showed an upward trend (Figure 2). According to the predicted results, the highest FPase activity of 1.45 U/gds was achieved at a moisture content of 53%, a fermentation time of 3 d, and a Tween-80 concentration of 0.15 mL/g (dry weight basis). Under these conditions, a validation experiment was conducted, and the measured enzyme activity was 1.41 U/gds, which is close to the predicted value and represents a 23.7% increase compared to the initial value. The quadratic regression model, analyzed by analysis of variance (ANOVA), showed an F-value of 31.23 and a p-value less than 0.0001, indicating a significant model and valid predicted results.

Table 5.
Box–Behnken design matrix for optimization of cellulase production by Trichoderma harzianum.
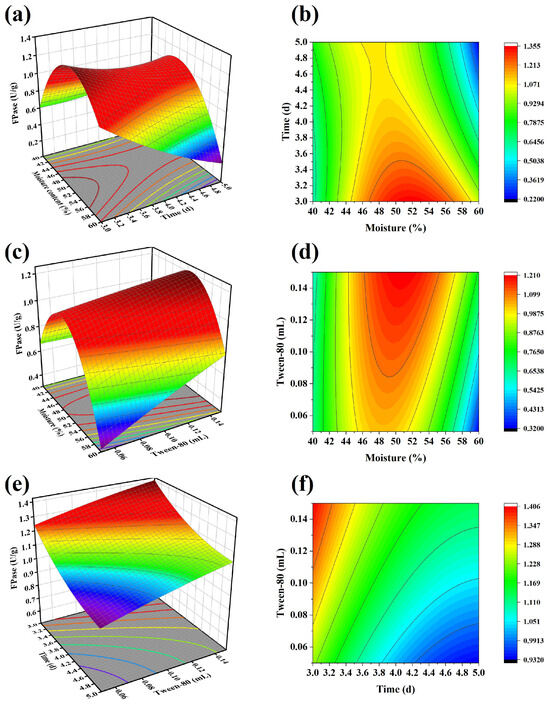
Figure 2.
Results of response surface optimization of enzyme production conditions for T. harz ianum. (a,b): Interaction effects of moisture content and time on FPase activity; (c,d): interaction effects of moisture content and Tween-80 addition on FPase activity; (e,f): interaction effects of time and Tween-80 on FPase activity.
3.3. Exploration of Optimal Reaction Conditions for Enzymolysis
The optimal reaction temperature and pH of the cellulase produced by Trichoderma harzianum were systematically evaluated through single-factor experiments. As shown in Figure 3a, FPase increased progressively with rising temperature and reached peak values at both 50 °C and 55 °C, with no significant difference in activity between these two temperature ranges (p > 0.05). In the pH gradient experiment (Figure 3b), FPase continued to increase with the increasing acidity of the reaction system, reaching the highest FPase at pH 4.5. In the optimized reaction system (50 °C, pH 4.5), the effects of four surfactants on enzymatic hydrolysis efficiency were tested (Figure 3c,d). The results showed that the reducing sugar yields in the PEG4000 and PEG6000 treatment groups were increased by 2.6% and 5.0%, respectively, compared to the control group (p < 0.05). A pre-incubation experiment was further designed to verify the stabilizing effect of surfactants on the enzyme system (Figure 3e,f). The results indicated that the reducing sugar and glucose yields in the PEG4000 pre-treated group were significantly higher than those in the untreated group, with increases of 5.66% and 3.21%, respectively.
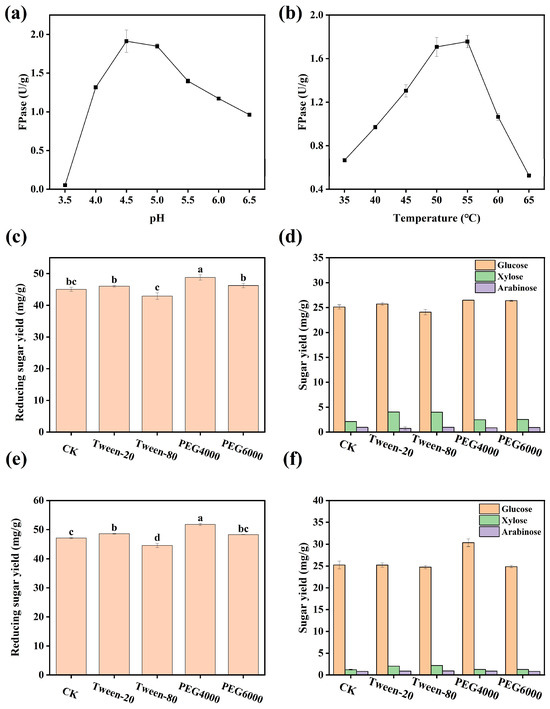
Figure 3.
Optimal conditions for cellulase activity. (a): Effect of temperature on FPase activity; (b): effect of pH on FPase activity; (c,d): effect of different Tween-80 concentrations on enzymatic hydrolysis; (e,f): effect of pre-incubation on enzymatic hydrolysis. Different letters indicate significant differences among groups (p < 0.05).
3.4. Effect of Trichoderma harzianum Fermentation on Protein Production from DGs
Figure 4a,b show the effects of different yeast ratios on the production of true protein and crude protein during SSF and SHF. The optimal fermentation strategy was identified as inoculating with Candida utilis/Saccharomyces cerevisiae/Rhodotorula marincola at a ratio of 1:1:1, using SSF. This approach yielded the highest true protein content of 14.42%, representing a 34.6% increase compared to the initial DGs. Figure 4c,d illustrate the impact of fermentation duration on true protein and crude protein production. As fermentation time extended, the contents of crude protein and true protein gradually increased, reaching their peaks on day 11 (crude protein 20.67%, true protein 15.16%), and then slightly decreased by day 15.
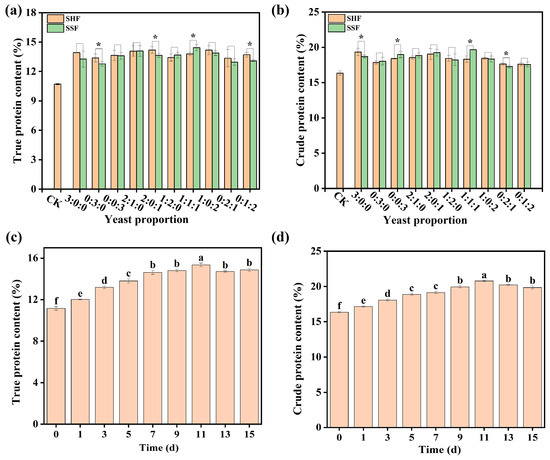
Figure 4.
Yield of crude and true protein under different fermentation conditions. (a): Effect of yeast ratio on true protein content during SHF and SSF; (b): effect of yeast ratio on crude protein content during SHF and SSF; (c): effect of fermentation duration on true protein content during simultaneous saccharification and fermentation; (d): effect of fermentation duration on crude protein content during simultaneous saccharification and fermentation. Asterisks indicate significant differences among the data. Different letters indicate significant differences among groups (p < 0.05).
3.5. Electronic Sensory Characterization of Protein Feed
Figure 5 shows the principal component analysis (PCA) plot of electronic nose data and the radar chart of electronic tongue data. As shown in Figure 5a, the cumulative contribution rate of principal component 1 and principal component 2 exceeded 99%, which meant that PCA could distinguish the differences in odor between different samples. There was some overlap after lactic acid fermentation of the Trichoderma harzianum protein feed. On principal component 1, the sensor with the greatest contribution was W5S, indicating differences in nitrogen oxides. On principal component 2, the sensor with the greatest contribution was W1W, indicating differences in sulfur compounds. Figure 5b shows the electronic tongue signals of DGs raw material and three types of fermented feed samples. Eight main flavor substances were detected, and there were no significant differences among the four samples in terms of saltiness, richness, umami, astringency, and aftertaste. Significant differences were only found in sourness and bitterness. The tasteless point for sourness was −13; values below this indicated no sourness, while values above it indicated the presence of sourness. After fermentation, the sourness of Trichoderma harzianum and Aspergillus niger–yeast fermented protein feed was below the tasteless value, indicating no sourness. The reduction in sourness might lead to an increase in bitterness, with the tasteless point for bitterness being 0 [26]. In comparison, the Trichoderma harzianum–yeast fermented feed exhibited the strongest bitterness. After lactic acid fermentation, the pH value decreased significantly, enhancing the sourness while reducing the bitterness, and the flavor of the feed was notably improved.
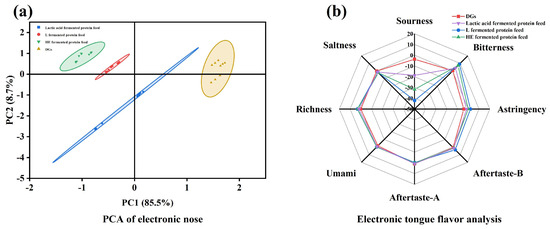
Figure 5.
Evaluation of protein feed flavor. (a): Principal component analysis (PCA) of electronic nose data; (b): radar chart of electronic tongue data.
3.6. Digestibility of Protein Feed
As shown in Figure 6a, the fermented protein feed using Trichoderma harzianum had the highest IVDMD (in vitro dry matter digestibility) and IVCPD (in vitro crude protein digestibility). Higher IVDMD indicated that more nutrients in the feed could be utilized by animals, providing them with more energy. Higher IVCPD indicated that the crude protein in the feed was more extensively broken down by rumen microorganisms. The breakdown of crude protein in the feed by rumen microorganisms produced VFA and ammonia. While VFA was beneficial for animal growth, the production of ammonia led to the loss of organic nitrogen and could become a source of air and water pollution. Figure 6b shows the changes in soluble protein content with in vitro digestion time. Over time, the soluble protein content increased in all four experimental groups. The DGs raw material group had the lowest protein content, with soluble protein levels lower than those of the Aspergillus niger and Trichoderma harzianum protein feed groups. At the end of fermentation, the soluble protein content of the Trichoderma harzianum–yeast fermented feed (0.31 mg/mL) was higher than that of the Aspergillus niger–yeast fermented feed (0.27 mg/mL). Gas production and methane yield are important indicators for evaluating rumen fermentation in ruminants, reflecting the extent to which substrates are utilized by rumen microbes. Figure 6c,d show that gas production increased over time, with the DG raw material having significantly higher gas production and cumulative methane yield than the two fermented feeds. The feed fermented with Trichoderma harzianum had the lowest gas production, indicating higher digestion efficiency compared to the others. Ammonia nitrogen mainly originates from the breakdown of proteins, and this ammonia can be absorbed by some microbes for further synthesis of microbial crude protein (MCP), while the remaining ammonia exists in the rumen fluid as NH3-N. Figure 6e,f show that ammonia nitrogen content first increased and then decreased over time, while MCP content showed an increasing trend. The Trichoderma harzianum–yeast fermented feed had significantly higher ammonia nitrogen and MCP content than the other groups.
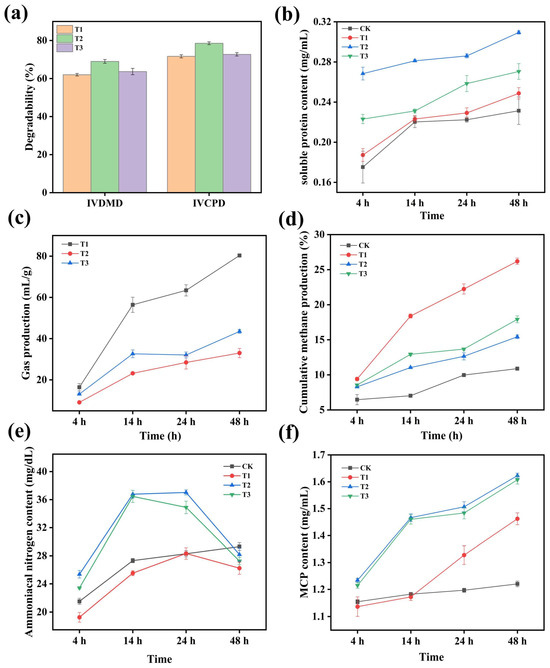
Figure 6.
In vitro simulated rumen digestion effects of distillery residue raw material and different fermented feeds (CK: blank control without dry matter. T1: Distillery residue raw material. T2: Trichoderma harzianum–yeast fermented feed. T3: Aspergillus niger–yeast fermented feed). (a): Degradation rates of nutrients in different fermented feeds; (b): changes in soluble protein content with in vitro digestion time; (c): changes in total gas production with in vitro digestion time; (d): dhanges in cumulative methane yield with in vitro digestion time; (e): changes in ammonia nitrogen content with in vitro digestion time; (f): changes in MCP content with in vitro digestion time.
4. Discussion
4.1. Feasibility Analysis of Cellulase Production by Trichoderma harzianum Fermentation
The RH content in DGs exceeds two-thirds and has a high inorganic content, which is poorly digestible. RH contains approximately 40% lignocellulose. If this component can be effectively degraded, the utilization rate of DGs will be significantly improved. According to reports by Cunha et al., agricultural waste used as an inducer to produce hydrolases can make lignocellulosic substrates more susceptible to fungal assimilation and enzymatic attack [27]. Therefore, this study aims to enhance the specific degradation capability of lignocellulose in DGs. Based on this, RH screened from DGs to formulate the enzyme-producing medium. It was found that when 40% RH was added to the mixed substrate, the highest FPase of 1.14 U/gds was obtained. Further, based on the results of response surface, the optimal conditions for enzyme production were obtained: a moisture content of 53%, a fermentation time of 3 d, and a Tween-80 content of 0.015 mL/g (dry weight basis). At these conditions, the highest enzyme activity reached 1.41 U/gds [13].
Cellulase is categorized into acidic cellulase, neutral cellulase, and alkaline cellulase. Most cellulases secreted by fungal have ideal activity in an acidic environment. Neutral and alkaline cellulases are also used in some studies, such as in the pulp and paper industry, animal feed, and the textile and laundry industries [28]. Different cellulases have their respective suitable catalytic temperatures and pH values. Therefore, it is necessary to investigate the physicochemical properties of enzymes produced by solid-state fermentation. The results showed that the cellulase produced by Trichoderma harzianum maintained high enzyme activity between pH 4.0 and 6.0, with enzyme activity gradually decreasing as the pH continued to rise. This cellulase maintained high enzyme activity at a temperature of 55 °C. To further improve the enzymatic hydrolysis efficiency of DGs, this study selected surfactants, including Tween-20, Tween-80, PEG4000, and PEG6000, as well as pre-incubation methods to optimize the enzymatic hydrolysis efficiency [29]. The enzymatic hydrolysis efficiency of the PEG4000 group had the highest improvement after pre-incubation, increasing by 10.75% compared to the original DGs. A previous study showed that non-catalytic proteins could replace enzymes adsorbed by lignin to reduce non-productive adsorption. The possible mechanism of action is that lignin may have stronger adsorption capacity than cellulose. Therefore, non-catalytic proteins reduced the non-productive adsorption of enzyme proteins on lignin and hemicellulose and prevented the blockage of enzyme catalytic sites on cellulose [30]. Tween and PEG had the same effect and could effectively enhance the enzymatic hydrolysis of lignocellulose.
4.2. Feasibility Analysis of Protein Feed Production by Trichoderma harzianum–Yeast Fermentation
According to previous studies, Saccharomyces cerevisiae could rapidly utilize glucose, Candida utilis could effectively utilize both glucose and xylose, and Rhodotorula marincola had a higher utilization efficiency for xylose and arabinose compared to the other two yeasts [6]. Therefore, a microbial community consisting of Rhodotorula marincola, Candida utilis, and Saccharomyces cerevisiae was constructed as the inoculum for protein production. Through a synergistic saccharification and fermentation system, it was expected to fully achieve the material conversion of DGs and increased the content of true protein. Firstly, the effects of yeast inoculation ratios on protein content were investigated by segmented saccharification fermentation and simultaneous saccharification fermentation. It was ultimately found that when Rhodotorula marincola/Candida utilis/Saccharomyces cerevisiae = 1:1:1, the true protein content reached a peak of 15.16% after 11 d of simultaneous saccharification fermentation, and the crude protein also reached a peak of 20.67% on the 11th day. Compared with SHF, SSF could promptly convert the reducing sugars generated during the enzymatic hydrolysis of DGs into microbial protein. This fermentation process could avoid sugar inhibition caused by the accumulation of reducing sugars, thereby increasing the true protein content [31]. The DG feed protein produced by this process had a high true protein content, which was 41.5% higher than that of directly fermented DG feed.
4.3. Performance Assessment of Protein Feed Produced by Trichoderma harzianum–Yeast Fermentation
This study first analyzed the flavors of fermented protein feed using an electronic nose and electronic tongue. The results showed that there were no significant differences among the three materials in terms of saltiness, richness, umami, astringency, and aftertaste, with significant differences only in sourness and bitterness. Specifically, the sourness values of the two fermented feeds were lower than that of the original DGs, while the bitterness values were significantly higher. After lactic acid fermentation, the flavor of the Trichoderma harzianum–yeast fermented protein feed was significantly improved, especially with an increase in sourness and a decrease in bitterness.
By comparing the in vitro simulated rumen digestion effects, it was found that the IVDMD (in vitro dry matter digestibility) and IVCPD (in vitro crude protein digestibility) levels and the soluble protein yields of the Trichoderma harzianum–yeast fermented feed were significantly higher than those of original DGs and the Aspergillus niger–yeast fermented feed. High DM (dry matter) and crude protein degradation rates in feed are generally associated with improved sensory characteristics (e.g., texture and odor), which are more preferred by animals. Furthermore, since most fermented DGs (distillers’ grains) are used as dietary supplements, their sourness or bitterness is further masked when mixed with other feed components, leading to increased feed intake [32]. In terms of gas production and cumulative methane yield, the Trichoderma harzianum–yeast fermented feed was significantly lower than the Aspergillus niger–yeast fermented feed and original DGs, indicating a higher utilization degree of the feed by rumen microbes [27]. In addition to the utilization efficiency of nutrients in the feed, lower methane production not only reduced greenhouse gas emissions but also improved the utilization efficiency of feed energy, which had positive implications for animal growth and production performance as well as the economic benefits of the livestock industry [33].
The ammonia nitrogen content in rumen fluid during in vitro digestion of feed is an important indicator in the digestion process of ruminant feed. The main source of ammonia nitrogen is the breakdown products of proteins. Therefore, the concentration of ammonia nitrogen can reflect the degree of protein breakdown in the feed [34]. Compared with the original DGs, the ammonia nitrogen levels in the Aspergillus niger and Trichoderma harzianum–yeast fermented feed groups were significantly (p < 0.05) higher than that in the control and original DGs groups. This might be related to the higher protein content in the Aspergillus niger and Trichoderma harzianum protein feeds. At the same time as the production of ammonia nitrogen, the pepsin in the rumen fluid rapidly utilized the nitrogen source, and rumen microbes also quickly converted ammonia nitrogen to microbial crude protein (MCP) [35]. The Trichoderma harzianum–yeast fermented feed group had the highest MCP content, reaching 1.62 mg/mL at 48 h. At the end of fermentation, the MCP concentration in the blank group was 1.22 mg/mL, significantly lower than that in the experimental groups. Therefore, DG feed increased the amount of nitrogen converted into microbial protein nitrogen after solid-state fermentation, which was conducive to improving the rumen microbes’ ability to digest and utilize nitrogen [36].
5. Conclusions
This study established a multi-stage biotransformation strategy for the valorization of DGs. A Trichoderma harzianum-derived cellulase system was employed for substrate pretreatment, with substrate induction and response surface methodology optimization synergistically enhancing cellulase production efficiency by 23.7%, thereby achieving a 10.75% improvement in reducing sugar yield from DGs. During solid-state fermentation, the functional synergism of three yeast strains was innovatively exploited to elevate the true protein content from 10% to 15.16% through targeted proteosynthesis. In vitro rumen simulation experiments demonstrated that the co-fermented protein feed exhibited 11.29% and 9.56% increases in dry matter and crude protein degradation rates, respectively, confirming enhanced nutrient bioavailability. Notably, a post-fermentation treatment with lactic acid bacteria effectively modulated organoleptic properties, mitigating bitterness while developing characteristic acidic aroma profiles. The developed three-stage processing framework (“enzymatic saccharification—protein fortification—palatability enhancement”) not only provides an eco-efficient solution for DGs biorefining but also establishes a universalizable technical paradigm for agricultural by-product valorization, demonstrating dual advantages in circular economy implementation and sustainable feed production.
Author Contributions
Conceptualization, X.B. and J.W.; methodology, X.W.; software, S.L. and Y.Y.; validation, R.S., S.W. and X.Z.; formal analysis, Z.W.; investigation, Y.C.; resources, X.Z.; data curation, Y.C.; writing—original draft preparation, X.B.; writing—review and editing, Z.W. and X.W.; visualization, J.X.; supervision, H.Y.; project administration, Y.C.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (52200178), the start-up fund of Zhengzhou University (32213091), National Engineering Research Center of Solid-State Brewing (2023HX16), Henan Province Science and Technology Research (12102310864 and 222102310142), the Key Research Project of the Higher Education Institutions of Henan Province, China (22A530010), and the Key Program for Collaborative and Innovation of Nanyang, Henan Province, China (21XTCX21001).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, J.; Wang, Z.; Shen, X.; Chen, R.; Peng, Y.; Cai, Y.; Zeng, S.; Liu, D.; Yang, J.; Zhuang, W.; et al. Solid-state fermentation through synthetic microbiome: An effective strategy for converting Chinese distillers’ grains into functional protein feed. Int. J. Food Microbiol. 2025, 435, 111154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Qin, H.; Huang, M.; Xi, B.; Mao, J.; Zhang, S. Comparing the antioxidation and bioavailability of polysaccharides from extruded and unextruded Baijiu vinasses via in vitro digestion and fecal fermentation. Int. J. Biol. Macromol. 2024, 276, 133681. [Google Scholar] [CrossRef]
- Andrade, L.C.L.; Putti, F.F.; Cremasco, C.P.; Gabriel Filho, L.R.A. New Paradigm for Vinasse Use as Fertilizer in Hydroponics. Sugar Tech 2022, 24, 1260–1271. [Google Scholar] [CrossRef]
- Fernández, F.J.; Sánchez-Arias, V.; Rodríguez, L.; Villaseñor, J. Feasibility of composting combinations of sewage sludge, olive mill waste and winery waste in a rotary drum reactor. Waste Manag. 2010, 30, 1948–1956. [Google Scholar] [CrossRef]
- Dennis, E.; Gertner, D.; Erickson, G. Economic Research on Ethanol Feed-Use Coproducts: A Review, Synthesis, and Path Forward. Animals 2024, 14, 1551. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Wang, Z.; Shen, C.; Liu, D.; Shen, X.; Weng, L.; He, Y.; Wang, S.; Wang, J.; et al. Pretreatment of Luzhou distiller’s grains for feed protein production using crude enzymes produced by a synthetic microbial consortium. Bioresour. Technol. 2023, 390, 129852. [Google Scholar] [CrossRef]
- Kong, S.; Wang, S.; He, Y.; Wang, N.; Wang, Z.; Weng, L.; Liu, D.; Zhao, X.; Chen, J.; Xu, J.; et al. Three-Stage Solid-State Fermentation Technology for Distillers’ Grain Feed Protein Based on Different Microorganisms Considering Oxygen Requirements. Fermentation 2024, 10, 550. [Google Scholar] [CrossRef]
- Braun, H.; Woitsch, L.; Hetzer, B.; Geisen, R.; Zange, B.; Schmidt-Heydt, M. Trichoderma harzianum: Inhibition of mycotoxin producing fungi and toxin biosynthesis. Int. J. Food Microbiol. 2018, 280, 10–16. [Google Scholar] [CrossRef]
- Lopez-Ramirez, N.; Volke-Sepulveda, T.; Gaime-Perraud, I.; Saucedo-Castañeda, G.; Favela-Torres, E. Effect of stirring on growth and cellulolytic enzymes production by Trichoderma harzianum in a novel bench-scale solid-state fermentation bioreactor. Bioresour. Technol. 2018, 265, 291–298. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Luo, L.; Wang, E.; Wang, R.; Liu, L.; Liu, J.; Yuan, H. Low-Cost Cellulase-Hemicellulase Mixture Secreted by Trichoderma harzianum EM0925 with Complete Saccharification Efficacy of Lignocellulose. Int. J. Mol. Sci. 2020, 21, 371. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, Z.; Cai, Y.; Zhao, Y.; Zhang, Y.; Gao, Y.; Cui, Z.; Wang, X. Accelerated biomethane production from lignocellulosic biomass: Pretreated by mixed enzymes secreted by Trichoderma viride and Aspergillus sp. Bioresour. Technol. 2020, 309, 123378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Plowman, J.E.; Tian, B.; Clerens, S.; On, S.L.W. The influence of growth conditions on MALDI-TOF MS spectra of winemaking yeast: Implications for industry applications. J. Microbiol. Methods 2021, 188, 106280. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J. Statistical optimization of sodium hydroxide pretreatment and enzymatic hydrolysis of corn stover powder for enhancing sugar production using response surface methodology. Biomass Convers. Biorefinery 2023, 13, 7111–7125. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, H.; Tao, Y.; Ma, Z.; Zheng, Z.; Ouyang, J. Addressing two major limitations in high-solids enzymatic hydrolysis by an ordered polyethylene glycol pre-incubated strategy: Rheological properties and lignin adsorption for enzyme. Bioresour. Technol. 2023, 390, 129895. [Google Scholar] [CrossRef]
- Goshadrou, A.; Lefsrud, M. Synergistic surfactant-assisted [EMIM]OAc pretreatment of lignocellulosic waste for enhanced cellulose accessibility to cellulase. Carbohydr. Polym. 2017, 166, 104–113. [Google Scholar] [CrossRef]
- Valles, A.; Álvarez-Hornos, F.J.; Martínez-Soria, V.; Marzal, P.; Gabaldón, C. Comparison of simultaneous saccharification and fermentation and separate hydrolysis and fermentation processes for butanol production from rice straw. Fuel 2020, 282, 118831. [Google Scholar] [CrossRef]
- Mateos, I.; Ranilla, M.J.; Ramos, M.; Saro, C.; Carro, M.D. Influence of rumen contents’ processing method on microbial populations in the fluid and subsequent in vitro fermentation of substrates of variable composition. Anim. Feed Sci. Technol. 2016, 220, 109–120. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Soller, H.; Steingass, H.; Menke, K.H. Energy and protein evaluation of tropical feedstuffs for whole tract and ruminal digestion by chemical analyses and rumen inoculum studies in vitro. Anim. Feed Sci. Technol. 1995, 52, 177–188. [Google Scholar] [CrossRef]
- Santamaría-Fernández, M.; Molinuevo-Salces, B.; Kiel, P.; Steenfeldt, S.; Uellendahl, H.; Lübeck, M. Lactic acid fermentation for refining proteins from green crops and obtaining a high quality feed product for monogastric animals. J. Clean. Prod. 2017, 162, 875–881. [Google Scholar] [CrossRef]
- Oesper, R.E. Kjeldahl and the determination of nitrogen. J. Chem. Educ. 1934, 11, 457. [Google Scholar] [CrossRef]
- Wang, G.; Tan, L.; Sun, Z.; Gou, Z.; Tang, Y.; Kida, K. Production of bioethanol from rice straw by simultaneous saccharification and fermentation of whole pretreated slurry using Saccharomyces cerevisiae KF-7. Environ. Prog. Sustain. Energy 2015, 34, 582–588. [Google Scholar] [CrossRef]
- Smith, V.R. A phenol-hypochlorite manual determination of ammonium-nitrogen in Kjeldahl digests of plant tissue. Commun. Soil Sci. Plant Anal. 1980, 11, 709–722. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Shen, C.; Wang, Z.; Liu, Y.; Meng, X.; Li, X.; Zhao, X.; Chen, J.; Xu, J.; et al. Biochar accelerates methane production efficiency from Baijiu wastewater: Some viewpoints considering direct interspecies electron transfer. Chem. Eng. J. 2024, 497, 154527. [Google Scholar] [CrossRef]
- Wu, J.; Ren, L.; Zhao, N.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Solid-state fermentation by Rhizopus oryzae improves flavor of wheat bran for application in food. J. Cereal Sci. 2022, 107, 103536. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Xie, C.; Yuan, R.; Su, L.; Li, D.; Zhang, C.; Yin, Y.; Wang, P.; Yang, R. Improving nutritional and sensory properties of rice bran by germination and solid-state fermentation with fungi. Food Biosci. 2024, 59, 103992. [Google Scholar] [CrossRef]
- Klop, G.; van Laar-Van Schuppen, S.; Pellikaan, W.F.; Hendriks, W.H.; Bannink, A.; Dijkstra, J. Changes in in vitro gas and methane production from rumen fluid from dairy cows during adaptation to feed additives in vivo. Animal 2017, 11, 591–599. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Zahoor; Chen, X.; Yu, Q.; Wang, Z.; Zhuang, X.; Yuan, Z. Effect of a Nonionic Surfactant on Enzymatic Hydrolysis of Lignocellulose Based on Lignocellulosic Features and Enzyme Adsorption. Acs Omega 2020, 5, 15812–15820. [Google Scholar] [CrossRef]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Tong, X.; Liu, K. An overview on the current status and future prospects in Aspergillus cellulase production. Environ. Res. 2024, 244, 117866. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, W.; Zhang, H.; Zhang, P.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y. Transformation of bacterial community structure in rumen liquid anaerobic digestion of rice straw. Environ. Pollut. 2021, 269, 116130. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, A.O.; Cheeke, P.R. Comparison of in vitro digestion of feed ingredients by rabbit cecal and bovine rumen fluids. Anim. Feed Sci. Technol. 1993, 41, 329–339. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Wang, R.; Ma, Z.; Long, D.; Mao, H.; Wen, J.; Bernard, L.A.; Beauchemin, K.A.; Tan, Z. Urea plus nitrate pretreatment of rice and wheat straws enhances degradation and reduces methane production in in vitro ruminal culture. J. Sci. Food Agric. 2018, 98, 5205–5211. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.; Su, X.; Wang, X.; Zhao, S.; Liu, L.; Luo, Y.; Liu, D.; Zheng, H.; et al. Metatranscriptomic Analyses of Plant Cell Wall Polysaccharide Degradation by Microorganisms in the Cow Rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef]
- Saminathan, M.; Wan Mohamed, W.N.; Md Noh, A.; Ibrahim, N.A.; Fuat, M.A.; Kumari Ramiah, S.; Jusoh, S.; Mat Dian, N.L.H. Effects of urea-treated oil palm frond on nutrient composition and in vitro rumen fermentation using goat rumen fluid. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1228–1237. [Google Scholar] [CrossRef]
- Schlageter-Tello, A.; Fahey, G.C.; Freel, T.; Koutsos, L.; Miller, P.S.; Weiss, W.P. ASAS-NANP symposium: Ruminant/nonruminant feed composition: Challenges and opportunities associated with creating large feed composition tables. J. Anim. Sci. 2020, 98, skaa240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).