Regulatory Effects of Cinnamon–Pepper–Chili Essential Oil Complex on Growth Performance, Immune Function, Complete Blood Count, and Intestinal Microbiota in Simmental CrossBred Cattle During the Late Fattening Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Animals
2.2. Feeding Procedure
2.3. Sample Collection and Measurement
2.3.1. Growth Performance Measurement
2.3.2. Measurement of Blood Parameters
2.3.3. Determination of Gut Microbiota
2.3.4. Statistical Analysis
3. Results
3.1. The Effect of Compound Essential Oils on the Growth Performance of Simmental Crossbred Cattle
3.2. Effect of Compound Essential Oil on Hematological Parameters in Simmental Crossbred Cattle
3.3. The Effect of Compound Essential Oils on Serum Indices in Simmental Crossbred Cattle
3.4. The Effect of Compound Essential Oils on the Intestinal Microbiota of Simmental Crossbred Cattle
3.4.1. Dilution Curve and OTUs Analysis
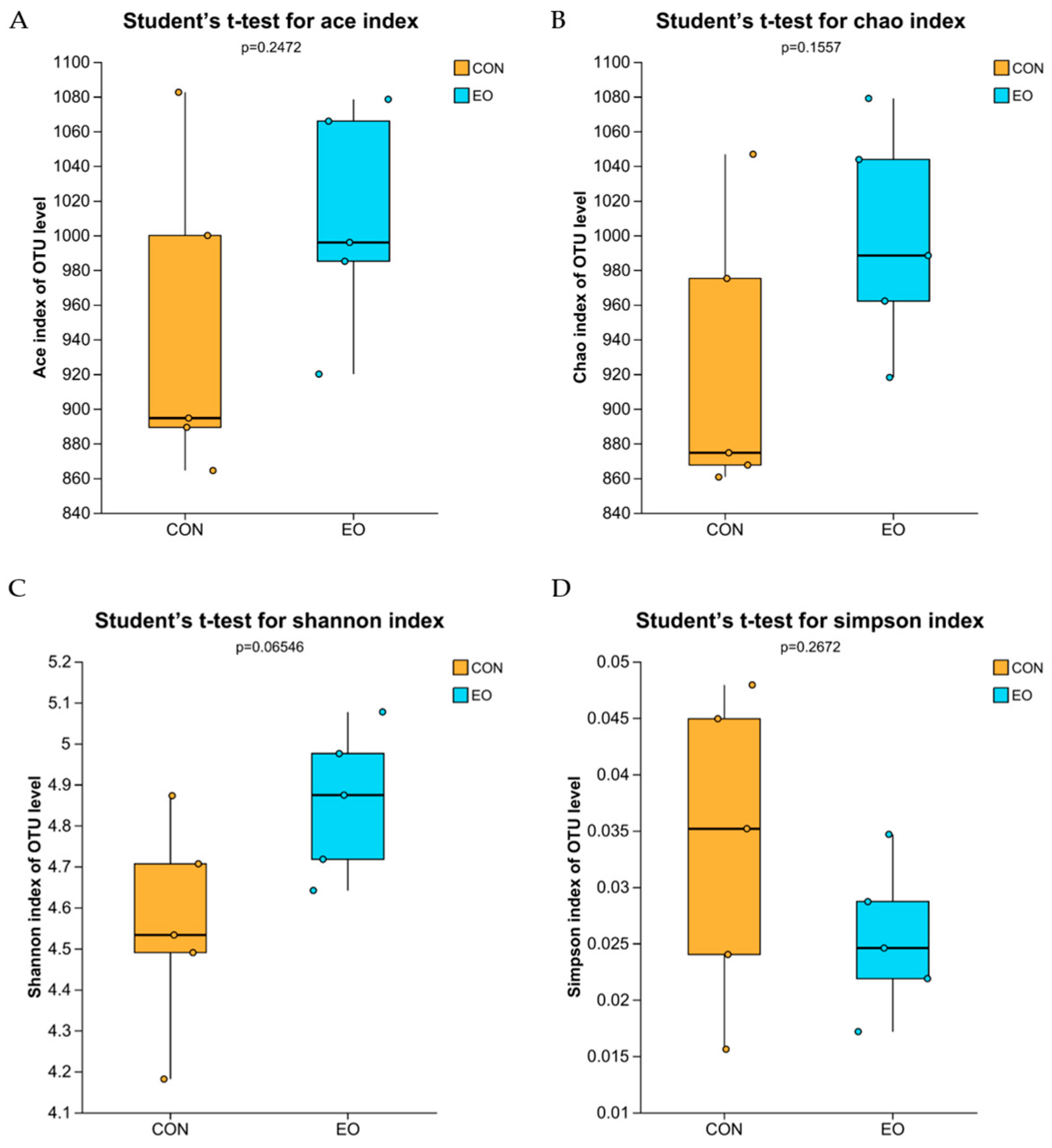
3.4.2. Alpha Diversity Analysis
3.4.3. Beta Diversity Analysis
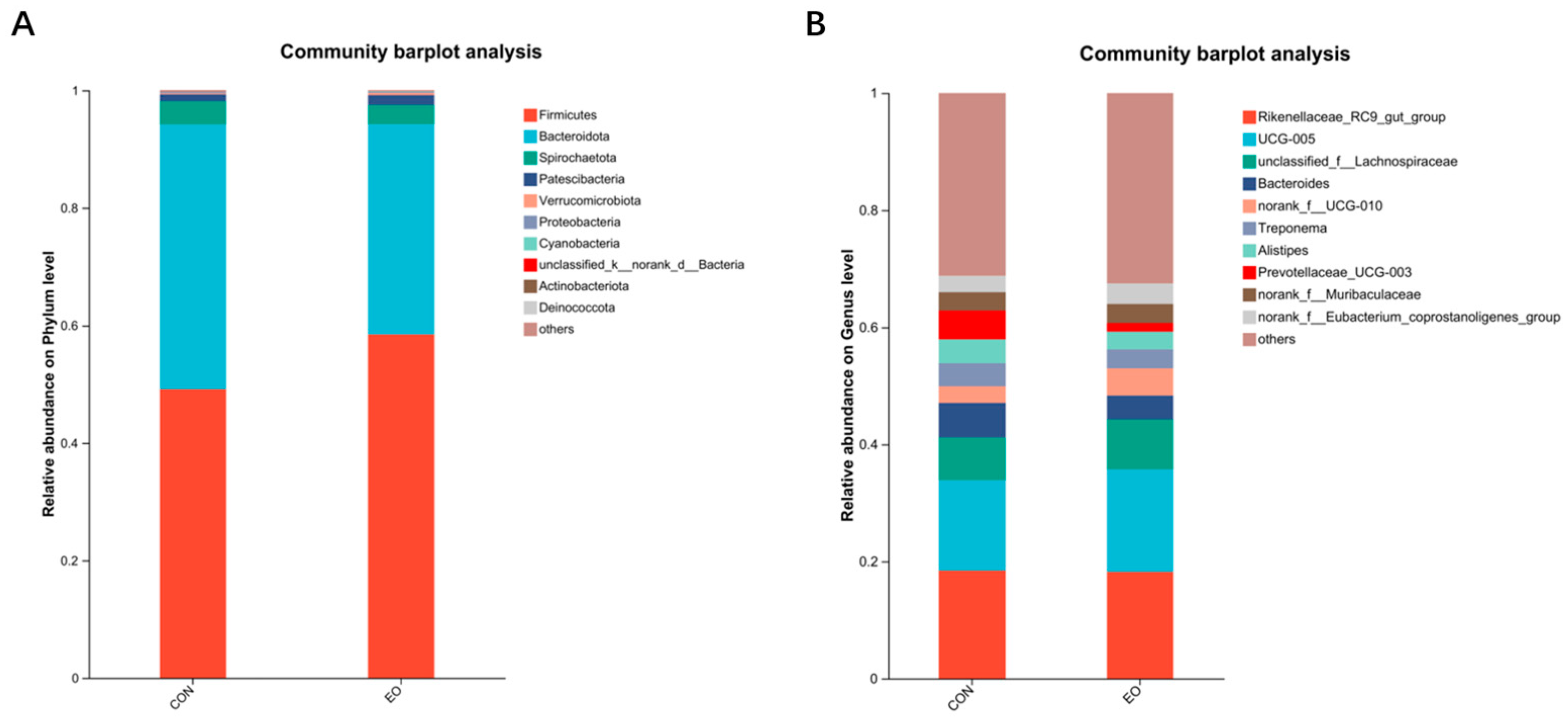
3.4.4. Analysis of Microbial Composition at the Phylum and Genus Levels
3.4.5. Species Difference Analysis
3.4.6. Functional Abundance Analysis of Gut Microbiota via KEGG Pathways
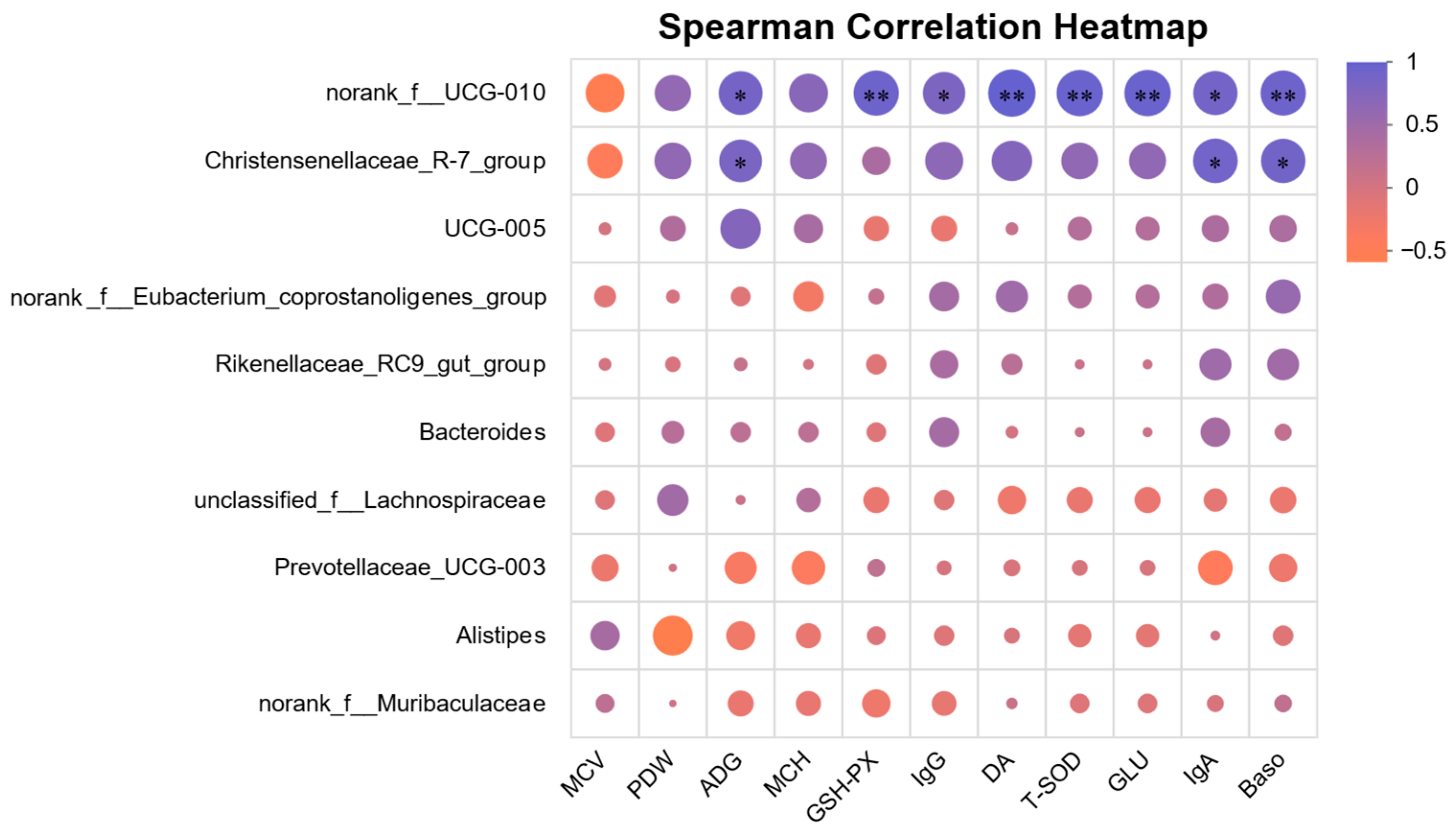
3.4.7. Association Analysis Between Gut Microbiota and Growth/Blood Parameters
4. Discussion
4.1. The Effects of Compound Essential Oils on the Growth Performance of Simmental Crossbred Cattle
4.2. Effects of Compound Essential Oils on Hematological Parameters in Simmental Crossbred Cattle
4.3. The Effect of Compound Essential Oils on Serum Parameters in Simmental Crossbred Cattle
4.4. The Impact of Compound Essential Oils on the Intestinal Microbiota of Simmental Crossbred Cattle
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). Food Balances. FAO Statistical Database. 2022. Available online: https://www.fao.org/faostat/zh/#data/FBS (accessed on 4 March 2025).
- Rich, K.M. What can Africa contribute to global meat demand? Opportunities and constraints. Outlook Agric. 2009, 38, 223–233. [Google Scholar] [CrossRef]
- Jouany, J.P.; Morgavi, D.P. Use of ‘natural’ products as alternatives to antibiotic feed additives in ruminant production. Animal 2007, 1, 1443–1466. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2023, 10, e22437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tasdemir, D.; Kaiser, M.; Demirci, B.; Demirci, F.; Baser, K.H.C. Antiprotozoal Activity of Turkish Origanum onites Essential Oil and Its Components. Molecules 2019, 24, 4421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, H.; Yang, M.; Li, Q.; Zhang, L.; Zhao, X. A comprehensive review of the main components of plant essential oils and the mechanisms responsible for the inhibitory effects on fungal growth and aflatoxin synthesis. Innov. Food Sci. Emerg. Technol. 2024, 96, 103747. [Google Scholar] [CrossRef]
- Cobellis, G.; Yu, Z.; Forte, C.; Acuti, G.; Trabalza-Marinucci, M. Dietary supplementation of Rosmarinus officinalis L. leaves in sheep affects the abundance of rumen methanogens and other microbial populations. J. Anim. Sci. Biotechnol. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives—Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nora, L.; Marcon, C.; Deolindo, G.L.; Signor, M.H.; Muniz, A.L.; Bajay, M.M.; Copetti, P.M.; Bissacotti, B.F.; Morsch, V.M.; da Silva, A.S. The Effects of a Blend of Essential Oils in the Milk of Suckling Calves on Performance, Immune and Antioxidant Systems, and Intestinal Microbiota. Animals 2024, 14, 3555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giacomelli, C.M.; Marchiori, M.S.; Nascimento, A.L.D.; de Vitt, M.G.; Molosse, V.L.; de Candido de Oliveira, F.; Wagner, R.; Milarch, C.F.; Vedovatto, M.; da Silva, A.S. Encapsulated pepper blend in the diet of confined Holstein bullocks: Effect on ruminal volatile fatty acid profiles, growth performance, and animal health. Trop. Anim. Health Prod. 2023, 55, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, X.; Li, X.; Chen, G.; Wang, C. Effect of Zanthoxylum bungeanum essential oil on rumen enzyme activity, microbiome, and metabolites in lambs. PLoS ONE 2022, 17, e0272310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, T.L.M.; Francis, B.B.G.; Ross, C.R.; Delver, J.J.; Francis, F.L.; Heldt, J.S.; Wall, E.H.; Rusche, W.C.; Smith, Z.K. Evaluation of a phytogenic blend fed with monensin on post-weaning growth performance, health, and sera metabolite responses during the initial 56 d feedlot receiving period in steer calves. Acta Agric. Scand. Sect. A—Anim. Sci. 2025, 74, 18–26. [Google Scholar] [CrossRef]
- He, Z.M.; Li, G.P.; Zhu, D.S. Mammalian experimental animals, sheep. In Laboratory Animals Management and Use Guidelines; Science Press: Beijing, China, 2016. [Google Scholar]
- Wu, J.; Bai, Y.; Lang, X.; Wang, C.; Shi, X.; Casper, D.P.; Zhang, L.; Liu, H.; Liu, T.; Gong, X.; et al. Dietary supplementation with oregano essential oil and monensin in combination is antagonistic to growth performance of yearling Holstein bulls. J. Dairy Sci. 2020, 103, 8119–8129. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, T.; Li, P.; Cheng, S.; Casper, D.P. Effects of Essential Oil and/or Encapsulated Butyrate on Fecal Microflora in Neonatal Holstein Calves. Animals 2023, 13, 3523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fugita, C.A.; do Prado, R.M.; Valero, M.V.; Bonafé, E.G.; Carvalho, C.B.; Guerrero, A.; Sañudo, C.; do Prado, I.N. Effect of the inclusion of natural additives on animal performance and meat quality of crossbred bulls (Angus × Nellore) finished in feedlot. Anim. Prod. Sci. 2017, 58, 2076–2083. [Google Scholar] [CrossRef]
- de Souza, K.A.; de Oliveira Monteschio, J.; Mottin, C.; Ramos, T.R.; de Moraes Pinto, L.A.; Eiras, C.E.; Guerrero, A.; do Prado, I.N. Effects of diet supplementation with clove and rosemary essential oils and protected oils (eugenol, thymol and vanillin) on animal performance, carcass characteristics, digestibility, and ingestive behavior activities for Nellore heifers finished in feedlot. Livest. Sci. 2019, 220, 190–195. [Google Scholar] [CrossRef]
- Su, M.; She, Y.; Deng, M.; Guo, Y.; Li, Y.; Liu, G.; Zhang, H.; Sun, B.; Liu, D. The Effect of Capsaicin on Growth Performance, Antioxidant Capacity, Immunity and Gut Micro-Organisms of Calves. Animals 2023, 13, 2309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, N.P.K.; Tran, K.N.; Nguyen, L.T.H.; Shin, H.-M.; Yang, I.-J. Effects of Essential Oils and Fragrant Compounds on Appetite: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mucha, W.; Witkowska, D. The Applicability of Essential Oils in Different Stages of Production of Animal-Based Foods. Molecules 2021, 26, 3798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalli, M.; Daoudi, N.E.; Abrigach, F.; Azizi, S.-E.; Bnouham, M.; Kim, B.; Gseyra, N. In vitro α-amylase and hemoglobin glycation inhibitory potential of Nigella sativa essential oil, and molecular docking studies of its principal components. Front. Pharmacol. 2022, 13, 1036129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Cao, P.; Zhu, T.; Wang, Y.; Wang, F.; Li, L.; Liu, X.; Zhang, Y. Combination of age-adjusted d-dimer, platelet distribution width and other factors predict preoperative deep venous thrombosis in elderly patients with femoral neck fracture. BMC Surg. 2024, 24, 426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, C.; Gao, J.; Wei, T.; Shen, W. Angiotensin II-Induced Erythrocyte Senescence Contributes to Oxidative Stress. Rejuvenation Res. 2022, 25, 30–38. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wang, Y.; Wang, R.; Hao, X.; Hu, Y.; Guo, T.; Zhang, J.; Wang, W.; Shi, X.; Han, S.; et al. Effects of a blend of cinnamaldehyde, eugenol and capsicum oleoresin (CEC) on growth performance, nutrient digestibility, immune response and antioxidant status of growing ewes. Livest. Sci. 2020, 234, 103982. [Google Scholar] [CrossRef]
- Karasuyama, H.; Mukai, K.; Obata, K.; Tsujimura, Y.; Wada, T. Nonredundant roles of basophils in immunity. Annu. Rev. Immunol. 2011, 29, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Zeng, H.; Han, Z. Effects of different combinations of antibacterial compound supplements in calf pellets on growth performance, health, blood parameters, and rumen microbiome of dairy calves. Front. Vet. Sci. 2024, 11, 1376758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Wu, Z.; Yu, B.; Chen, J.; Yang, C.; Guo, Y.; Sun, B. Dietary Capsaicin Supplementation Mitigates Calving-Induced Stress and Enhances Antioxidant Capacity, Immune Function, and Gut Microbiota in Periparturient Dairy Cows. Antioxidants 2024, 14, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Dong, B.; Friesen, M.; Liu, S.; Zhu, C.; Yang, C. Capsaicin Attenuates Lipopolysaccharide-Induced Inflammation and Barrier Dysfunction in Intestinal Porcine Epithelial Cell Line-J2. Front. Physiol. 2021, 12, 715469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasierski, M.; Szulczyk, B. Beneficial Effects of Capsaicin in Disorders of the Central Nervous System. Molecules 2022, 27, 2484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Ren, L.; Zhong, X.; Ding, Y.; Liu, T.; Liu, Z.; Yang, X.; Cui, L.; Yang, L.; Fan, Y.; et al. D2-Like Receptors Mediate Dopamine-Inhibited Insulin Secretion via Ion Channels in Rat Pancreatic β-Cells. Front. Endocrinol. 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ge, C.; Luo, X.; Lv, Y.; Wu, L.; Hu, Z.; Huang, W.; Zhan, S.; Shen, X.; Hui, C.; Yu, D.; et al. Essential oils ameliorate the intestinal damages induced by nonylphenol exposure by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Chemosphere 2024, 362, 142571. [Google Scholar] [CrossRef] [PubMed]
- da Silva Pereira, M.; Alcantara, L.M.; de Freitas, L.M.; de Oliveira Ferreira, A.L.; Leal, P.L. Microbial Rumen proteome analysis suggests Firmicutes and Bacteroidetes as key producers of lignocellulolytic enzymes and carbohydrate-binding modules. Braz. J. Microbiol, 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gavande, P.V.; Basak, A.; Sen, S.; Lepcha, K.; Murmu, N.; Rai, V.; Mazumdar, D.; Saha, S.P.; Das, V.; Ghosh, S. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 2021, 11, 3032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szeligowska, N.; Cholewińska, P.; Czyż, K.; Wojnarowski, K.; Janczak, M. Inter and intraspecies comparison of the level of selected bacterial phyla in in cattle and sheep based on feces. BMC Vet. Res. 2021, 17, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin Can Alleviate Metabolism Disorders in ob/ob Mice by Partially Restoring Leptin-related Pathways Mediated by Gut Microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhao, H.; Li, Q.; Tsechoe, D.; Yuan, H.; Su, G.; Yang, J. Environmental factors influence yak milk composition by modulating short-chain fatty acid metabolism in intestinal microorganisms. LWT 2022, 163, 113608. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277, Erratum in Front. Immunol. 2019, 10, 1486. https://doi.org/10.3389/fimmu.2019.01486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Ingredient Composition | Content | Nutrient Level | Content |
|---|---|---|---|
| Corn | 33.0 | DM | 63.28 |
| Soybean meal | 7.30 | CP | 13.30 |
| Rapeseed meal | 4.30 | EE | 3.20 |
| Wheat bran | 3.10 | NDF | 34.40 |
| Silage corn | 20.80 | ADF | 21.30 |
| Corn stover | 13.40 | Ca | 0.80 |
| Wheat straw | 8.90 | P | 0.41 |
| Alfalfa hay | 4.50 | ||
| NaHCO3 | 0.30 | ||
| NaCl | 0.30 | ||
| 5%Premix 1 | 0.60 |
| Index | Treatment | Content | |
|---|---|---|---|
| CON | EO | ||
| Initial weight | 442.00 ± 73.10 | 443.70 ± 75.83 | 0.960 |
| Final weight | 515.10 ± 73.38 | 538.70 ± 90.51 | 0.530 |
| Average daily gain | 1.74 ± 0.27 b | 2.26 ± 0.55 a | 0.020 |
| Index | Treatment | p-Value | ||
|---|---|---|---|---|
| CON | EO | |||
| WBC (109/L) | 0 d | 7.43 ± 0.86 | 7.27 ± 0.96 | 0.762 |
| 42 d | 7.76 ± 1.04 | 7.76 ± 0.94 | 0.998 | |
| NEU (109/L) | 0 d | 3.25 ± 0.65 | 3.23 ± 0.52 | 0.970 |
| 42 d | 3.05 ± 0.62 | 3.89 ± 1.12 | 0.135 | |
| LYM (109/L) | 0 d | 4.02 ± 0.58 | 3.98 ± 0.58 | 0.908 |
| 42 d | 4.56 ± 1.10 | 4.41 ± 1.18 | 0.832 | |
| MONO (109/L) | 0 d | 0.44 ± 0.09 | 0.44 ± 0.11 | 0.978 |
| 42 d | 0.27 ± 0.04 | 0.30 ± 0.06 | 0.396 | |
| EOS (109/L) | 0 d | 0.41 ± 0.10 | 0.43 ± 0.12 | 0.766 |
| 42 d | 0.15 ± 0.03 | 0.19 ± 0.06 | 0.187 | |
| BASO (109/L) | 0 d | 0.07 ± 0.02 | 0.06 ± 0.04 | 0.858 |
| 42 d | 0.06 ± 0.02 b | 0.10 ± 0.02 a | 0.009 | |
| NEU% | 0 d | 38.60 ± 8.23 | 39.01 ± 5.81 | 0.923 |
| 42 d | 39.24 ± 7.03 | 40.50 ± 8.09 | 0.779 | |
| LYM% | 0 d | 48.16 ± 4.57 | 49.70 ± 9.72 | 0.732 |
| 42 d | 54.91 ± 7.39 | 55.03 ± 15.80 | 0.987 | |
| MONO% | 0 d | 2.77 ± 0.82 | 2.94 ± 0.40 | 0.659 |
| 42 d | 3.54 ± 0.60 | 3.20 ± 0.65 | 0.371 | |
| EOS% | 0 d | 3.16 ± 0.36 | 2.82 ± 0.75 | 0.340 |
| 42 d | 1.88 ± 0.51 | 3.22 ± 1.52 | 0.067 | |
| BASO% | 0 d | 0.69 ± 0.16 | 0.70 ± 0.20 | 0.915 |
| 42 d | 0.74 ± 0.24 | 1.05 ± 0.42 | 0.145 | |
| RBC (109/L) | 0 d | 7.81 ± 1.07 | 7.25 ± 0.82 | 0.336 |
| 42 d | 7.47 ± 0.82 | 8.02 ± 1.07 | 0.339 | |
| HGB (g/L) | 0 d | 135.87 ± 13.33 | 138.21 ± 11.44 | 0.751 |
| 42 d | 141.44 ± 11.42 | 138.67 ± 14.64 | 0.722 | |
| HCT (%) | 0 d | 35.94 ± 3.74 | 37.13 ± 4.30 | 0.618 |
| 42 d | 31.31 ± 2.05 | 30.02 ± 3.00 | 0.405 | |
| MCV (fL) | 0 d | 40.56 ± 1.89 | 43.51 ± 3.59 | 0.105 |
| 42 d | 42.30 ± 3.39 a | 37.58 ± 2.05 b | 0.015 | |
| MCH (pg) | 0 d | 18.59 ± 1.44 | 17.86 ± 1.00 | 0.335 |
| 42 d | 17.35 ± 1.09 b | 19.06 ± 1.49 a | 0.047 | |
| MCHC (g/L) | 0 d | 460.94 ± 5.73 | 458.46 ± 8.40 | 0.563 |
| 42 d | 451.22 ± 7.35 | 462.17 ± 9.77 | 0.053 | |
| RDW-CV (%) | 0 d | 20.73 ± 0.71 | 21.15 ± 0.77 | 0.350 |
| 42 d | 20.60 ± 0.58 | 20.27 ± 0.64 | 0.367 | |
| RDW-SD (fL) | 0 d | 29.81 ± 3.38 | 31.80 ± 2.01 | 0.245 |
| 42 d | 30.63 ± 2.25 | 28.65 ± 2.69 | 0.197 | |
| PLT (109/L) | 0 d | 385.13 ± 48.39 | 419.21 ± 47.14 | 0.245 |
| 42 d | 405.56 ± 53.88 | 367.83 ± 52.04 | 0.246 | |
| MPV (fL) | 0 d | 6.08 ± 0.04 | 6.25 ± 0.27 | 0.149 |
| 42 d | 5.76 ± 0.24 | 5.58 ± 0.20 | 0.204 | |
| PDW (fL) | 0 d | 15.51 ± 0.29 | 15.23 ± 0.30 | 0.130 |
| 42 d | 14.73 ± 0.22 b | 15.02 ± 0.20 a | 0.039 | |
| PCT (%) | 0 d | 0.97 ± 0.19 | 1.22 ± 0.30 | 0.123 |
| 42 d | 0.23 ± 0.03 | 0.21 ± 0.04 | 0.146 | |
| Index | Treatment | p-Value | ||
|---|---|---|---|---|
| CON | EO | |||
| Blood immune parameters | ||||
| IgA (μg/mL) | 0 d | 1117.06 ± 13.31 | 1123.52 ± 13.78 | 0.428 |
| 42 d | 980.95 ± 142.18 b | 1296.01 ± 306.46 a | 0.045 | |
| IgG (mg/mL) | 0 d | 1.24 ± 0.29 | 1.24 ± 0.23 | 0.974 |
| 42 d | 2.32 ± 0.48 b | 3.61 ± 1.19 a | 0.033 | |
| IgM (μg/mL) | 0 d | 708.76 ± 190.68 | 693.63 ± 81.41 | 0.862 |
| 42 d | 753.17 ± 373.75 | 782.59 ± 384.60 | 0.896 | |
| IL-6 (pg/mL) | 0 d | 99.63 ± 9.55 | 87.90 ± 17.40 | 0.178 |
| 42 d | 78.51 ± 26.49 | 74.79 ± 32.13 | 0.831 | |
| IFN-γ (pg/mL) | 0 d | 301.66 ± 24.90 | 291.42 ± 37.16 | 0.785 |
| 42 d | 394.01 ± 139.98 | 395.32 ± 166.71 | 0.989 | |
| TNF-α (pg/mL) | 0 d | 81.23 ± 8.82 | 77.41 ± 18.15 | 0.652 |
| 42 d | 88.08 ± 20.83 | 67.62 ± 27.91 | 0.180 | |
| Blood antioxidant indicators | ||||
| T-SOD (U/mL) | 0 d | 190.25 ± 11.04 | 189.57 ± 19.86 | 0.943 |
| 42 d | 162.17 ± 19.97 b | 187.22 ± 15.33 a | 0.035 | |
| T-AOC (U/mL) | 0 d | 5.53 ± 2.18 | 4.98 ± 1.45 | 0.618 |
| 42 d | 4.63 ± 3.23 | 5.18 ± 3.49 | 0.781 | |
| GSH-PX (U/mL) | 0 d | 108.30 ± 23.66 | 112.45 ± 17.67 | 0.738 |
| 42 d | 111.41 ± 9.02 b | 128.54 ± 15.03 a | 0.038 | |
| MDA (nmol/mL) | 0 d | 2.51 ± 0.46 | 2.73 ± 0.27 | 0.323 |
| 42 d | 3.41 ± 0.30 | 3.11 ± 0.21 | 0.083 | |
| Biochemical index | ||||
| BUN (mmol/L) | 0 d | 2.77 ± 1.00 | 2.13 ± 0.64 | 0.213 |
| 42 d | 3.16 ± 0.74 | 3.04 ± 0.68 | 0.768 | |
| GLU (mmol/L) | 0 d | 3.64 ± 0.39 | 3.98 ± 0.59 | 0.275 |
| 42 d | 2.18 ± 0.48 B | 3.82 ± 0.67 A | 0.001 | |
| CHO (mmol/L) | 0 d | 2.53 ± 0.17 | 2.73 ± 0.65 | 0.503 |
| 42 d | 2.90 ± 0.74 | 2.69 ± 0.34 | 0.542 | |
| TG (mmol/L) | 0 d | 0.16 ± 0.05 | 0.14 ± 0.04 | 0.542 |
| 42 d | 0.16 ± 0.04 | 0.15 ± 0.04 | 0.654 | |
| ACTH (pg/mL) | 0 d | 28.19 ± 12.12 | 29.68 ± 11.08 | 0.828 |
| 42 d | 16.05 ± 4.07 | 27.46 ± 19.80 | 0.197 | |
| GH (ng/mL) | 0 d | 5.13 ± 1.70 | 4.39 ± 2.04 | 0.511 |
| 42 d | 6.16 ± 2.46 | 8.25 ± 3.19 | 0.232 | |
| IGF-1 (ng/mL) | 0 d | 112.16 ± 39.72 | 105.75 ± 25.84 | 0.747 |
| 42 d | 71.76 ± 26.45 | 107.73 ± 57.27 | 0.193 | |
| Gas (pg/mL) | 0 d | 102.29 ± 40.74 | 106.77 ± 25.59 | 0.824 |
| 42 d | 120.57 ± 34.21 | 122.63 ± 63.15 | 0.954 | |
| CCK (ng/mL) | 0 d | 1366.14 ± 207.48 | 1378.08 ± 192.49 | 0.920 |
| 42 d | 1241.35 ± 481.71 | 1420.18 ± 443.91 | 0.519 | |
| DA (pg/mL) | 0 d | 240.73 ± 25.94 | 258.62 ± 10.18 | 0.147 |
| 42 d | 212.97 ± 109.41 b | 374.23 ± 93.65 a | 0.021 | |
| # | Df | SumsOfSqs | MeanSqs | F.Model | R2 | Pr (>F) |
|---|---|---|---|---|---|---|
| group_factor | 1 | 0.02772 | 0.02772 | 4.06921 | 0.33715 | 0.034 |
| Residuals | 8 | 0.05450 | 0.00681 | - | 0.66284 | - |
| Total | 9 | 0.08223 | - | - | 1 | - |
| Index | Treatment | p-Value | |
|---|---|---|---|
| CON | EO | ||
| gate level | |||
| p__Firmicutes | 49.120 ± 6.830 | 58.490 ± 6.684 | 0.144 |
| p__Bacteroidota | 45.080 ± 5.088 a | 35.760 ± 4.946 b | 0.037 |
| p__Spirochaetota | 3.960 ± 6.318 | 3.259 ± 5.995 | 0.531 |
| p__Patescibacteria | 1.174 ± 0.353 | 1.701 ± 0.538 | 0.144 |
| p__Verrucomicrobiota | 0.186 ± 0.111 | 0.363 ± 0.417 | 0.531 |
| p__Proteobacteria | 0.235 ± 0.215 | 0.196 ± 0.145 | 0.999 |
| p__Cyanobacteria | 0.086 ± 0.068 | 0.117 ± 0.061 | 0.463 |
| p__unclassified_k__norank_d__Bacteria | 0.082 ± 0.033 | 0.069 ± 0.031 | 0.674 |
| p__Actinobacteriota | 0.070 ± 0.065 | 0.049 ± 0.034 | 0.600 |
| p__Deinococcota | 0.003 ± 0.005 | 0.000 ± 0.000 | 0.180 |
| genus level | |||
| g__Rikenellaceae_RC9_gut_group | 18.440 ± 8.036 | 18.220 ± 6.025 | 0.835 |
| g__UCG-005 | 15.460 ± 5.113 | 17.520 ± 2.661 | 0.531 |
| g__unclassified_f__Lachnospiraceae | 7.276 ± 2.412 | 8.531 ± 5.884 | 0.835 |
| g__Bacteroides | 5.915 ± 6.554 | 4.078 ± 1.154 | 0.835 |
| g__norank_f__UCG-010 | 2.803 ± 0.716 b | 4.613 ± 1.134 a | 0.012 |
| g__Treponema | 3.960 ± 6.318 | 3.259 ± 5.995 | 0.531 |
| g__Alistipes | 4.098 ± 1.315 | 3.041 ± 1.111 | 0.404 |
| g__Prevotellaceae_UCG-003 | 4.913 ± 5.919 | 1.486 ± 0.955 | 0.403 |
| g__norank_f__Muribaculaceae | 3.096 ± 1.060 | 3.213 ± 1.144 | 0.834 |
| g__norank_f__Eubacterium_coprostanoligenes_group | 2.799 ± 1.832 | 3.487 ± 1.901 | 0.676 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, T.; Wu, J.; Cheng, Y.; Ma, Y.; Chen, W.; Chen, H.; Liu, Y.; Wang, Y. Regulatory Effects of Cinnamon–Pepper–Chili Essential Oil Complex on Growth Performance, Immune Function, Complete Blood Count, and Intestinal Microbiota in Simmental CrossBred Cattle During the Late Fattening Stage. Fermentation 2025, 11, 303. https://doi.org/10.3390/fermentation11060303
Zhang T, Liu T, Wu J, Cheng Y, Ma Y, Chen W, Chen H, Liu Y, Wang Y. Regulatory Effects of Cinnamon–Pepper–Chili Essential Oil Complex on Growth Performance, Immune Function, Complete Blood Count, and Intestinal Microbiota in Simmental CrossBred Cattle During the Late Fattening Stage. Fermentation. 2025; 11(6):303. https://doi.org/10.3390/fermentation11060303
Chicago/Turabian StyleZhang, Tao, Ting Liu, Jianping Wu, Yining Cheng, Yannan Ma, Wen Chen, Huan Chen, Yunyun Liu, and Yunbo Wang. 2025. "Regulatory Effects of Cinnamon–Pepper–Chili Essential Oil Complex on Growth Performance, Immune Function, Complete Blood Count, and Intestinal Microbiota in Simmental CrossBred Cattle During the Late Fattening Stage" Fermentation 11, no. 6: 303. https://doi.org/10.3390/fermentation11060303
APA StyleZhang, T., Liu, T., Wu, J., Cheng, Y., Ma, Y., Chen, W., Chen, H., Liu, Y., & Wang, Y. (2025). Regulatory Effects of Cinnamon–Pepper–Chili Essential Oil Complex on Growth Performance, Immune Function, Complete Blood Count, and Intestinal Microbiota in Simmental CrossBred Cattle During the Late Fattening Stage. Fermentation, 11(6), 303. https://doi.org/10.3390/fermentation11060303






