Determination of the Optimal Biotechnological Parameters for Industrial Production of Protein Hydrolysates for Animal Feed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extruded Soybean Meal (ESBM)
2.2. The Enzyme Complex
2.3. Obtaining Hydrolysate Samples Using Various Technological Modes
2.4. Selection and Application of Preservatives
2.5. Free Amino Acid Content Analysis
2.6. Analysis of the Lysine and Methionine Contents in the Composition of the Obtained Hydrolysate
2.7. Statistical Analysis
3. Results
3.1. Comparison of the Hydrolysis Parameter Conditions
3.2. Evaluation of Preservation Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESBM | Extruded soybean meal |
| GOST | Government Standard |
| CE | Capillary electrophoresis |
References
- Dezhina, I.G.; Arutyunyan, A.G. Development of Russian Biotechnologies for the Cattle Breeding (Evaluation Based on Patent Analysis). ECO 2023, 7, 149–171. [Google Scholar] [CrossRef]
- Vasilevich, F.I.; Bachinskaya, V.M.; Deltsov, A.A. The effect of feed additives based on protein hydrolysates on the quality and safety of quail meat. Vet. Med. 2019, 10, 51–54. [Google Scholar] [CrossRef]
- Vasilevich, F.I.; Bachinskaya, V.M.; Deltsov, A.A. The effectiveness of the use of protein hydrolysates in poultry. Vet. Med. 2019, 8, 8–11. [Google Scholar] [CrossRef]
- Watanabe, T. Strategies for further development of aquatic feeds. Fish. Sci. 2002, 68, 242–252. [Google Scholar] [CrossRef]
- Mezenova, O.Y.; Pyanov, D.S.; Agafonova, S.V.; Mezenova, N.Y.; Volkov, V.V. Designing balanced feeds for industrial aquaculture using protein hydrolysates of fish by–products. Fisheries 2021, 4, 81–88. [Google Scholar] [CrossRef]
- Bachinskaya, V.M.; Deltsov, A.A.; Gonchar, D.V. The use of protein hydrolysates in rabbit breeding and their effect on meat quality and safety. Russ. J. Probl. Vet. Sanit. Hyg. Ecol. 2021, 1, 26–29. [Google Scholar] [CrossRef]
- Shurbina, M.Y.; Valeeva, R.T. Hydrolysates of waste from processing of vegetable raw materials for the production of feed yeast. Probl. Dev. Mod. Soc. 2023, 1, 440–442. [Google Scholar]
- Muranova, T.A.; Zinchenko, D.V.; Miroshnikov, A.I. Hydrolysates of soy proteins for starter feeds of aquaculture: Behavior of proteins during fermentolysis, compositional analysis of hydrolysates. Bioorganic Chem. 2019, 4, 380–390. [Google Scholar] [CrossRef]
- Boltovsky, V.S. Directions for improving the production processes of feed yeast by processing hydrolysates obtained by acid hydrolysis of plant raw materials. Proc. BSTU Ser. 2 Chem. Technol. Biotechnol. Geoecology 2021, 2, 13–18. [Google Scholar] [CrossRef]
- Komleva, V.S.; Kuchin, N.N. Canning of flattened corn grain. Bull. NGIEI 2022, 12, 7–17. [Google Scholar] [CrossRef]
- Starberg, E.S.; Penzin, A.; Starberg, M.A.; Borodin, A. Technology for obtaining enrichment additive on soybean raw materials. Food Process. Ind. 2025, 1, 52653. [Google Scholar] [CrossRef]
- Shulgin, I.V. Prospects of bio-processing carbohydrates contained in by-products of soy processing into products with increased added value. Technol. Prod. Healthy Nutr. 2021, 2, 825–830. [Google Scholar]
- Pakhomov, V.I.; Braginets, S.; Bakhchevnikov, O. Technological module for the production of feed additives from green plant matter. J. VNIIMZH 2019, 1, 82–85. [Google Scholar] [CrossRef]
- Shaaban, M.; Belyshkina, M.E. Non-traditional sources of protein in animal nutrition. Agrar. Sci. 2025, 392, 69–75. [Google Scholar] [CrossRef]
- Hou, Y.; Zhenlong, W.; Zhaolai, D.; Genhu, W.; Guoyao, W. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. Bioact. Pept. Food 2022, 1, 209–232. [Google Scholar] [CrossRef]
- Mutlu, R.N.; Jayaraman, K.; Kıymaz, T.B.; Güleryüz, D.; Böncü, E.; Eroğlu, E.; Gökalp, İ. Optimization of aluminum hydrolysis reactions and reactor design for continuous hydrogen production using aluminum wire feeding. Int. J. Hydrogen Energy 2024, 52, 1390–1403. [Google Scholar] [CrossRef]
- Hien, B.T.T.; Diem, P.T.; Tung, L.A.; Huong, T.T.; Hoang, N.H.; Bat, N.K.; Nghia, N.V. Optimizing enzymatic hydrolysis for feed production from catfish by-products. Foods Raw Mater. 2022, 1, 19–26. [Google Scholar] [CrossRef]
- Theodosi Palimeri, D. Hydrolysis Optimization of By-Products from the Potato Processing Industry and Biomethane Production from Starch Hydrolysates. Sustainability 2023, 20, 14860. [Google Scholar] [CrossRef]
- Pratiwy, F.M.; Maulida, Y. Hydrolysis Enzyme of Alternative Ingredients for Fish Feed: A Review. Asian J. Fish. Aquat. Res. 2022, 6, 95–101. [Google Scholar] [CrossRef]
- Nebi, H.B.; Lalisa, D. Complete Animal Feed Production and Method of Feed Conservation. Int. J. Agric. Agribus. 2024, 1, 133–141. [Google Scholar]
- Min, J.H.; Lee, Y.J.; Kang, H.J.; Moon, N.R.; Park, Y.K.; Joo, S.T.; Jung, Y.H. Characterization of Yeast Protein Hydrolysate for Potential Application as afeed additive. Food Sci. Anim. Resour. 2024, 44, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M. Hydrolyzed proteins: Availability, bioactivity, safety, applications in feed production (review). Anim. Husb. Fodd. Prod. 2024, 107, 309–323. [Google Scholar] [CrossRef]
- Singh, R.; Jain, R.; Soni, P.; Santos-Villalobos, S.; Chattaraj, S.; Roy, D.; Gaur, A. Graphing the green route: Enzymatic hydrolysis in sustainable decomposition. Curr. Res. Microb. Sci. 2024, 1, 100281. [Google Scholar] [CrossRef] [PubMed]
- Hartoyo, B.; Widyastuti, T.; Rahayu, S.; Santosa, R.S. Study of protein hydrolysis and peptide antioxidants activity of chicken slaughterhouse waste and its potential for feed additives. Anim. Prod. 2022, 24, 97–103. [Google Scholar] [CrossRef]
- Klein, T.; Eckhard, U.; Dufour, A.; Solis, N.; Overall, C.M. Proteolytic Cleavage Mechanisms, Function, and «Omic» Approaches for a Near-Ubiquitous Posttranslational Modification. Chem. Rev. 2018, 118, 1137–1168. [Google Scholar] [CrossRef]
- Bento de Carvalho, T.; Silva, B.N.; Tomé, E.; Teixeira, P. Preventing fungal spoilage from raw materials to final product: Innovative preservation techniques for fruit fillings. Foods 2024, 13, 2669. [Google Scholar] [CrossRef]
- Harvey, H.J.; Hendry, A.C.; Archer, D.B.; Avery, S.V. Evaluating the potential of natural product combinations with sorbic acid for improving preservative action against food-spoilage yeasts. Fungal Biol. 2023, 127, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Stingelin, G.M.; Scherer, R.S.; Machado, A.C.; Piva, A.; Grilli, E.; Penha Filho, R.C. The use of thymol, carvacrol and sorbic acid in microencapsules to control Salmonella Heidelberg, S. Minnesota and S. Typhimurium in broilers. Front. Vet. Sci. 2022, 9, 1046395. [Google Scholar] [CrossRef]
- Kukhlevskaya, Y. Feed preservatives: The key to agricultural profitability. Eff. Anim. Husb. 2024, 3, 47–54. [Google Scholar]
- GOST 32195-2013 (ISO 13903:2005); Feed, Compound Feed—Method for Determining the Content of Amino Acids. Standardinform: Moscow, Russia, 2013.
- Ali, Z.; Bhaskar, S.B. Basic statistical tools in research and data analysis. Indian J. Anaesth. 2016, 60, 662–669. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, Z.; Fu, W.; Pan, M.; Ren, X.; Zhang, X.; Rao, Z. Research and Prospects of Enzymatic Hydrolysis and Microbial Fermentation Technologies in Protein Raw Materials for Aquatic Feed. Fermentation 2024, 10, 648. [Google Scholar] [CrossRef]
- Kulig, D.; Krol-Kilinska, Z.; Bobak, L.; Zarowska, B.; Jarmoluk, A.; Zimoch-Korzycka, A. Functional properties of chitosan oligomers obtained by enzymatic hydrolysis. Polymers 2023, 15, 3801. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.S.; Singh, P.N.; Singh, S.K. Fungal alkaline proteases and their potential applications in different industries. Front. Microbiol. 2023, 14, 1138401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, X.; Ding, Y.; Zheng, K.; Wang, K.; Lu, F.; Liu, Y. Engineering a Protease K for Efficient Degradation of Wool Scale Layer Using a Substrate Pocket Modification. Fermentation 2025, 11, 51. [Google Scholar] [CrossRef]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef]
- Samosyuk, V.V.; Shakhov, V.A. Preparation of nano feed mixture for intensive fattening. Mach. Technol. Livest. Prod. 2023, 3, 45–49. [Google Scholar] [CrossRef]
- Bryukhanov, A.Y.; Popov, V.D.; Vasilev, E.V.; Shalavina, E.V.; Uvarov, R.A. Analysis and solutions to environmental problems in livestock farming. Agric. Mach. Technol. 2021, 15, 48–55. [Google Scholar] [CrossRef]
- Sokolov, D.V.; Bolkhonov, B.A.; Zhamsaranova, S.D.; Lebedeva, S.N.; Bazhenova, B.A. Hydrolysis of Soy Protein. Food Processing. Tech. Technol. 2023, 53, 86–96. (In Russian) [Google Scholar] [CrossRef]
- Liewen, M.B.; Marth, E.H. Growth and inhibition of microorganisms in the presence of sorbic acid: A review. J. Food Prot. 1985, 48, 364–375. [Google Scholar] [CrossRef]
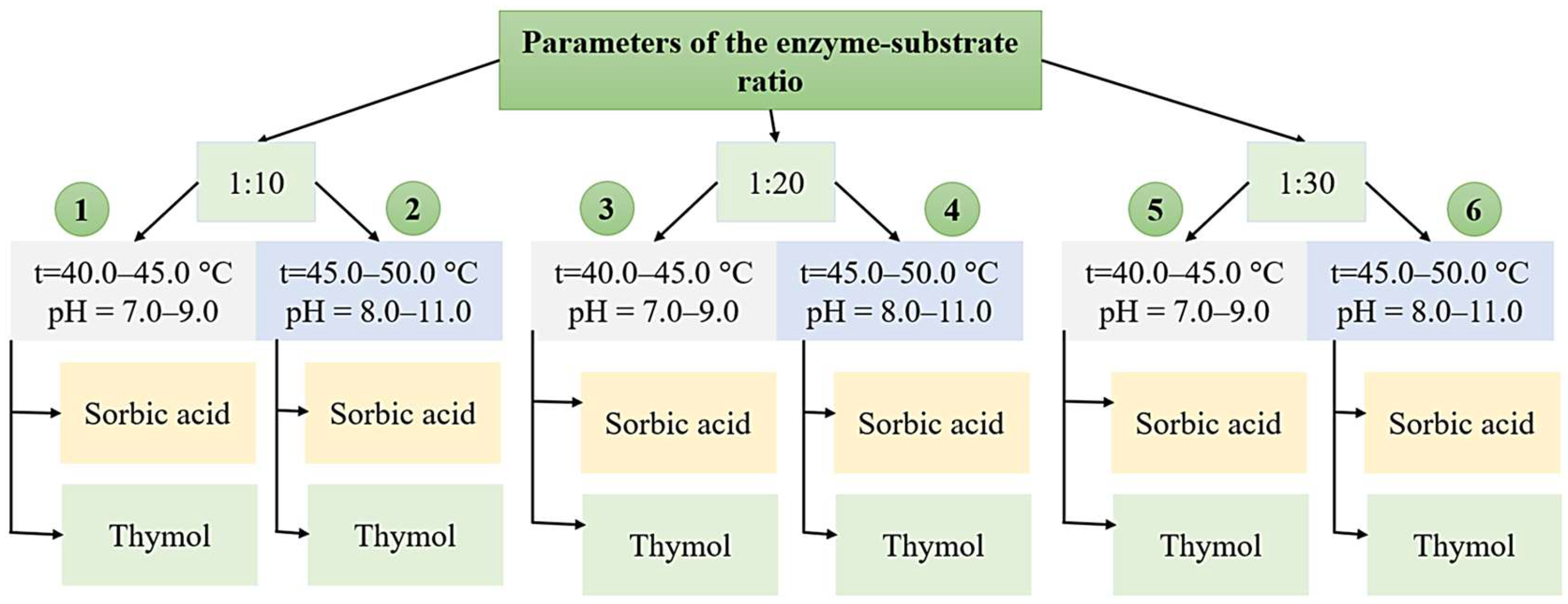
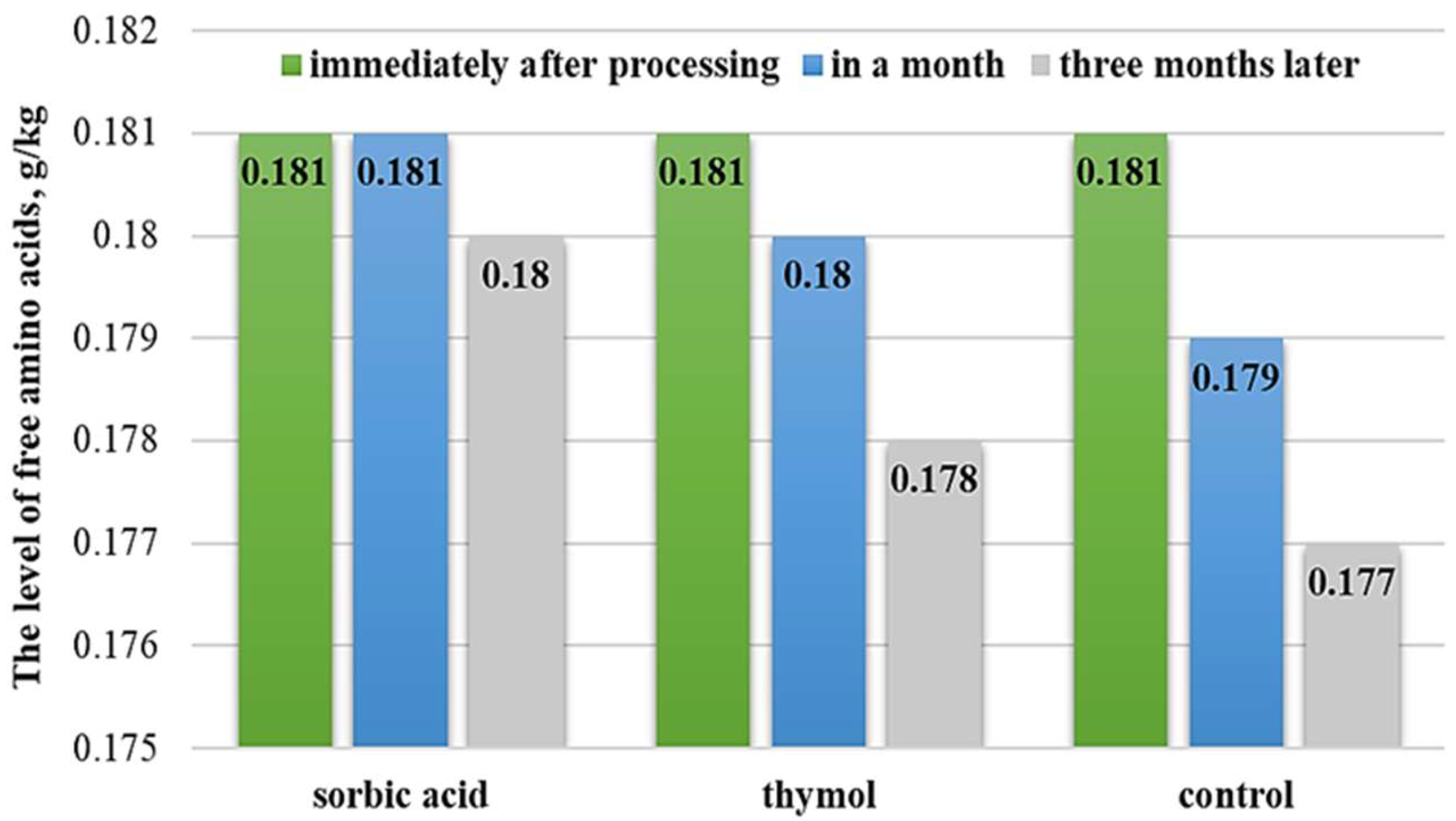
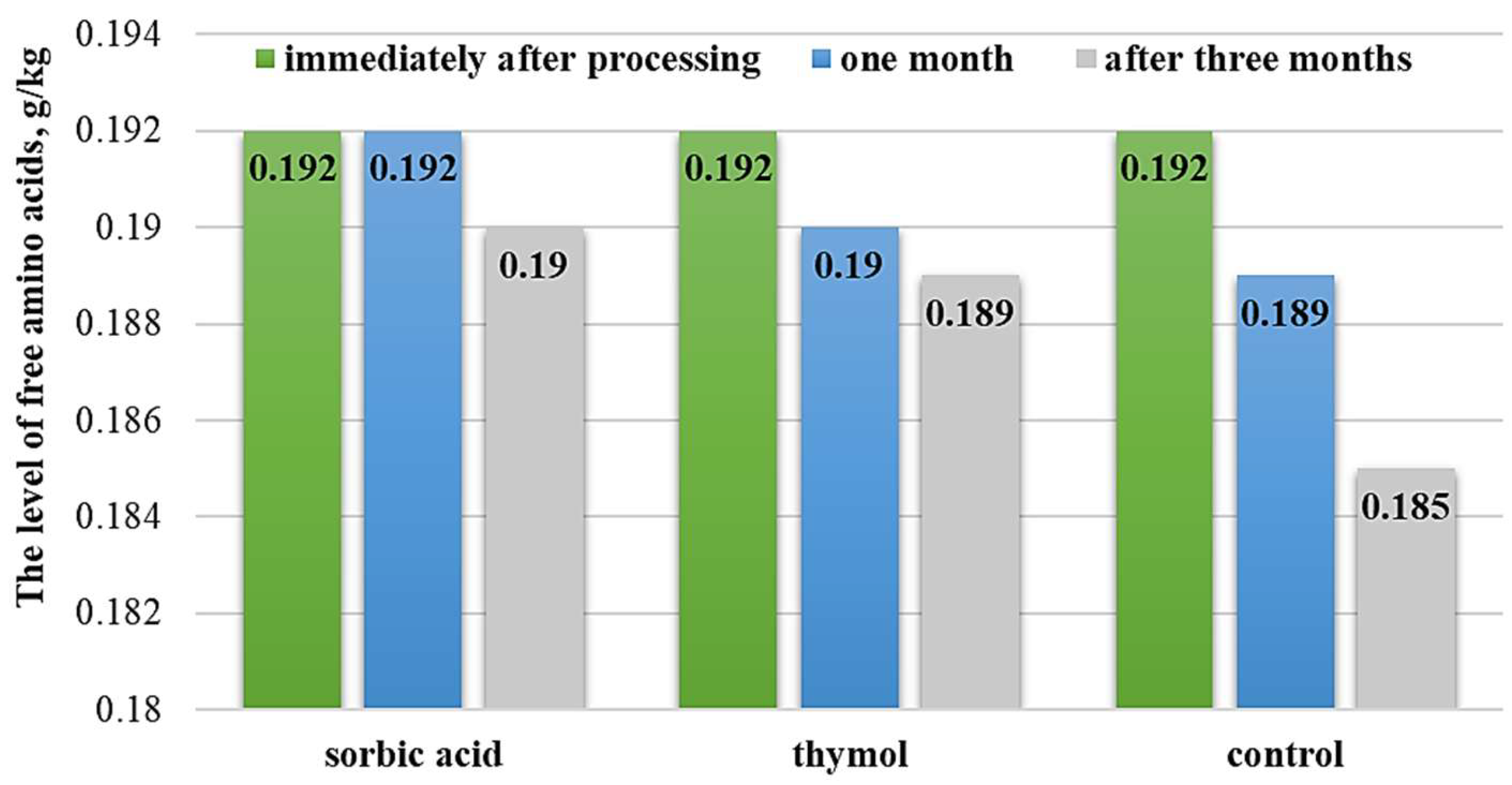
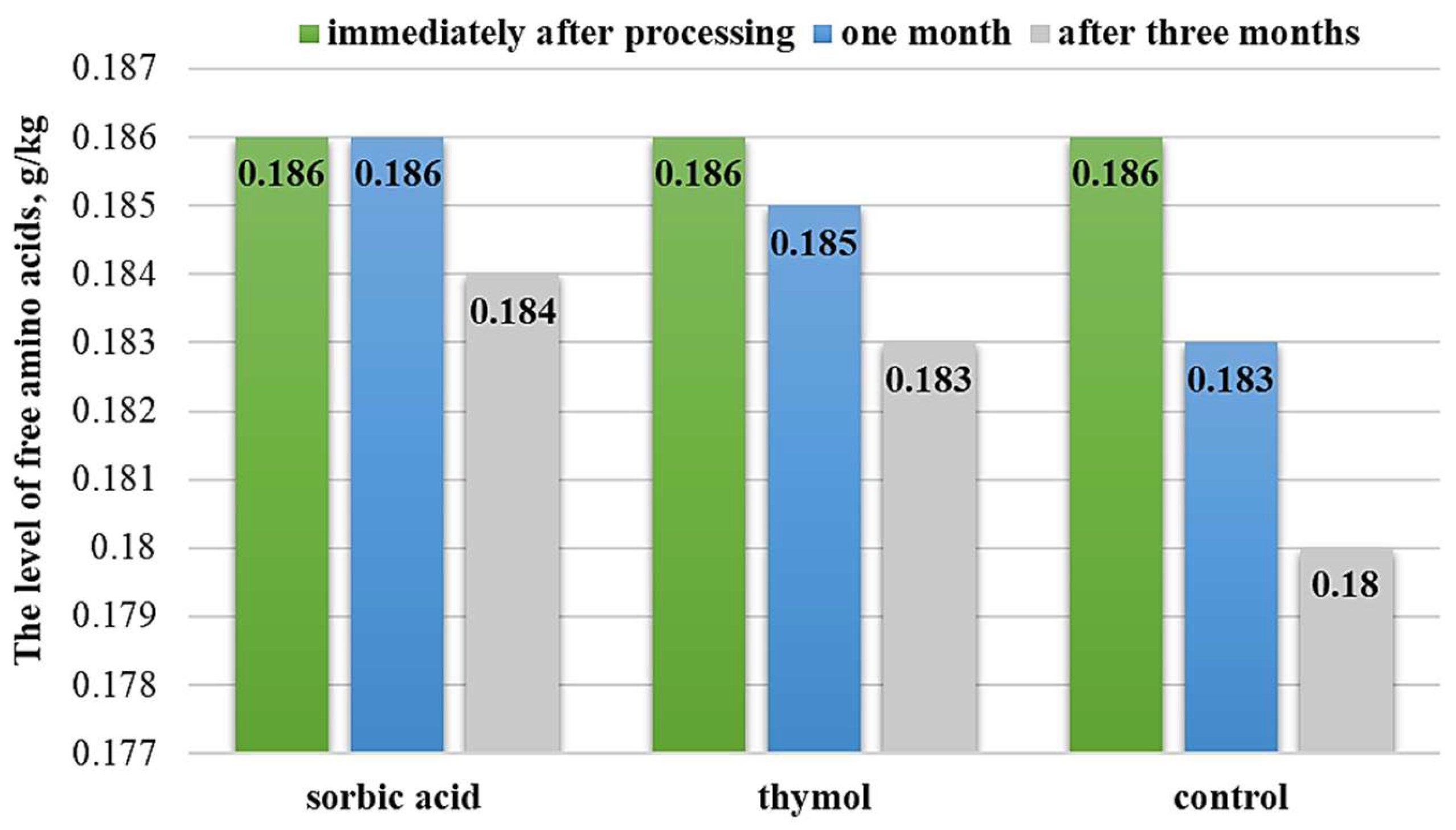
| Samples | Total Free Amino Acid Content, g/kg | % to the Initial Value | Amine Nitrogen Content, g/kg | % to the Initial Value | |
|---|---|---|---|---|---|
| The Initial Value | 0.120 ± 0.14 | 2.4 ± 0.19 | |||
| 1:10 | 1 (t = 40.0–45.0 °C; pH = 7.0–9.0) | 0.179 ± 0.41 * | 54.2 ± 0.32 | 2.4 ± 0.14 | 0 |
| 2 (t = 45.0–50.0 °C; pH = 8.0–11.0) | 0.182 ± 0.47 ** | 64.2 ± 0.61 | 2.4 ± 0.17 | 0 | |
| 1:20 | 3 (t = 40.0–45.0 °C; pH = 7.0–9.0) | 0.189 ± 0.37 ** | 57.5 ± 0.42 | 2.5 ± 0.16 | 8.0 ± 0.21 |
| 4 (t = 45.0–50.0 °C; pH = 8.0–11.0) | 0.195 ± 0.51 ** | 61.5 ± 0.57 | 2.5 ± 0.47 | 8.0 ± 0.23 | |
| 1:30 | 5 (t = 40.0–45.0 °C; pH = 7.0–9.0) | 0.185 ± 0.27 ** | 54.2 ± 0.33 | 2.7 ± 0.22 * | 12.5 ± 0.77 |
| 6 (t = 45.0–50.0 °C; pH = 8.0–11.0) | 0.187 ± 0.44 ** | 64.2 ± 0.47 | 2.8 ± 0.37 * | 14.3 ± 0.51 | |
| Samples | Lysine, g/kg | % to the Initial Value | Methionine, g/kg | % to the Initial Value | |
|---|---|---|---|---|---|
| The Initial Value | 0.042 ± 0.04 | 0.030 ± 0.09 | |||
| 1:10 | 1 (t = 40.0–45.0 °C; pH = 7.0–9.0) | 0.069 ± 0.05 * | 64.3 ±0.05 | 0.050 ± 0.05 * | 66.7 ± 0.05 |
| 2 (t = 45.0–50.0 °C; pH = 8.0–11.0) | 0.070 ±0.05 ** | 66.7 ± 0.05 | 0.052 ± 0.05 ** | 57.7 ± 0.05 | |
| 1:20 | 3 (t = 40.0–45.0 °C; pH = 7.0–9.0) | 0.069 ± 0.02 * | 64.3 ± 0.02 | 0.046 ± 0.02 * | 53.3 ± 0.02 |
| 4 (t = 45.0–50.0 °C; pH = 8.0–11.0) | 0.071 ± 0.02 ** | 59.2 ± 0.02 | 0.051 ± 0.01 ** | 58.8 ± 0.02 | |
| 1:30 | 5 (t = 40.0–45.0 °C; pH = 7.0–9.0) | 0.068 ± 0.07 * | 61.9 ± 0.07 | 0.045 ± 0.07 * | 50.0 ± 0.07 |
| 6 (t = 45.0–50.0 °C; pH = 8.0–11.0) | 0.069 ± 0.07 * | 60.9 ± 0.07 | 0.049 ± 0.06 * | 61.2 ± 0.06 | |
| Preservative and Storage Period | Enzyme–Substrate Ratio | |||
|---|---|---|---|---|
| 1:10 | 1:20 | 1:30 | ||
| Control | Immediately after preparation | 0.070 ± 0.07 | 0.070 ± 0.03 | 0.069 ± 0.09 |
| In a month | 0.068 ± 0.07 | 0.068 ± 0.03 | 0.066 ± 0.04 | |
| In three months | 0.065 ± 0.09 | 0.066 ± 0.08 | 0.063 ± 0.07 | |
| Thymol | In a month | 0.069 ± 0.08 * | 0.069 ± 0.07 * | 0.067 ± 0.03 * |
| In three months | 0.068 ± 0.04 * | 0.067 ± 0.05 * | 0.067 ± 0.09 * | |
| Sorbic acid | In a month | 0.070 ± 0.03 ** | 0.070 ± 0.04 ** | 0.069 ± 0.09 ** |
| In three months | 0.069 ± 0.07 * | 0.069 ± 0.09 * | 0.068 ± 0.03 ** | |
| Preservative and Storage Period | Enzyme–Substrate Ratio | |||
|---|---|---|---|---|
| 1:10 | 1:20 | 1:30 | ||
| Control | Immediately after preparation | 0.061 ± 0.03 | 0.062 ± 0.07 | 0.060 ± 0.06 |
| In a month | 0.059 ± 0.07 | 0.060 ± 0.09 | 0.059 ± 0.06 | |
| In three months | 0.058 ± 0.07 | 0.059 ± 0.07 | 0.057 ± 0.03 | |
| Thymol | In a month | 0.060 ± 0.04 * | 0.061 ± 0.03 * | 0.059 ± 0.07 * |
| In three months | 0.059 ± 0.03 * | 0.060 ± 0.07 * | 0.059 ± 0.09 * | |
| Sorbic acid | In a month | 0.061 ± 0.09 ** | 0.062 ± 0.04 ** | 0.060 ± 0.07 ** |
| In three months | 0.061 ± 0.07 ** | 0.061 ± 0.09 * | 0.060 ± 0.03 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belyshkina, M.; Kobozeva, T.; Zagoruiko, M.; Serebryakova, O.; Shaaban, M.; Ananeva, T.; Bashmakov, I. Determination of the Optimal Biotechnological Parameters for Industrial Production of Protein Hydrolysates for Animal Feed. Fermentation 2025, 11, 209. https://doi.org/10.3390/fermentation11040209
Belyshkina M, Kobozeva T, Zagoruiko M, Serebryakova O, Shaaban M, Ananeva T, Bashmakov I. Determination of the Optimal Biotechnological Parameters for Industrial Production of Protein Hydrolysates for Animal Feed. Fermentation. 2025; 11(4):209. https://doi.org/10.3390/fermentation11040209
Chicago/Turabian StyleBelyshkina, Marina, Tamara Kobozeva, Mikhail Zagoruiko, Oksana Serebryakova, Maisoon Shaaban, Tatiana Ananeva, and Igor Bashmakov. 2025. "Determination of the Optimal Biotechnological Parameters for Industrial Production of Protein Hydrolysates for Animal Feed" Fermentation 11, no. 4: 209. https://doi.org/10.3390/fermentation11040209
APA StyleBelyshkina, M., Kobozeva, T., Zagoruiko, M., Serebryakova, O., Shaaban, M., Ananeva, T., & Bashmakov, I. (2025). Determination of the Optimal Biotechnological Parameters for Industrial Production of Protein Hydrolysates for Animal Feed. Fermentation, 11(4), 209. https://doi.org/10.3390/fermentation11040209










