A Comparison of Static Aeration and Conventional Turning Windrow Techniques: Physicochemical and Microbial Dynamics in Wine Residue Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Composting Process and Experimental Design
2.3. Sampling
2.4. Physicochemical Analysis
2.5. DNA Extraction and Sequencing
2.6. Microbiological Data Processing
3. Results
3.1. Physicochemical
3.2. Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Organization of Vine and Wine. State of the World Vine and Wine Sector in 2023; International Organization of Vine and Wine: Dijon, France, 2023. [Google Scholar]
- Servicio Agrícola y Ganadero. Producción de Vinos 2023; Servicio Agrícola y Ganadero: Santiago, Chile, 2023. [Google Scholar]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0. A Global Snapshot of Solid Waste Management to 2050; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Bless, A.; Davila, F.; Plant, R. A genealogy of sustainable agriculture narratives: Implications for the transformative potential of regenerative agriculture. Agric. Hum. Values 2023, 40, 1379–1397. [Google Scholar] [CrossRef]
- Shah, A.M.; Khan, I.M.; Shah, T.I.; Bangroo, S.A.; Kirmani, N.A.; Nazir, S.; Malik, A.R.; Aezum, A.M.; Mir, Y.H.; Hilal, A.; et al. Soil Microbiome: A Treasure Trove for Soil Health Sustainability under Changing Climate. Land 2022, 11, 1887. [Google Scholar] [CrossRef]
- Mondini, C.; Fornasier, F.; Sinicco, T.; Sivilotti, P.; Gaiotti, F.; Mosetti, D. Organic amendment effectively recovers soil functionality in degraded vineyards. Eur. J. Agron. 2018, 101, 210–221. [Google Scholar] [CrossRef]
- Cooperband, L. The Art and Science of Composting: A Resource for Farmers and Compost Producers; Center for Integrated Agricultural Systems: Madison, WI, USA, 2002. [Google Scholar]
- Ministerio del Medio Ambiente MMA. Ley 20920 Establece Marco para la Gestión de Residuos, la Responsabilidad Extendida del Productor y Fomento al Reciclaje; Diario Oficial; Ministerio del Medio Ambiente MMA: Santiago, Chile, 2016; pp. 17–20. [Google Scholar]
- Comisión Económica para América Latina y el Caribe (CEPAL), Ministerio del Medio Ambiente, and Compromiso Empresarial para el Reciclaje (CEMPRE). Encuesta a Municipios Sobre Gestión de Residuos Sólidos Domiciliarios 2019; ECLAC: Santiago, Chile, 2020. [Google Scholar]
- Echeverría-Vega, A.; Espinoza-Mondaca, A.; Arqueros-Sanhueza, E.; Mellado-Quintanilla, D.; Roa-Roco, R.; González, A.; Morales-Vera, R. Management of industrial wine residues: Physicochemical, bacterial and fungal dynamics during composting processes. Discov. Appl. Sci. 2024, 6, 354. [Google Scholar] [CrossRef]
- Temel, F.A. Evaluation of the influence of rice husk amendment on compost quality in the composting of sewage sludge. Bioresour. Technol. 2023, 373, 128748. [Google Scholar] [CrossRef]
- Tortosa, G.; Alburquerque, J.A.; Ait-Baddi, G.; Cegarra, J. The production of commercial organic amendments and fertilisers by composting of two-phase olive mill waste (‘alperujo’). J. Clean. Prod. 2012, 26, 48–55. [Google Scholar] [CrossRef]
- Liu, X.; Rong, X.; Yang, J.; Li, H.; Hu, W.; Yang, Y.; Jiang, G.; Xiao, R.; Deng, X.; Xie, G.; et al. Community succession of microbial populations related to C–N–P–S biological transformations regulates product maturity during cow-manure-driven composting. Bioresour. Technol. 2023, 369, 128493. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Lyamlouli, K. Olive mill waste sludge: From permanent pollution to a highly beneficial organic biofertilizer: A critical review and future perspectives. Ecotoxicol. Environ. Saf. 2023, 259, 114997. [Google Scholar] [CrossRef]
- Di Piazza, S.; Houbraken, J.; Meijer, M.; Cecchi, G.; Kraak, B.; Rosa, E.; Zotti, M. Thermotolerant and thermophilic mycobiota in different steps of compost maturation. Microorganisms 2020, 8, 880. [Google Scholar] [CrossRef]
- Torres, V.M.; Ochoa-Álvarez, N.A.; Nieto-Garibay, A.; Murillo-Amador, B.; Lavastida, P.G.; Alfonso, P. Inactivation of pathogens in poultry residues through composting. Rev. Investig. Vet. Peru 2023, 34, e24488. [Google Scholar] [CrossRef]
- Sundberg, C. Improving Compost Process Efficiency by Controlling Aeration, Temperature and pH. Ph.D. Thesis, Swedish University of Agricultural Sciences, Upsala, Sweden, 2005. [Google Scholar]
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.O.; Iroh, I.N.; Ezeogu, L.I. Composting technology in waste stabilization: On the methods, challenges and future prospects. J. Environ. Manag. 2017, 190, 140–157. [Google Scholar] [CrossRef]
- Martins, G.L.; de Souza, A.J.; Mendes, L.W.; Gontijo, J.B.; Rodrigues, M.M.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Physicochemical and bacterial changes during composting of vegetable and animal-derived agro-industrial wastes. Bioresour. Technol. 2023, 376, 128842. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.P.; Martins, L.F.; Pereira, R.V.; Thomas, A.M.; Barbosa, D.; Lemos, L.N.; Silva, G.M.M.; Moura, L.M.S.; Epamino, G.W.C.; Digiampietri, L.A.; et al. Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics. Sci. Rep. 2016, 6, 38915. [Google Scholar] [CrossRef]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic bacteria and their thermozymes in composting processes: A review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef]
- Orozco, V.H.V. Diseño y automatización de un sistema de aireación forzada para el co-compostaje de residuos hortícolas en la comunidad de Gatazo Cantón Colta; Escuela Superior Politécnica de Chimborazo: Riobamaba, Ecuador, 2015. [Google Scholar]
- Then, Y.H.; Lai, J.C.; Then, Y.L. Study of forced aeration system for fruit and vegetable waste composting. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1195, 012059. [Google Scholar] [CrossRef]
- Román, P.; Martínez, M.M.; Pantoja, A. Farmer’s Compost Handbook; FAO: Santiago, Chile, 2015; Available online: http://www.fao.org/3/a-i3388e.pdf (accessed on 30 March 2023).
- Arslan, E.I.; Ünlü, A.; Topal, M. Determination of the Effect of Aeration Rate on Composting of Vegetable-Fruit Wastes. Clean 2011, 39, 1014–1021. [Google Scholar] [CrossRef]
- HMS. Ewon Industrial Remote Connectivity and IIoT. Available online: https://www.hms-networks.com/ewon (accessed on 3 November 2024).
- Instituto Nacional de Normalización INN. Norma chilena oficia NCh 2880 of 2004. 2004. Available online: www.inn.cl (accessed on 30 April 2023).
- US Composting Council. TMECC 02.01 Field Sampling of Compost Materials. In Test Methods for the Examination of Composting and Compost; US Compost Council: Raleigh, NC, USA, 2001; p. 26. Available online: http://soiltestlab.com/wp-content/uploads/2012/10/TMECC-Field-Sampling-Protocol.pdf (accessed on 30 April 2023).
- Hill, G.B.; Baldwin, S.A.; Vinnerås, B. Evaluation of Solvita compost stability and maturity tests for assessment of quality of end-products from mixed latrine style compost toilets. Waste Manag. 2013, 33, 1602–1606. [Google Scholar] [CrossRef]
- Qiagen. Qiagen DNeasy PowerSoil Pro Kits. 2020. Available online: https://www.qiagen.com/us/products/discovery-and-translational-research/dna-rna-purification/dna-purification/microbial-dna/dneasy-powersoil-pro-kit (accessed on 2 November 2024).
- National Cancer Institute Informatics Technology for Cancer Research and Chan Zuckerberg Initiative. QIIME 2. Available online: https://qiime2.org (accessed on 3 November 2024).
- UNITE Community. UNITE rDNA ITS Based Identification of Eukaryotes and Their Communication via DOIs. Available online: https://www.re3data.org/repository/r3d100011316 (accessed on 3 November 2024).
- Kõljalg, U.; Nilsson, H.R.; Schigel, D.; Tedersoo, L.; Larsson, K.-H.; May, T.W.; Taylor, A.F.S.; Jeppesen, T.S.; Frøslev, T.G.; Lindahl, B.D.; et al. The taxon hypothesis paradigm—On the unambiguous detection and communication of taxa. Microorganisms 2020, 8, 1910. [Google Scholar] [CrossRef]
- SILVA Team. SILVA 138-SSU-NR99. Available online: https://www.arb-silva.de/documentation/release-138/ (accessed on 3 November 2024).
- Pruesse, E.; Peplies, J.; Glöckner, F.O. SINA: Accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 2012, 28, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- PRIMER-e. PRIMER-e Robust Multivariate Statistical Analysis. Available online: https://www.primer-e.com (accessed on 3 November 2024).
- Khater, E.S.G. Some Physical and Chemical Properties of Compost. Int. J. Waste Resour. 2015, 5, 1000172. [Google Scholar] [CrossRef]
- Huerta-Pujol, O.; Soliva, M.; Martínez-Farré, F.X.; Valero, J.; López, M. Bulk density determination as a simple and complementary tool in composting process control. Bioresour. Technol. 2010, 101, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Agnew, J.M.; Leonard, J.J. The physical properties of compost. Compost Sci Util. 2003, 11, 238–264. [Google Scholar] [CrossRef]
- Morales-Vera, R.; Echeverría-Vega, A.; Espinoza, A.; Roco, R.R.; Gonzalez, A.; Schober, D.; Tramon, S. Compost of vitiviniculture residues. Closing the loop of the wine industry. BIO Web Conf. 2023, 56, 01034. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Alami, I.T. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Sundberg, C.; Smårs, S.; Jönsson, H. Low pH as an inhibiting factor in the transition from mesophilic to thermophilic phase in composting. Bioresour. Technol. 2004, 95, 145–150. [Google Scholar] [CrossRef]
- Wang, C.; Dong, D.; Wang, H.; Müller, K.; Qin, Y.; Wang, H.; Wu, W. Metagenomic analysis of microbial consortia enriched from compost: New insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef]
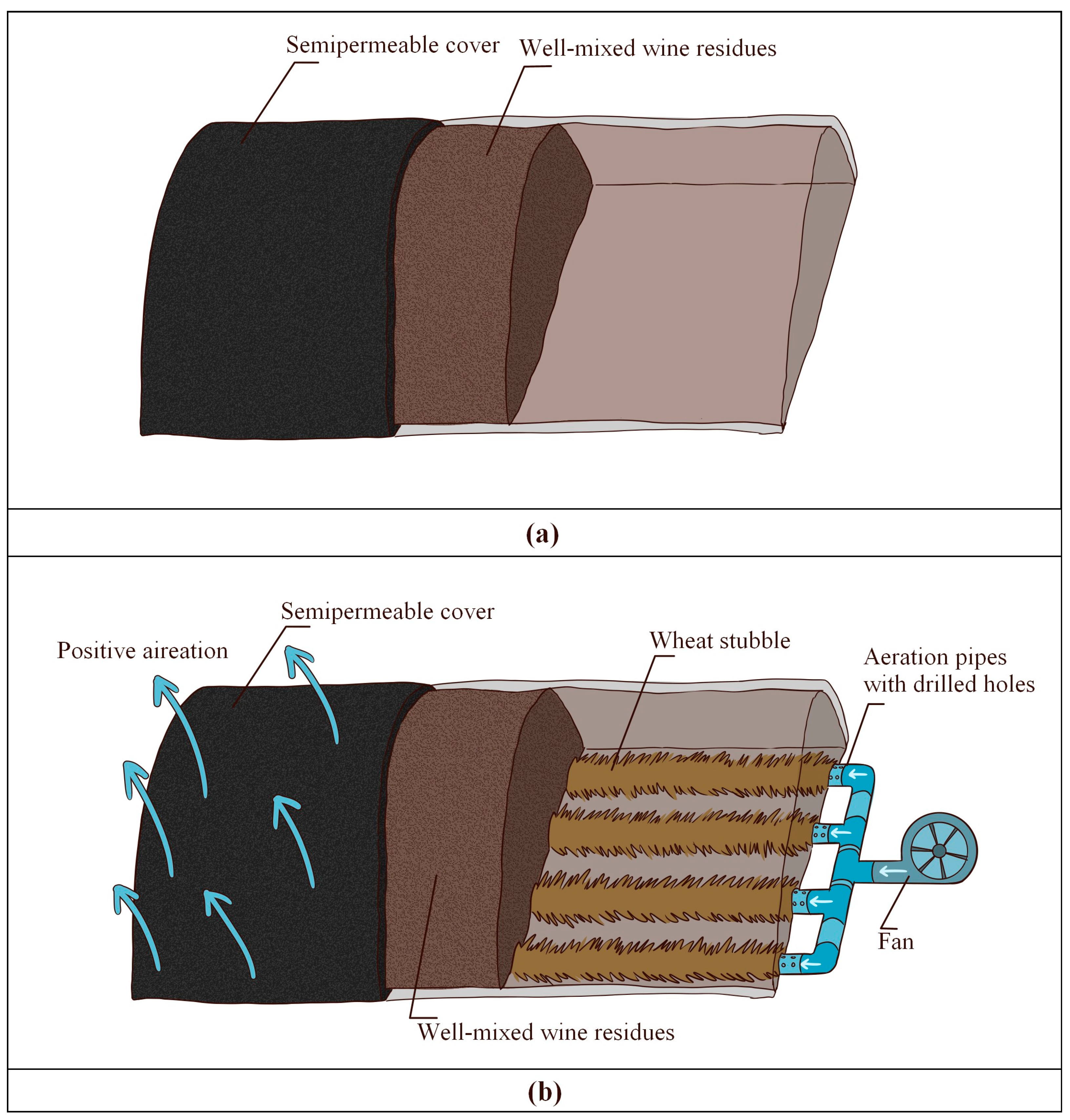
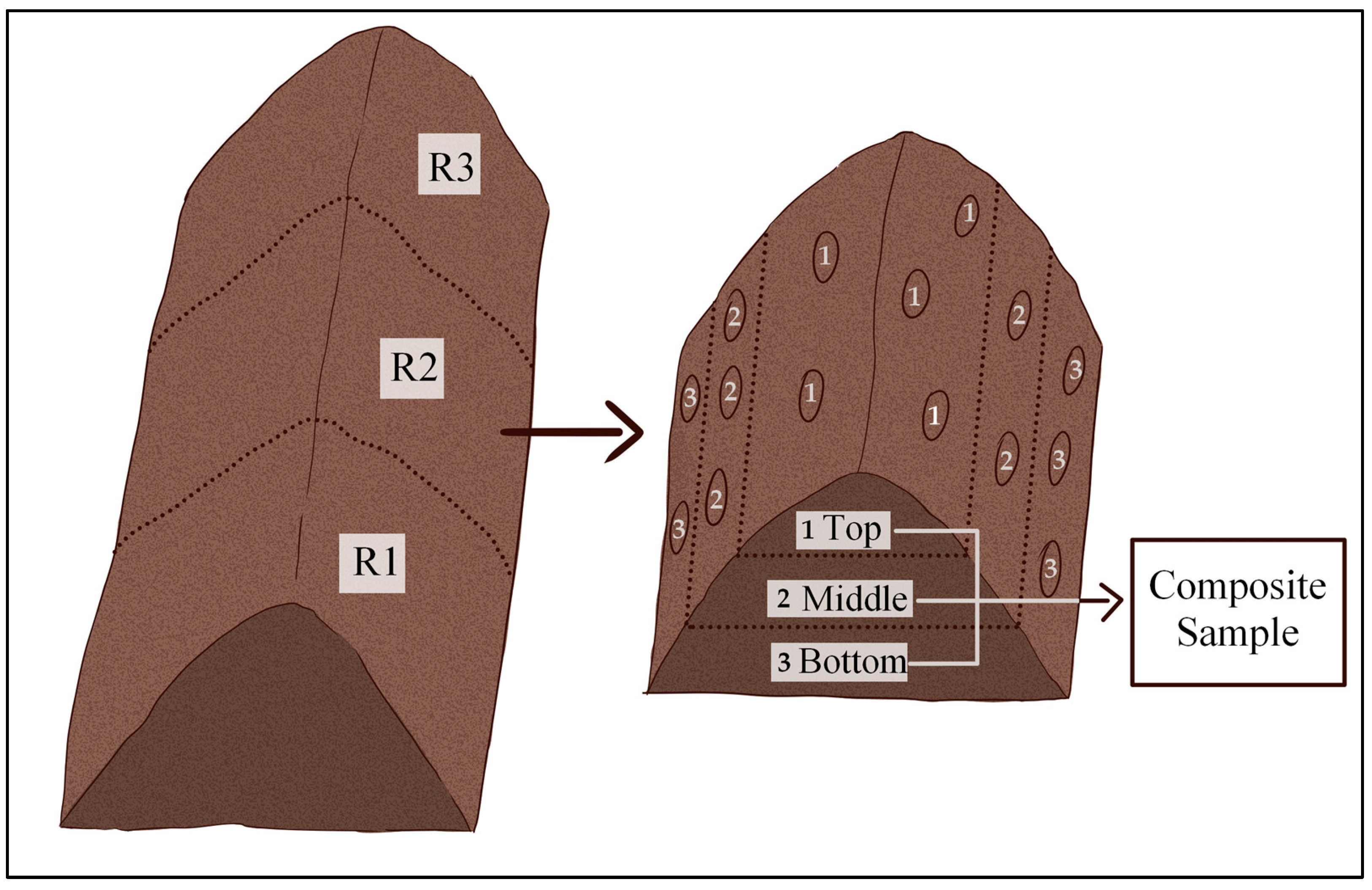


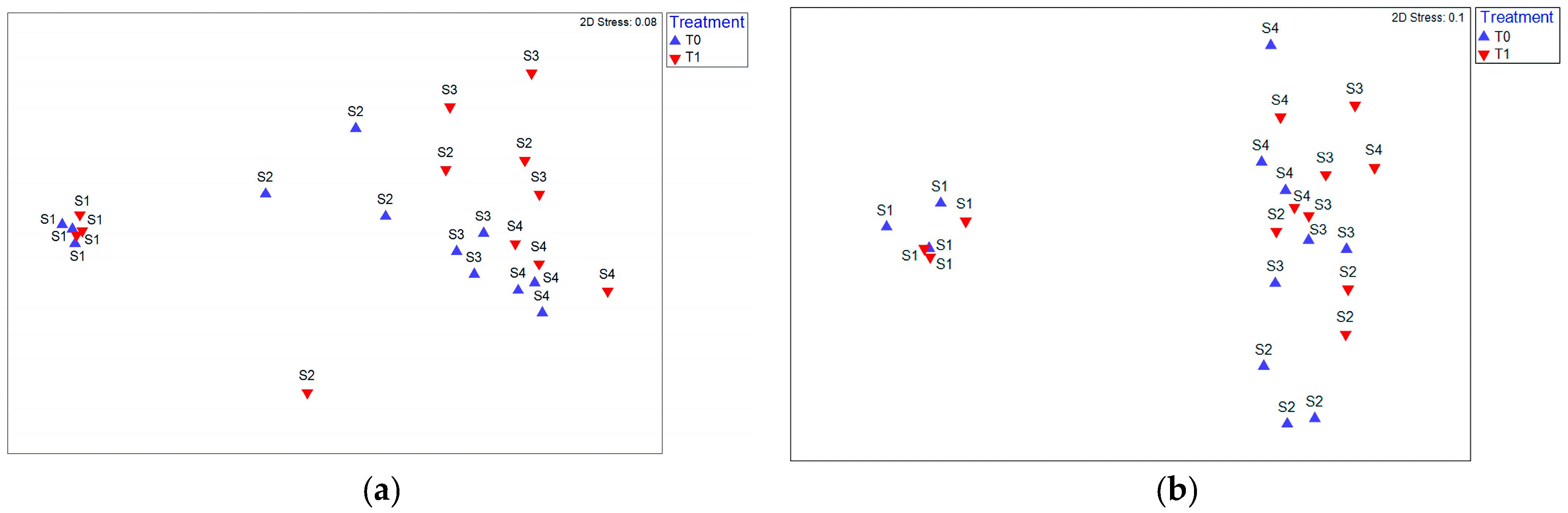
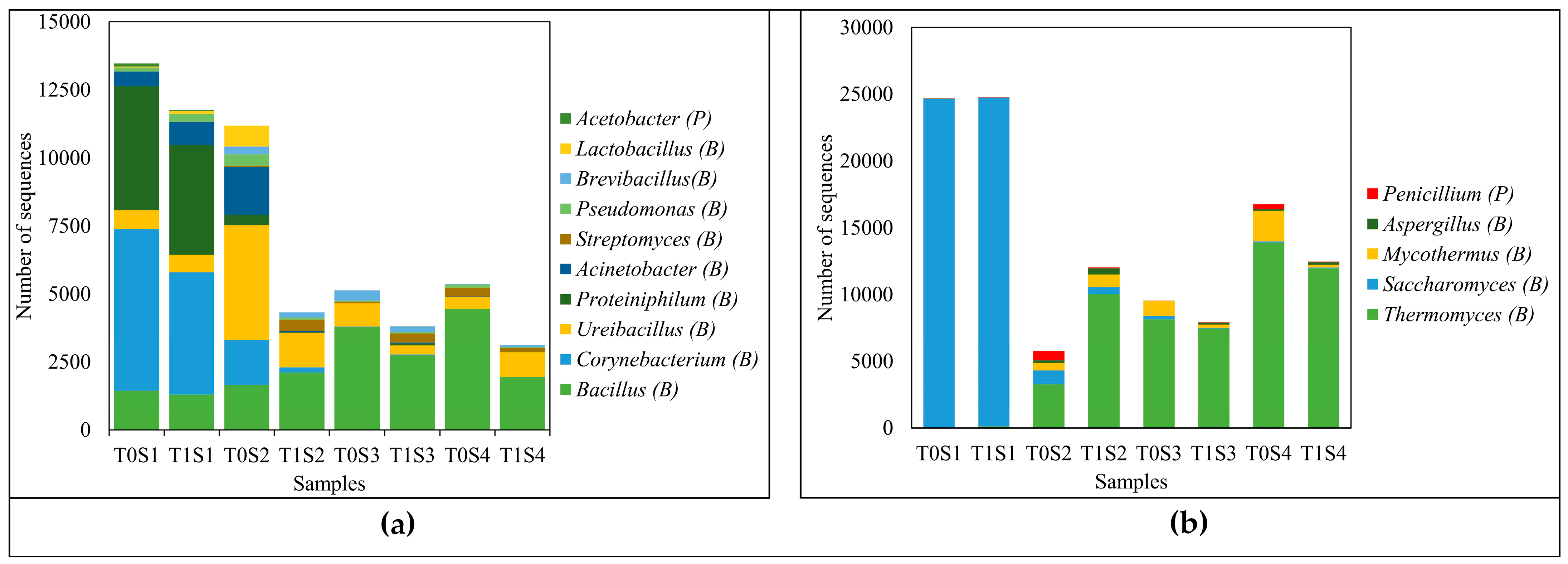
| Treatment | pH | Organic Matter (%) | C/N | NH4/NO3 | N (%) | P2O5 (%) | K2O (%) | NH4 (mg/kg) | NO3 (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| T0 | 5.9 ± 0.2 | 78.6 ± 1.2 | 27.2 ± 2.3 | 3.1 ± 0.7 | 1.6 ± 0.1 | 1 ± 0 | 2.7 ± 0.1 | 425 ± 76 | 142 ± 10 |
| T1 | 5.6 ± 0 | 82.6 ± 0.7 | 28.0 ± 1 | 2.3 ± 0.2 | 1.6 ± 0 | 0.9 ± 0 | 2.6 ± 0 | 353 ± 57 | 151 ± 30 |
| P | T | pH | C:N | N (%) | P (%) | K (%) | NH4 (mg/kg) | NO3 (mg/kg) | NH4:NO3 |
|---|---|---|---|---|---|---|---|---|---|
| M | T0 | 6 ± 0.2 | 27.2 ± 2.4 | 1.6 ± 0.2 | 1 ± 0.1 | 2.8 ± 0.1 | 452.7 ± 76.6 | 142 ± 10.7 | 3.1 ± 0.8 |
| T1 | 5.7 ± 0.1 | 28 ± 1.1 | 1.6 ± 0.1 | 0.9 ± 0 | 2.6 ± 0.1 | 353.7 ± 57.7 | 151 ± 30.8 | 2.4 ± 0.2 | |
| T | T0 | 9.1 ± 0.1 | 24.3 ± 2.4 | 1.7 ± 0.1 | 1 ± 0.1 | 2.2 ± 0.1 | 374.7 ± 33 | 237 ± 8.9 | 1.6 ± 0.2 |
| T1 | 8.9 ± 0.1 | 18.9 ± 0.4 | 2.2 ± 0.1 | 1.1 ± 0.1 | 2.9 ± 0.2 | 482.3 ± 48.5 | 305.3 ± 16.6 | 1.6 ± 0.3 | |
| L | T0 | 8.7 ± 0.1 | 15.3 ± 0.6 | 2.4 ± 0 | 1.5 ± 0 | 3.1 ± 0 | 460.7 ± 98.2 | 243.7 ± 25.4 | 1.9 ± 0.4 |
| T1 | 8.5 ± 0.1 | 17.1 ± 1.5 | 2.3 ± 0.1 | 1.2 ± 0.1 | 3.1 ± 0 | 328.3 ± 31.4 | 242.7 ± 13 | 1.4 ± 0.2 | |
| S | T0 | 8.1 ± 0.1 | 12.7 ± 0.2 | 2.5 ± 0 | 2 ± 0.1 | 3.1 ± 0.1 | 459.7 ± 24.8 | 142.3 ± 14.4 | 3.3 ± 0.6 |
| T1 | 7.9 ± 0.1 | 13.5 ± 0.5 | 2.4 ± 0 | 1.9 ± 0 | 3.1 ± 0.1 | 575.3 ± 73.2 | 113 ± 12.5 | 5.3 ± 1.2 |
| Treatment | Bacteria | Fungi |
|---|---|---|
| T0S1 | 4.59 ± 0.01 | 0.54 ± 0.31 |
| T1S1 | 4.68 ± 0.07 | 0.70 ± 0.10 |
| T0S2 | 4.57 ± 0.25 | 2.50 ± 0.37 |
| T1S2 | 4.38 ± 0.42 | 2.32 ± 0.67 |
| T0S3 | 4.43 ± 0.28 | 2.27 ± 0.41 |
| T1S3 | 5.09 ± 0.16 | 1.94 ± 0.16 |
| T0S4 | 4.49 ± 0.48 | 1.75 ± 0.31 |
| T1S4 | 4.33 ± 0.38 | 1.95 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Vera, R.; Echeverría-Vega, A.; Ríos-Rozas, H.; Barrera-Valenzuela, F.; Mellado-Quintanilla, D.; Piesche, M.; Roa-Roco, R.; Tramon, S. A Comparison of Static Aeration and Conventional Turning Windrow Techniques: Physicochemical and Microbial Dynamics in Wine Residue Composting. Fermentation 2025, 11, 197. https://doi.org/10.3390/fermentation11040197
Morales-Vera R, Echeverría-Vega A, Ríos-Rozas H, Barrera-Valenzuela F, Mellado-Quintanilla D, Piesche M, Roa-Roco R, Tramon S. A Comparison of Static Aeration and Conventional Turning Windrow Techniques: Physicochemical and Microbial Dynamics in Wine Residue Composting. Fermentation. 2025; 11(4):197. https://doi.org/10.3390/fermentation11040197
Chicago/Turabian StyleMorales-Vera, Rodrigo, Alex Echeverría-Vega, Hernán Ríos-Rozas, Francisca Barrera-Valenzuela, Denisse Mellado-Quintanilla, Matthias Piesche, Rosa Roa-Roco, and Sebastian Tramon. 2025. "A Comparison of Static Aeration and Conventional Turning Windrow Techniques: Physicochemical and Microbial Dynamics in Wine Residue Composting" Fermentation 11, no. 4: 197. https://doi.org/10.3390/fermentation11040197
APA StyleMorales-Vera, R., Echeverría-Vega, A., Ríos-Rozas, H., Barrera-Valenzuela, F., Mellado-Quintanilla, D., Piesche, M., Roa-Roco, R., & Tramon, S. (2025). A Comparison of Static Aeration and Conventional Turning Windrow Techniques: Physicochemical and Microbial Dynamics in Wine Residue Composting. Fermentation, 11(4), 197. https://doi.org/10.3390/fermentation11040197









