A Novel Food Wastewater Treatment Approach: Developing a Sustainable Fungicide for Agricultural Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewaters (SH, CW, and WL)
2.2. Analytical Methods
2.3. Anaerobic Experiments
2.4. Antifungal Application of VFAs
2.4.1. Fungal Isolate, Reagents
2.4.2. UV and Heat Pretreatments
2.4.3. In Vitro Tests of Mycelial Growth Inhibition
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the Substrates
3.2. VFA Production and Composition
3.3. Antifungal Application of VFAs
3.3.1. Influence of Pretreatment on the VFA Reduction and Contamination
3.3.2. Antifungal Activity: In Vitro Growth Inhibition Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CW | Cheese whey |
| WL | Wine lees |
| SH | Slaughterhouse |
| VFAs | Volatile fatty acids |
| TCOD | Total chemical oxygen demand |
| COD | Chemical oxygen demand |
| pH | Potential hydrogen |
| AD | Anaerobic digestion |
| TSs | Total solids |
| VSs | Volatile solids |
| TKN | Total Kjeldahl nitrogen |
| N-NH4+ | Ammonium |
| R1 | Reactor 1 |
| R2 | Reactor 2 |
| ANOVA | Analysis of variance |
| DNA | Deoxyribonucleic acid |
References
- Shrivastava, V.; Ali, I.; Marjub, M.M.; Rene, E.R.; Soto, A.M.F. Wastewater in the food industry: Treatment technologies and reuse potential. Chemosphere 2022, 293, 133553. [Google Scholar]
- Buleca, J.; Kováč, V.; Kočanová, D. Cluster analysis of beef production distribution in Europe. Potravinarstvo 2018, 12, 789. [Google Scholar]
- Hocquette, J.-F.; Ellies-Oury, M.-P.; Lherm, M.; Pineau, C.; Deblitz, C.; Farmer, L. Current situation and future prospects for beef production in Europe—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1017. [Google Scholar]
- Iten, M.; Fernandes, U.; Oliveira, M.C. Framework to assess eco-efficiency improvement: Case study of a meat production industry. Energy Rep. 2021, 7, 7134–7148. [Google Scholar]
- Djekic, I. Environmental impact of meat industry-current status and future perspectives. Procedia Food Sci. 2015, 5, 61–64. [Google Scholar]
- Kupusovic, T.; Midzic, S.; Silajdzic, I.; Bjelavac, J. Cleaner production measures in small-scale slaughterhouse industry-case study in Bosnia and Herzegovina. J. Clean. Prod. 2007, 15, 378–383. [Google Scholar]
- Mulimi, L.K.; Home, P.G.; Chacha, J.S.; Siringi, D.O. Investigating electrocoagulation as an alternative treatment method for wastewater from Slaughterhouses in Kenya. In Proceedings of the Sustainable Research and Innovation Conference, Pretoria, South Africa, 20–24 June 2022; pp. 143–151. [Google Scholar]
- Budiyono, B.; Seno, J.; Sunarso, S. Study on slaughterhouse wastes potency and characteristic for biogas production. Int. J. Waste Resour. (IJWR) 2011, 1, 4–7. [Google Scholar]
- Awodi, I.; Shallangwa, G.; Okon, I.; Ekwumemgbo, P. Application of Green Synthesized Iron Nanoparticles for Treatment of Slaughterhouse Wastewater from Abattoir in Zaria, Nigeria J. Mater. Environ. Sci. 2023, 14, 1226–1235. [Google Scholar]
- Gottardo, M.; Bolzonella, D.; Tuci, G.A.; Valentino, F.; Majone, M.; Pavan, P.; Battista, F. Producing volatile fatty acids and polyhydroxyalkanoates from foods by-products and waste: A review. Bioresour. Technol. 2022, 361, 127716. [Google Scholar]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Walsh, G. The biotechnological potential of whey. Rev. Environ. Sci. Bio/Technol. 2016, 15, 479–498. [Google Scholar]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.C.; Russo, M.; Novellino, E. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A novel approach for the valorization of wine lees as a source of compounds able to modify wine properties. LWT 2021, 136, 110274. [Google Scholar]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.; de Souza Oliveira, R.P.; Domínguez, J. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Moldes, A.B.; Domínguez, J.M. Tartaric acid recovery from distilled lees and use of the residual solid as an economic nutrient for Lactobacillus. J. Agric. Food Chem. 2006, 54, 7904–7911. [Google Scholar]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar]
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental sustainability of anaerobic digestion of household food waste. J. Environ. Manag. 2019, 236, 798–814. [Google Scholar]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of palladium (ii) with a magnetic sorbent based on polyvinyl alcohol gel, metallic iron, and an environmentally friendly polydentate phosphazene-containing extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Zeng, Z.; Gao, Y.; Liu, C.; Sun, X. Separation of lithium and transition metals from the leachate of spent lithium-ion battery by extraction-precipitation with p-tert-butylphenoxy acetic acid. Hydrometallurgy 2021, 206, 105768. [Google Scholar]
- Zacharof, M.-P.; Lovitt, R. Recovery of volatile fatty acids (VFA) from complex waste effluents using membranes. Water Sci. Technol. 2014, 69, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Masse, L.; Massé, D.; Pellerin, Y. The effect of pH on the separation of manure nutrients with reverse osmosis membranes. J. Membr. Sci. 2008, 325, 914–919. [Google Scholar] [CrossRef]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef]
- Evangelisti, S.; Lettieri, P.; Borello, D.; Clift, R. Life cycle assessment of energy from waste via anaerobic digestion: A UK case study. Waste Manag. 2014, 34, 226–237. [Google Scholar] [CrossRef]
- Yu, Q.; Li, H.; Deng, Z.; Liao, X.; Liu, S.; Liu, J. Comparative assessment on two full-scale food waste treatment plants with different anaerobic digestion processes. J. Clean. Prod. 2020, 263, 121625. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.-M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A glimpse of the world of volatile fatty acids production and application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The potential of fatty acids and their derivatives as antifungal agents: A review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P. Community ecology of fungal pathogens causing wheat head blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar]
- Pereira, L.T.P.; Putnik, P.; Iwase, C.H.T.; de Oliveira Rocha, L. Deoxynivalenol: Insights on genetics, analytical methods and occurrence. Curr. Opin. Food Sci. 2019, 30, 85–92. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B.; Kaczynski, P.; Konecki, R. Multifactorial wheat response under Fusarium culmorum, herbicidal, fungicidal and biostimulator treatments on the biochemical and mycotoxins status of wheat. J. Saudi Soc. Agric. Sci. 2021, 20, 443–453. [Google Scholar]
- Dweba, C.; Figlan, S.; Shimelis, H.; Motaung, T.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Sinha, M.; Singh, H.; Patel, R.S.; Ghosh, S.; Sardana, K.; Ghosh, S.; Sengupta, S. Mechanistic insight into the antifungal effects of a fatty acid derivative against drug-resistant fungal infections. Front. Microbiol. 2020, 11, 2116. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- Molinuevo-Salces, B.; da Silva-Lacerda, V.; García-González, M.C.; Riaño, B. Production of Volatile Fatty Acids from Cheese Whey and Their Recovery Using Gas-Permeable Membranes. Recycling 2024, 9, 65. [Google Scholar] [CrossRef]
- Gutiérrez-Santa Ana, A.; Carrillo-Cerda, H.A.; Rodriguez-Campos, J.; Kirchmayr, M.R.; Contreras-Ramos, S.M.; Velázquez-Fernández, J.B. Volatile emission compounds from plant growth-promoting bacteria are responsible for the antifungal activity against F. solani. 3 Biotech 2020, 10, 292. [Google Scholar]
- Al Smadi, B.M.; Al-Hayek, W.; Abu Hajar, H.A. Treatment of Amman Slaughterhouse Wastewater by Anaerobic Baffled Reactor. Int. J. Civ. Eng. 2019, 17, 1445–1454. [Google Scholar] [CrossRef]
- Estikomah, S.A.; Masykuri, M. Cheese Whey Wastewater: Characterization and Value. Kne Soc. Sci. 2023, 8, 465–474. [Google Scholar]
- Koda, E.; Miszkowska, A.; Sieczka, A. Levels of Organic Pollution Indicators in Groundwater at the Old Landfill and Waste Management Site. Appl. Sci. 2017, 7, 638. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Physicochemical and Nutritional Characterization of Winemaking Lees: A New Food Ingredient. Agronomy 2020, 10, 996. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liang, N.Y.; Curtis, J.M.; Gänzle, M.G. Characterization of linoleate 10-hydratase of Lactobacillus plantarum and novel antifungal metabolites. Front. Microbiol. 2016, 7, 1561. [Google Scholar] [CrossRef]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated Batch Fermentation of Cheese Whey for Semi-Continuous Lactic Acid Production Using Mixed Cultures at Uncontrolled pH. Sustainability 2019, 11, 3330. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese Whey Fermentation into Volatile Fatty Acids in an Anaerobic Sequencing Batch Reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar]
- Lanfranchi, A.; Desmond-Le Quéméner, E.; Magdalena, J.A.; Cavinato, C.; Trably, E. Conversion of wine lees and waste activated sludge into caproate and heptanoate: Thermodynamic and microbiological insights. Bioresour. Technol. 2024, 408, 131126. [Google Scholar]
- Villegas-Rodríguez, S.B.; Arreola-Vargas, J.; Buitrón, G. Influence of pH and temperature on the performance and microbial community during the production of medium-chain carboxylic acids using winery effluents as substrate. Environ. Sci. Pollut. Res. 2024, 1–10. [Google Scholar] [CrossRef]
- Shelomi, M. Mitigation Strategies against Food Safety Contaminant Transmission from Black Soldier Fly Larva Bioconversion. Animals 2024, 14, 1590. [Google Scholar] [CrossRef]
- Xu, S.; Chen, H. Mild heat treatment achieved better inactivation of Salmonella and preservation of almond quality than ultraviolet light and chemical sanitizers. Int. J. Food Microbiol. 2023, 399, 110253. [Google Scholar]
- Kaya, Z.; Yıldız, S.; Ünlütürk, S. Effect of UV-C irradiation and heat treatment on the shelf life stability of a lemon–melon juice blend: Multivariate statistical approach. Innov. Food Sci. Emerg. Technol. 2015, 29, 230–239. [Google Scholar]
- Fenoglio, D.; Ferrario, M.; Schenk, M.; Guerrero, S. Effect of pilot-scale UV-C light treatment assisted by mild heat on E. coli, L. plantarum and S. cerevisiae inactivation in clear and turbid fruit juices. Storage study of surviving populations. Int. J. Food Microbiol. 2020, 332, 108767. [Google Scholar]
- Gouma, M.; Álvarez, I.; Condón, S.; Gayán, E. Pasteurization of carrot juice by combining UV-C and mild heat: Impact on shelf-life and quality compared to conventional thermal treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 102362. [Google Scholar]
- Cárdenas-Laverde, D.; Barbosa-Cornelio, R.; Coy-Barrera, E. Antifungal activity against Fusarium oxysporum of botanical end-products: An integration of chemical composition and antifungal activity datasets to identify antifungal bioactives. Plants 2021, 10, 2563. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Ramos-Sánchez, M.D.C.; Pérez-Lebeña, E.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Martín-Ramos, P. Antifungal activity against Fusarium culmorum of stevioside, Silybum marianum seed extracts, and their conjugate complexes. Antibiotics 2020, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.D.; Achimón, F.; Dambolena, J.S.; Pizzolitto, R.P.; Zygadlo, J.A. Trans-2-hexen-1-ol as a tool for the control of Fusarium verticillioides in stored maize grains. J. Stored Prod. Res. 2019, 82, 123–130. [Google Scholar]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar] [PubMed]
- Watanabe, H.; Horinouchi, H.; Muramoto, Y.; Ishii, H. Occurrence of azoxystrobin-resistant isolates in Passalora fulva, the pathogen of tomato leaf mould disease. Plant Pathol. 2017, 66, 1472–1479. [Google Scholar]
- Chauhan, S.; Singh, R.P. In vitro evaluation of minimum inhibitory concentration (mic) of fungicides against Rhizoctonia solani f. sp. Sasakii Exner causing banded leaf and sheath blight disease in maize. Pharma Innov. J. 2022, 11, 2014–2019. [Google Scholar]
- Colley, T.; Sehra, G.; Chowdhary, A.; Alanio, A.; Kelly, S.L.; Kizawa, Y.; Rapeport, G. In vitro and in vivo efficacy of a novel and long-acting fungicidal azole, PC1244, on Aspergillus fumigatus infection. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar]
- Worawong, K.; Borlace, G.N.; Aiemsaard, J. Antifungal activities of azole drugs in combination with clove essential oil against Microsporum gallinae. ScienceAsia 2023, 49, 337–343. [Google Scholar]

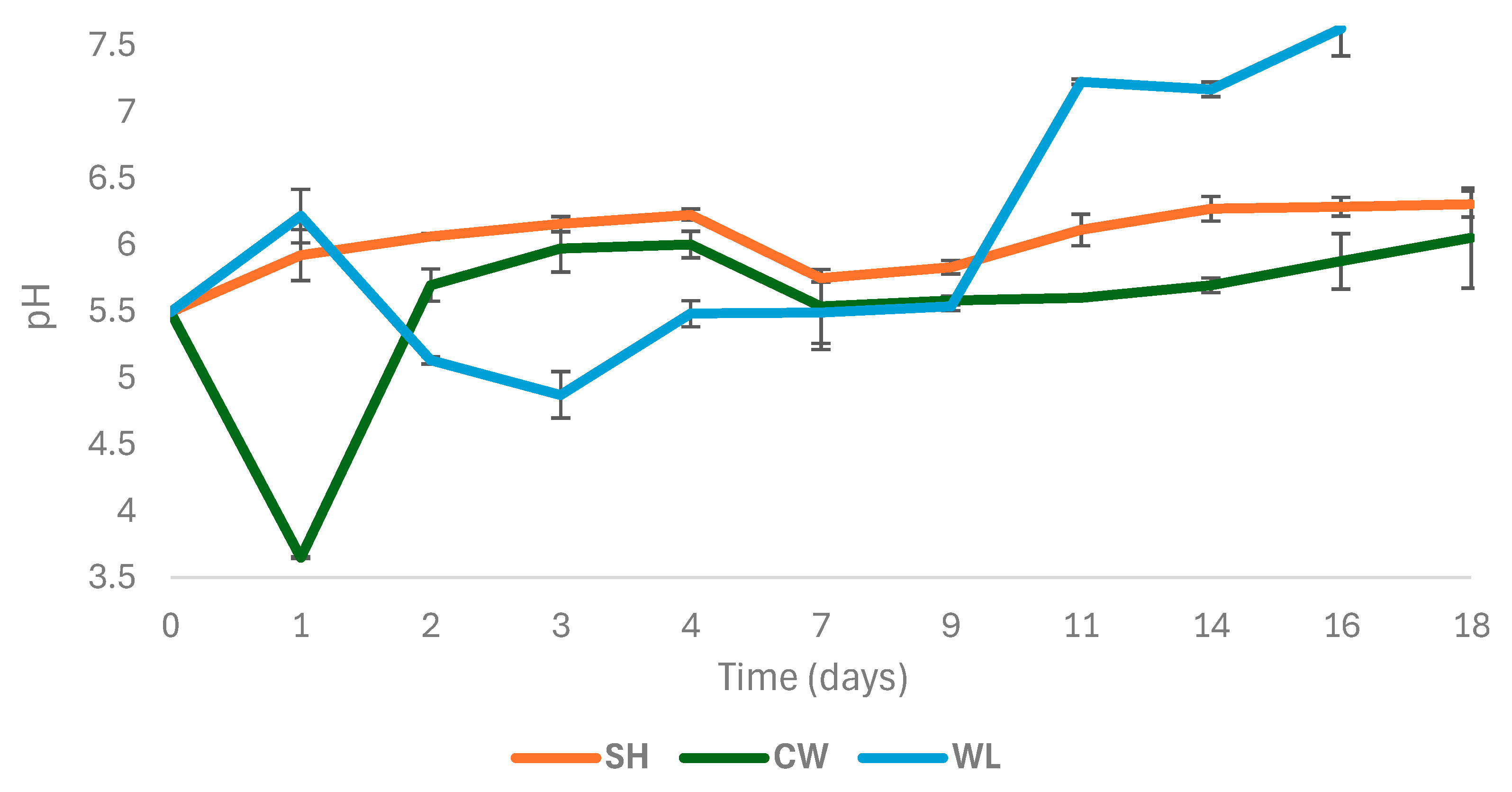
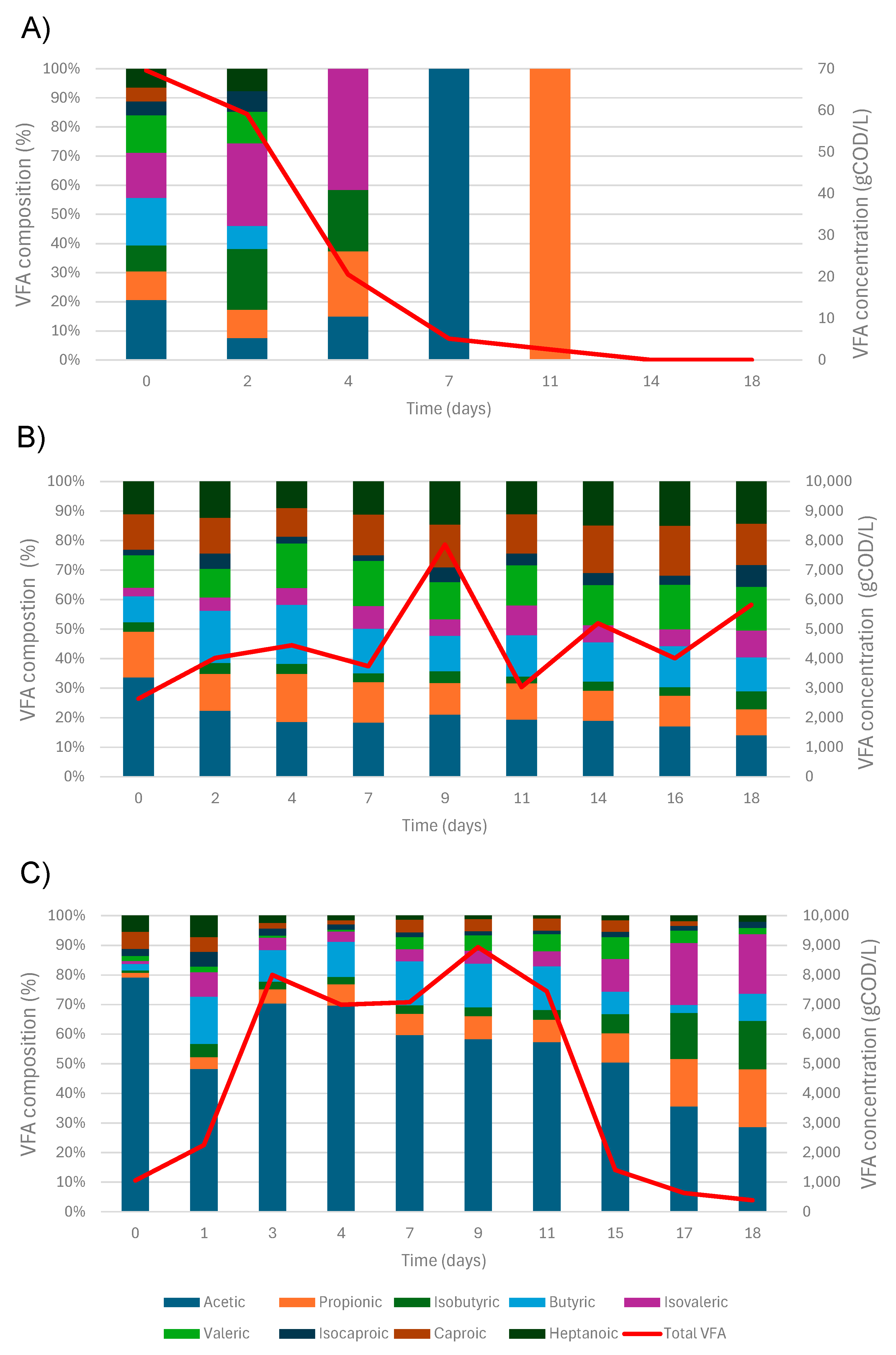

| Parameter | Unit | SH | CW | WL | AS |
|---|---|---|---|---|---|
| pH | - | 7.76 (0.00) | 5.94 (0.00) | 3.56 (0.00) | 7.73 (0.00) |
| Conductivity | mS cm−1 | 2.20 (0.00) | 5.73 (0.00) | 2.08 (0.00) | 7.01 (0.00) |
| TS | % | 2.20 (0.84) | 6.22 (0.41) | 28.07 (0.57) | 2.21 (0.23) |
| VS | % | 1.40 (0.03) | 5.83 (0.30) | 24.73 (0.45) | 1.48 (0.12) |
| Alkalinity | mg L−1 CaCO3 | - | 950 (0.00) | - | 1000 (0.00) |
| N-NH4+ | mg L−1 | 68 (0.00) | 1762 (0.10) | 1125 (0.02) | 1206 (0.15) |
| TKN | mg L−1 | 192 (0.01) | 2706 (0.00) | 16,540 (1.20) | 2361 (0.10) |
| TCOD | mg−1 | 1310 | 7460 | 17,980 | 22,800 |
| Protein | mg L−1 | 775 (0.00) | 5513 (0.00) | 96,340 (0.00) | 7220 (0.00) |
| Experiment | TCOD Initial (mg L−1) | TCOD Final (mg L−1) |
|---|---|---|
| SH | 696 (255) | 608 (88) |
| CW | 8725 (248) | 6525 (1789) |
| Acetic | Propionic | Isobutyric | Butyric | Isovaleric | Valeric | Isocaproic | Caproic | Heptanoic | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| CW_original | 1248 | 1263 | 617 | 11,267 | 1878 | 229 | 169 | 251 | 169 | 17,092 |
| CW_heat | 1716 | 1365 | 566 | 11,099 | 1726 | 199 | 121 | 209 | 46 | 17,047 |
| CW_UV | 1124 | 1089 | 531 | 10,241 | 1675 | 156 | 123 | 215 | 39 | 15,194 |
| WL-original | 2257 | 608 | 258 | 2493 | 494 | 212 | 133 | 305 | 37 | 6797 |
| WL-heat | 1580 | 464 | 225 | 1879 | 428 | 181 | 130 | 290 | 39 | 5216 |
| WL-UV | 1498 | 415 | 207 | 1723 | 385 | 161 | 105 | 251 | 36 | 4779 |
| Effective Concentration | CW No Treatment | CW UV | CW Heat | WL No Treatment | WL UV | WL Heat |
|---|---|---|---|---|---|---|
| EC50 | 2000 | 696 | 780 | 1036 | 685 | 682 |
| EC90 | 5780 | 3832 | 2202 | 4936 | 3145 | 1880 |
| Compound Class | Specific Compound | Target Fungus | MFC | MIC | MFC/MIC Ratio | Reference |
|---|---|---|---|---|---|---|
| Fatty Acids | ||||||
| FAME extract | Mixed fatty acids | Aspergillus fumigatus | 16,000 | 8000 | 2.0 | [55] |
| FAME extract | Mixed fatty acids | Aspergillus niger | 16,000 | 8000 | 2.0 | [55] |
| Hydroxy fatty acids | Various | Multiple fungi | 10–100 | - | - | [28] |
| Strobilurins | ||||||
| Azoxystrobin | Pure compound | Passalora fulva (sensitive) | - | 0.031–0.5 | - | [56] |
| Azoxystrobin | Pure compound | Passalora fulva (resistant) | - | 8–32 | - | [56] |
| Azoxystrobin | Pure compound | Rhizoctonia solani | - | 10 (100% inhibition) | - | [57] |
| Tebuconazole + Trifloxystrobin | Combined formulation | Rhizoctonia solani | - | 10 (100% inhibition) | - | [57] |
| Azoxystrobin + Difenoconazole | Combined formulation | Rhizoctonia solani | - | 14 (100% inhibition) | - | [57] |
| Azoles | ||||||
| PC1244 | Novel triazole | Aspergillus fumigatus | 0.14 | 0.064 | 2.2 | [58] |
| Posaconazole | Triazole | Aspergillus fumigatus | 0.42 | 0.125 | 3.4 | [58] |
| Voriconazole | Triazole | Aspergillus fumigatus | >32 | 1.67 | >19 | [58] |
| Clotrimazole | Imidazole | Microsporum gallinae | 1.00 | 0.50 | 2.0 | [59] |
| Ketoconazole | Imidazole | Microsporum gallinae | 1.00 | 0.50 | 2.0 | [59] |
| Miconazole | Imidazole | Microsporum gallinae | 1.00 | 0.50 | 2.0 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshemese, Z.; Buzón-Durán, L.; García-González, M.C.; Deenadayalu, N.; Molinuevo-Salces, B. A Novel Food Wastewater Treatment Approach: Developing a Sustainable Fungicide for Agricultural Use. Fermentation 2025, 11, 189. https://doi.org/10.3390/fermentation11040189
Tshemese Z, Buzón-Durán L, García-González MC, Deenadayalu N, Molinuevo-Salces B. A Novel Food Wastewater Treatment Approach: Developing a Sustainable Fungicide for Agricultural Use. Fermentation. 2025; 11(4):189. https://doi.org/10.3390/fermentation11040189
Chicago/Turabian StyleTshemese, Zikhona, Laura Buzón-Durán, María Cruz García-González, Nirmala Deenadayalu, and Beatriz Molinuevo-Salces. 2025. "A Novel Food Wastewater Treatment Approach: Developing a Sustainable Fungicide for Agricultural Use" Fermentation 11, no. 4: 189. https://doi.org/10.3390/fermentation11040189
APA StyleTshemese, Z., Buzón-Durán, L., García-González, M. C., Deenadayalu, N., & Molinuevo-Salces, B. (2025). A Novel Food Wastewater Treatment Approach: Developing a Sustainable Fungicide for Agricultural Use. Fermentation, 11(4), 189. https://doi.org/10.3390/fermentation11040189







