Functional Component Production Capabilities in Milk Fermentation of Some Featured Lactic Acid Bacteria Species for Use in Different Food Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Activation and Preparation of Featured Strains
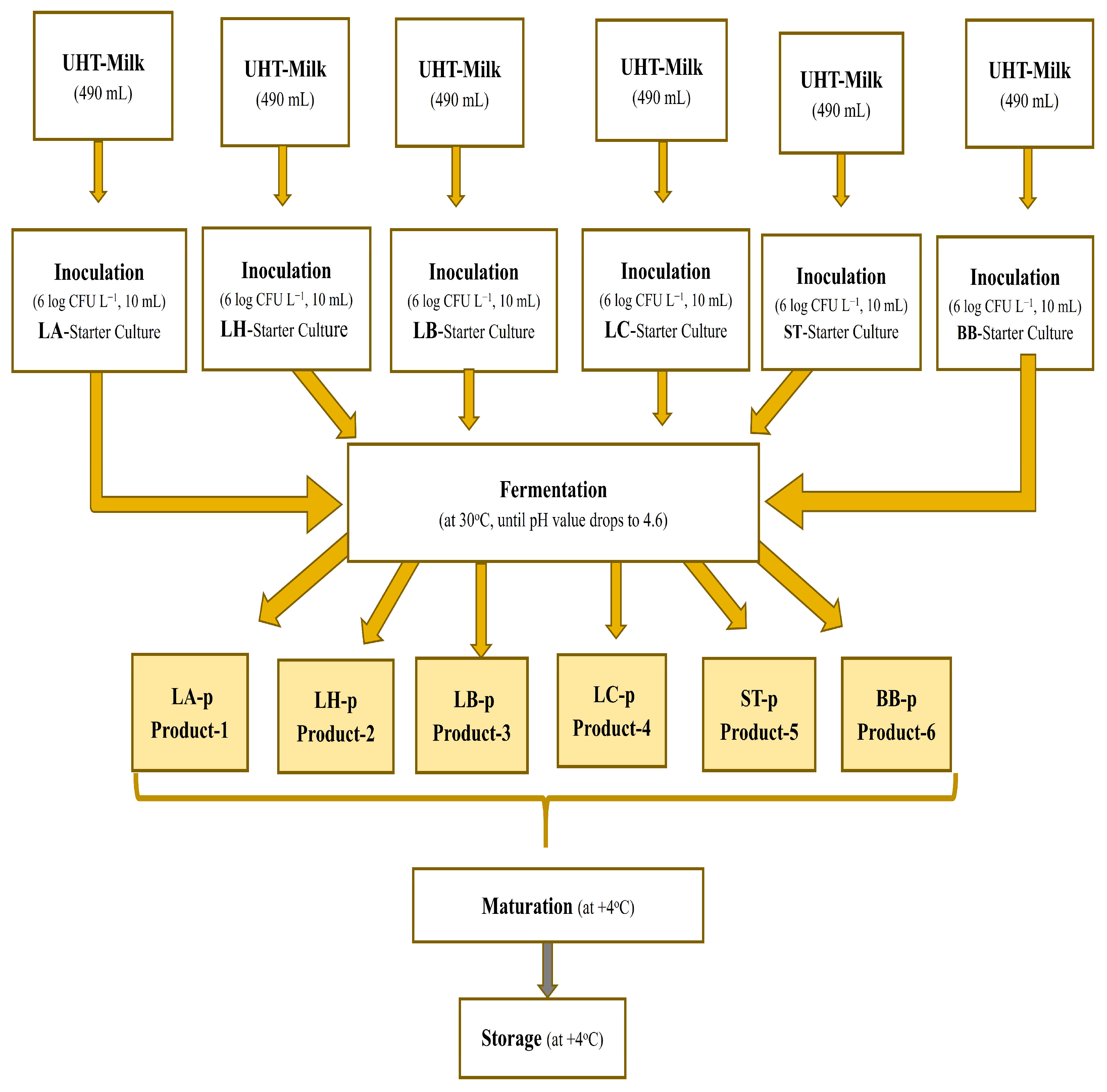
2.3. Production of Fermented Products with Six Featured Strains
2.4. Lactic Acid Bacteria Count and Physicochemical Analysis
2.5. Rheological Analysis
2.6. Organic Acid and Sugar Analysis
2.7. Fat-Soluble Vitamin Analysis
2.8. Preparation of Standard Solutions and Calibration Standards
2.9. Linearity and Sensitivity
2.10. Volatile Compound Analysis
2.11. Sensory Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Lactic Acid Bacteria Counts and Physicochemical Properties of Fermented Products
3.2. Organic Acid Profiles of Fermented Samples
3.3. Sugar Profiles of Fermented Samples
3.4. Vitamin A, D, E, K Contents of Fermented Samples
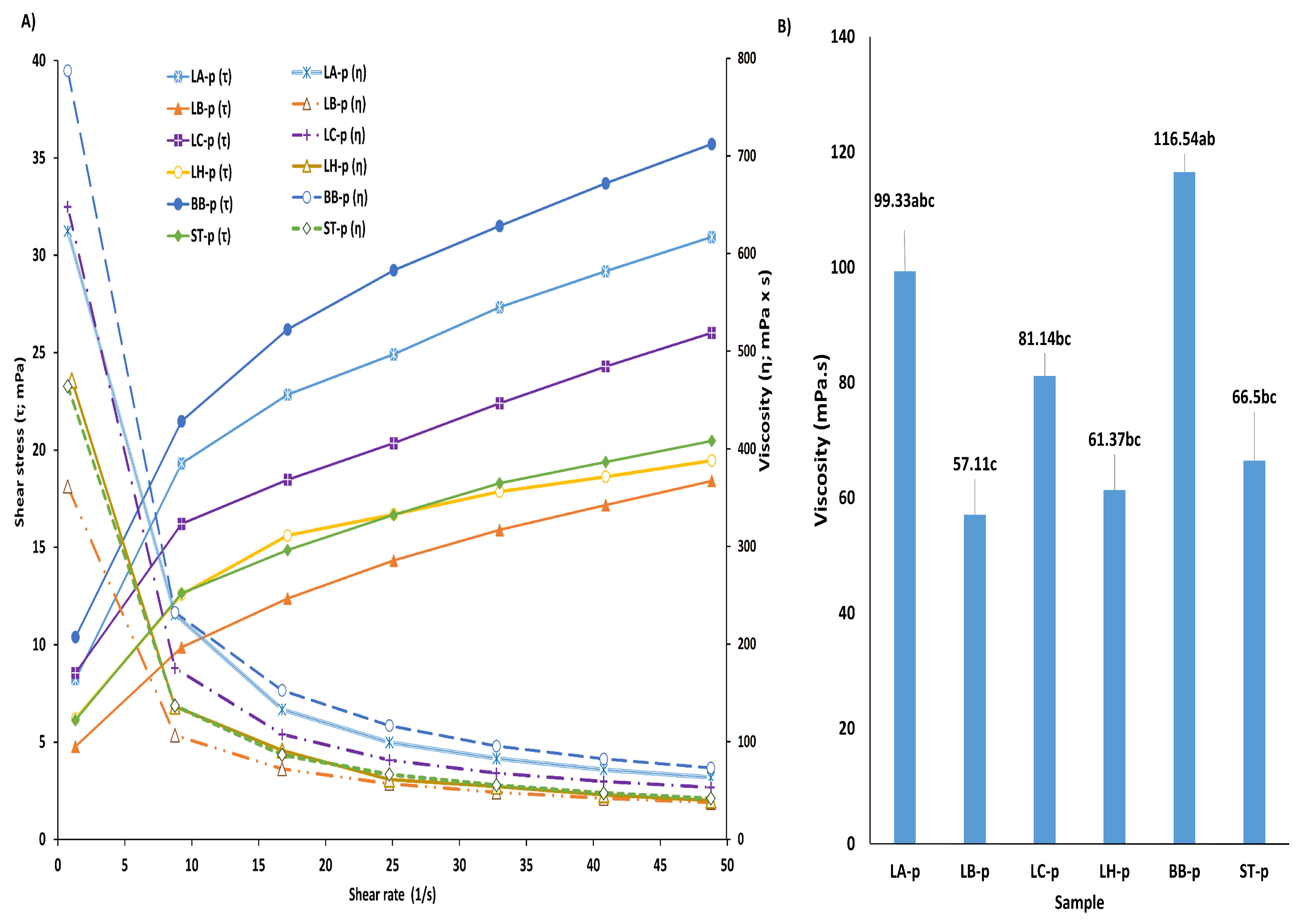
3.5. Rheological Properties of Fermented Samples
3.6. The Volatile Compounds Related to the Aroma Profiles of the Fermented Samples
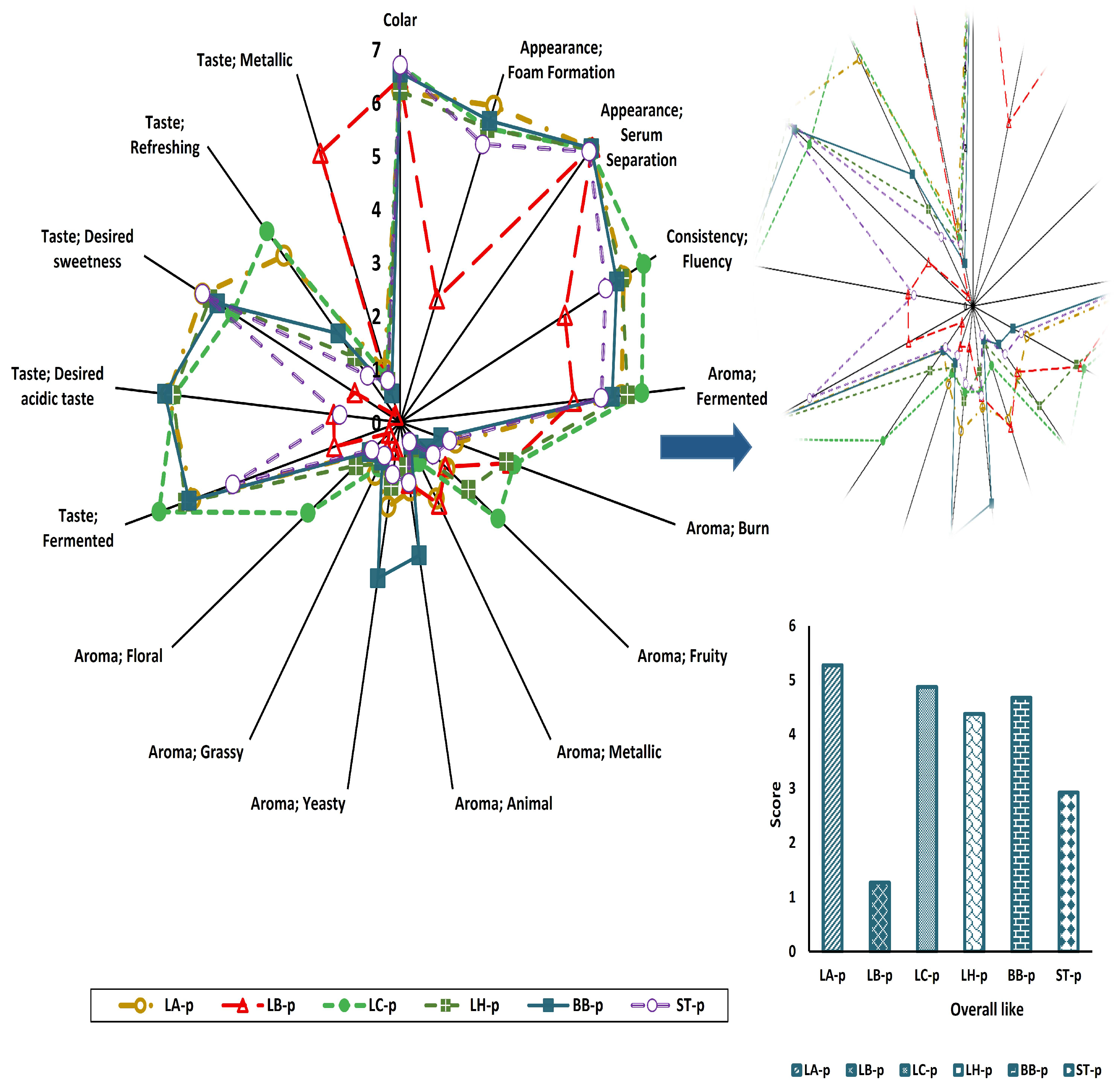
3.7. Sensory Profiles of Fermented Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Sarkar, P.; Ray, R.C.; Singh, S.P.; Rai, A.K. Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10730–10748. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented foods: An update on evidence-based health benefits and future perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- Bangar, S.P.; Tomar, M.; Singh, R.B.; Singh, P.; Kumar, M.; Chaudhary, V.; Trif, M.; Prajapati, U.; Dey, A.; Maqsood, S. Fermented dairy products: A food matrix to deliver bioactive compounds. J. Funct. Foods 2022, 89, 104906. [Google Scholar]
- Dopazo, V.; Musto, L.; de Melo Nazareth, T.; Lafuente, C.; Meca, G.; Luz, C. Revalorization of rice bran as a potential ingredient for reducing fungal contamination in bread by lactic acid bacterial fermentation. Food Biosci. 2024, 58, 103703. [Google Scholar] [CrossRef]
- Gizachew, S.; Van Beeck, W.; Spacova, I.; Dekeukeleire, M.; Alemu, A.; Woldemedhin, W.M.; Mariam, S.H.; Lebeer, S.; Engidawork, E. Antibacterial and Immunostimulatory Activity of Potential Probiotic Lactic Acid Bacteria Isolated from Ethiopian Fermented Dairy Products. Fermentation 2023, 9, 258. [Google Scholar] [CrossRef]
- Doo, H.; Kwak, J.; Keum, G.B.; Ryu, S.; Choi, Y.; Kang, J.; Lee, J.H. Lactic acid bacteria in Asian fermented foods and their beneficial roles in human health. Food Sci. Biotechnol. 2024, 33, 2021–2033. [Google Scholar] [CrossRef]
- Joshi, T.J.; Salini, S.V.; Mohan, L.; Nandagopal, P.; Arakal, J.J. Functional metabolites of probiotic lactic acid bacteria in fermented dairy products. Food Hum. 2024, 3, 100341. [Google Scholar]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The role of Lactobacillus in inflammatory bowel disease: From actualities to prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar]
- Kang, M.J.; Baek, K.R.; Lee, Y.R.; Kim, G.H.; Seo, S.O. Production of vitamin K by wild-type and engineered microorganisms. Microorganisms 2022, 10, 554. [Google Scholar] [CrossRef]
- Khromova, N.Y.; Epishkina, J.M.; Karetkin, B.A.; Khabibulina, N.V.; Beloded, A.V.; Shakir, I.V.; Panfilov, V.I. The combination of in vitro assessment of stress tolerance ability, autoaggregation, and vitamin B-producing ability for new probiotic strain introduction. Microorganisms 2022, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Bhushan, B.; Kokkiligadda, A.; Kumar, V.; Behare, P.; Tomar, S.K. Vitamin B12 biofortification of soymilk through optimized fermentation with extracellular B12 producing Lactobacillus isolates of human fecal origin. Curr. Res. Food Sci. 2021, 4, 646–654. [Google Scholar]
- LeBlanc, J.G.; Laiño, J.E.; del Valle, M.J.; de Giori, G.S.; Sesma, F.; Taranto, M.P. B-group vitamins production by probiotic lactic acid bacteria. In Biotechnology of Lactic Acid Bacteria: Novel Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 279–296. [Google Scholar]
- Altuncu, V.; Kaymaz, A.; Ertekin Filiz, B.; Çubuk Demiralay, E.; Kök Taş, T. Evaluation of MENAQUINONE-7 and fat-soluble vitamin production by starter cultures during fermentation in dairy products using RPLC method. Food Sci. Nutr. 2024, 12, 9822–9833. [Google Scholar]
- Minari, G.D.; Piazza, R.D.; Sass, D.C.; Contiero, J. EPS Production by Lacticaseibacillus casei Using Glycerol, Glucose, and Molasses as Carbon Sources. Microorganisms 2024, 12, 1159. [Google Scholar] [CrossRef]
- Ali, M.S.; Darwish, A.A.; Hassan, M.A.; Hekal, S.A.; Abdel-Mobdy, A.E.; Bakry, A.M. Utilisation of Lacticaseibacillus casei ATCC 393-derived exopolysaccharide for camel milk yoghurt production and its potential anticancer and hepatoprotective properties. Int. J. Dairy Technol. 2024, 77, 792–803. [Google Scholar]
- Silva, L.A.; Lopes Neto, J.H.P.; Cardarelli, H.R. Exopolysaccharides produced by Lactobacillus plantarum: Technological properties, biological activity, and potential application in the food industry. Ann. Microbiol. 2019, 69, 321–328. [Google Scholar]
- Hu, S.M.; Zhou, J.M.; Zhou, Q.Q.; Li, P.; Xie, Y.Y.; Zhou, T.; Gu, Q. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus rhamnosus ZFM231 isolated from milk. LWT 2021, 147, 111561. [Google Scholar]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar]
- Wen, L.; Yang, L.; Chen, C.; Li, J.; Fu, J.; Liu, G.; Cao, Y. Applications of multi-omics techniques to unravel the fermentation process and the flavor formation mechanism in fermented foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 8367–8383. [Google Scholar]
- Kayitesi, E.; Onojakpor, O.; Moyo, S.M. Highlighting the impact of lactic-acid-bacteria-derived flavours or aromas on sensory perception of African fermented cereals. Fermentation 2023, 9, 111. [Google Scholar] [CrossRef]
- Duran, F.E.; Özdemir, N.; Güneşer, O.; Kök-Taş, T. Prominent strains of kefir grains in the formation of volatile compound profile in milk medium; the role of Lactobacillus kefiranofaciens subsp. kefiranofaciens, Lentilactobacillus kefiri and Lentilactobacillus parakefiri. Eur. Food Res. Technol. 2022, 248, 975–989. [Google Scholar]
- Gao, H.; Yang, B.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, H.; Chen, W. Linoleic acid induces different metabolic modes in two Bifidobacterium breve strains with different conjugated linoleic acid-producing abilities. LWT 2021, 142, 110974. [Google Scholar]
- Guo, S.; Chen, M.; Wu, T.; Liu, K.; Zhang, H.; Wang, J. Probiotic Bifidobacterium animalis ssp. lactis Probio-M8 improves the properties and organic acid metabolism of fermented goat milk. J. Dairy Sci. 2022, 105, 9426–9438. [Google Scholar] [PubMed]
- ICH. Validation of Analytical Procedures: Text and MethodologyQ2 (R1) Harmonized Tripartite Guideline; ICH: Geneva, Switzerland, 2005. [Google Scholar]
- ISO 6658; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- Rezaei, A.; Alirezalu, K.; Damirchi, S.A.; Hesari, J.; Papademas, P.; Domínguez, R.; Lorenzo, J.M.; Yaghoubi, M. Effect of Pasteurization and Ripening Temperature on Chemical and Sensory Characteristics of Traditional Motal Cheese. Fermentation 2020, 6, 95. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Shah, N.P. Functional Cultures and Health Benefits. In Probiotic Dairy Products, 2nd ed.; Tamime, A.Y., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 27–58. [Google Scholar]
- Vodnar, D.C.; Paucean, A.; Dulf, F.V.; Socaciu, C. HPLC characterization of lactic acid formation and FTIR fingerprint of probiotic bacteria during fermentation processes. Not. Bot. Horti Agrobot. 2010, 38, 109–113. [Google Scholar]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar]
- Ranadheera, R.D.; Baines, S.K.; Adams, M.C. Importance of Food in Probiotic Efficacy. Food Res. Int. 2012, 48, 356–361. [Google Scholar]
- Siddiqi, M.; Tarrah, A.; Chen, Z.H.; LaPointe, G. Phenotypic Differentiation of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus Isolates Found in Yogurt Starter Cultures. Fermentation 2024, 10, 601. [Google Scholar] [CrossRef]
- Özcelik, S.; Kuley, E.; Özogul, F. Formation of lactic, acetic, succinic, propionic, formic and butyric acid by lactic acid bacteria. LWT 2016, 73, 536–542. [Google Scholar]
- Li, H.; Pajor, A.M. Functional characterization of CitM, the Mg2+-citrate transporter. J. Membr. Biol. 2002, 185, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavor Formation by Lactic Acid Bacteria and Biochemical Flavor Profiling of Cheese Products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, K.V.; Glazunova, O.A.; Savinova, O.S.; Shabaev, A.V.; Fedorova, T.V. Changes in composition of some bioactive molecules upon inclusion of Lacticaseibacillus paracasei probiotic strains into a standard yogurt starter culture. Foods 2023, 12, 4238. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology, 3rd ed.; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Chramostová, K.; Štětina, J.; Čurda, L. Effect of Cultivation Conditions on Organic Acid Production by Probiotic Strains. Czech J. Food Sci. 2014, 32, 521–526. [Google Scholar]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc: Characteristics, Use in Dairy Technology and Prospects in Functional Foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Ollilainen, V.; Heinonen, M.; Linkola, E.; Varo, P.; Koivistoinen, P. Carotenoids and Retinoids in Finnish Foods: Dairy Products and Eggs. J. Dairy Sci. 1989, 72, 2257–2265. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Kaushik, S.; Wander, R.; Leonard, S.; German, B.; Traber, M.G. Removal of Fat from Cow’s Milk Decreases the Vitamin E Contents of the Resulting Dairy Products. Lipids 2001, 36, 73–78. [Google Scholar] [CrossRef]
- Elder, S.J.; Haytowitz, D.B.; Howe, J.; Peterson, J.W.; Booth, S.L. Vitamin K Contents of Meat, Dairy, and Fast Food in the U.S. Diet. J. Agric. Food Chem. 2006, 54, 463–467. [Google Scholar] [PubMed]
- Zhou, T.; Huo, R.; Kwok, L.Y.; Li, C.; Ma, Y.; Mi, Z.; Chen, Y. Effects of Applying Lactobacillus helveticus H9 as Adjunct Starter Culture in Yogurt Fermentation and Storage. J. Dairy Sci. 2019, 102, 223–235. [Google Scholar] [PubMed]
- Santos, A.; San Mauro, M.; Sanchez, A.; Torres, J.M.; Marquina, D. The Antimicrobial Properties of Different Strains of Lactobacillus spp. Isolated from Kefir. Syst. Appl. Microbiol. 2003, 26, 434–443. [Google Scholar] [CrossRef]
- Khedr, O.M.; El-Sonbaty, S.M.; Moawed, F.S.; Kandil, E.I.; Abdel-Maksoud, B.E. Lactobacillus acidophilus ATCC 4356 Exopolysaccharides Suppresses Mediators of Inflammation through the Inhibition of TLR2/STAT-3/P38-MAPK Pathway in DEN-Induced Hepatocarcinogenesis in Rats. Nutr. Cancer 2022, 74, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from Lactic Acid Bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic Viability and Physico-Chemical and Sensory Properties of Plain and Stirred Fruit Yogurts Made from Goat’s Milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar]
- Joyner, H.S.; Daubert, C.R. Rheological Principles for Food Analysis. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 207–220. [Google Scholar]
- Penna, A.L.B.; Buriti, F.C.A.; de Oliveira, M.N. Rheological properties and microstructure of fat-free plain yogurt prepared with whey protein concentrate. Int. J. Dairy Technol. 2000, 53, 131–136. [Google Scholar]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Aznar, R.; Ferrer, S.; Zúñiga, M. Rheological properties and exopolysaccharide characterization of nonropy lactic acid bacteria isolated from wine. J. Agric. Food Chem. 2013, 61, 6538–6548. [Google Scholar]
- FEMA. Flavor and Extract Manufacturers Association (FEMA). FEMA. 2025. Available online: https://www.femaflavor.org/ (accessed on 24 February 2025).
- Zareba, S.; Ziarno, M.; Obiedzinski, M.W. Volatile compound profile in fermented and unfermented milk inoculated with Lactobacillus casei. Int. Dairy J. 2014, 39, 182–189. [Google Scholar]
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Chen, W. Volatile flavor compounds profile and fermentation characteristics of milk fermented by Lactobacillus delbrueckii subsp. bulgaricus strains. Front. Microbiol. 2019, 10, 2183. [Google Scholar]
- Li, T.; Tian, Y.; Zhao, B.; Zhang, H.; Chen, W.; Chen, H. Comparative study of volatile compounds in fermented milk produced by Lactobacillus plantarum or Bifidobacterium animalis subsp. lactis combined with Streptococcus thermophilus. Molecules 2019, 24, 4184. [Google Scholar]


| Fermented Samples | LAB (log CFU/mL) | Solid (%) | pH | Titration Acidity (%) | Incubation Time (hour) |
|---|---|---|---|---|---|
| * LA-p | 9.17 ± 0.03 ab** | 9.70 ± 0.22 b | 4.62 ± 0.01 a | 0.90 ± 0.05 ab | 18 |
| LB-p | 9.52 ± 0.28 a | 9.98 ± 0.05 a | 4.62 ± 0.02 a | 0.84 ± 0.11 b | 25 |
| LC-p | 9.23 ± 0.09 ab | 9.78 ± 0.04 ab | 4.63 ± 0.02 a | 0.98 ± 0.09 a | 20 |
| LH-p | 8.58 ± 0.15 c | 10.02 ± 0.04 a | 4.62 ± 0.03 a | 0.86 ± 0.07 b | 28 |
| BB-p | 9.16 ± 0.02 ab | 9.66 ± 0.32 b | 4.61 ± 0.02 a | 0.82 ± 0.07 b | 16 |
| ST-p | 9.05 ± 0.08 bc | 9.92 ± 0.03 a | 4.62 ± 0.02 a | 0.76 ± 0.04 c | 28 |
| (A) Organic Acid Profiles of the Fermented Samples ** (g/100 mL) | |||||||||
| Fermented Samples | Lactic Acid | Acetic Acid | Sitric Acid | Formic Acid | |||||
| * LA-p | 0.744 ± 0.028 ab** | 0.543 ± 0.065 c | 0.098 ± 0.035 c | 0.152 ± 0.015 ab | |||||
| LB-p | 0.637 ± 0.004 bc | 0.523 ± 0.003 c | 0.166 ± 0.001 a | 0.163 ± 0.014 a | |||||
| LC-p | 0.783 ± 0.066 a | 0.559 ± 0.032 b | 0.068 ± 0.001 d | 0.131 ± 0.011 b | |||||
| LH-p | 0.668 ± 0.016 bc | 0.551 ± 0.028 b | 0.125 ± 0.007 b | 0.134 ± 0.006 b | |||||
| BB-p | 0.722 ± 0.044 b | 0.607 ± 0.044 a | 0.166 ± 0.009 a | 0.164 ± 0.009 a | |||||
| ST-p | 0.574 ± 0.044 c | 0.594 ± 0.004 a | 0.168 ± 0.012 a | 0.139 ± 0.008 b | |||||
| (B) Sugar Profiles of the Fermented Samples ** (g/100 mL) | |||||||||
| Fermented Samples | Glucose | Lactose | Galactose | ||||||
| * LA-p | 0.674 ± 0.098 a | 1.963 ± 0.322 ab | 0.124 ± 0.008 bc | ||||||
| LB-p | 0.672 ± 0.333 a | 1.867 ± 0.617 b | 0.276 ± 0.069 a | ||||||
| LC-p | 0.453 ± 0.222 c | 1.704 ± 0.351 b | 0.093 ± 0.003 c | ||||||
| LH-p | 0.453 ± 0.180 c | 2.151 ± 0.393 a | 0.118 ± 0.007 bc | ||||||
| BB-p | 0.456 ± 0.207 c | 1.878 ± 0.361 b | 0.103 ± 0.004 c | ||||||
| ST-p | 0.592 ± 0.258 b | 2.085 ± 0.610 ab | 0.184 ± 0.028 b | ||||||
| (C) Vitamin A, D, E, K1, and K2 Contents of the Fermented Samples ** (µg/mL) | |||||||||
| Fermented Samples | Vitamin A | Vitamin D | Vitamin E | Vitamin K1 | Vitamin K2 | ||||
| * LA-p | 31.083 ± 0.041 ax | 3.723 ± 0.043 e | 1.009 ± 0.006 b | 0.232 ± 0.002 d | 0.070 ± 0.008 a | ||||
| LB-p | 16.326 ± 0.013 d | 5.070 ± 0.012 c | 0.949 ± 0.002 c | 0.704 ± 0.009 b | 0.030 ± 0.003 c | ||||
| LC-p | 23.893 ± 0.009 b | 7.073 ± 0.044 a | 1.087 ± 0.005 b | 0.803 ± 0.006 a | 0.014 ± 0.006 d | ||||
| LH-p | 16.549 ± 0.054 d | 3.854 ± 0.008 e | 0.976 ± 0.002 c | 0.644 ± 0.011 c | 0.048 ± 0.001 b | ||||
| BB-p | 20.119 ± 0.014 c | 4.667 ± 0.102 d | 1.135 ± 0.004 a | 0.805 ± 0.015 a | 0.028 ± 0.006 c | ||||
| ST-p | 13.253 ± 0.066 e | 6.448 ± 0.017 b | 0.972 ± 0.007 c | 0.869 ± 0.003 a | 0.029 ± 0.008 c | ||||
| Calibration Data | Linearity Range (μg/mL) | Slope (μg/mL) | Intercept (μg/mL) | r | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| Compounds | ||||||
| Vitamin A | 1.0–50 | 12,789 | 13,913 | 0.999 | 0.269 | 0.977 |
| Vitamin D | 0.5–15 | 44,423 | 1567.7 | 0.999 | 0.090 | 0.300 |
| Vitamin E | 0.25–7.5 | 48,611 | 54,328 | 0.999 | 0.062 | 0.208 |
| Vitamin K1 | 0.15–5.0 | 23,001 | −2111 | 0.999 | 0.040 | 0.132 |
| Vitamin K2 (MK-7) | 0.02–0.5 | 111,582 | −1804.3 | 0.999 | 0.003 | 0.011 |
| Lactic Acid | 500–3000 | 574.30 | −35,601 | 0.999 | 374.834 | 1249.450 |
| Acetic Acid | 250–1250 | 547.01 | 5814.7 | 0.999 | 21.023 | 70.077 |
| Sitric Acid | 50–300 | 1416.30 | −40,274 | 0.999 | 10.139 | 33.798 |
| Formic Acid | 100–500 | 908.21 | −2836.50 | 0.999 | 18.907 | 63.023 |
| Glucose | 100–2500 | 1255.40 | −60,924 | 0.999 | 25.263 | 84.543 |
| Lactose | 500–7500 | 1755.30 | −79,311 | 0.999 | 76.322 | 254.407 |
| Galactose | 100–1250 | 2237.50 | −252,460 | 0.999 | 12.143 | 40.477 |
| Chemical Names | Sample | |||||
|---|---|---|---|---|---|---|
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| Acetaldehyde | 1.64 ± 0.08 a* | 0.21 ± 0.09 c | 1.28 ± 0.49 a | n.d. | 0.60 ± 0.11 b | 0.40 ± 0.06 bc |
| Benzaldehyde | n.d. | 0.23 ± 0.08 | 0.13 ± 0.18 | n.d. | 2.68 ± 1.92 a | 0.15 ± 0.21 c |
| 2-Methylbutanal | 0.76 ± 0.04 f | 2.24 ± 0.15 d | 5.06 ± 0.18 c | 6.10 ± 0.18 b | 7.39 ± 0.38 a | 1.26 ± 0.21 e |
| 3-Methylbutanal | 0.24 ± 0.02 b | n.d. | 0.19 ± 0.07 c | n.d. | 0.20 ± 0.09 c | 0.36 ± 0.03 a |
| Nonanal | 0.11 ± 0.15 c | 0.50 ± 0.71 a | 0.29 ± 0.05 b | n.d. | 0.50 ± 0.21 a | 0.15 ± 0.02 c |
| Octanal | n.d. | n.d. | 0.11 ± 0.04 | n.d. | n.d. | n.d. |
| Total | 2.74 | 3.18 | 7.06 | 6.10 | 11.37 | 2.32 |
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| 2(5H)-Furanone | n.d. | n.d. | 0.11 ± 0.05 | n.d. | n.d. | n.d. |
| Diacetyl | 0.14 ± 0.02 b | n.d. | n.d. | n.d. | n.d. | 0.54 ± 0.07 a |
| Acetoin | 1.10 ± 0.36 b | 0.15 ± 0.21 b | 0.29 ± 0.01 c | 2.35 ± 0.25 a | 0.05 ± 0.07 | 1.17 ± 0.04 b |
| 3-Methyl-2-butanone | n.d. | 0.25 ± 0.03 c | 0.32 ± 0.06 b | n.d. | n.d. | 0.50 ± 0.07 a |
| 2-Heptanone | 0.49 ± 0.09 c | 1.31 ± 0.05 b | 1.46 ± 0.16 b | 3.37 ± 0.71 | 3.47 ± 1.53 a | 1.57 ± 0.21 b |
| 2-Nonanone | 0.50 ± 0.07 e | 1.00 ± 0.08 d | 1.71 ± 0.18 c | 6.27 ± 0.55 a | 3.58 ± 1.55 b | 1.41 ± 0.09 cd |
| 2-Nonen-4-one | n.d. | n.d. | 0.44 ± 0.04 | n.d. | n.d. | n.d. |
| 2-Pentanone | n.d. | 0.11 ± 0.15 c | n.d. | 0.72 ± 0.11 a | 0.34 ± 0.17 b | n.d. |
| Acetone | n.d. | 0.44 ± 0.02 b | 0.34 ± 0.13 bc | 0.27 ± 0.07 c | 0.59 ± 0.04 a | 0.30 ± 0.09 c |
| 2-Tridecanone | n.d. | n.d. | 0.09 ± 0.13 | 0.24 ± 0.04 | n.d. | n.d. |
| 3-Undecen-2-one | n.d. | n.d. | n.d. | 0.27 ± 0.09 | n.d. | n.d. |
| 2,3-Pentanedione | n.d. | n.d. | n.d. | n.d. | n.d. | 0.71 ± 0.02 |
| Methyl undecyl ketone | n.d. | 0.27 ± 0.08 b | 0.12 ± 0.07 c | 0.14 ± 0.02 c | 0.39 ± 0.05 a | 0.40 ± 0.06 a |
| Nonyl methyl ketone | 0.26 ± 0.07 d | 0.87 ± 0.05 c | 0.71 ± 0.18 c | 1.20 ± 0.22 b | 1.16 ± 0.68 a | 1.07 ± 0.52 b |
| Total | 2.52 | 4.43 | 5.52 | 14.91 | 9.65 | 7.76 |
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| 1-Butanol | n.d. | n.d. | n.d. | n.d. | n.d. | 0.66 ± 0.03 a |
| 3-Methyl-1-butanol | 44.42 ± 3.15 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-Propanol | n.d. | n.d. | n.d. | n.d. | n.d. | 0.45 ± 0.09 |
| Isobutyl alcohol | 4.20 ± 2.94 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-Furanmethanol | n.d. | 0.10 ± 0.15 d | 1.84 ± 0.90 a | 0.10 ± 0.14 b | 0.60 ± 0.02 c | n.d. |
| Phenethyl alcohol | 77.68 ± 3.04 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Capryl alcohol | 0.34 ± 0.08 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethanol | 64.07 ± 5.23 a | 0.58 ± 0.27 c | 0.24 ± 0.08 d | 0.12 ± 0.07 e | 0.46 ± 0.07 c | 1.30 ± 0.14 b |
| Hexanol | 0.76 ± 0.07 | n.d. | n.d. | n.d. | n.d. | 3.64 ± 0.08 |
| Lauryl alcohol | n.d. | 0.18 ± 0.05 | n.d. | n.d. | n.d. | n.d. |
| Nonan-2-ol | n.d. | n.d. | 0.32 ± 0.01 | n.d. | n.d. | n.d. |
| Total | 191.47 | 0.86 | 2.40 | 0.22 | 1.06 | 6.05 |
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| Acetic acid | n.d. | 1.02 ± 0.44 a | 0.55 ± 0.06 b | n.d. | n.d. | 0.67 ± 0.05 b |
| Butanoic acid | 2.96 ± 0.18 d | 10.46 ± 1.58 a | 6.41 ± 0.81 c | 8.14 ± 1.01 b | 11.14 ± 6.25 a | 7.09 ± 2.25 b |
| Total | 2.96 | 11.47 | 6.96 | 8.14 | 11.14 | 7.76 |
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| Isoamyl acetate | 0.67 ± 0.94 | n.d. | n.d. | n.d. | n.d. | n.d. |
| (Z)-5-Dodecenyl acetate | n.d. | n.d. | n.d. | 0.19 ± 0.27 | 0.22 ± 0.02 | n.d. |
| Acetic acid, 2-phenylethyl ester | 1.21 ± 0.71 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl butyrate | 1.51 ± 0.13 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl decanoate | 2.10 ± 0.98 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl Octanoate | 1.27 ± 0.80 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total | 6.76 | 0.00 | 0.00 | 0.19 | 0.22 | 0.00 |
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| β-Myrcene | n.d. | n.d. | 0.19 ± 0.04 b | 0.29 ± 0.09 a | 0.31 ± 0.06 a | n.d. |
| D-Limonene | n.d. | n.d. | 0.14 ± 0.08 c | 0.77 ± 0.03 a | 0.46 ± 0.07 b | 0.17 ± 0.03 c |
| Total | 0.00 | 0.00 | 0.33 | 1.06 | 0.77 | 0.17 |
| BB-p | LA-p | LB-p | LC-p | LH-p | ST-p |
| 2,5-Dimethylpyrazine | n.d. | n.d. | 0.25 ± 0.01 b | 0.21 ± 0.07 b | 0.35 ± 0.04 a | n.d. |
| Methylpyrazine | n.d. | n.d. | 0.32 ± 0.01 b | 0.21 ± 0.09 c | 0.42 ± 0.02 a | n.d. |
| Total | 0 | 0 | 0.57 | 0.42 | 0.77 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kök Taş, T.; Duran, F.E.; Özdemir, N.; Çubuk Demiralay, E. Functional Component Production Capabilities in Milk Fermentation of Some Featured Lactic Acid Bacteria Species for Use in Different Food Processes. Fermentation 2025, 11, 165. https://doi.org/10.3390/fermentation11040165
Kök Taş T, Duran FE, Özdemir N, Çubuk Demiralay E. Functional Component Production Capabilities in Milk Fermentation of Some Featured Lactic Acid Bacteria Species for Use in Different Food Processes. Fermentation. 2025; 11(4):165. https://doi.org/10.3390/fermentation11040165
Chicago/Turabian StyleKök Taş, Tuğba, Fatma Ecemnur Duran, Nilgün Özdemir, and Ebru Çubuk Demiralay. 2025. "Functional Component Production Capabilities in Milk Fermentation of Some Featured Lactic Acid Bacteria Species for Use in Different Food Processes" Fermentation 11, no. 4: 165. https://doi.org/10.3390/fermentation11040165
APA StyleKök Taş, T., Duran, F. E., Özdemir, N., & Çubuk Demiralay, E. (2025). Functional Component Production Capabilities in Milk Fermentation of Some Featured Lactic Acid Bacteria Species for Use in Different Food Processes. Fermentation, 11(4), 165. https://doi.org/10.3390/fermentation11040165






