Bioprospecting the Endophytic Fungus, Lasiodiplodia theobromae MMPI, for the Integrated Production of Mycoprotein and Exocellular (1→6)-β-Glucan
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation, Soy Molasses’ Clarification, and Rice Bran Extraction
2.2. Submerged Fermentation for the Co-Production of Mycelial Biomass and Lasiodiplodan
2.3. Analytical Methods
2.4. Characterization of Mycelial Biomass and Lasiodiplodan
2.4.1. Proximal Composition
2.4.2. Assessment of Total Phenolics, Antioxidant Activity, and Bioactive Compounds
2.4.3. Characterization of Mycelial Biomass and Lasiodiplodan by Scanning Electron Microscopy, Thermal Analysis, X-Ray Diffraction, and FT-IR Spectroscopy
3. Results and Discussions
3.1. Co-Production of Mycelial Biomass and Exocellular Lasiodiplodan by Lasiodiplodia theobromae MMPI
3.2. Validation of Predictive Models and Kinetic Study of the Cultivation of Lasiodiplodia theobromae MMPI in Media Based on Soybean Molasses and Sucrose
3.3. Proximal Composition and Profiles of Amino and Fatty Acids of the Mycelial Biomass from Lasiodiplodia theobromae MMPI
3.4. Profile of Phenolic Compounds in Extracts of Mycelial Biomass from Lasiodiplodia theobromae MMPI
3.5. Antioxidant Potential of Lasiodiplodia theobromae MMPI Biomass Extracts
3.6. Morphological Aspects of Mycelial Biomass and Lasiodiplodan
3.7. Thermal Profiles of Mycelial Biomass and Lasiodiplodan Samples
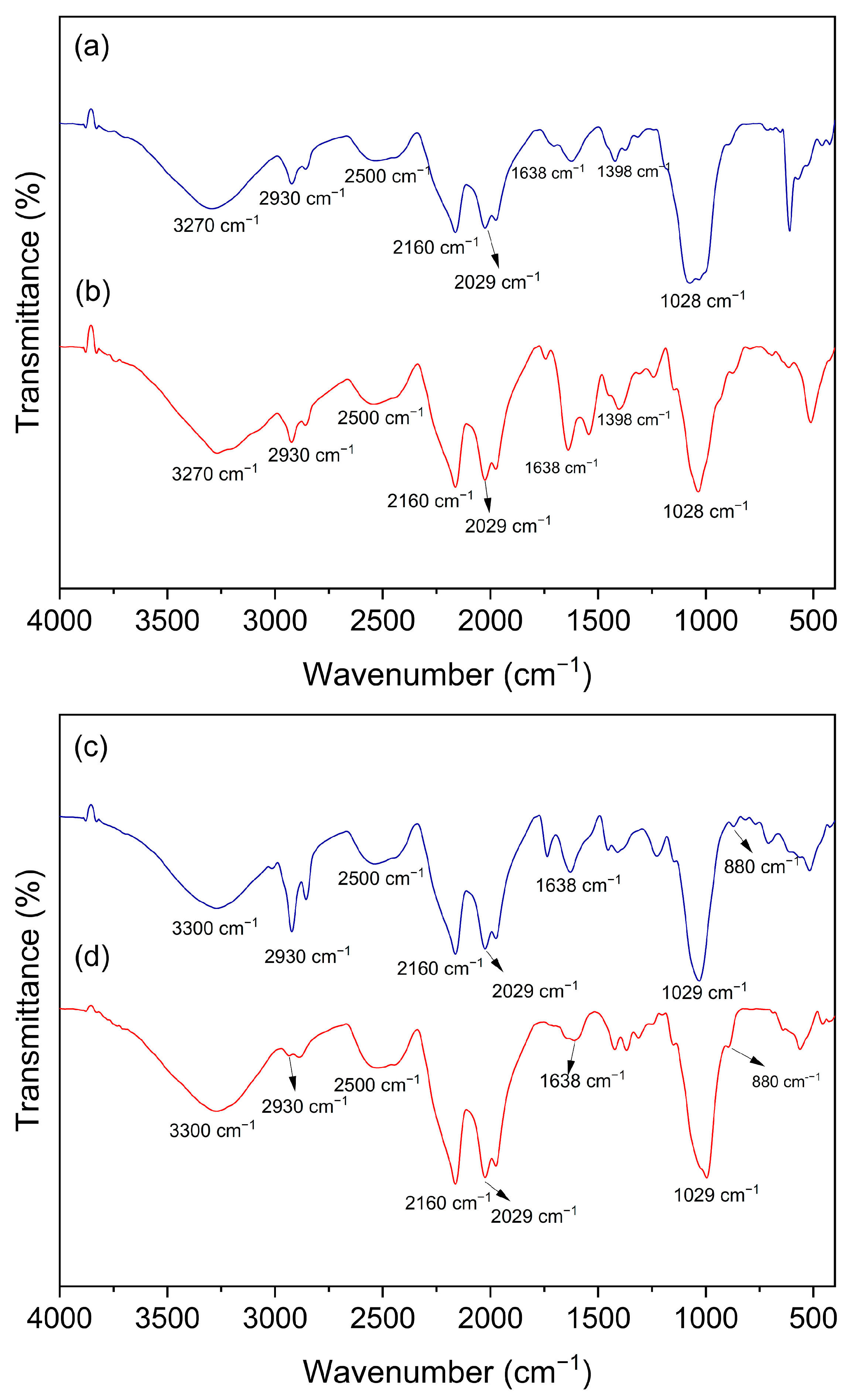
3.8. Infrared Spectroscopy
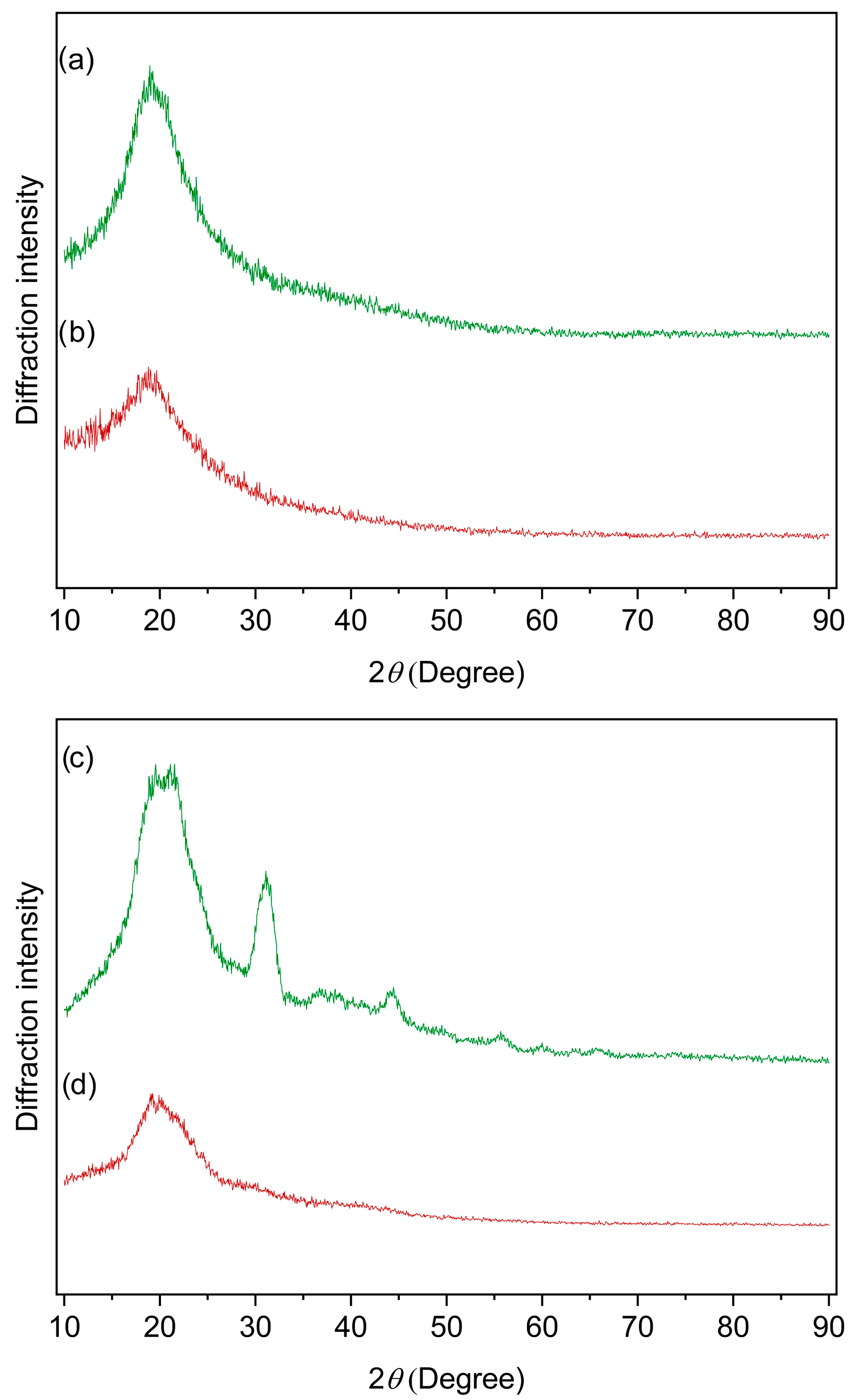
3.9. Diffractometric Profiles of Mycelial Biomass and Lasiodiplodan Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMM | Soybean molasses medium |
| SBM | Sucrose-based medium |
| RBE | Rice bran extract |
References
- Pexas, G.; Doherty, B.; Kyriazakis, I. The Future of Protein Sources in Livestock Feeds: Implications for Sustainability and Food Safety. Front. Sustain. Food Syst. 2023, 7, 1188467. [Google Scholar] [CrossRef]
- Gil, M.; Rudy, M.; Duma-Kocan, P.; Stanisławczyk, R.; Krajewska, A.; Dziki, D.; Hassoon, W.H. Sustainability of Alternatives to Animal Protein Sources, a Comprehensive Review. Sustainability 2024, 16, 7701. [Google Scholar] [CrossRef]
- United Nations. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 15 March 2022).
- Kumar, R.; Raj, T.; Næss, G.; Sørensen, M.; Dhawan, V. Opportunities and Challenges in Single-cell Protein Production Using Lignocellulosic Material. Biofuels Bioprod. Biorefining 2024, 18, 310–321. [Google Scholar] [CrossRef]
- USDA. Livestock and Products Annual; USDA: Washington, DC, USA, 2022.
- USDA. Poultry and Products Semi-Annual; USDA: Washington, DC, USA, 2024.
- Foreign Agricultural Service Production—Pork. Available online: https://fas.usda.gov/data/production/commodity/0113000 (accessed on 15 March 2022).
- Bahar, N.H.A.; Lo, M.; Sanjaya, M.; Van Vianen, J.; Alexander, P.; Ickowitz, A.; Sunderland, T. Meeting the Food Security Challenge for Nine Billion People in 2050: What Impact on Forests? Glob. Environ. Change 2020, 62, 102056. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; Volume 4. [Google Scholar]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate Change and Future of Agri-Food Production. In Future Foods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–79. [Google Scholar]
- Stoffel, F.; de Santana, W.O.; Gregolon, J.G.N.; Kist, T.B.L.; Fontana, R.C.; Camassola, M. Production of Edible Mycoprotein Using Agroindustrial Wastes: Influence on Nutritional, Chemical and Biological Properties. Innov. Food Sci. Emerg. Technol. 2019, 58, 102227. [Google Scholar] [CrossRef]
- Cunha, M.A.A.; Santos, V.A.Q.; Calegari, G.C.; Sánchez Luna, W.N.; Marin, S.L.A.; Dekker, R.F.H.; Barbosa-Dekker, A.M. Structure and Biological Properties of Lasiodiplodan: An Uncommon Fungal Exopolysaccharide of the (1→6)-β-D-Glucan Type. In Extracellular Sugar-Based Biopolymers Matrices. Biologically-Inspired Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 409–432. ISBN 978-3-030-12918-7. [Google Scholar]
- Calegari, G.C.; Santos, V.A.Q.; Barbosa-Dekker, A.M.; Busso, C.; Dekker, R.F.H.; Alves da Cunha, M.A. Sulfonated (1→6)-β-D-Glucan (Lasiodiplodan): Preparation, Characterization and Bioactive Properties. Food Technol. Biotechnol. 2019, 57, 490–502. [Google Scholar] [CrossRef]
- Heringer, H.C.E.; Kuhn Marchioro, M.L.; Meneguzzi, D.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Alves da Cunha, M.A. Valorization of Spent Brewers Yeast in the Integrated Production of the Fungal Exopolysaccharide (1→6)-β-D-Glucan (Lasiodiplodan) and Single-Cell Protein. Biocatal. Agric. Biotechnol. 2023, 54, 102971. [Google Scholar] [CrossRef]
- Sivieri, K.; de Oliveira, S.M.; de Marquez, A.S.; Pérez-Jiménez, J.; Diniz, S.N. Insights on β-Glucan as a Prebiotic Coadjuvant in the Treatment of Diabetes Mellitus: A Review. Food Hydrocoll. Health 2022, 2, 100056. [Google Scholar] [CrossRef]
- Carlson, J.; Erickson, J.; Hess, J.; Gould, T.; Slavin, J. Prebiotic Dietary Fiber and Gut Health: Comparing the in Vitro Fermentations of Beta-Glucan, Inulin and Xylooligosaccharide. Nutrients 2017, 9, 1361. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell Wall Glucans of Fungi. A Review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Vogel, H.J. A Convenient Growth Medium for Neurospora (Medium And). Microb. Genet. Bull. 1956, 13, 42–47. [Google Scholar]
- Acosta, S.B.P.; Marchioro, M.L.K.; Santos, V.A.Q.; Calegari, G.C.; Lafay, C.B.B.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; da Cunha, M.A.A. Valorization of Soybean Molasses as Fermentation Substrate for the Production of Microbial Exocellular β-Glucan. J. Polym. Environ. 2020, 28, 2149–2160. [Google Scholar] [CrossRef]
- da Silva, D.D.V.; Cândido, E.D.J.; de Arruda, P.V.; da Silva, S.S.; Felipe, M.d.G.d.A. New Cultive Medium for Bioconversion of C5 Fraction from Sugarcane Bagasse Using Rice Bran Extract. Braz. J. Microbiol. 2014, 45, 1469–1475. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 20th ed.; Latimer, G., Jr., Ed.; AOAC International: Rockville, MD, USA, 2016; Volume 52, ISBN 0935584870. [Google Scholar]
- Park, Y.K.; Koo, M.H.; Ikegaki, M.; Contado, J. Comparison of the Flavonoid Aglycone Contents of Apis mellifera Propolis from Various Regions of Brazil. Arq. Biol. Technol. 1997, 40, 97–106. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 5, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and Antioxidant Activity of Two Low-Molecular-Weight Polysaccharides Purified from the Fruiting Bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the Antioxidant Activity of Total Flavonoids Extract from Persimmon (Diospyros kaki L.) Leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Khatun, M.H.A.; Sajib, M.A.M.; Rahman, M.M.; Rahman, M.S.; Roy, M.; Miah, M.N.; Ahmed, K.U. Effect of Wheat Bran Supplement with Sugarcane Bagasse on Growth, Yield and Proximate Composition of Pink Oyster Mushroom (Pleurotus djamor). Am. J. Food Sci. Technol. 2015, 3, 150–157. [Google Scholar]
- El-Gayar, K.; Essa, A.; Abada, E. Whey Fermentation for Protease Production Using Bacillus thuringiensis Isolated from Mangrove Rhizosphere Soil in Jazan, Saudi Arabia. Pol. J. Environ. Stud. 2020, 29, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, N.B.; Yılmaz, N.; Kocabıyık, H.; Uygur, A. The Effect of Infrared Stabilized Rice Bran Substitution on B Vitamins, Minerals and Phytic Acid Content of Pan Breads: Part II. J. Cereal Sci. 2014, 59, 162–166. [Google Scholar] [CrossRef]
- Manzoor, A.; Pandey, V.K.; Dar, A.H.; Fayaz, U.; Dash, K.K.; Shams, R.; Ahmad, S.; Bashir, I.; Fayaz, J.; Singh, P.; et al. Rice Bran: Nutritional, Phytochemical, and Pharmacological Profile and Its Contribution to Human Health Promotion. Food Chem. Adv. 2023, 2, 100296. [Google Scholar] [CrossRef]
- Singh, U.; Gautam, A.; Singha, T.K.; Tiwari, A.; Tiwari, P.; Sahai, V.; Sharma, S. Mass Production of Pleurotus eryngii Mycelia under Submerged Culture Conditions with Improved Minerals and Vitamin D2. LWT 2020, 131, 109665. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Suhonen, A.; Ritala, A.; Oksman-Caldentey, K.M. The Role of Single Cell Protein in Cellular Agriculture. Curr. Opin. Biotechnol. 2022, 75, 102686. [Google Scholar] [CrossRef]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of Single Cell Protein from Orange Peel Residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Karimi, S.; Mahboobi Soofiani, N.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Filamentous Fungal Biomass Cultivated on Vinasse as an Alternative Nutrient Source of Fish Feed: Protein, Lipid, and Mineral Composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- Bardhan, P.; Gohain, M.; Daimary, N.; Kishor, S.; Chattopadhyay, P.; Gupta, K.; Chaliha, C.; Kalita, E.; Deka, D.; Mandal, M. Microbial Lipids from Cellulolytic Oleaginous Fungus Penicillium citrinum PKB20 as a Potential Feedstock for Biodiesel Production. Ann. Microbiol. 2019, 69, 1135–1146. [Google Scholar] [CrossRef]
- Altun, R.; Esim, N.; Aykutoglu, G.; Baltaci, M.O.; Adiguzel, A.; Taskin, M. Production of Linoleic Acid-Rich Lipids in Molasses-Based Medium by Oleaginous Fungus Galactomyces geotrichum TS61. J. Food Process. Preserv. 2020, 44, e14518. [Google Scholar] [CrossRef]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from Yeasts and Fungi: Physiology, Production and Analytical Considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef]
- Ali, T.H.; El-Gamal, M.S.; El-Ghonemy, D.H.; Awad, G.E.; Tantawy, A.E. Improvement of Lipid Production from an Oil-Producing Filamentous Fungus, Penicillium brevicompactum NRC 829, through Central Composite Statistical Design. Ann. Microbiol. 2017, 67, 601–613. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic Acid Inhibits Prostate Cancer Cell Proliferation and Metastasis by Suppressing the PI3K/Akt Pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef]
- van Rooijen, M.; Mensink, R. Palmitic Acid Versus Stearic Acid: Effects of Interesterification and Intakes on Cardiometabolic Risk Markers—A Systematic Review. Nutrients 2020, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Inocêncio, E.S.; Buarque, F.S.; Ferreira, L.F.R.; Soares, C.M.F.; Lima, Á.S.; Souza, R.L.d. Exploring the Potential of Licuri (Syagrus coronata) Using Sustainable Techniques and Solvents for Extracting Bioactive Compounds. Sustainability 2025, 17, 1507. [Google Scholar] [CrossRef]
- Vamanu, E. Antioxidant Properties of Mushroom Mycelia Obtained by Batch Cultivation and Tocopherol Content Affected by Extraction Procedures. BioMed Res. Int. 2014, 2014, 974804. [Google Scholar] [CrossRef]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of Ultrasonic Extraction to Obtain Erinacine A and Polyphenols with Antioxidant Activity from the Fungal Biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the Antioxidant Activity of Aqueous and Methanol Extracts of Pleurotus ostreatus in Different Growth Stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Muszyńska, B.; Sułkowska-Ziaja, K.; Kała, K.; Pawlik, A.; Stefaniuk, D.; Matuszewska, A.; Piska, K.; Pękala, E.; Kaczmarczyk, P.; et al. Medicinal Potential of Mycelium and Fruiting Bodies of an Arboreal Mushroom Fomitopsis officinalis in Therapy of Lifestyle Diseases. Sci. Rep. 2020, 10, 20081. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, C.; Jin, C.; Bao, Z.; Yang, G.; Jin, Y. Catechin from Green Tea Had the Potential to Decrease the Chlorpyrifos Induced Oxidative Stress in Larval Zebrafish (Danio rerio). Pestic. Biochem. Physiol. 2022, 182, 105028. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Disease: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Xu, H.J.; Zhang, Q.Y.; Wang, L.H.; Zhang, C.R.; Li, Y.; Zhang, Y.G. Growth Performance, Digestibility, Blood Metabolites, Ruminal Fermentation, and Bacterial Communities in Response to the Inclusion of Gallic Acid in the Starter Feed of Preweaning Dairy Calves. J. Dairy Sci. 2022, 105, 3078–3089. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.A.; Yang, J.; Ijaz, M.U.; Liu, Q.; Suleria, H.A.R.; Song, Y. Antioxidants Potential of the Filamentous Fungi (Mucor circinelloides). Nutrients 2017, 9, 1101. [Google Scholar] [CrossRef]
- Couttolenc, A.; Medina, M.E.; Trigos, Á.; Espinoza, C. Antioxidant Capacity of Fungi Associated with Corals and Sponges of the Reef System of Veracruz, Mexico. Electron. J. Biotechnol. 2022, 55, 40–46. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Nissola, C.; Marchioro, M.L.K.; de Souza Leite Mello, E.V.; Guidi, A.C.; de Medeiros, D.C.; da Silva, C.G.; de Mello, J.C.P.; Pereira, E.A.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; et al. Hydrogel Containing (1→6)-β-D-Glucan (Lasiodiplodan) Effectively Promotes Dermal Wound Healing. Int. J. Biol. Macromol. 2021, 183, 316–330. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Alaguprathana, M.; Poonkothai, M.; Ameen, F.; Ahmad Bhat, S.; Mythili, R.; Sudhakar, C. Sodium Hydroxide Pre-Treated Aspergillus flavus Biomass for the Removal of Reactive Black 5 and Its Toxicity Evaluation. Environ. Res. 2022, 214, 113859. [Google Scholar] [CrossRef]
- Popa, R.M.; Fetea, F.; Socaciu, C. ATR-FTIR-MIR Spectrometry and Pattern Recognition of Bioactive Volatiles in Oily versus Microencapsulated Food Supplements: Authenticity, Quality, and Stability. Molecules 2021, 26, 4837. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, K.; Li, Y.; Feng, Q. Fourier Transform Infrared Spectroscopy Analysis of the Active Components in Serum of Rats Treated with Zuogui Pill. J. Tradit. Chin. Med. Sci. 2015, 2, 264–269. [Google Scholar] [CrossRef][Green Version]
- Tan, C.S.; Leow, S.Y.; Ying, C.; Tan, C.J.; Yoon, T.L.; Jingying, C.; Yam, M.F. Comparison of FTIR Spectrum with Chemometric and Machine Learning Classifying Analysis for Differentiating Guan-Mutong a Nephrotoxic and Carcinogenic Traditional Chinese Medicine with Chuan-Mutong. Microchem. J. 2021, 163, 105835. [Google Scholar] [CrossRef]
- Olugbemide, A.D.; Oberlintner, A.; Novak, U.; Likozar, B. Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion. Sustainability 2021, 13, 10504. [Google Scholar] [CrossRef]
- Luna WN, S.; Santos, V.A.; Teixeira, S.D.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; da Cunha, M.A.A. O-Acetylated (1→6)-β-D-Glucan (Lasiodiplodan): Chemical Derivatization, Characterization and Antioxidant Activity. J. Pharm. Pharmacol. 2018, 6, 320–332. [Google Scholar] [CrossRef][Green Version]
- Arunprasath, T.; Sudalai, S.; Meenatchi, R.; Jeyavishnu, K.; Arumugam, A. Biodegradation of Triphenylmethane Dye Malachite Green by a Newly Isolated Fungus Strain. Biocatal. Agric. Biotechnol. 2019, 17, 672–679. [Google Scholar] [CrossRef]
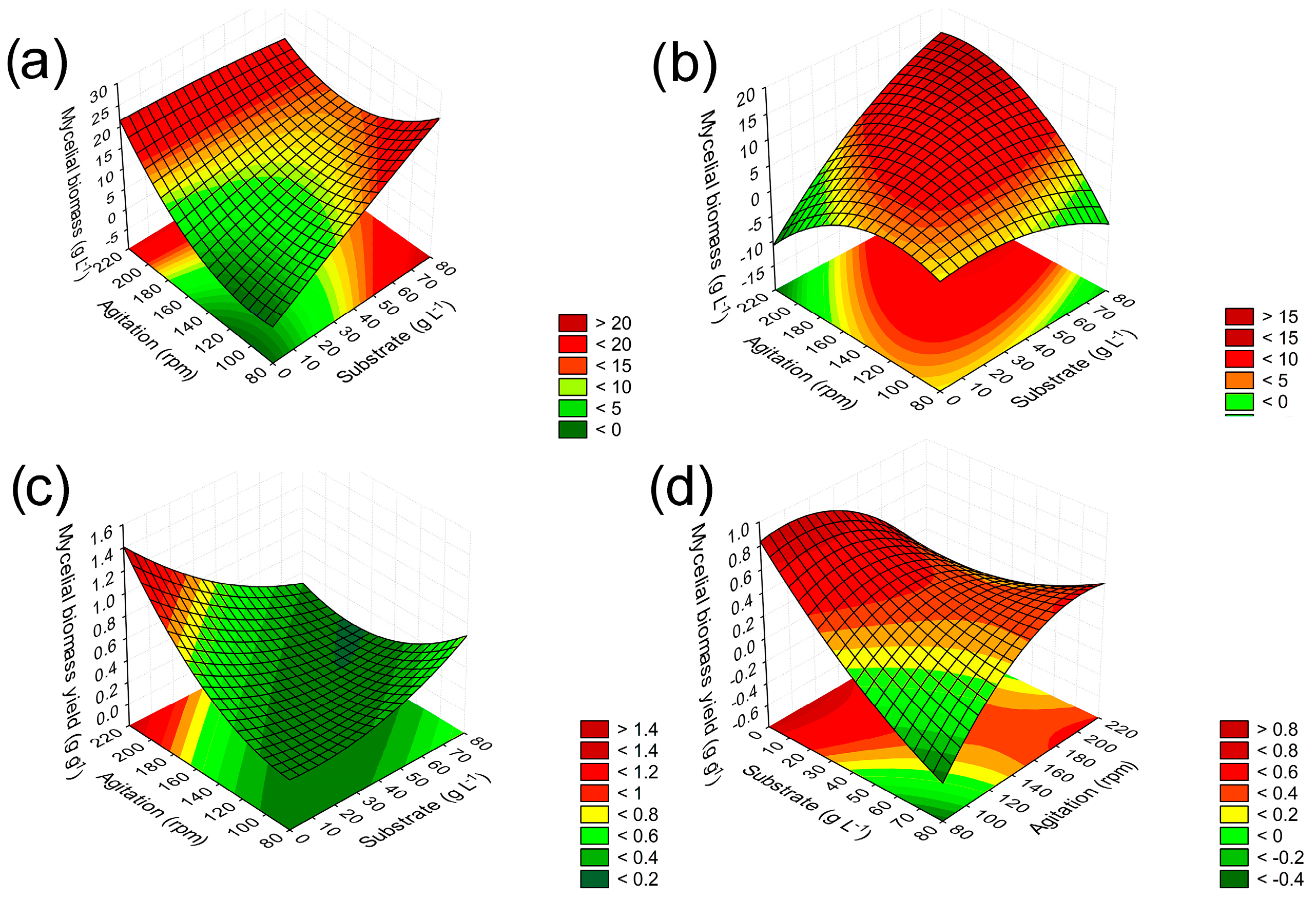
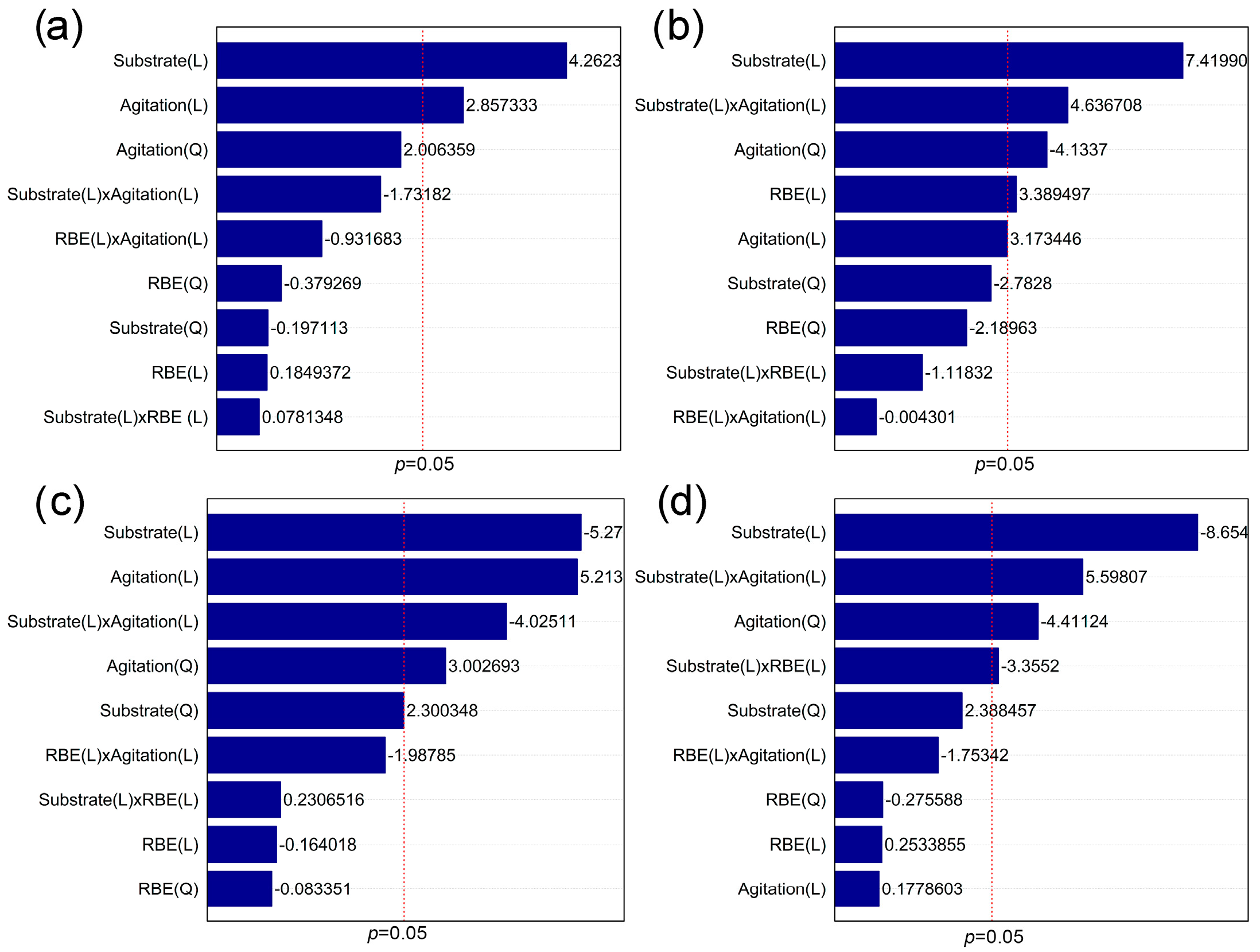




| Runs | Variable Levels | Values Obtained | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soy Molasses Medium | Sucrose-Based Medium | ||||||||||
| X1 | X2 | X3 | PX (g L−1) | PLas (g L−1) | YP/S (g g−1) | YX/S (g g−1) | PX (g L−1) | PLas (g L−1) | YP/S (g g−1) | YX/S (g g−1) | |
| 1 | −1 | −1 | −1 | 3.450 | 2.640 | 0.154 | 0.202 | 3.160 | 2.267 | 0.290 | 0.404 |
| 2 | −1 | −1 | 1 | 10.627 | 1.520 | 0.095 | 0.667 | 4.633 | 0.827 | 0.062 | 0.348 |
| 3 | −1 | 1 | −1 | 6.580 | 1.120 | 0.060 | 0.353 | 8.360 | 3.333 | 0.287 | 0.721 |
| 4 | −1 | 1 | 1 | 9.783 | 1.840 | 0.099 | 0.527 | 6.073 | 1.627 | 0.104 | 0.387 |
| 5 | 1 | −1 | −1 | 10.077 | 3.487 | 0.069 | 0.199 | 6.493 | 5.700 | 0.178 | 0.203 |
| 6 | 1 | −1 | 1 | 10.583 | 1.893 | 0.037 | 0.205 | 11.400 | 1.813 | 0.056 | 0.354 |
| 7 | 1 | 1 | −1 | 12.637 | 0.667 | 0.013 | 0.241 | 6.207 | 1.653 | 0.036 | 0.136 |
| 8 | 1 | 1 | 1 | 10.837 | 2.267 | 0.044 | 0.211 | 14.860 | 0.400 | 0.009 | 0.334 |
| 9 | −1.68 | 0 | 0 | 2.443 | 0.427 | 0.074 | 0.426 | 3.183 | 0.240 | 0.049 | 0.653 |
| 10 | 1.68 | 0 | 0 | 16.627 | 0.533 | 0.009 | 0.282 | 11.103 | 1.013 | 0.024 | 0.262 |
| 11 | 0 | −1.68 | 0 | 10.223 | 1.120 | 0.030 | 0.272 | 6.497 | 0.720 | 0.047 | 0.426 |
| 12 | 0 | 1.68 | 0 | 8.157 | 0.800 | 0.021 | 0.215 | 8.823 | 1.013 | 0.034 | 0.292 |
| 13 | 0 | 0 | −1.68 | 8.927 | 1.093 | 0.029 | 0.236 | 5.937 | 0.800 | 0.025 | 0.184 |
| 14 | 0 | 0 | 1.68 | 18.490 | 2.320 | 0.067 | 0.538 | 5.997 | 1.493 | 0.056 | 0.227 |
| 15 (C) | 0 | 0 | 0 | 8.110 | 0.560 | 0.016 | 0.230 | 11.205 | 0.760 | 0.029 | 0.423 |
| 16 (C) | 0 | 0 | 0 | 9.315 | 0.800 | 0.022 | 0.259 | 10.930 | 1.560 | 0.060 | 0.424 |
| 17 (C) | 0 | 0 | 0 | 9.475 | 0.520 | 0.014 | 0.251 | 9.425 | 1.000 | 0.037 | 0.351 |
| 18 (C) | 0 | 0 | 0 | 8.785 | 0.280 | 0.007 | 0.230 | 8.985 | 0.720 | 0.027 | 0.335 |
| Variables | Levels (real values) | ||||||||||
| −1.68 | −1 | 0 | 1 | 1.68 | |||||||
| X1 substrate (g L−1) | 6.4 | 20 | 40 | 60 | 73.6 | ||||||
| X2 RBE (%) | 1.6 | 5 | 10 | 15 | 18.4 | ||||||
| X3 agitation (rpm) | 99.6 | 120 | 150 | 180 | 200.4 | ||||||
| Soy Molasses Medium (SMM) | Sucrose (SBM) | |||||
|---|---|---|---|---|---|---|
| Variables | Critical Value | Experimental Value | Recovery (%) | Critical Value | Experimental Value | Recovery (%) |
| Substrate (g L−1) | 64.43 | 64.43 | - | 40.68 | 40.68 | |
| RBE (%) | 8.81 | 8.81 | - | 3.44 | 3.44 | |
| Agitation (rpm) | 152.83 | 152.83 | - | 161.22 | 161.22 | |
| PX (g L−1) | 12.13 | 12.44 | 102.6 | 7.90 | 10.09 | 127.7 |
| YX/S (g g−1) | 0.196 | 0.214 | 109.2 | 0.378 | 0.328 | 86.8 |
| Fermentation Parameters | Cultivation Medium | |
|---|---|---|
| SMM * | SBM ** | |
| PX (g L−1) | 12.440 a | 10.087 a |
| PF (g L−1) | 0.573 a | 0.547 a |
| TRS (g L−1) | 6.275 b | 9.964 a |
| YP/S (g g−1) | 0.010 a | 0.018 a |
| YX/S (g g−1) | 0.214 a | 0.328 a |
| Ye (g g−1) | 0.046 a | 0.054 a |
| YC (%) | 90.261 a | 75.507 b |
| QX (g L−1 h−1) | 0.130 a | 0.105 a |
| QP (g L−1 h−1) | 0.006 a | 0.006 a |
| QS (g L−1 h−1) | 0.606 a | 0.320 b |
| Proximal Composition # | |||||
|---|---|---|---|---|---|
| SMM * | SBM ** | SMM * | SBM ** | ||
| Moisture (% at 105 °C) | 9.5 | 9.7 | Dietary fiber | 7.5 | 17.0 |
| Crude protein | 16.27 | 19.88 | Total carbohydrates | 24.67 | 50.96 |
| Total fat | 43.77 | 7.56 | Mineral residue (ash) | 5.79 | 12.57 |
| Caloric value (Kcal 100 g−1) | 557.69 | 351.4 | |||
| Essential Amino Acids ## | |||||
| SMM * | SBM ** | SMM * | SBM ** | ||
| Histidine | 20.28 | 0.00 | Phenylalanine | 38.11 | 7.04 |
| Isoleucine | 49.17 | 16.10 | Threonine | 43.64 | 7.04 |
| Leucine | 97.11 | 39.24 | Tryptophan | 8.60 | 6.04 |
| Lysine | 91.58 | 19.11 | Valine | 61.46 | 18.61 |
| Methionine | 25.20 | 7.04 | |||
| Non-essential amino acids ## | |||||
| SMM * | SBM ** | SMM * | SBM ** | ||
| Aspartic acid | 119.85 | 30.18 | Tyrosine | 29.50 | 2.01 |
| Glutamic acid | 159.19 | 37.22 | Glycine | 51.63 | 8.05 |
| Alanine | 76.83 | 15.59 | Proline | 1.23 | 0.00 |
| Arginine | 86.05 | 11.07 | Serine | 71.30 | 11.57 |
| Cystine | 15.98 | 0 | |||
| Monounsaturated fatty acids (MUFA) # | |||||
| SMM * | SBM ** | SMM * | SBM ** | ||
| Elaidic acid (C18:1n9t) | 0.01 | - | Palmitoleic acid (C16:1n7) (ω -7) | 0.08 | 0.05 |
| Oleic acid (C18:1n9c) (ω-9) | 5.12 | 1.85 | cis-11-Eicosenoic acid (C20:1n9) | 0.04 | 0.01 |
| Polyunsaturated fatty acids (PUFA) # | |||||
| SMM * | SBM ** | SMM * | SBM ** | ||
| Linoleic acid (C18:2n6c) (ω-6) | 24.38 | 2.24 | Linoleic acid (C18:2n6t) | 0.02 | - |
| α-Linolenic acid (C18:3n3) (ω-3) | 2.99 | 0.16 | cis-11,14-Eicosadienoic acid (C20:2) | 0.04 | - |
| Saturated fatty acids (SFA) # | |||||
| SMM * | SBM ** | SMM * | SBM ** | ||
| Mystic acid (C14:0) | 0.04 | 0.03 | Caprylic acid (C8:0) | 0.12 | 0.00 |
| Pentadecanoic acid (C15:0) | 0.04 | 0.01 | Arachidic acid (C20:0) | 0.06 | 0.05 |
| Palmitic acid (C16:0) | 8.81 | 2.05 | Heneicosanoic acid (C21:0) | 0.02 | 0.00 |
| Margaric acid (C17:0) | 0.07 | 0.01 | Behenic acid (C22:0) | 0.14 | 0.03 |
| Stearic acid (C18:0) | 1.60 | 1.03 | Tricosanoic acid (C23:0) | 0.05 | 0.01 |
| Lignoceric acid (C24:0) | 0.14 | 0.03 | |||
| Total lipids # | |||||
| SMM * | SBM ** | SMM * | SBM ** | ||
| Monounsaturated | 5.25 | 1.91 | Saturated | 11.09 | 3.25 |
| Polyunsaturated | 27.43 | 2.40 | Trans lipids | 0.03 | 0.00 |
| Unsaturated | 32.68 | 4.31 | Total lipids | 43.77 | 7.56 |
| Antioxidant Assay | Antioxidant Capacity | |
|---|---|---|
| SMM | SBM | |
| ABTS (mmol TEq g−1) | 713.90 a | 741.89 a |
| DPPH (mmol TEq g−1) | 180.72 b | 187.95 a |
| OH (% reduction) | 84.56 a | 92.10 a |
| FRAP (mmol ferrous sulfate g−1) | 244.74 a | 230.81 a |
| TAC (mmol AAE g−1) | 147.72 a | 84.81 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchioro, M.L.K.; Candeia, G.A.P.B.; Bertoleti, L.M.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; da Cunha, M.A.A. Bioprospecting the Endophytic Fungus, Lasiodiplodia theobromae MMPI, for the Integrated Production of Mycoprotein and Exocellular (1→6)-β-Glucan. Fermentation 2025, 11, 166. https://doi.org/10.3390/fermentation11040166
Marchioro MLK, Candeia GAPB, Bertoleti LM, Barbosa-Dekker AM, Dekker RFH, da Cunha MAA. Bioprospecting the Endophytic Fungus, Lasiodiplodia theobromae MMPI, for the Integrated Production of Mycoprotein and Exocellular (1→6)-β-Glucan. Fermentation. 2025; 11(4):166. https://doi.org/10.3390/fermentation11040166
Chicago/Turabian StyleMarchioro, Marcelo Luis Kuhn, Gabrielli Aline Pietro Bom Candeia, Luana Malaquias Bertoleti, Aneli M. Barbosa-Dekker, Robert F. H. Dekker, and Mário Antônio Alves da Cunha. 2025. "Bioprospecting the Endophytic Fungus, Lasiodiplodia theobromae MMPI, for the Integrated Production of Mycoprotein and Exocellular (1→6)-β-Glucan" Fermentation 11, no. 4: 166. https://doi.org/10.3390/fermentation11040166
APA StyleMarchioro, M. L. K., Candeia, G. A. P. B., Bertoleti, L. M., Barbosa-Dekker, A. M., Dekker, R. F. H., & da Cunha, M. A. A. (2025). Bioprospecting the Endophytic Fungus, Lasiodiplodia theobromae MMPI, for the Integrated Production of Mycoprotein and Exocellular (1→6)-β-Glucan. Fermentation, 11(4), 166. https://doi.org/10.3390/fermentation11040166





