Abstract
Argentina ranks worldwide among the top ten wine producers, known for its diverse terroirs and Malbec as its emblematic varietal. Typically, the winemaking process involves alcoholic fermentation, led by yeasts, and malolactic fermentation (MLF), primarily driven by lactic acid bacteria (LAB). Oenococcus oeni and Lactiplantibacillus plantarum are recognised as the best-adapted LAB species for this process. Our previous research focused on a winery located in the southwest of Buenos Aires Province, a scarcely studied re-emerging region of Argentina, which showed a low relative abundance of LAB and incomplete MLF in various vintages. The current study involved the isolation, identification, typing, and use of native strains from the above-mentioned region to formulate a malolactic fermentation starter (MLFS) and to evaluate the strains’ malolactic performance at pilot-scale, implantation capacity and impact on wine aromatic profiles using HS-SPME-GC-FID/MS. Two selected autochthonous strains (Lpb. plantarum UNQLp1001 and a O. oeni UNQOe1101) from the re-emerging region successfully implanted in Malbec wine, achieving faster and more efficient MLF compared to spontaneous MLF. Moreover, the MLFS seems to have influenced the aromatic profile, reducing relative concentrations of alcohols, contributing to the decrease in the bitter and herbaceous notes, and increasing some esters (ethyl acetate, 2-phenethyl acetate, ethyl octanoate), that could enhance floral and fruity, notes. Expanding the availability of candidate strains to formulate native MLFS is a crucial technological tool for the wine industry. Thus, we propose the use of Lpb. plantarum UNQLp1001 and O. oeni UNQOe1101 as potential MLFS in Malbec wines from somewhat similar wine-producing regions. Additionally, the local winery can access a cost-effective MLFS with native LAB strains, enabling a more controlled MLF that preserves regional typicity. Moreover, these strains could enable technology transfer, potentially becoming the first malolactic starters in the region.
1. Introduction
The winemaking process usually involves both the alcoholic fermentation (AF), led by yeasts, and the malolactic fermentation (MLF), carried out by lactic acid bacteria (LAB). To expedite and regulate alcoholic fermentation (AF), it is common practice to employ strains of Saccharomyces yeasts as starter cultures. Particularly, Saccharomyces cerevisiae stands out for its high fermentative capacity and resilience to winemaking conditions [1]. Moreover, during MLF, LAB can contribute positively to wine flavour through decarboxylation of malic acid, with acidity reduction and other numerous enzymatic reactions. Among the LABs involved in MLF, the best adapted are Oenococcus oeni and Lactiplantibacillus plantarum [2,3,4].
Although MLF can occur spontaneously through the action of native LAB from the grapes and cellar, it carries risks such as an increase in the volatile acidity, consumption of residual sugars, and formation of undesirable metabolites such as biogenic amines [5,6,7]. To prevent this, a possible strategy is a guided MLF through the inoculation of MLF starter (MLFS) cultures, comprising autochthonous LAB, which are better adapted to a given wine, thus capable of preserving the terroir [2,8].
The development and use of MLFS depend on the characterisation, propagation, and conservation of suitable LAB strains for this purpose. Since MLF typically occurs after AF, the proliferation of LAB and the efficiency of MLF can be affected, for instance, by the addition of SO2 and by the production and release of ethanol, fatty acids, peptides, polysaccharides, and other molecules by yeasts (thoroughly reviewed by Virdis, Sumby [8]). Additionally, since different yeast strains vary in their nutrient requirements, the availability of nutrients for LAB will also influence the progression of MLF; a low nutrient demand by yeasts tends to favour the efficiency of MLF [9].
Argentina ranks worldwide among the “top 10” wine-producing countries, and its vineyards stretch over 3800 km from north to south (22° to 45° parallels south) and from east to west, ranging from the desertic regions of the Andes mountains, with some vineyards at altitudes exceeding 3300 m.a.s.l., to the more humid Pampa region at sea level. Variations in thermal amplitude, sunlight exposure, winds, and water availability, among other factors, result in an immense diversity of terroirs. The recent expansion of the Argentine viticulture frontier explores new horizons in search of wines that can meet consumer demands for a set of attributes, experiences, and sensations associated with these different terroirs. However, certain climatic challenges faced by the main wine regions, including water scarcity, rising temperatures, and drought conditions, have led to a decline in the total vineyard area in Argentina since 2015 [10].
Red grape varieties dominate Argentine wine production, with Malbec standing out as a particular highlight, achieving international recognition and acclaim. Malbec is a red grape variety originally from Cahors, Bordeaux (southwestern France), where it is known as Cot or Auxerrois. In Argentina, this grape variety exhibits distinct characteristics from its original terroir, influenced by differences in climate, soil, genetic traits of the plants, vineyard management, and winemaking methods. Malbec is renowned for its deep, intense colour and fruity flavour and aroma.
Previous findings by our research team, based on microbial diversity analysis using Next-Generation Sequencing (NGS) over three vintages (2017–2019), uncovered a notable scarcity of LAB and a prevalence of acetic acid bacteria (AAB) in Malbec wine samples from the winery under investigation (Saldungaray, Buenos Aires, Argentina). Additionally, the microbial diversity of wine seems to have decreased through the years. Nevertheless, it is plausible that LAB may be present at various stages of winemaking, albeit in limited numbers and with low diversity, as incomplete MLFs were observed during different vintages [11,12]. In light of these observations, the necessity arose to devise strategies aimed at modulating the bacterial community’s diversity to promote the optimal progression of MLF. Thus, it was proposed than an MLFS be employed, which is designed to enhance the presence and concentration of LAB, thereby facilitating a more controlled and efficient MLF.
The current study outlines the isolation of autochthonous LAB strains from both Malbec musts and wines from a re-emerging wine-producing region (Saldungaray, Buenos Aires, Argentina), with the aim to employ them as MLFS. Both a spontaneous MLF and a guided MLF were conducted, and the malolactic performance in Malbec wines was assessed on a pilot scale within the winery. The implantation capacity of the autochthonous MLFS formulated in this work was studied as a measure of its ability to guide the fermentation process. Additionally, the aromatic profiles of the wines obtained were compared using gas chromatography coupled with mass spectrometry.
2. Materials and Methods
2.1. LAB Isolation
The bacterial isolates were obtained from samples of Malbec must and wine at different stages of fermentation, from the Saldungaray winery (38°12′54.5″ S 61°46′36.3″ W, 194 m.a.s.l.), province of Buenos Aires, Argentina, from the 2019 vintage: must, final alcoholic fermentation (AF) and malolactic fermentation (MLF). Several enrichment cycles were carried out in MRS broth (Man Rogosa and Sharpe, Biokar Diagnostics, Allonne, France), and MLO according to Maicas et al. (1999) [13], both supplemented with 20 mg/L of nystatin (local provider) and 20 mg/L of cycloheximide (Merck SA, Darmstadt, Germany), for 7 days at 28 °C, under microaerophilic conditions. For each enrichment cycle, wine samples were incubated under the above-mentioned conditions in MRS to promote the growth of Lpb. plantarum strains, and in MLO to promote the growth of O. oeni strains. Axenic cultures were obtained by successive exhaustion streaks on solid media. Pure cultures were subjected to catalase testing and microscopic observation after Gram staining. Gram-positive, non-sporulating, catalase-negative isolates were selected as possible LAB.
Sovereign Rights Over Natural Resources: The LAB strains obtained are genetic resources belonging to the Province of Buenos Aires. They are deposited in the strain collection of the Molecular Microbiology Laboratory (IMBA–DCyT–UNQ) and registered with the Direction of Flora and Fauna—Ministry of Agrarian Development—Province of Buenos Aires. Their preservation follows the protocols of the Convention on Biological Diversity and the Nagoya Protocol, which safeguard the ownership and use of genetic resources from the regions where they were isolated (DISPO-2025-16-GDEBA-DFYFMDAGP).
2.2. Identification and Typing of Strains
The isolates were grown for 72 h (at 28 °C, in microaerophilic conditions) in the corresponding medium (MRS or MLO, depending on the origin of each isolate), to extract genomic DNA, according to Bravo-Ferrada et al. (2013) [14]. The integrity and quality of the DNA obtained were evaluated by electrophoresis in a 1% agarose gel, stained with GelRed (Genbiotech, Ciudad Autónoma de Buenos Aires, Argentina), and by measuring the absorbances at 260, 280, and 230 nm nanodrop (NanoDrop® ND-1000 Thermo Scientific, Wilmington, NC, USA). Each isolate was molecularly typed at the strain level, using Random Amplified Polymorphic-DNA (RAPD-PCR), with the primer M13 [15], according to Bravo-Ferrada et al. (2013) [14]. Those that showed unique electrophoretic patterns (different strains) were identified at the species level by sequencing a fragment of the 16S rRNA gene with the primers pA16SF and pH16SR [16] and comparing the sequences based on data (Genbank, NCBI).
2.3. Pilot-Scale Vinification
2.3.1. Preparation of Inocula for Malolactic Fermentation
Two strains from the Province of Buenos Aires were used for the formulation of an MLFS, a Lpb. plantarum (UNQLp1001) and a O. oeni (UNQOe1101). The microorganisms were grown to stationary phase in a volume of 200 mL, in order to obtain the necessary biomass to inoculate a 50 L tank, and to reach a final concentration of the order of 106 CFU/mL. The cell pellet was washed and centrifuged at 5000 rpm for 10 min and suspended in a physiological solution (0.9% w/v sodium chloride). The resulting biomass was subjected to a new centrifugation (5000 rpm, 10 min). In order to preserve the viability and metabolic activity of the strains, the cells underwent a pre-adaptation process through a suspension in the acclimation medium (composition: 50 g/L of MRS, 40 g/L of D (-) fructose, 20 g/L of D (-) glucose, 4.5 g/L of malic acid, 1 g/L of Tween 80, 0.1 mg/L of pyridoxine, 6% ethanol v/v, pH 4.6) [17], for 48 h, prior to inoculation.
2.3.2. Fermentation Process
The pilot-scale vinification was conducted after the 2020 harvest (between March and April 2020, at the onset of the SARS-CoV-2 pandemic, when Argentina was under strict quarantine lockdown) at the Saldungaray winery.
Once the Malbec grapes were harvested, and after destemming, macerating, and crushing, 180 L of must was obtained and placed in a fermentation tank (FT) (Formingplast, Ranelagh, Buenos Aires, Argentina) to carry out the AF. The commercial alcoholic fermentation starter usually employed in the winery was added (20 g/hL) (Uvaferm BC®, Lallemand Inc., Montreal, QC, Canada, Saccharomyces cerevisiae cerevisiae ex bayanus). Sulfiting was performed during vatting at a concentration of 5 g/hL (Química Palumbo, Mendoza, Argentina), and after MLF at a concentration of 8 g/hL.
The progress of AF was monitored by measuring Baumé degrees, as an indirect estimate of sugar consumption. Once Baumé degrees decreased to zero, the wine was kept for 48 h. This is a common practice in wineries, ensuring that the consumption of residual sugar is complete. Then, the wine was devatted, and recovered by pressing (Table S1).
To evaluate the performance of the MLFS, at the end of AF, the FT was divided into two 50 L tanks (Formingplast, Ranelagh, Buenos Aires, Argentina). One of them was inoculated with the MLFS in order to carry out a guided MLF (FT-A), whereas in the other, MLF was performed spontaneously (FT-B). The evolution of the fermentation process during AF as well as during MLF, was controlled by collecting samples at different times (1, 5, 8, 9, 10, 13, 16, 20, 23, 26, and 29 days), and by analysing the viability of the inoculated LAB and the concentration of L-malic acid.
2.3.3. LAB Viability Control
During the AF phase, samples were collected on days 1, 5, 8, 9, and 10. Additionally, during the MLF phase, the samples were obtained from FT-A (guided MLF) and FT-B (spontaneous MLF) on days 13, 16, 20, 23, and 26. The survival of LAB during the fermentation process was assessed by viable count in triplicate on MRS and MLO media, using the seeding technique according to Corral et al. (2012) [18]. The plates were incubated aerobically at 28 °C, and counts were conducted at 48 h and 7 days to confirm the appearance of slow-growing colonies. Colonies obtained were analysed through catalase testing and Gram staining. The results were expressed in colony-forming units per millilitre (CFU/mL).
2.3.4. Implantation Capacity of the Inoculated Cultures
Twenty colonies were randomly selected from each MRS and MLO plate corresponding to FT-A. Colonies identified as catalase-negative, Gram-positive cocci or bacilli were chosen and preserved at −20 °C until analysis. Isolates were cultured in the respective medium (MRS or MLO, depending on the origin of the colonies). Total genomic DNA extraction was performed following Bravo-Ferrada et al. (2013) [14], quantified using a Nanodrop spectrophotometer (ND-1000, Thermo Scientific, Wilmington, NC, USA), and its integrity was verified through 1.0% (w/v) agarose gel electrophoresis.
The implantation capacity was analysed using RAPD-PCR according to Bravo-Ferrada et al. (2013) [14] with the M13 primer [15] based on the genomic DNA obtained. PCR products were separated by 2.0% (w/v) agarose gel electrophoresis, using a 100 bp ladder (Productos Bio-Lógicos, Bernal, Buenos Aires, Argentina).
The assessment of the percentage implantation capacity of the strains was carried out by comparing the RAPD profiles of each colony analysed with the RAPD profiles of each inoculated strain, based on the presence or absence of the same RAPD pattern. The threshold to consider that a strain was successfully implanted was that at least 60% of the profiles of the isolates should be identical to those of the strains present in the MLFS [19].
2.3.5. Evaluation of L-Malic and Acetic Acids During Malolactic Fermentation (MLF)
The concentration of L-malic acid in the samples corresponding to different stages of the MLF (days 10, 13, 20, and 29) from each FT (A: guided MLF and B: spontaneous MLF) was determined using an enzymatic kit (L-Malic acid, BioSystems, Barcelona, Spain) in quintuplicate. Additionally, the concentration of acetic acid at the end of the fermentation process (day 29) was determined using an enzymatic kit (Acetic-acid, BioSystems, Barcelona, Spain) for FT-A and FT-B.
Briefly, the conversion of both L-malic acid and acetic acid by specific enzymes is monitored directly by measuring the absorbance (344 and 340 nm, respectively) resulting from the generation of a by-product of the reaction (NADH):
The concentration of both L-malic acid and acetic acid was calculated by following the manufacturer’s instructions (BioSystems, Barcelona, Spain).
2.3.6. Analysis of Aromatic Profiles
To assess the difference in aromatic profiles among the obtained wines, the volatile compound content of the final wine samples from FT-A (guided MLF) and FT-B (spontaneous MLF) were analysed and quantified according to Brizuela et al. (2018) [20]. The analysis took place at the Centre for Research and Technical Assistance to the Industry—CIATI (Villa Regina, Río Negro, Argentina).
Different techniques were employed based on the nature of the compounds sought.
Headspace solid-phase microextraction combined with gas chromatography with flame ionisation (HS-SPME-GC-FID) was used to evaluate: Ethyl octanoate, benzyl acetate, 3-methyl-1-butanol, isobutyl acetate, n-pentanol, 2-furaldehyde, benzyl alcohol, ethyl acetate, ethyl decanoate, alpha-terpineol, 2-methyl-1-propanol, isoamyl acetate, nerol, diethyl succinate, geraniol, 2-phenylethyl acetate, 1-hexanol, limonene, ethyl butyrate, hexyl acetate, and ethyl hexanoate.
Headspace solid-phase microextraction combined with gas chromatography with mass spectrometry (HS-SPME-GC-MS) was used to evaluate the following: beta-damascenone, beta-ionone, alpha-ionone, ethyl propanoate, beta-pinene, t-2-hexenol, citronellol, terpinen-4-ol, eugenol, c-3-hexenol, alpha-pinene, guaiacol (2-methoxyphenol), 4-ethylphenol, 4-ethylguaiacol, ethyl 2-methylbutyrate, ethyl isovalerate, phenylethanol, 2-methyl-1-butanol, and butyl acetate.
2.4. Statistical Analysis
The bacterial counts and L-malic acid consumption through time were analysed by repeated measurements ANOVA (RM-ANOVA) and Tukey’s post hoc test (p < 0.05). Evaluation of the remaining L-malic acid at the end of AF and at the end of MLF was analysed by means of one-way ANOVA and Tukey’s post hoc test (p < 0.05). The remaining acetic acid and volatile compounds were analysed by t-test (p < 0.05). All statistical analyses were performed using Graphpad Prism 8.0.2 (© 1992–2019 GraphPad Software, Inc., San Diego, CA, USA).
3. Results and Discussion
3.1. Isolation, Identification and Typing of Indigenous Strains
The Saldungaray winery is located in the southwest region of the Province of Buenos Aires, an area considered resurgent for Argentine wine production, with vineyards approximately 20 years old. We have conducted studies on soil microbial diversity and the fermentative processes associated with the Malbec varietal from this winery since 2017. The results from Next-Generation Sequencing (NGS) studies revealed a low percentage relative abundance of LAB and a consistent presence of acetic acid bacteria (AAB) throughout the fermentation process. Additionally, MLF in these wines tends to be incomplete [11,12].
In this study, successive rounds of enrichment were conducted to obtain LAB isolates from must and Malbec 2019 wines, an approach selected due to the aforementioned low relative abundance of LAB. A total of 76 isolates were successfully obtained (24 from must, 13 from Alcoholic Fermentation, and 39 from Malolactic Fermentation), presumptively assigned as LAB. Typing through RAPD-PCR allowed the differentiation of nine unique strains, subsequently identified through 16S rRNA gene sequencing and comparison with the Genbank database (NCBI, NIH). The strains identified were three strains of Lactiplantibacillus plantarum (UNQLP1001, UNQLp1002, UNQLp1003), two of Oenococcus oeni (UNQOe1101 and UNQOe1102), two of Pediococcus sp. (UNQPsp1203 and UNQPsp1204), and two of Leuconostoc mesenteroides (UNQLm1201 and UNQLm1202) (Table S2 and Figure S1). Subsequent experiments were carried out with the O. oeni and Lpb. plantarum strains, as these species have shown better adaptation to the stressful conditions of wine and have exhibited the potential to positively contribute to the organoleptic characteristics of the final product [2,3,8,21,22].
The relative abundance of each strain was assessed in relation to the total isolates obtained. The O. oeni strains UNQOe1101 and UNQOe1102 accounted for 21.21% and 1.52%, respectively, and the Lpb. plantarum strains UNQLP1001, UNQLp1002, and UNQLp1003 accounted for 34.85%, 3.03%, and 1.52%, respectively. The high relative abundance of O. oeni UNQOe1101 and Lpb. plantarum UNQLP1001 suggested a greater tolerance to fermentation conditions and some adaptation to laboratory cultivation. Consequently, Lpb. plantarum UNQLP1001 and O. oeni UNQOe1101 were the strains selected to formulate an autochthonous malolactic starter (MLFS).
3.2. Pilot-Scale Winemaking
The winemaking process was carried out as described in Section 2.3.2, and its progression was monitored through analyses of the survival and implantation of the inoculated bacteria, consumption of L-malic acid, and final concentration of acetic acid.
3.2.1. Viability Control and Implantation of LAB
LAB counts in MLO were higher compared to those obtained in MRS (Figure 1), highlighting the former as the most suitable medium for monitoring LAB viability during the fermentation process. In the case of samples from the spontaneous MLF tank (FT-B), LAB colonies were only observed in MLO toward the end of the process (days 20, 23, and 26).
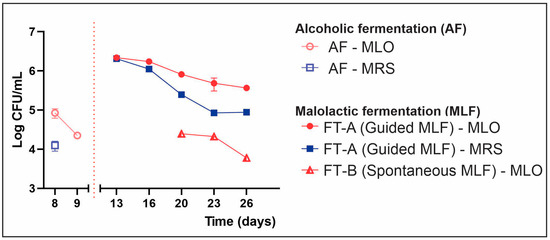
Figure 1.
Viable counts on MRS and MLO agar through alcoholic fermentation (AF) and malolactic fermentation (MLF). FT-A: guided MLF. FT-B: spontaneous MLF. Guided-FT was inoculated with autochthonous strains: Lpb. plantarum UNQLp1001 and O. oeni UNQOe1101. RM-ANOVA showed significant differences for treatments, time and interaction (in all cases, p < 0.00001).
By day 8 of the AF, native LAB counts, which probably come from raw material and work surfaces, were around 105 CFU/mL and rapidly declining. This decrease can be attributed to the addition of sulphite and yeast proliferation. After the completion of AF and following inoculation (FT-A), LAB counts around 106 CFU/mL were observed in the sample taken on day 13, gradually declining over time (Figure 1). This pattern aligns with observations made by other researchers in winemaking processes [20,23,24] and could be attributed to nutrient deficiency and adverse environmental conditions, such as a high ethanol concentration.
On the other hand, in samples from FT-B (spontaneous MLF), LABs were only found toward the end of fermentation (day 20), in the order of 104 CFU/mL (Figure 1). These values were similar to those found toward the end of AF, during which the LAB concentration declined due to increased ethanol (Table S1) and the presence of sulphite. The native LAB biota seems to reappear on day 20, when wine conditions become more favourable (decrease in sulphite, yeast lysis and release of growth factors, etc.), suggesting that the native microbial community needs to adapt to proliferate under these stressful conditions.
Moreover, it has been reported that the stressful conditions of wine can induce the state of “viable but non-culturable” (VBNC), at which bacteria are metabolically active but lose the ability to grow [25,26]. This could explain the absence of native LAB at the beginning of MLF in the FT-B, which was conducted spontaneously. Millet and Lonvaud-Funel [27] demonstrated that both AAB and LAB could be induced into the VBNC state by oxygen deprivation and inhibitory concentrations of sulphite, respectively. Once the stressful conditions in the environment are alleviated (oxygen restitution for AAB and reduction in sulphite concentration for LAB), bacteria can return to a viable state and proliferate. These findings may explain what was observed in this study, as the stressful conditions the LAB faced at the end of AF could have induced the VBNC state.
In contrast, in the FT-A, counts of the inoculated LAB were obtained from the beginning of MLF. This is because they underwent prior acclimation (incubation in a medium with a high content of glucose and fructose and sublethal concentrations of ethanol), which favours their viability and metabolic activity under the stressful conditions of wine. These adaptations are related to changes in the composition and properties of the bacterial plasmatic membrane, such as modifications in the fatty acid composition and protein profiles [17,28,29,30,31].
The high LAB counts recorded in FT-A, compared to the non-inoculated one (FT-B), provided an initial indication of survival by the inoculated strains under winemaking conditions. To confirm that the microorganisms recovered in the culture media were indeed the LAB inoculated in Malbec wine, their implantation capacity was analysed using RAPD-PCR with the M13 primer. The implantation percentage was calculated by relating the number of electrophoretic profiles identical to those of the inoculated strains (Lpb. plantarum UNQLP1001, and O. oeni UNQOe1101) to the total number of different profiles. Figure 2 shows that all electrophoretic patterns found in FT-A corresponded to Lpb. plantarum UNQLP1001 or O. oeni UNQOe1101, with no profiles different from those of the inoculated strains. This result confirms their implantation and demonstrates their adaptation capacity to the conditions of the wine from which they originated.
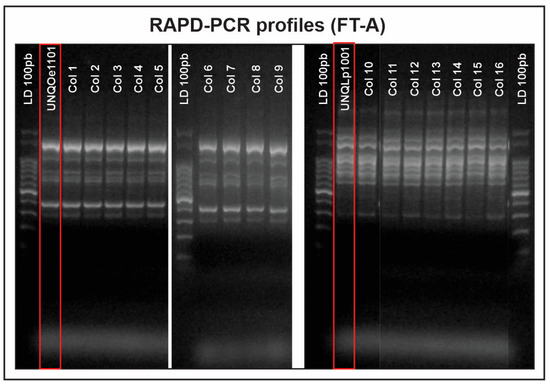
Figure 2.
Implantation analysis carried out by RAPD-PCR in Guided-MLF tank (FT-A), inoculated with Lpb. plantarum UNQLP1001 and O. oeni UNQOe1101. The electrophoretic profiles of the strains inoculated (marked in red in the figure) were compared with the profiles obtained for the isolates from the MLF. LD: Ladder. Col 1–16: number assigned to colonies growing in MRS or MLO.
3.2.2. Evaluation of L-Malic and Acetic Acids During MLF
To assess the performance of the inoculated strains as malolactic starters, in addition to survival and implantation, the consumption of L-malic acid over time was measured under each studied condition.
Figure 3A illustrates the evolution of L-malic acid consumption, starting from the initial must (1.88 g/L ± 0.16). Toward the end of AF (day 13), a consumption of L-malic acid of 39.89% was observed. This could be attributed to the well-known demalicating activity of the Saccharomyces cerevisiae Uvaferm BC® yeast (Lallemand Inc.), the AF starter used in the Saldungaray winery. Additionally, other possibly present non-Saccharomyces yeasts could contribute to L-malic acid consumption during AF [32,33], although it is worth mentioning that previous NGS studies had demonstrated an almost absolute dominance of S. cerevisiae in prior harvests from the winery under study [12].
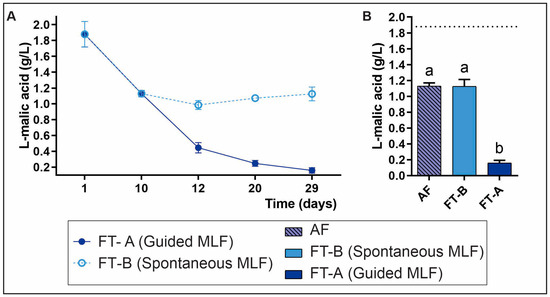
Figure 3.
Consumption of L-malic acid during the fermentation process (A). RM-ANOVA showed significant differences for treatments, time and interaction (in all cases, p < 0.00001). The evaluation of the remaining L-malic acid at the end of AF-guided MLF (inoculated with Lpb. plantarum UNQLp1001 and O. oeni UNQOe1101) and spontaneous MLF (B). Data were compared using one-way ANOVA followed by the Tukey test (p < 0.05). The dashed horizontal line (X = 1.88) represents the L-malic acid at the beginning of alcoholic fermentation (must). Letters a and b represent statistical differences.
Figure 3B shows the comparison between the consumption of L-malic acid at the end of AF and toward the end of MLF (day 29). FT-A exhibited a nearly total consumption of L-malic acid (91.49%), with a remaining concentration of 0.16 g/L. The L-malic acid consumed after AF represented 51.59% of the total consumption, showing significant differences (p < 0.05) compared to FT-B (spontaneous MLF), and the remaining L-malic acid at the end of AF. It can be considered that the MLF in FT-A was complete, given that in an optimal MLF, it is desirable for the L-malic acid concentration to reach values below 0.5 g/L by the end of the process [29,34]. It is worth noting that residual malic acid in FT-A had reached levels below 0.5 g/L at 48 h after MLFS inoculation (day 12 of the fermentation process) (Figure 3A), with residual enzymatic activity persisting until day 29 of the process when the L-malic acid concentration was close to 0. This outcome underscores the remarkable efficiency of these strains under the conditions assayed, which could allow a significant reduction in the time required to complete the MLF and, consequently, a decrease in costs associated with the process.
Additionally, the absence of L-malic acid consumption in FT-B (spontaneous MLF) suggests that the native bacterial community, revealed by viable counts in the uninoculated tank, was unable to conduct spontaneous MLF. It also confirms that the consumption of L-malic acid in the inoculated tank can be exclusively attributed to the metabolic activity of the added strains.
These results prove the implantation and ability to conduct a complete MLF of the strains Lpb. plantarum UNQLP1001 and O. oeni UNQOe1101, suggesting their better adaptation to this wine and fermentation conditions. On the other hand, the lack of success in the spontaneous MLF by native LAB (FT-B) could be attributed to the presence of inhibitory metabolites produced by yeasts, to which this native microbiota may be particularly sensitive. Other authors also found partial degradation of L-malic acid when studying the interaction between yeast and LAB, especially when LAB were subjected to inhibitory conditions caused by yeast metabolites [35]. Therefore, the success of the strains used in the MLFS to complete the MLF, could be related to the selection process, looking for the best-adapted ones and the concentration of the inoculum (107 CFU/mL).
When acetic acid production was evaluated at the end of MLF (day 29) (Figure 4), a baseline production of acetic acid, around 0.18 g/L, can be attributed to the presence of AAB, whose persistence during the fermentation process we have previously demonstrated [11,12]. AABs are concerning due to their potential detrimental capacity, as they can alter the volatile acidity of the wine by producing a significant amount of acetic acid. It is worth mentioning that relatively high concentrations of acetic acid have been reported in bottled wines from this winery. However, it did not exceed the 0.8 g/L limit established by the Argentine regulatory institute [36]. Surprisingly, FT-A showed an acetic acid concentration of 0.4 g/L, which was significantly higher than FT-B. This increase in acetic acid concentration could be attributed to the heterofermentative metabolism of O. oeni, which is capable of producing lactic acid and ethanol or lactic acid and acetic acid, depending on the redox potential of the environment. On the other hand, the species Lpb. plantarum has a homofermentative metabolism, not related to an increase in volatile acidity [8,37,38,39]. Further studies on the metabolism of O. oeni UNQOe1101 are needed to assess its acetic acid-producing capability in order to re-evaluate the inclusion of this specific strain in future MLFS.
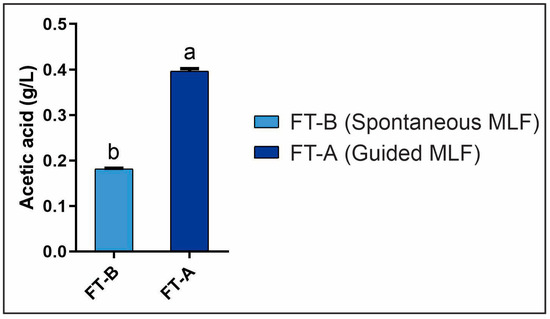
Figure 4.
Evaluation of the remaining acetic acid (day 29) in spontaneous MLF (FT-B), and in guided MLF (FT-A) inoculated with autochthonous strains: Lpb. plantarum UNQLp1001 and O. oeni UNQOe1101. Data were compared pairwise using t-test; different letters indicate significant differences (p < 0.05).
3.2.3. Analysis of the Aromatic Profiles of the Wines Obtained
The content of volatile compounds was analysed by HS-SPME-GC-MS/FID. Samples from FT-A and FT-B were examined at the end of the fermentation process (day 29).
The concentrations of 37 compounds were analysed, including 13 alcohols, 12 esters, 8 terpenes, and 4 aldehydes and ketones. Compounds exceeded the sensory threshold, meaning those whose aroma would be perceptible in the wines, included: 3-methylbutanol, phenethyl alcohol, isobutanol, 2-methylbutanol, 1-hexanol, 2-phenethyl acetate, ethyl acetate, isoamyl acetate, ethyl hexanoate, ethyl octanoate, ethyl butyrate, nerol, and β-damascenone (Figure 5). Meanwhile, other compounds were either below the sensory threshold established for each of them or were below the detection limit of the technique (Table S3).
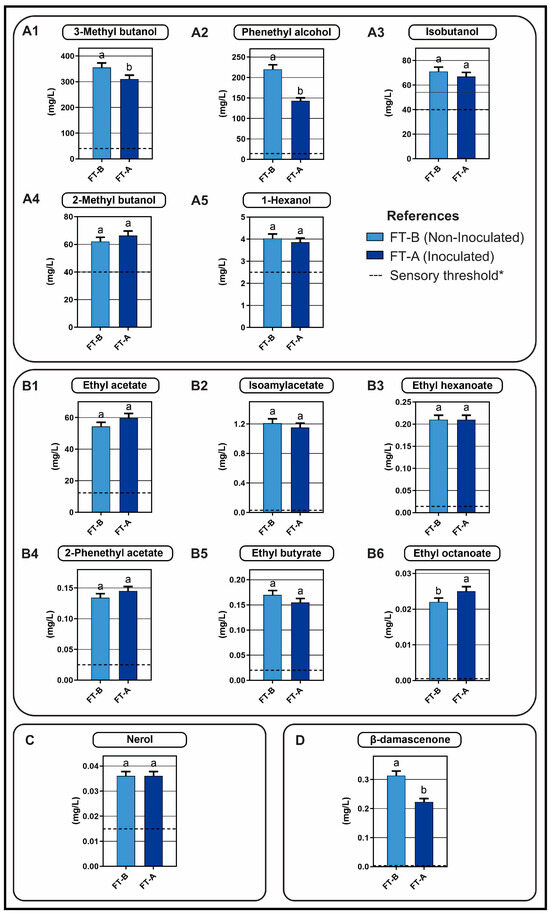
Figure 5.
Volatile compounds measured by HS-SPME-GC-FID/MS that exceeded the sensory threshold at the end of the fermentation process (day 29). The compounds were classified as alcohols (A1–A5), esters (B1–B6), terpenes (C), and aldehydes and ketones (D). Data were compared pairwise using t-test; different letters indicate significant differences (p < 0.05). * Sensory thresholds and attributes of the chemical components according to other authors [40,41,42,43,44,45]: (A1). Chocolate, malt, bitter; (A2). Sweet floral; (A3). Bitter; (A4). Malt; (A5). Freshly cut grass; (B1). Pineapple, fruity, balsamic solvent; (B2). Banana; (B3). Green apple; (B4). Fruity, floral, honeyed; (B5). Pineapple; (B6). Banana, sweet, pineapple; (C). Roses and thyme; (D). Blackberry, fruit.
Alcohols represented around 90% of the aromatic compounds found in each FT, esters accounted for 7–9%, and there was a content of less than 1% (and lower than 1 mg/L) of terpenes, aldehydes, ketones, and other volatile compounds (Table 1). In FT-A, we observed a slight increase in esters and a reduction in alcohols compared to the FT-B. Several authors have shown that the decrease in the content of C6 alcohols (herbaceous notes) and the increase in esters (fruity aroma) during MLF improve the taste of wine [20,40].

Table 1.
Relative abundance of aromatic compound types detected by GC-SM.
Higher alcohols or fusel alcohols (with more than two carbon atoms) constitute the major group of aromatic compounds found in alcoholic beverages and are by-products of amino acid fermentation by yeasts [46,47]. They are characterised by their strong and spicy smell and taste and can significantly influence the flavour and character of wine. A moderate content in wines, between 400 and 500 mg/L, contributes to its desirable complexity, while higher contents (close to 1 g/L) are considered negative [48]. These compounds are present in higher concentrations in red varietals compared to whites and are particularly important in grape distillates, where they are more concentrated [46]. Although none of the samples analysed reached these values, which were considered negative, FT-B showed a higher concentration than FT-A.
Previous studies on the aromatic profiles of Malbec wines using similar methodologies have focused on traditional wine regions in Argentina, such as the Cuyo region Provinces of Mendoza, San Juan, and La Rioja in the central west of Argentina. These studies have highlighted significant differences among wines, even when grouped by geographical proximity or climatic characteristics [49]. Goldner et al. (2009) [50] analysed the aromatic characteristics of 23 young, non-commercial Malbec wines from different wine-growing areas in Mendoza. They identified 17 volatile compounds using GC-MS and correlated the results with sensory profiling, characterising these wines with sensory attributes such as herbaceous, fruity, sweet/spicy, citrus, floral, and cooked/matured. Additionally, King et al. (2014) [49] conducted a comparison between Malbec varietals from typical wine-growing regions in Mendoza and California, USA. Using GC-MS, these authors identified 60 aromatic compounds and performed sensory analyses, concluding that the Malbec wines studied were characterised by aromas of red fruits, nuts, cooked vegetables, chocolate, earth/mushrooms, soy, oxidation, acidic taste, and astringent sensation in the mouth, regardless of the region of origin. Likewise, compounds exceeding sensory thresholds were detected in our study that could contribute to some of the mentioned attributes. Furthermore, King et al. (2014) [49] did not detect guaiacol, 4-ethyl guaiacol, geraniol, or furfural in any of the Malbec wines they studied, which agrees with our results, with the exception of geraniol, a compound that was detected in our fermentation tanks (FTs) but without exceeding the sensory threshold. Nerol (detected in concentrations exceeding the sensory threshold), like geraniol, are monoterpenes contributing positive notes of roses, citrus fruits, and/or thyme to the aroma [41]. In contrast, guaiacol, 4-ethyl guaiacol, and furfural are compounds that are sought to be minimised in wine. The first two are phenolic compounds related to negative or defective aromas, providing nuances of leather or animal (bretty flavours), often associated with the metabolism of non-Saccharomyces yeasts, such as Brettanomyces, especially during the ageing period [42]. Furanic compounds are formed through carbohydrate degradation and are associated with non-enzymatic browning reactions, such as the Maillard reaction, which involves sugar degradation in an acidic environment, leading to caramelisation, and these compounds are used as markers for thermal treatments in food [51]. The concentration of furanic compounds, such as furfural and hydroxymethylfurfural, is a matter of controversy, as, besides contributing unwanted caramel-like aromas [52], they have been shown to have carcinogenic activity in rats and mice. Therefore, the EFSA limited their concentration to a maximum of 25 mg/kg [53]. It is noteworthy that furfural remained undetectable in the Malbec wines analysed in the fermentation tanks.
In the present study, alcohols were the predominant compounds, with a decrease in FT-A, compared to the FT-B, (Table 1). A more detailed analysis, comparing the concentrations of individual compounds, reveals a statistically significant decrease in 3-methylbutanol, along with a reduction in phenethyl alcohol in FT-A compared with FT-B (Figure 5). No significant differences were detected between FTs in the concentrations of other alcohols (isobutanol, 2-methylbutanol, and hexanol).
It is also essential to remark that the winemaking processes were conducted in the presence of native microbiota, and the metabolism of these microorganisms could contribute to the development of the aromatic profile through synergistic/antagonistic interactions with the strains in the MLFS. Additionally, both fermentation processes (FT-A and FT-B) came from a controlled alcoholic fermentation achieved by adding a commercial Saccharomyces cerevisiae yeast starter, which also contributes to the aromatic profiles obtained.
Other compounds relevant to the wine aroma are esters generated through chemical esterification or direct synthesis [54], which can be formed in both alcoholic fermentation (produced by yeast) and malolactic fermentation (produced by LAB) [8,55]. In both FTs analysed, esters related to sweet and fruity aromas were found to exceed the sensory threshold and contribute desirable aromatic characteristics to the wine (isoamyl acetate, ethyl hexanoate, 2-phenethyl acetate, ethyl butyrate, and ethyl octanoate) [40,42,43]. The contribution of ethyl acetate to the wine aroma and flavour has been debated by various authors. Some indicate that it could contribute to suppressing the perception of other esters or consider it an off-flavour [43], while others classify it as a positive compound as long as its concentration does not exceed 200 mg/L [56,57], as is the case in this study where the concentration does not exceed that value but is above the sensory threshold.
The comparison of ester concentrations between FT-A and FT-B (Figure 5), showed that only the concentration of ethyl octanoate increased significantly in FT-A. Meanwhile, no statistically significant modifications were observed in the concentrations of the other esters that exceeded the threshold. These results align with reports from other authors regarding the ability of LAB to contribute to the wine ester content in a strain-specific manner [58,59].
Interestingly, although some esters were found in concentrations below the perception threshold (diethyl succinate, isobutyl acetate, ethyl 2-hexyl acetate, butyl acetate, and benzyl acetate), a synergistic effect between different esters or between esters and other compounds could occur, contributing to the modification of the wine aroma [60].
4. Conclusions
This study represents an initial approach to the use of autochthonous LAB strains from a re-emerging wine-growing region as malolactic fermentation starters, conducted through a pilot-scale trial in a relevant environment.
The native strains assessed here were able to successfully implant, achieving a faster and more efficient MLF compared to the spontaneous MLF assayed, and evaluated under identical conditions.
The volatile compounds analysed appear to have been influenced by the MLFS compared to the spontaneous MLF, showing variations in major components: a slight but significant decrease in the relative concentration of alcohols and an increase in esters. Previous research has related these aromatic compounds exceeding the sensory threshold in the Malbec wines with certain scents such as bitter, floral, fruity, and herbaceous notes. Further assays are needed to ascertain the degree in which these native strains change the aromatic profile of Malbec wines, as suggested here.
Enhancing the availability of candidate strains to formulate native MLFS from a region is a crucial technological tool aimed at improving the wine industry. Our findings support the employment of the strains Lpb. plantarum UNQLP1001 and O. oeni UNQOe1101 as potential MLFS in Malbec wines from somewhat similar wine-producing regions. Additionally, the Saldungaray winery can directly access a less expensive MLFS formulated with native LAB strains, allowing for more controlled MLF that preserves the regional typicity of their wines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11030140/s1, Table S1: Evolution of density (Baumé degrees) throughout the AF. Table S2: List of isolates from Malbec must and wine (Saldungaray, Buenos Aires, Argentina) and their corresponding GenBank Access Numbers. Figure S1: Phylogenetic tree generated using UPGMA with bootstrapping = 1000 (MEGA 12.0.9 © 1993-2025), comparing the partial sequences of the 16S rRNA gene of the strains obtained, and compared to the Lpb. plantarum NR_042394 sequence. Table S3: List of compounds analyzed by HS-SPME-GC-FID/MS, categorized based on whether they were detected or exceeded the sensory threshold.
Author Contributions
Conceptualization, L.D.; data curation, G.A.R. and L.D.; funding acquisition, L.C.S. and L.D.; investigation, G.A.R.; methodology, G.A.R., N.E.F. and N.S.B.; project administration, L.C.S. and L.D.; resources, L.C.S.; supervision, L.D.; writing—original draft, G.A.R.; writing—review and editing, G.A.R., A.C.G., L.C.S. and L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the Comisión de Investigaciones Científicas de la Provincia de Buenos Aires (CIC PBA; project PIT-AP-BA number 173/16), Universidad Nacional de Quilmes (Programa Microbiología Molecular Básica y Aplicada–Resolution (R) numbers 990/19, 797/20, 647/21, 918/22, 689/23), Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCyT; PICT-2021-GRFTI-00350, PICT 2019 N°0008, PICT 2021 Apli CatI 0013), and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP-11220200100394CO), Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Parra family, owners of the Saldungaray winery, for granting us access to their vineyards, and their staff, for their technical assistance in field sampling. Also, to the Directors of Flora and Fauna, Ministry of Agrarian Development, Government of the Province of Buenos Aires, for authorizing access to provincial genetic resources and their use for academic and research purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AAB | Acetic acid bacteria |
| AF | Alcoholic fermentation |
| FT | Fermentation tank |
| GC-MS | Gas Chromatography and Mass Spectrometry |
| LAB | Lactic acid bacteria |
| MLF | Malolactic fermentation |
| MLFS | Malolactic fermentation starter |
| NGS | Next-Generation Sequencing |
References
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Selection and Characterisation of Oenococcus oeni and Lactobacillus plantarum South African Wine Isolates for Use as Malolactic Fermentation Starter Cultures; Stellenbosch University: Stellenbosch, South Africa, 2011. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Stasinou, V.; Tzamourani, A.; Kotseridis, Y.; Dimopoulou, M. Malolactic fermentation—Theoretical advances and practical considerations. Fermentation 2022, 8, 521. [Google Scholar] [CrossRef]
- Renouf, V.; Vayssieres, L.C.; Claisse, O.; Lonvaud-Funel, A. Genetic and phenotypic evidence for two groups of Oenococcus oeni strains and their prevalence during winemaking. Appl. Microbiol. Biotechnol. 2009, 83, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Manera, C.; Olguin, N.T.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Delfederico, L.; Bibiloni, H.; Caballero, A.C.; Semorile, L.; La Hens, D.V. Survival and implantation of indigenous psychrotrophic Oenococcus oeni strains during malolactic fermentation in a Patagonian Pinot noir wine. LWT 2019, 108, 353–360. [Google Scholar] [CrossRef]
- Marcobal, Á.; Martín-Álvarez, P.J.; Polo, M.C.; Muñoz, R.; Moreno-Arribas, M. Formation of biogenic amines throughout the industrial manufacture of red wine. J. Food Prot. 2006, 69, 397–404. [Google Scholar] [CrossRef]
- Mira de Orduna, R.; Liu, S.Q.; Patchett, M.; Pilone, G. Kinetics of the arginine metabolism of malolactic wine lactic acid bacteria Lactobacillus buchneri CUC-3 and Oenococcus oeni Lo111. J. Appl. Microbiol. 2000, 89, 547–552. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Guilloux-Benatier, M.; Le Fur, Y.; Feuillat, M. Influence of fatty acids on the growth of wine microorganisms Saccharomyces cerevisiae and Oenococcus oeni. J. Ind. Microbiol. Biotechnol. 1998, 20, 144–149. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). State of the World Vitivinicultural Sector in 2019; OIV: Dijon, France, 2021. [Google Scholar]
- Rivas, G.A.; Guillade, A.C.; Semorile, L.C.; Delfederico, L. Influence of climate on soil and wine bacterial diversity on a vineyard in a non-traditional wine region in Argentina. Front. Microbiol. 2021, 12, 726384. [Google Scholar] [CrossRef]
- Rivas, G.A.; Semorile, L.; Delfederico, L. Microbial diversity of the soil, rhizosphere and wine from an emerging wine-producing region of Argentina. LWT 2022, 153, 112429. [Google Scholar] [CrossRef]
- Maicas, S.; González-Cabo, P.; Ferrer, S.; Pardo, I. Production of Oenococcus oeni biomass to induce malolactic fermentation in wine by control of pH and substrate addition. Biotechnol. Lett. 1999, 21, 349–353. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Delfederico, L.; Valdés La Hens, D.; Caballero, A.; Semorile, L. Patagonian red wines: Selection of Lactobacillus plantarum isolates as potential starter cultures for malolactic fermentation. World J. Microbiol. Biotechnol. 2013, 29, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Stenlid, J.; Karlsson, J.-O.; Högberg, N. Intraspecific genetic variation in Heterobasidion annosum revealed by amplification of minisatellite DNA. Mycol. Res. 1994, 98, 57–63. [Google Scholar] [CrossRef]
- Rodas, A.M.; Ferrer, S.; Pardo, I. 16S-ARDRA, a tool for identification of lactic acid bacteria isolated from grape must and wine. Syst. Appl. Microbiol. 2003, 26, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Semorile, L. Effect of acclimation medium on cell viability, membrane integrity and ability to consume malic acid in synthetic wine by oenological Lactobacillus plantarum strains. J. Appl. Microbiol. 2014, 116, 360–367. [Google Scholar] [CrossRef]
- Corral-Lugo, A.; Morales-García, Y.E.; Pazos-Rojas, L.A.; Ramírez-Valverde, A.; Martínez-Contreras, R.D.; Muñoz-Rojas, J. Cuantificación de bacterias cultivables mediante el método de” Goteo en Placa por Sellado (o estampado) Masivo”. Ann. Microbiol. 2012, 14, 147–156. [Google Scholar]
- Aymerich, T.; Martin, B.; Garriga, M.; Vidal-Carou, M.; Bover-Cid, S.; Hugas, M. Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J. Appl. Microbiol. 2006, 100, 40–49. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; Pozo-Bayón, M.Á.; Semorile, L.; Tymczyszyn, E.E. Changes in the volatile profile of Pinot noir wines caused by Patagonian Lactobacillus plantarum and Oenococcus oeni strains. Food Res. Int. 2018, 106, 22–28. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Borneman, A.R. Genomic variations of Oenococcus oeni strains and the potential to impact on malolactic fermentation and aroma compounds in wine. Appl. Microbiol. Biotechnol. 2011, 92, 441–447. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Franco-Luesma, E.; Bravo-Ferrada, B.M.; Pérez-Jiménez, M.; Semorile, L.; Tymczyszyn, E.E.; Pozo-Bayon, M. Influence of Patagonian Lactiplantibacillus plantarum and Oenococcus oeni strains on sensory perception of Pinot Noir wine after malolactic fermentation. Aust. J. Grape Wine Res. 2021, 27, 118–127. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Lavilla, M.; Amárita, F. Technological characterisation of potential malolactic starters from Rioja Alavesa winemaking region. LWT 2020, 134, 109916. [Google Scholar] [CrossRef]
- Capozzi, V.; Di Toro, M.R.; Grieco, F.; Michelotti, V.; Salma, M.; Lamontanara, A.; Russo, P.; Orrù, L.; Alexandre, H.; Spano, G. Viable But Not Culturable (VBNC) state of Brettanomyces bruxellensis in wine: New insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016, 59, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kioroglou, D. Analysis of Microbial Populations in Wines Through NGS Methodologies. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 2020. [Google Scholar]
- Millet, V.; Lonvaud-Funel, A. The viable but non-culturable state of wine micro-organisms during storage. Lett. Appl. Microbiol. 2000, 30, 136–141. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Brizuela, N.; Gerbino, E.; Gómez-Zavaglia, A.; Semorile, L.; Tymczyszyn, E.E. Effect of protective agents and previous acclimation on ethanol resistance of frozen and freeze-dried Lactobacillus plantarum strains. Cryobiology 2015, 71, 522–528. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Bravo-Ferrada, B.M.; La Hens, D.V.; Hollmann, A.; Delfederico, L.; Caballero, A.; Tymczyszyn, E.E.; Semorile, L. Comparative vinification assays with selected Patagonian strains of Oenococcus oeni and Lactobacillus plantarum. LWT 2017, 77, 348–355. [Google Scholar] [CrossRef]
- Chu-Ky, S.; Tourdot-Marechal, R.; Marechal, P.-A.; Guzzo, J. Combined cold, acid, ethanol shocks in Oenococcus oeni: Effects on membrane fluidity and cell viability. Biochim. Biophys. Acta (BBA) Biomembr. 2005, 1717, 118–124. [Google Scholar] [CrossRef]
- Da Silveira, M.G.; Golovina, E.A.; Hoekstra, F.A.; Rombouts, F.M.; Abee, T. Membrane fluidity adjustments in ethanol-stressed Oenococcus oeni cells. Appl. Environ. Microbiol. 2003, 69, 5826–5832. [Google Scholar] [CrossRef]
- van Wyk, N.; Scansani, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Pretorius, I.S.; Rauhut, D.; von Wallbrunn, C. The use of Hanseniaspora occidentalis in a sequential must inoculation to reduce the malic acid content of wine. Appl. Sci. 2022, 12, 6919. [Google Scholar] [CrossRef]
- Vicente, J.; Kelanne, N.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Yang, B.; Benito, S. Combined use of Schizosaccharomyces pombe and a Lachancea thermotolerans strain with a high malic acid consumption ability for wine production. Fermentation 2023, 9, 165. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Brizuela, N.; La Hens, D.V.; Tymczyszyn, E.; Semorile, L. Growth and consumption of L-malic acid in wine-like medium by acclimated and non-acclimated cultures of Patagonian Oenococcus oeni strains. Folia Microbiol. 2016, 61, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Ciani, M. The inhibitory activity of wine yeast starters on malolactic bacteria. Ann. Microbiol. 2007, 57, 61–66. [Google Scholar] [CrossRef]
- Instituto Nacional de Vitivinicultura (INV); Secretaría de Agricultura; Ganadería y Pesca; Ministerio de Economía. Legislación y Avisos Oficiales; RESOL-2018-2-APN-INV, MPYT12; INV: Mendoza, Argentina, 2021. [Google Scholar]
- Cibrario, A.; Peanne, C.; Lailheugue, M.; Campbell-Sills, H.; Dols-Lafargue, M. Carbohydrate metabolism in Oenococcus oeni: A genomic insight. BMC Genom. 2016, 17, 984. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Pretorius, N. Evaluation of Citrate Metabolism in Oenococcus oeni and Lactobacillus plantarum. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene content of wine from the aromatic grape variety ‘Irsai Oliver’ (Vitis vinifera L.) depends on maceration time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Santos, J.P.; Lozano, J.; Aleixandre, M.; Arroyo, T.; Cabellos, J.M.; Gil, M.; del Carmen Horrillo, M. Threshold detection of aromatic compounds in wine with an electronic nose and a human sensory panel. Talanta 2010, 80, 1899–1906. [Google Scholar] [CrossRef]
- Synos, K.; Reynolds, A.; Bowen, A. Effect of yeast strain on aroma compounds in Cabernet franc icewines. LWT Food Sci. Technol. 2015, 64, 227–235. [Google Scholar] [CrossRef]
- Rodriguez-Campos, J.; Escalona-Buendía, H.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and its importance to wine aroma. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Amerine, M.; Berg, H.; Kunkee, R.; Ough, C.; Singleton, V.; Webb, A. The Technology of Wine Making, 4th ed.; AVI Publishing Co.: Westport, CT, USA, 1980; pp. 50–75. [Google Scholar]
- Catania, C.; Avagnina, S. Curso Superior de Degustación de Vinos; INTA EEA: Mendoza, Argentina; INTA: Ciudad de Buenos Aires, Argentina, 2007. [Google Scholar]
- King, E.S.; Stoumen, M.; Buscema, F.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H.; Boulton, R.B. Regional sensory and chemical characteristics of Malbec wines from Mendoza and California. Food Chem. 2014, 143, 256–267. [Google Scholar] [CrossRef]
- Goldner, M.C.; Zamora, M.C.; Di Leo Lira, P.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.; Ferreira, A.; Cacho, J.; Marques, J. Evolution of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Res. Int. 2011, 44, 71–76. [Google Scholar] [CrossRef]
- Hale, M.D.; Mccafferty, K.; Larmie, E.; Newton, J.; Swan, J.S. The influence of oak seasoning and toasting parameters on the composition and quality of wine. Am. J. Enol. Vitic. 1999, 50, 495–502. [Google Scholar] [CrossRef]
- Delgado de la Torre, M.P.; Priego-Capote, F.; Luque de Castro, M.a.D. Evaluation of the composition of vine shoots and oak chips for oenological purposes by superheated liquid extraction and high-resolution liquid chromatography–time-of-flight/mass spectrometry analysis. J. Agric. Food Chem. 2012, 60, 3409–3417. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2024. [Google Scholar]
- Maarman, B.C. Interaction Between Wine Yeast and Malolactic Bacteria and the Impact on Wine Aroma and Flavour. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Guerrini, L.; Masella, P.; Angeloni, G.; Sacconi, A.; Calamai, L.; Parenti, A. Effects of a small increase in carbon dioxide pressure during fermentation on wine aroma. Foods 2020, 9, 1496. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, C.; Dubourdieu, D. Examples of perceptive interactions involved in specific “red-” and “black-berry” aromas in red wines. J. Agric. Food Chem. 2009, 57, 3702–3708. [Google Scholar] [CrossRef]
- Gammacurta, M.; Lytra, G.; Marchal, A.; Marchand, S.; Barbe, J.C.; Moine, V.; de Revel, G. Influence of lactic acid bacteria strains on ester concentrations in red wines: Specific impact on branched hydroxylated compounds. Food Chem. 2018, 239, 252–259. [Google Scholar] [CrossRef]
- Sumby, K.M.; Jiranek, V.; Grbin, P.R. Ester synthesis and hydrolysis in an aqueous environment, and strain specific changes during malolactic fermentation in wine with Oenococcus oeni. Food Chem. 2013, 141, 1673–1680. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.-C.; de Revel, G. Characterization of fruity aroma modifications in red wines during malolactic fermentation. J. Agric. Food Chem. 2012, 60, 12371–12383. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).