Construction of Efficient Multienzyme Cascade Reactions for D-Tagatose Biosynthesis from D-Fructose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strains, Plasmids and Primers
2.3. Gene Editing by CRISPR-Cas9
2.4. Expression and Purification of Enzymes
2.5. Whole-Cell Biotransformation and Determination of Catalytic Activity
2.6. Optimization of Whole-Cell Reaction Conditions
2.7. Analytical Approaches
3. Results and Discussion
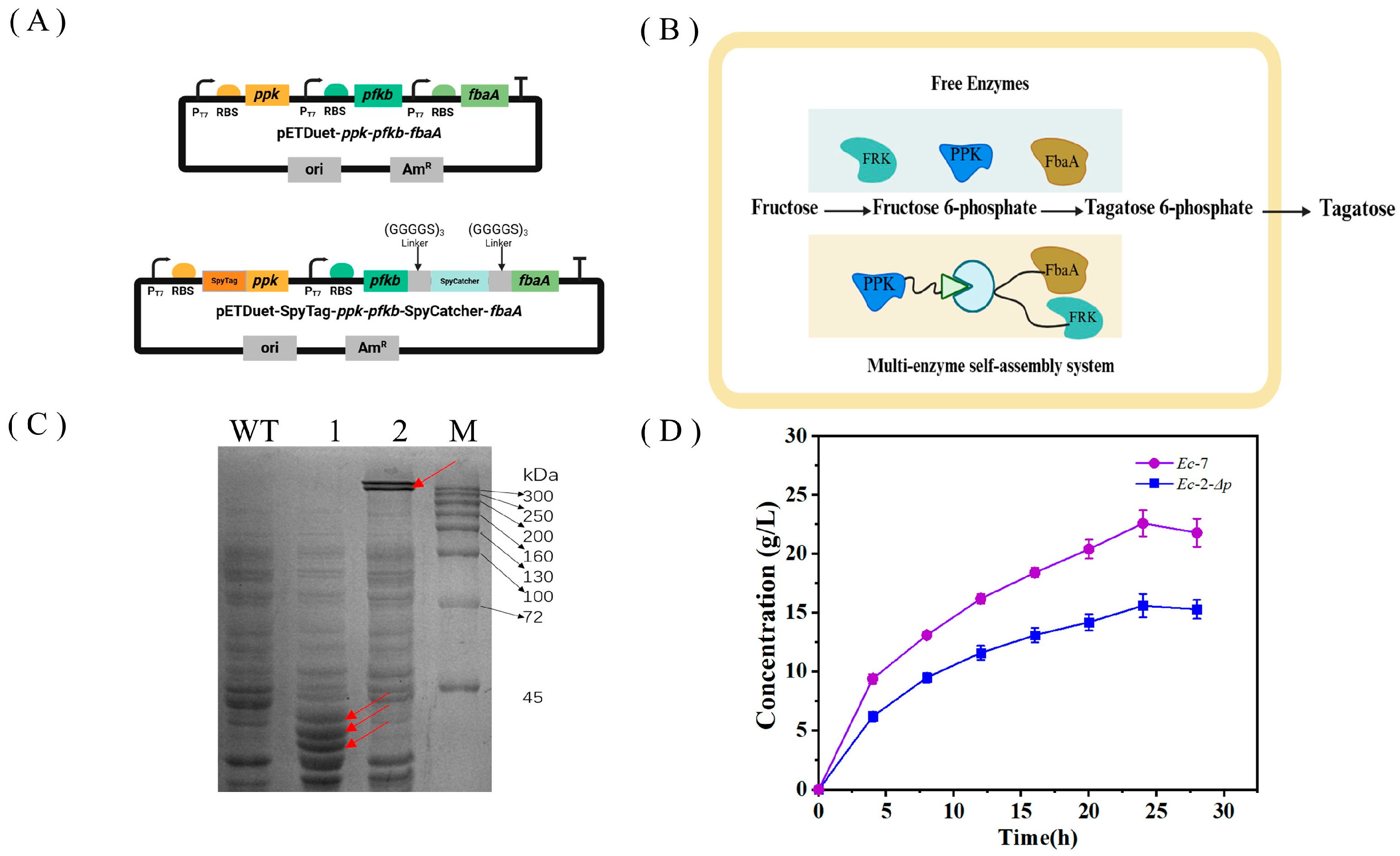
3.1. Construction of a Multienzyme Cascade Pathway for Producing D-Tagatose from D-Fructose
3.2. Optimizing D-Tagatose Production by Transcriptional Fine-Tuning
3.3. Block of the Alternative Pathway of Fructose-6-Phosphate to Enhance D-Tagatose Production
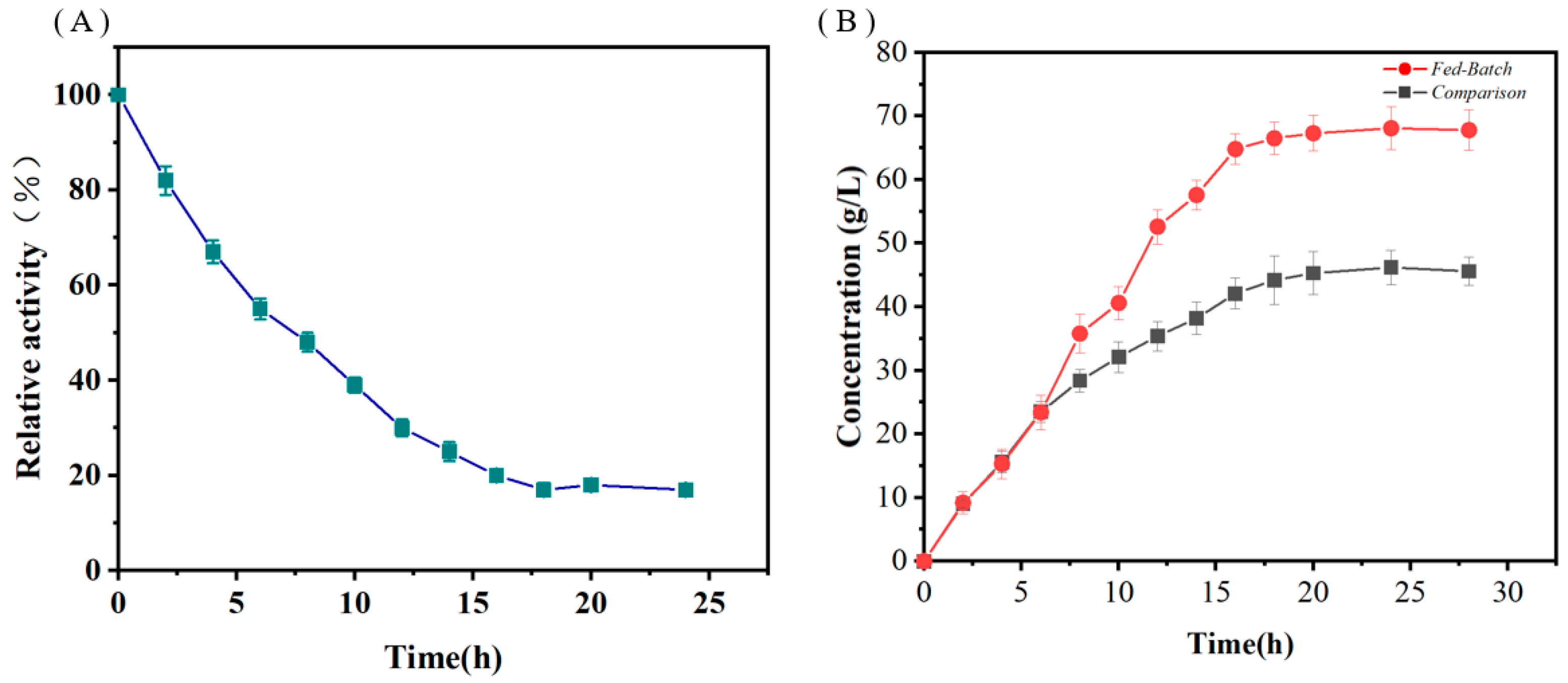
3.4. Effects of Biotransformation Conditions on Whole-Cell Catalytic Activity
3.5. Improving Whole-Cell Catalytic Efficiency by Constructing Multienzyme Complex
3.6. Improving D-Tagatose Production Using the Fed-Batch Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, P.; Wang, Q.; Ren, K.; Zhang, Z.; Xu, T.; Xu, M.; Zhang, X.; Rao, Z. Advances and Prospects of d-Tagatose Production Based on a Biocatalytic Isomerization Pathway. Catalysts 2023, 13, 1437. [Google Scholar] [CrossRef]
- Jayamuthunagai, J.; Gautam, P.; Srisowmeya, G.; Chakravarthy, M. Biocatalytic production of D-tagatose: A potential rare sugar with versatile applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zabed, H.M.; Yun, J.; Yuan, J.; Zhang, Y.; Wang, Y.; Qi, X. Two-stage biosynthesis of D-tagatose from milk whey powder by an engineered Escherichia coli strain expressing L-arabinose isomerase from Lactobacillus plantarum. Bioresour. Technol. 2020, 305, 123010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, Z.; Ma, M.; Zhao, G.; Chang, R.; Si, H.; Dai, M. A novel Lactococcus lactis l-arabinose isomerase for d-tagatose production from lactose. Food Biosci. 2022, 48, 101765. [Google Scholar] [CrossRef]
- Aburto, C.; Vera, C.; Arenas, F.; Illanes, A.; Guerrero, C.J. One-pot production of tagatose using l-arabinose isomerase from Thermotoga maritima and β-galactosidase from Aspergillus oryzae. LWT 2024, 194, 115787. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Z.; Tang, B.; Li, S.; Gao, J.; Chi, B.; Xu, H. Construction and co-expression of polycistronic plasmids encoding thermophilic L-arabinose isomerase and hyperthermophilic β-galactosidase for single-step production of D-tagatose. Biochem. Eng. J. 2016, 109, 28–34. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, J.; Liu, P.; Li, X.; Ouyang, J. Elegant and efficient biotransformation for dual production of D-tagatose and bioethanol from cheese whey powder. J. Agric. Food Chem. 2019, 67, 829–835. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, W.; Zhang, T.; Jiang, B.; Mu, W. L-arabinose isomerases: Characteristics, modification, and application. Trends Food Sci. Technol. 2018, 78, 25–33. [Google Scholar] [CrossRef]
- Lim, B.C.; Kim, H.J.; Oh, D.K. High production of D-tagatose by the addition of boric acid. Biotechnol Prog. 2007, 23, 824–828. [Google Scholar] [CrossRef]
- Shin, K.-C.; Lee, T.-E.; Seo, M.-J.; Kim, D.W.; Kang, L.-W.; Oh, D.-K. Development of tagaturonate 3-epimerase into tagatose 4-epimerase with a biocatalytic route from fructose to tagatose. ACS Catal. 2020, 10, 12212–12222. [Google Scholar] [CrossRef]
- Jeon, E.J.; Lee, Y.-M.; Choi, E.J.; Kim, S.-B.; Jeong, K.J. Production of tagatose by whole-cell bioconversion from fructose using corynebacterium glutamicum. Biotechnol. Bioprocess Eng. 2023, 28, 419–427. [Google Scholar] [CrossRef]
- Zhang, G.; An, Y.; Zabed, H.M.; Yun, J.; Parvez, A.; Zhao, M.; Zhang, C.; Ravikumar, Y.; Li, J.; Qi, X. Rewiring Bacillus subtilis and bioprocess optimization for oxidoreductive reaction-mediated biosynthesis of D-tagatose. Bioresour. Technol. 2023, 389, 129843. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Zhang, G.-C.; Kwak, S.; Oh, E.J.; Yun, E.J.; Chomvong, K.; Cate, J.H.D.; Jin, Y.-S. Overcoming the thermodynamic equilibrium of an isomerization reaction through oxidoreductive reactions for biotransformation. Nat. Commun. 2019, 10, 1356. [Google Scholar] [CrossRef] [PubMed]
- Sperl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar] [CrossRef]
- Sun, D.; Liu, X.; Zhu, M.; Chen, Y.; Li, C.; Cheng, X.; Zhu, Z.; Lu, F.; Qin, H.-M. Efficient biosynthesis of high-value succinic acid and 5-hydroxyleucine using a multienzyme cascade and whole-cell catalysis. J. Agric. Food Chem. 2019, 67, 12502–12510. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hong, S.-H.; Kim, K.-R.; Oh, D.-K. High-yield production of pure tagatose from fructose by a three-step enzymatic cascade reaction. Biotechnol. Lett. 2017, 39, 1141–1148. [Google Scholar] [CrossRef]
- Liu, S.; Tu, W.; Ni, Y.; Guo, Y.; Han, R. Novel In Vitro Multienzyme Cascade for Efficient Synthesis of d-Tagatose from Sucrose. Catalysts 2023, 13, 1515. [Google Scholar] [CrossRef]
- Han, P.; Wang, X.; Li, Y.; Wu, H.; Shi, T.; Shi, J. Synthesis of a healthy sweetener D-tagatose from starch catalyzed by semiartificial cell factories. J. Agric. Food Chem. 2023, 71, 3813–3820. [Google Scholar] [CrossRef]
- Dai, Y.; Li, M.; Jiang, B.; Zhang, T.; Chen, J. Whole-cell biosynthesis of d-tagatose from maltodextrin by engineered Escherichia coli with multi-enzyme co-expression system. Enzym. Microb. Technol. 2021, 145, 109747. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Wen, Q.; Miao, R.; Zhang, B.; Yan, Z.; Ying, H.; Liu, D.; Wang, J. Rational design of a novel halotolerant ATP regeneration system for biocatalytic CTP production. J. Chem. Technol. Biotechnol. 2023, 98, 1025–1031. [Google Scholar] [CrossRef]
- Du, Z.; Liu, Z.; Tan, Y.; Niu, K.; Guo, W.; Jia, Y.; Fang, X. Lacto-N-biose synthesis via a modular enzymatic cascade with ATP regeneration. iScience 2021, 24, 102236. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, T.; Wang, Z.; Li, H.; Wei, X.; Chen, J.; Niu, D.; Liu, J. Phosphorylation-driven production of D-Allulose from D-glucose by coupling with an ATP regeneration system. J. Agric. Food Chem. 2022, 70, 15539–15547. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, C.; Zheng, L.; Jiang, B.; Zhang, T.; Chen, J. Enhanced biosynthesis of d-tagatose from maltodextrin through modular pathway engineering of recombinant Escherichia coli. Biochem. Eng. J. 2022, 178, 108303. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Li, Q.; Chen, J.; Niu, D.; Liu, J. Reconstruction of a cofactor self-sufficient whole-cell biocatalyst system for efficient biosynthesis of allitol from D-glucose. J. Agric. Food Chem. 2022, 70, 3775–3784. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Zhang, Y.-H.; Ren, H.; Wang, Y.-L.; Jiang, W.; Fang, B.-S. Strategies and perspectives of assembling multi-enzyme systems. Crit. Rev. Biotechnol. 2017, 37, 1024–1037. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Zhong, X.; Ma, Y.; Zhang, X.; Zhang, J.; Wu, B.; Hollmann, F.; Wang, Y. More efficient enzymatic cascade reactions by spatially confining enzymes via the SpyTag/SpyCatcher technology. Mol. Catal. 2022, 521, 112188. [Google Scholar] [CrossRef]
- Chen, R.; Wan, L.; Zhu, Y.; Liu, Y.; Zhang, W.; Mu, W.J.B. Spatial organization of pathway enzymes via self-assembly to improve 2′-fucosyllactose biosynthesis in engineered Escherichia coli. Biotechnol. Bioeng. 2023, 120, 524–535. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Cheng, P.; Zhang, C.; Tang, L.; Du, L.; Li, J.; Ou, Z. Construction of bi-enzyme self-assembly clusters based on SpyCatcher/SpyTag for the efficient biosynthesis of (R)-Ethyl 2-hydroxy-4-phenylbutyrate. Biomolecules 2023, 13, 91. [Google Scholar] [CrossRef]
- Caescu, C.I.; Vidal, O.; Krzewinski, F.; Artenie, V.; Bouquelet, S.J. Bifidobacterium longum requires a fructokinase (Frk; ATP: D-fructose 6-phosphotransferase, EC 2.7. 1.4) for fructose catabolism. J. Bacteriol. 2004, 186, 6515–6525. [Google Scholar] [CrossRef]
- Gao, H.; Li, M.; Wang, Q.; Liu, T.; Zhang, X.; Yang, T.; Xu, M.; Rao, Z. A high-throughput dual system to screen polyphosphate kinase mutants for efficient ATP regeneration in L-theanine biocatalysis. Biotechnol. Biofuels Bioprod. 2023, 16, 122. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, M.; Fang, M.; Wang, Q.; Lu, R.; Zhang, H.; Xu, M.; Zhang, X.; Rao, Z. Heterologous Expression of Thermotolerant α-Glucosidase in Bacillus subtilis 168 and Improving Its Thermal Stability by Constructing Cyclized Proteins. Fermentation 2022, 8, 498. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.; Masone, B.S.; Ribbe, J.J.B.D.; Protocols, R. One-step purification of recombinant proteins with the 6xHis tag and Ni-NTA resin. Mol. Biotechnol. 1996, 4, 491–510. [Google Scholar]
- Saito, Y.; Akazawa-Ogawa, Y.; Matsumura, A.; Saigoh, K.; Itoh, S.; Sutou, K.; Kobayashi, M.; Mita, Y.; Shichiri, M.; Hisahara, S.; et al. Oxidation and interaction of DJ-1 with 20S proteasome in the erythrocytes of early stage Parkinson’s disease patients. Sci. Rep. 2016, 6, 30793. [Google Scholar] [CrossRef]
- Neville, N.; Roberge, N.; Jia, Z. Polyphosphate kinase 2 (PPK2) enzymes: Structure, function, and roles in bacterial physiology and virulence. Int. J. Mol. Sci. 2022, 23, 670. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Hong, S.-H.; An, J.-U.; Kim, K.-R.; Kim, D.-E.; Kang, L.-W.; Oh, D.-K. Structure-based prediction and identification of 4-epimerization activity of phosphate sugars in class II aldolases. Sci. Rep. 2017, 7, 1934. [Google Scholar] [CrossRef]
- Yoo, H.-W.; Jung, H.; Sarak, S.; Kim, Y.C.; Park, B.G.; Kim, B.-G.; Patil, M.D.; Yun, H. Multi-enzymatic cascade reactions with Escherichia coli-based modules for synthesizing various bioplastic monomers from fatty acid methyl esters. Green Chem. 2022, 24, 2222–2231. [Google Scholar] [CrossRef]
- Kim, D.; Sathesh-Prabu, C.; JooYeon, Y.; Lee, S.K. High-level production of 4-hydroxyvalerate from levulinic acid via whole-cell biotransformation decoupled from cell metabolism. J. Agric. Food Chem. 2019, 67, 10678–10684. [Google Scholar] [CrossRef]
- Nishizaki, T.; Tsuge, K.; Itaya, M.; Doi, N.; Yanagawa, H. Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl. Environ. Microbiol. 2007, 73, 1355–1361. [Google Scholar] [CrossRef]
- Parschat, K.; Schreiber, S.; Wartenberg, D.; Engels, B.; Jennewein, S.M. High-titer de novo biosynthesis of the predominant human milk oligosaccharide 2′-fucosyllactose from sucrose in Escherichia coli. ACS Synth. Biol. 2020, 9, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Tu, W.; Liu, S.; Ji, Y.; Schwaneberg, U.; Guo, Y.; Ni, Y. Novel multienzyme cascade for efficient synthesis of d-allulose from inexpensive sucrose. Food Biosci. 2023, 56, 103303. [Google Scholar] [CrossRef]
- Kulmer, S.T.; Gutmann, A.; Lemmerer, M.; Nidetzky, B. Biocatalytic cascade of polyphosphate kinase and sucrose synthase for synthesis of nucleotide-activated derivatives of glucose. Adv. Synth. Catal. 2017, 359, 292–301. [Google Scholar] [CrossRef]
- Iwamoto, S.; Motomura, K.; Shinoda, Y.; Urata, M.; Kato, J.; Takiguchi, N.; Ohtake, H.; Hirota, R.; Kuroda, A. Use of an Escherichia coli recombinant producing thermostable polyphosphate kinase as an ATP regenerator to produce fructose 1,6-diphosphate. Appl. Environ. Microbiol. 2007, 73, 5676–5678. [Google Scholar] [CrossRef]
- Aalbers, F.S.; Fraaije, M.W. Enzyme fusions in biocatalysis: Coupling reactions by pairing enzymes. ChemBioChem 2019, 20, 20–28. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Wang, H.; Xu, J.; Zhang, W. Efficient biosynthesis of high-value 5-Hydroxytryptophan using a multienzyme cascade. Mol. Catal. 2023, 546, 113274. [Google Scholar] [CrossRef]
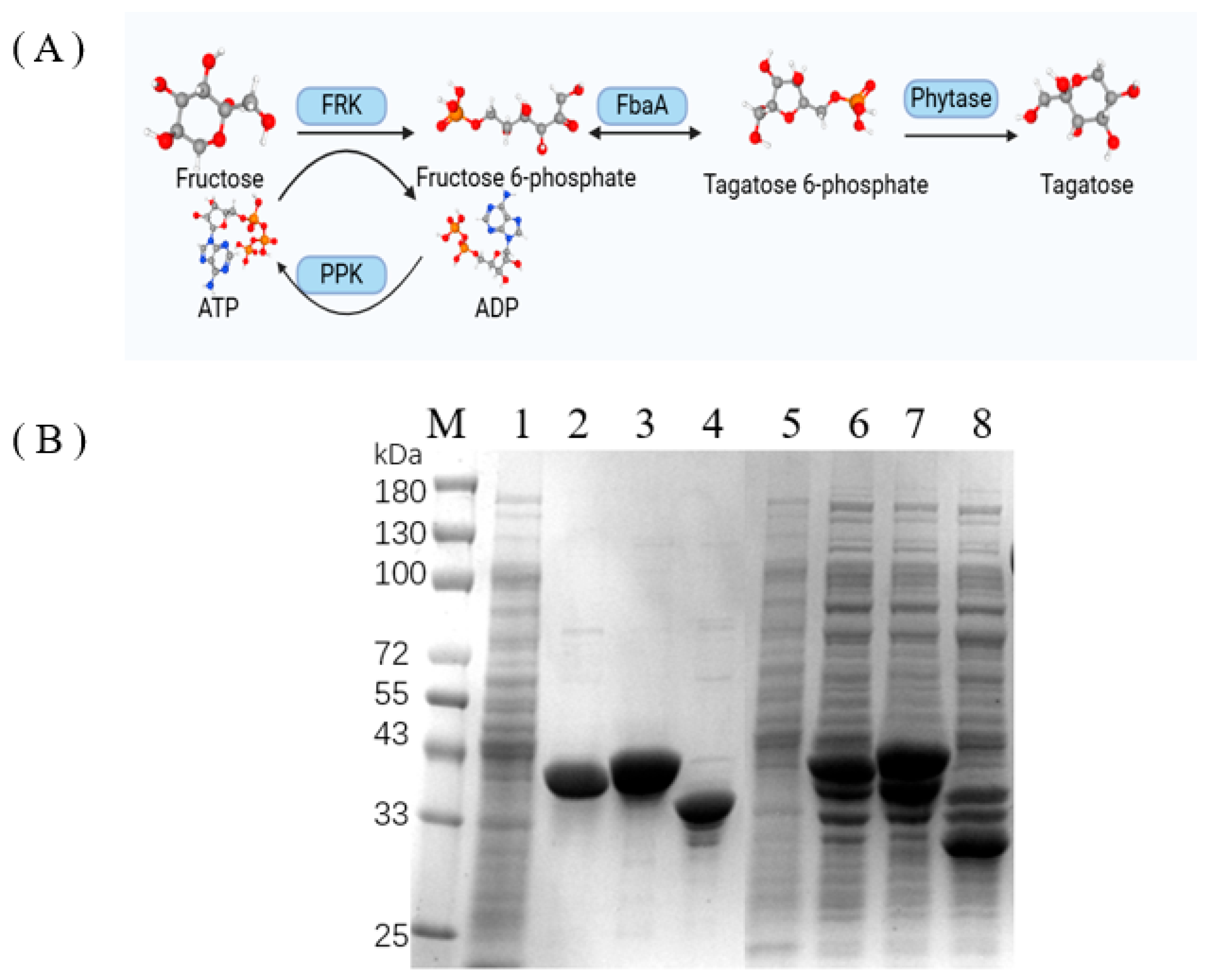
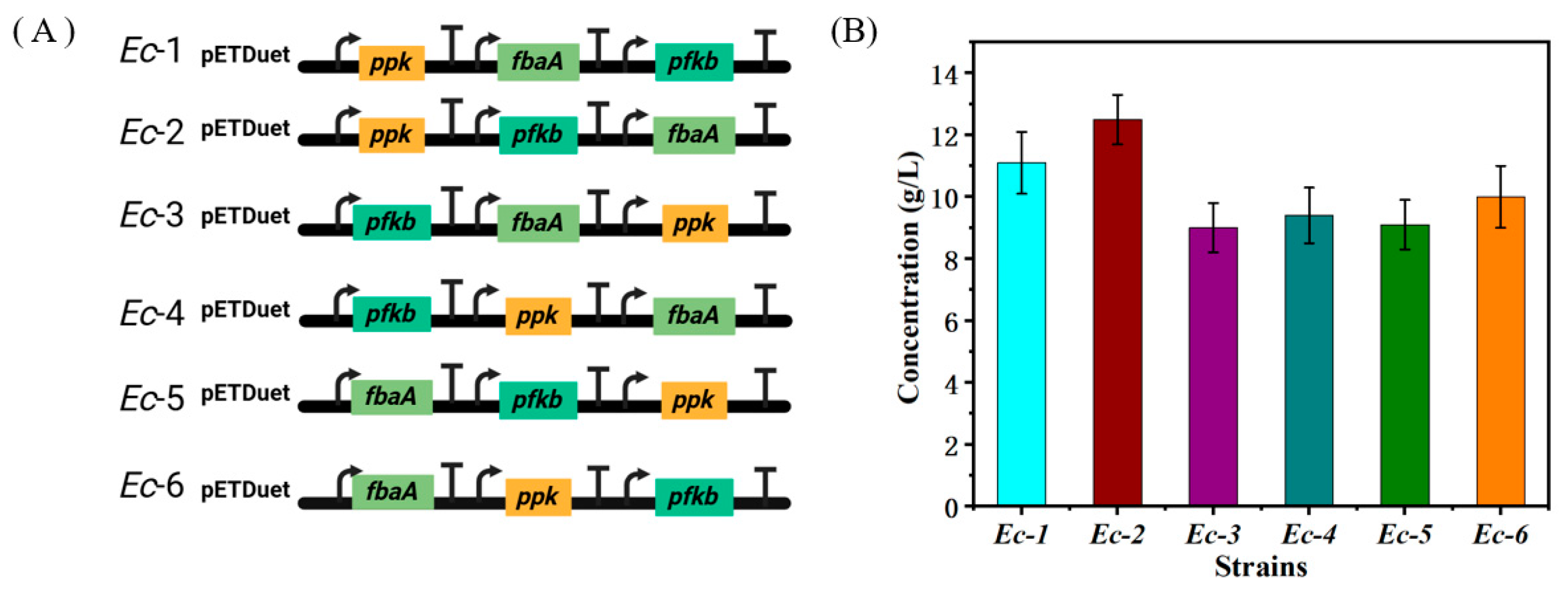
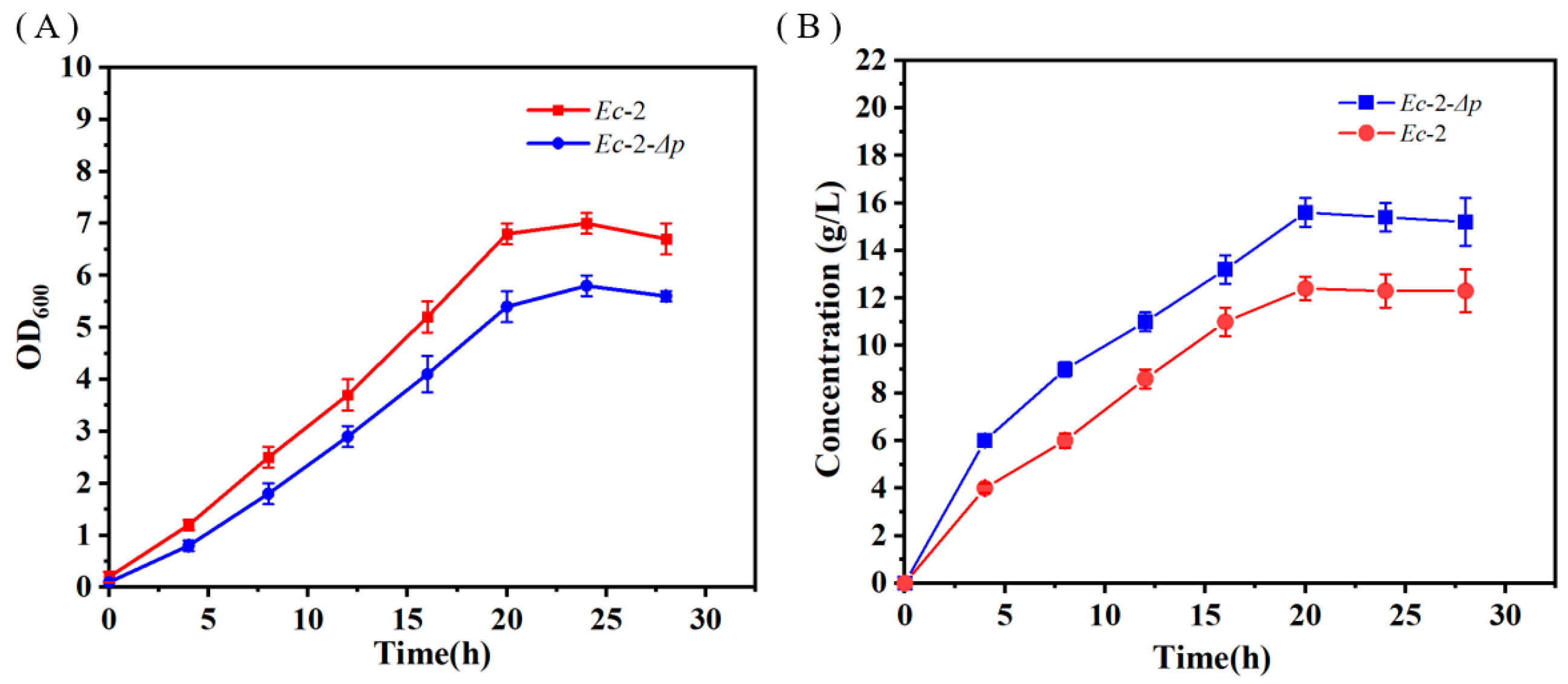
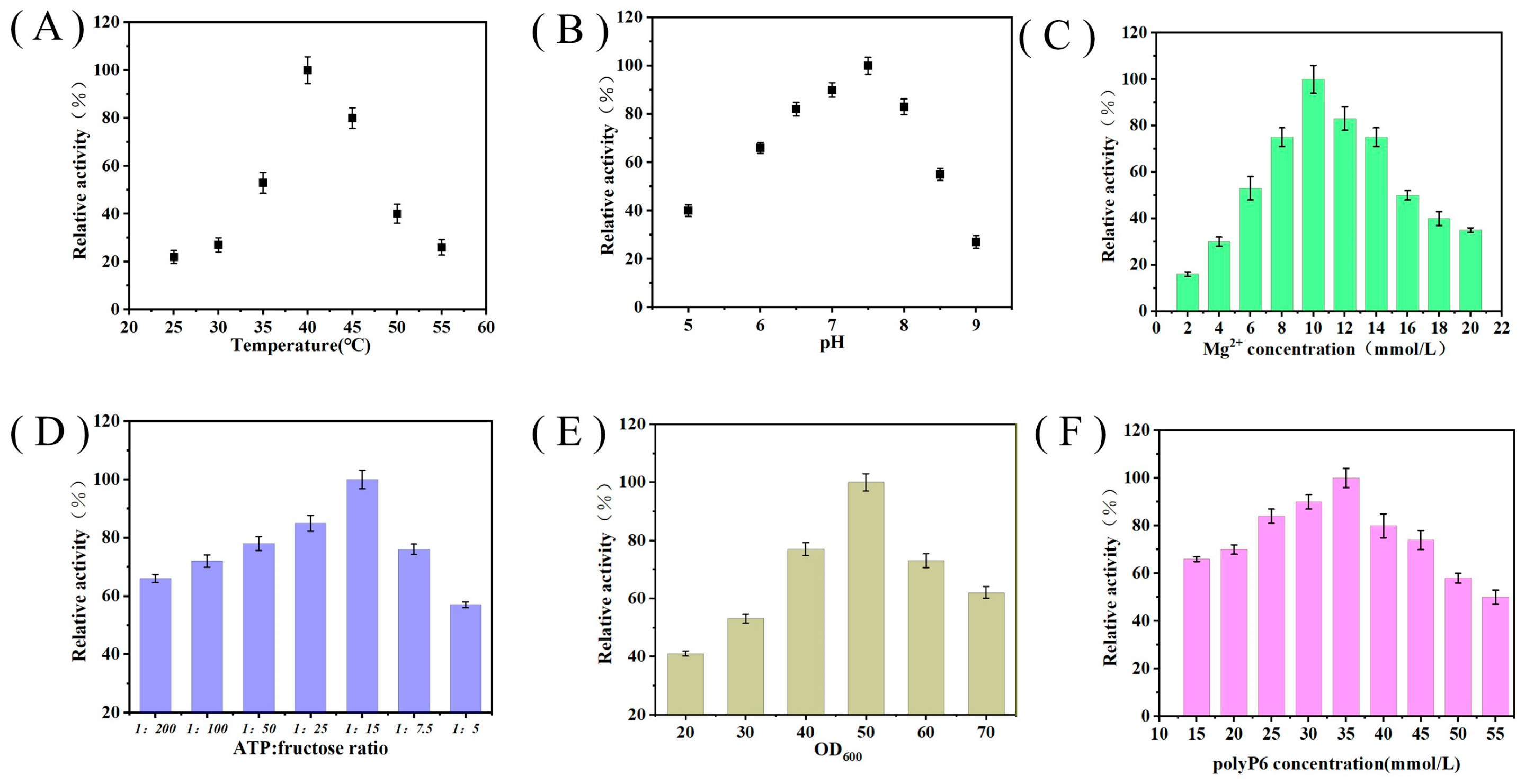
| Name | Description | Source |
|---|---|---|
| Strains | ||
| E. coli BL21(DE3) | The expressing host for recombinant protein | Lab stock |
| E. coli JM109 | General cloning host | Lab stock |
| Ec-pfkb | E. coli BL21 with plasmid pET28a-pfkb | This work |
| Ec-fbaA | E. coli BL21 with plasmid pET28a-fbaA | This work |
| Ec-ppk | E. coli BL21 with plasmid pET28a-ppk | This work |
| Ec-1 | E. coli BL21 with plasmid pETDuet-ppk-fbaA-pfkb | This work |
| Ec-2 | E. coli BL21 with plasmid pETDuet-ppk-pfkb-fbaA | This work |
| Ec-3 | E. coli BL21 with plasmid pETDuet-pfkb-fbaA-ppk | This work |
| Ec-4 | E. coli BL21 with plasmid pETDuet-pfkb-ppk-fbaA | This work |
| Ec-5 | E. coli BL21 with plasmid pETDuet-fbaA-pfkb-ppk | This work |
| Ec-6 | E. coli BL21 with plasmid pETDuet-fbaA-ppk-pfkb | This work |
| Ec-2-Δp | E. coli BL21 with plasmid pETDuet-ppk-pfkb-fbaA, Δpfka | This work |
| Ec-7 | E. coli BL21 with plasmid pETDuet-ppk-SpyTag-pfkb-SpyCatcher-fbaA, Δpfka | This work |
| Plasmids | ||
| pET28a | Kanr, lacIq, PT7, expression vector | Lab stock |
| pETDuet | Ampr, lacIq, PT7, expression vector | Lab stock |
| pET28a-pfkb | pET28a containing pfkb | This work |
| pET28a-fbaA | pET28a containing fbaA | This work |
| pET28a-ppk | pET28a containing ppk | This work |
| pETDuet-ppk-fbaA-pfkb | pETDuet containing ppk-fbaA-pfkb | This work |
| pETDuet-ppk-pfkb-fbaA | pETDuet containing ppk-pfkb-fbaA | This work |
| pETDuet-pfkb-fbaA-ppk | pETDuet containing pfkb-fbaA-ppk | This work |
| pETDuet-pfkb-ppk-fbaA | pETDuet containing pfkb-ppk-fbaA | This work |
| pETDuet-fbaA-pfkb-ppk | pETDuet containing fbaA-pfkb-ppk | This work |
| pETDuet-fbaA-ppk-pfkb | pETDuet containing fbaA-ppk-pfkb | This work |
| pETDuet-ppk-SpyTag-pfkb-SpyCatcher-fbaA | pETDuet containing ppk-SpyTag-pfkb-SpyCatcher-fbaA | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, P.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Hu, R.; Xu, M.; Rao, Z.; Zhang, X. Construction of Efficient Multienzyme Cascade Reactions for D-Tagatose Biosynthesis from D-Fructose. Fermentation 2025, 11, 139. https://doi.org/10.3390/fermentation11030139
Miao P, Wang Q, Ren K, Xu T, Zhang Z, Hu R, Xu M, Rao Z, Zhang X. Construction of Efficient Multienzyme Cascade Reactions for D-Tagatose Biosynthesis from D-Fructose. Fermentation. 2025; 11(3):139. https://doi.org/10.3390/fermentation11030139
Chicago/Turabian StyleMiao, Peiyu, Qiang Wang, Kexin Ren, Tongtong Xu, Zigang Zhang, Runxin Hu, Meijuan Xu, Zhiming Rao, and Xian Zhang. 2025. "Construction of Efficient Multienzyme Cascade Reactions for D-Tagatose Biosynthesis from D-Fructose" Fermentation 11, no. 3: 139. https://doi.org/10.3390/fermentation11030139
APA StyleMiao, P., Wang, Q., Ren, K., Xu, T., Zhang, Z., Hu, R., Xu, M., Rao, Z., & Zhang, X. (2025). Construction of Efficient Multienzyme Cascade Reactions for D-Tagatose Biosynthesis from D-Fructose. Fermentation, 11(3), 139. https://doi.org/10.3390/fermentation11030139








